Characterization of Sodium Alginate—Locust Bean Gum Films Reinforced with Daphnetin Emulsions for the Development of Active Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
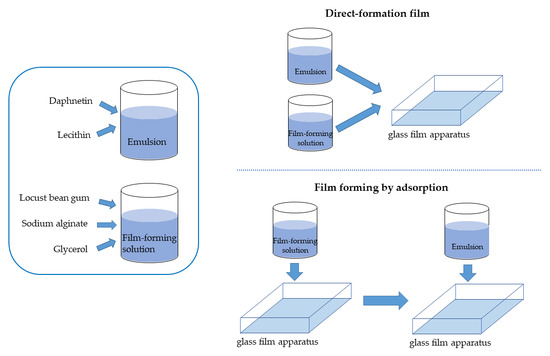
2.2. Preparation of Active Film
2.3. Thickness Detection
2.4. Color Difference
2.5. Measurement of Light Transmittance
2.6. Determination of Mechanical Properties
2.7. Determination of Oxygen Transmission Rate
2.8. Determination of Water Vapor Transmission Rate
2.9. Determination of Water Solubility
2.10. Determination of Antioxidant Activity
2.11. Determination of Bacterial Inhibition
2.12. Scanning Electron Microscopy (SEM)
2.13. Statistical Analysis
3. Results and Discussion
3.1. Mechanical Properties of Cling Film
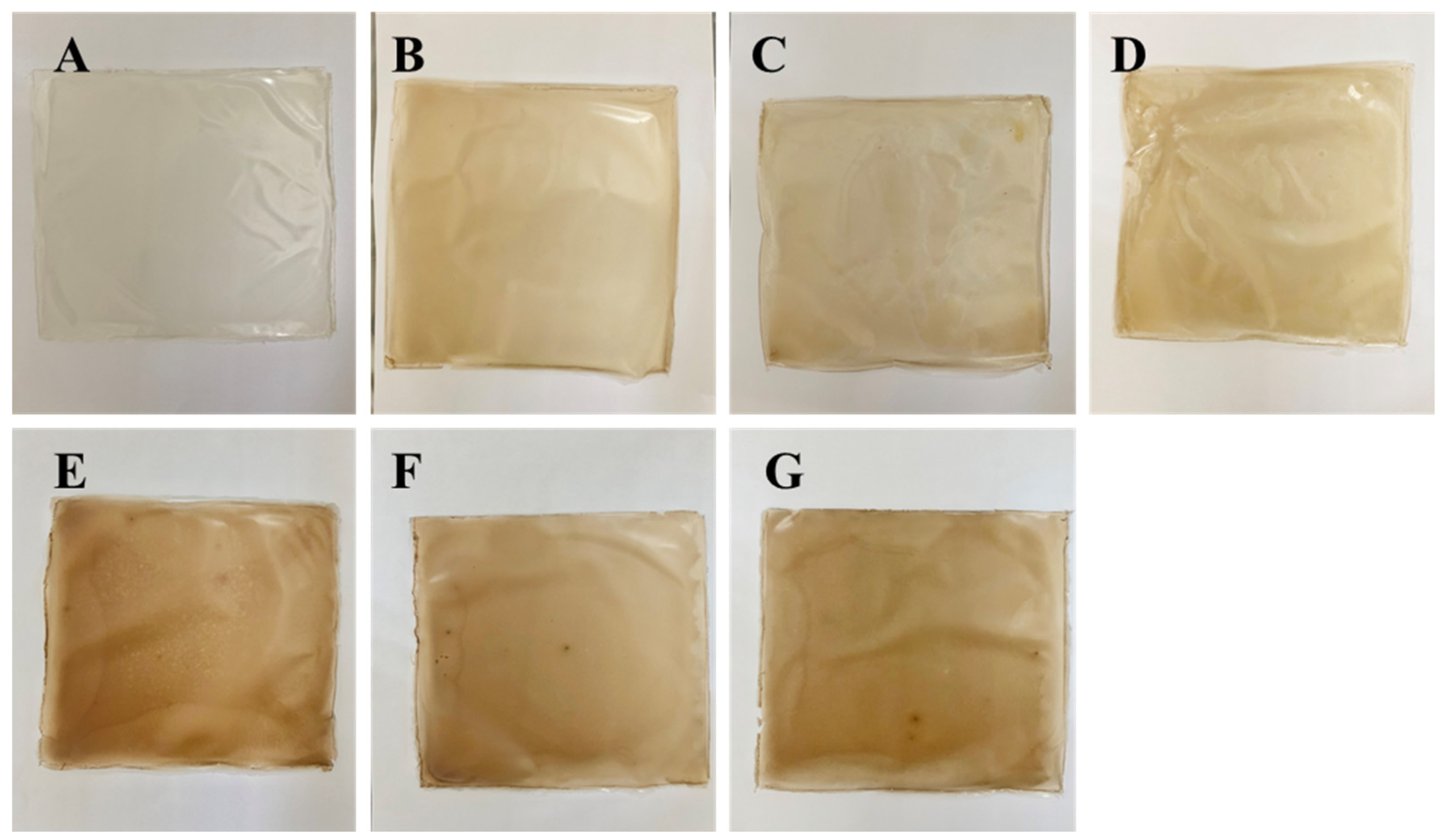
3.2. Optical Properties of Cling Film
3.3. Barrier Performance and Water Solubility
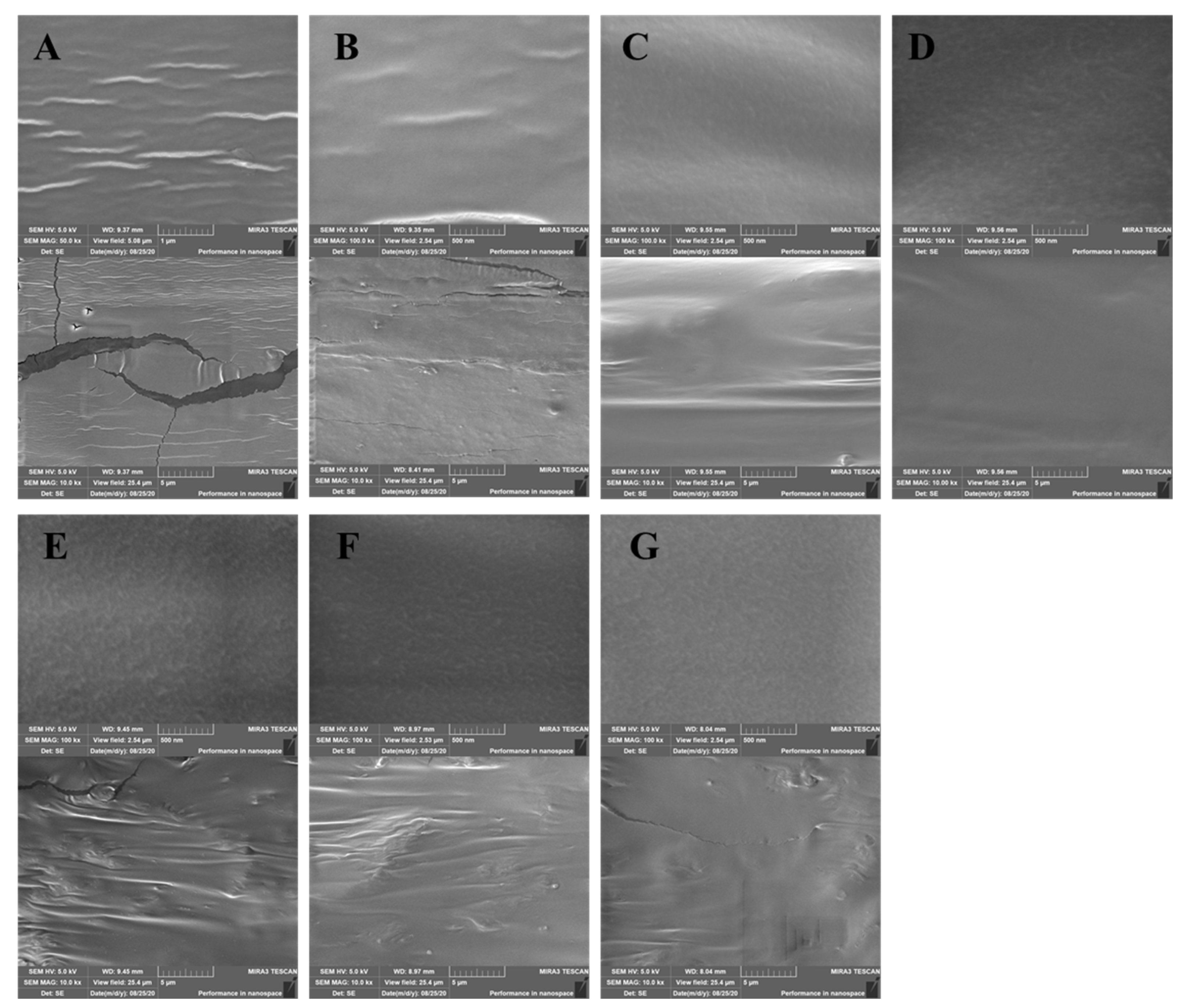
3.4. SEM Analysis
3.5. Antioxidant Activity
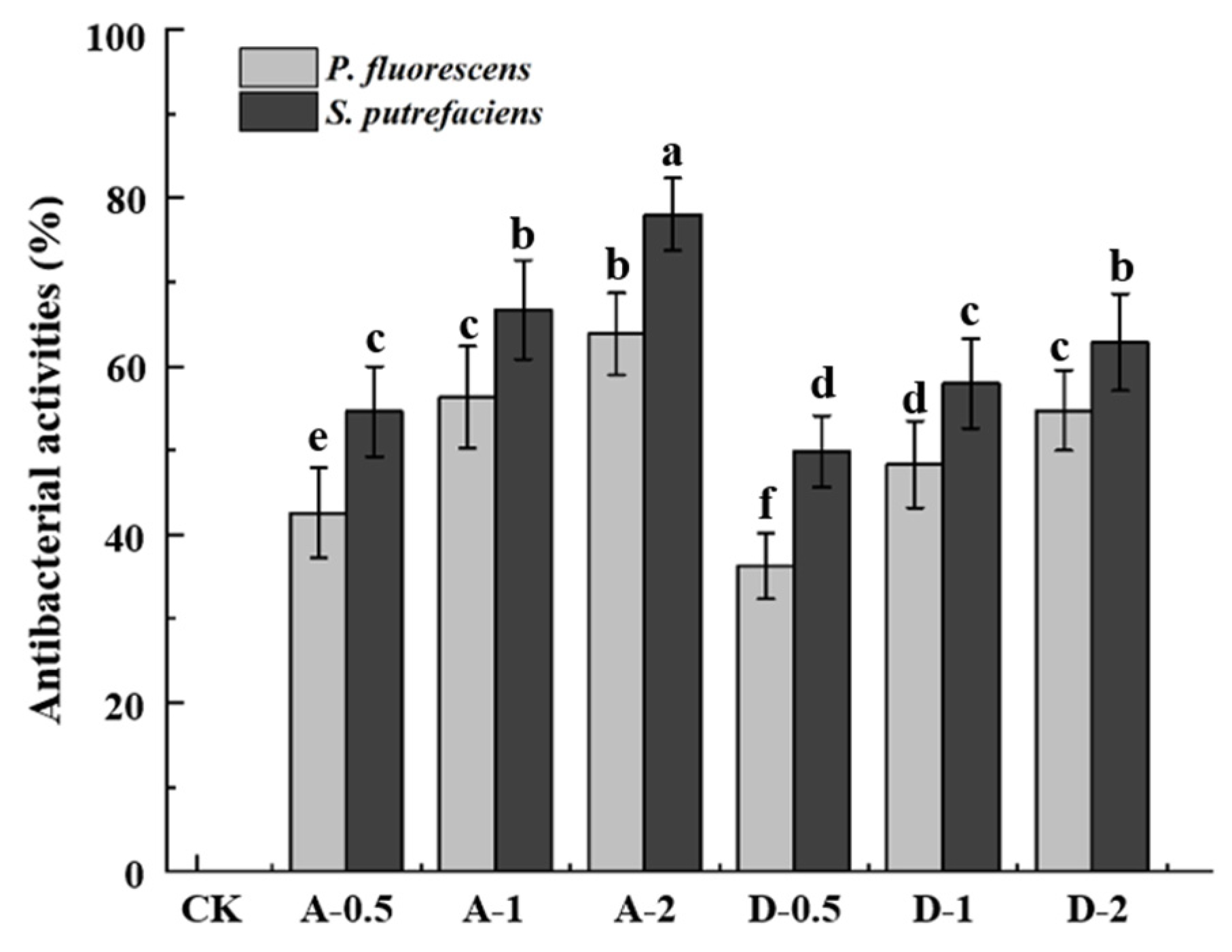
3.6. Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aga, M.B.; Dar, A.H.; Nayik, G.A.; Panesar, P.S.; Allai, F.; Khan, S.A.; Shams, R.; Kennedy, J.F.; Altaf, A. Recent insights into carrageenan-based bio-nanocomposite polymers in food applications: A review. Int. J. Biol. Macromol. 2021, 192, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.F.; Xu, T.; Gao, C.C.; Liu, X.Y.; Zhang, N.; Feng, X.; Liu, X.X.; Shen, X.C.; Tang, X.Z. Evaluations of physicochemical and biological properties of pullulan-based films incorporated with cinnamon essential oil and Tween 80. Int. J. Biol. Macromol. 2019, 122, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, A.; Hong, L.; Long, Z.; Brody, A.L.; Zhenxing, L.; Qazi, I.M.; Tushar, R.P.; Liangtao, L. A comprehensive review on the application of active packaging technologies to muscle foods. Food Control 2017, 82, 163–178. [Google Scholar] [CrossRef]
- Samira, D.; Vali Hosseini, S.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Costa, S.M.; Ferreira, D.P.; Teixeira, P.; Ballesteros, L.F.; Teixeira, J.A.; Fangueiro, R. Active natural-based films for food packaging applications: The combined effect of chitosan and nanocellulose. Int. J. Biol. Macromol. 2021, 177, 241–251. [Google Scholar] [CrossRef]
- Valdés, A.; Martínez, C.; Garrigos, M.C.; Jimenez, A. Multilayer Films Based on Poly(lactic acid)/Gelatin Supplemented with Cellulose Nanocrystals and Antioxidant Extract from Almond Shell By-Product and Its Application on Hass Avocado Preservation. Polymers 2021, 13, 3615. [Google Scholar] [CrossRef]
- Akamatsu, K.; Ide, Y.; Inabe, T.; Nakao, S. Preparation of Monodisperse Calcium Alginate Micro-/Nanospheres via Shirasu Porous Glass Membrane Emulsification Followed by Classification Using Microfiltration Membranes. Ind. Eng. Chem. Res. 2018, 57, 9465–9470. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Avena-Bustillos, R.J.; Wen-Xian, D.; Teofilo, R.F.; Soares, N.F.F.; McHugh, T.H. Optimal antimicrobial formulation and physical-mechanical properties of edible films based on acai and pectin for food preservation. Food Packag. Shelf Life 2014, 2, 38–49. [Google Scholar] [CrossRef]
- Bilal, H.; Shahid Chatha, S.A.; Ijaz Hussain, A.; Mahmood Zia, K.; Naseem, A. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Xiao, Q.; Gu, X.H.; Tan, S. Drying process of sodium alginate films studied by two-dimensional correlation ATR-FTIR spectroscopy. Food Chem. 2014, 164, 179–184. [Google Scholar] [CrossRef]
- Kandasamy, S.; Anbazhagan, S.; Anand Mariadoss, A.V.; Hu, X.; Myeong-Hyeon, W. Physical and bioactivities of biopolymeric films incorporated with cellulose, sodium alginate and copper oxide nanoparticles for food packaging application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef]
- Sen, F.; Uzunsoy, I.; Basturk, E.; Kahraman, M.V. Antimicrobial agent-free hybrid cationic starch/sodium alginate polyelectrolyte films for food packaging materials. Carbohydr. Polym. 2017, 170, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Chalitangkoon, J.; Wongkittisin, M.; Monvisade, P. Silver loaded hydroxyethylacryl chitosan/sodium alginate hydrogel films for controlled drug release wound dressings. Int. J. Biol. Macromol. 2020, 159, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Sen, K.K. Chitosan—Locust bean gum interpenetrating polymeric network nanocomposites for delivery of aceclofenac. Int. J. Biol. Macromol. 2017, 102, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Kaity, S.; Ghosh, A. Comparative bio-safety and in vivo evaluation of native or modified locust bean gum-PVA IPN microspheres. Int. J. Biol. Macromol. 2015, 72, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Wei, C.; Maoshen, C.; Feifei, X.; Jianguo, M.; Fang, Z. Film-forming properties of guar gum, tara gum and locust bean gum. Food Hydrocoll. 2020, 98, 105007. [Google Scholar] [CrossRef]
- Han, Y.; Yu, M.; Wang, L. Physical and antimicrobial properties of sodium alginate/carboxymethyl cellulose films incorporated with cinnamon essential oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuo, S.; He, L.; Cheng, C.; Zhu, B.; Lu, Y.; Wu, Q.; Shang, W.; Ge, W.; Shi, L. Daphnetin prevents methicillin-resistant Staphylococcus aureus infection by inducing autophagic response. Int. Immunopharmacol. 2019, 72, 195–203. [Google Scholar] [CrossRef]
- Lv, X.; Li, Y.; Xiao, Q.; Li, D. Daphnetin activates the Nrf2-dependent antioxidant response to prevent arsenic-induced oxidative insult in human lung epithelial cells. Chem.-Biol. Interact. 2019, 302, 93–100. [Google Scholar] [CrossRef]
- Liu, W.; Mei, J.; Xie, J. Effect of locust bean gum-sodium alginate coatings incorporated with daphnetin emulsions on the quality of Scophthalmus maximus at refrigerated condition. Int. J. Biol. Macromol. 2021, 170, 129–139. [Google Scholar] [CrossRef]
- Talón, E.; Lampi, A.-M.; Vargas, M.; Chiralt, A.; Jouppila, K.; González-Martínez, C. Encapsulation of eugenol by spray-drying using whey protein isolate or lecithin: Release kinetics, antioxidant and antimicrobial properties. Food Chem. 2019, 295, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Rubini, K.; Boanini, E.; Menichetti, A.; Bonvicini, F.; Gentilomi, G.A.; Montalti, M.; Bigi, A. Quercetin loaded gelatin films with modulated release and tailored anti-oxidant, mechanical and swelling properties. Food Hydrocoll. 2020, 109, 106089. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Sun, Y.; Wang, X.; Li, L. Effect of α-tocopherol antioxidant on rheological and physicochemical properties of chitosan/zein edible films. LWT 2020, 118, 108799. [Google Scholar] [CrossRef]
- Tang, Z.-P.; Chen, C.-W.; Xie, J. Development of antimicrobial active films based on poly(vinyl alcohol) containing nano-TiO2 and its application in macrobrachium rosenbergii packaging. J. Food Process. Preserv. 2018, 42, e13702. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Lopez-Sanchez, P.; Yang, X. Characterizations of bacterial cellulose nanofibers reinforced edible films based on konjac glucomannan. Int. J. Biol. Macromol. 2020, 145, 634–645. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Zheng, X.; Liu, S.; Lu, K.; Tang, K.; Liu, J. Heat sealable soluble soybean polysaccharide/gelatin blend edible films for food packaging applications. Food Packag. Shelf Life 2020, 24, 100485. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, S.; Lu, R.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Li, S.; Liu, Y. Preparation, characterization, and 3D printing verification of chitosan/halloysite nanotubes/tea polyphenol nanocomposite films. Int. J. Biol. Macromol. 2021, 166, 32–44. [Google Scholar] [CrossRef]
- Aguilar-Sanchez, R.; Munguia-Perez, R.; Reyes-Jurado, F.; Rhode Navarro-Cruz, A.; Soledad Cid-Perez, T.; Hernandez-Carranza, P.; del Carmen Beristain-Bauza, S.; Enrique Ochoa-Velasco, C.; Avila-Sosa, R. Structural, Physical, and Antifungal Characterization of Starch Edible Films Added with Nanocomposites and Mexican Oregano (Lippia berlandieri Schauer) Essential Oil. Molecules 2019, 24, 2340. [Google Scholar] [CrossRef] [Green Version]
- Wangprasertkul, J.; Siriwattanapong, R.; Harnkarnsujarit, N. Antifungal packaging of sorbate and benzoate incorporated biodegradable films for fresh noodles. Food Control 2021, 123, 107763. [Google Scholar] [CrossRef]
- Biao, Y.; Yuxuan, C.; Qi, T.; Ziqi, Y.; Yourong, Z.; McClements, D.J.; Chongjiang, C. Enhanced performance and functionality of active edible films by incorporating tea polyphenols into thin calcium alginate hydrogels. Food Hydrocoll. 2019, 97, 105197. [Google Scholar] [CrossRef]
- Pan, J.; Lian, H.; Jia, H.; Li, S.; Hao, R.; Wang, Y.; Zhang, X.; Dong, X. Ultrasound treatment modified the functional mode of gallic acid on properties of fish myofibrillar protein. Food Chem. 2020, 320, 126637. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Miao, J.; Wu, J.-L.; Chen, S.-F.; Zhang, Q.-Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Cheikh, D.; Martín-Sampedro, R.; Majdoub, H.; Darder, M. Alginate bionanocomposite films containing sepiolite modified with polyphenols from myrtle berries extract. Int. J. Biol. Macromol. 2020, 165, 2079–2088. [Google Scholar] [CrossRef]
- Chatkitanan, T.; Harnkarnsujarit, N. Effects of nitrite incorporated active films on quality of pork. Meat Sci. 2021, 172, 108367. [Google Scholar] [CrossRef] [PubMed]
- Leelaphiwat, P.; Pechprankan, C.; Siripho, P.; Bumbudsanpharoke, N.; Harnkarnsujarit, N. Effects of nisin and EDTA on morphology and properties of thermoplastic starch and PBAT biodegradable films for meat packaging. Food Chem. 2022, 369, 130956. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lei, Y.; Zhu, R.; Zhao, M.; Lu, J.; Xiao, D.; Jiao, C.; Zhang, Z.; Shen, G.; Li, S. Preparation and characterization of bioactive edible packaging films based on pomelo peel flours incorporating tea polyphenol. Food Hydrocoll. 2019, 90, 41–49. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Development of ultrasound treated polyvinyl alcohol/tea polyphenol composite films and their physicochemical properties. Ultrason. Sonochem. 2019, 51, 386–394. [Google Scholar] [CrossRef]
- Wongphan, P.; Khowthong, M.; Supatrawiporn, T.; Harnkarnsujarit, N. Novel edible starch films incorporating papain for meat tenderization. Food Packag. Shelf Life 2022, 31, 100787. [Google Scholar] [CrossRef]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and plasticization effects of carvacrol in biodegradable poly(lactic acid) and poly(butylene adipate terephthalate) blend films for bakery packaging. LWT 2021, 152, 112356. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Laorenza, Y.; Harnkarnsujarit, N. Carvacrol, citral and α-terpineol essential oil incorporated biodegradable films for functional active packaging of Pacific white shrimp. Food Chem. 2021, 363, 130252. [Google Scholar] [CrossRef] [PubMed]

| Samples | Thickness (mm) | TS (KN/m) | EAB (%) |
|---|---|---|---|
| CK | 0.101 ± 0.002 a | 0.76 ± 0.02 d | 8.9 ± 0.49 b |
| A-0.5 | 0.103 ± 0.003 a | 2.07 ± 0.09 a | 11.3 ± 0.53 b |
| A-1 | 0.101 ± 0.001 a | 1.81 ± 0.03 b | 10.4 ± 0.64 b |
| A-2 | 0.098 ± 0.001 a | 1.75 ± 0.05 b | 9.9 ± 0.57 b |
| D-0.5 | 0.113 ± 0.003 a | 1.31 ± 0.05 c | 18.7 ± 0.63 a |
| D-1 | 0.110 ± 0.003 a | 1.53 ± 0.02 c | 19.2 ± 0.56 a |
| D-2 | 0.112 ± 0.003 a | 1.40 ± 0.07 c | 17.2 ± 0.66 a |
| Samples | Brightness (L*) | Degree of Red-Green (a*) | Degree of Yellow-Blue (b*) | Total Color Difference (ΔE) | Transparency (mm−1) |
|---|---|---|---|---|---|
| CK | −1.93 ± 0.14 c | −0.85 ± 0.03 c | +7.23 ± 0.30 c | 28.36 ± 2.64 f | 1.03 ± 0.03 e |
| A-0.5 | −11.34 ± 0.37 b | +1.57 ± 0.11 b | +15.69 ± 0.11 b | 188.62 ± 6.78 e | 2.78 ± 0.14 c |
| A-1 | −13.70 ± 0.39 b | +1.53 ± 0.12 b | +16.15 ± 0.45 b | 225.43 ± 11.92 d | 2.02 ± 0.04 d |
| A-2 | −15.65 ± 0.42 b | +1.98 ± 0.08 b | +24.64 ± 0.76 a | 427.99 ± 17.43 c | 1.93 ± 0.11 d |
| D-0.5 | −23.92 ± 1.25 a | +5.76 ± 0.15 a | +25.13 ± 0.68 a | 618.43 ± 21.97 a | 5.04 ± 0.07 a |
| D-1 | −23.40 ± 1.24 a | +6.15 ± 0.21 a | +21.59 ± 0.74 a | 525.76 ± 22.24 b | 5.09 ± 0.11 a |
| D-2 | −24.48 ± 1.27 a | +6.13 ± 0.19 a | +23.63 ± 0.83 a | 597.61 ± 22.51 a | 4.17 ± 0.12 b |
| Samples | Water Vapor Permeability (g·mm/m2·kPa·h) | Oxygen Permeability (g/m2·h) | Water Solubility (%) |
|---|---|---|---|
| CK | 11.20 ± 0.43 e | 8.11 ± 0.40 c | 0.349 ± 0.02 a |
| A-0.5 | 13.83 ± 0.47 c | 8.41 ± 0.33 b | 0.287 ± 0.00 b |
| A-1 | 14.24 ± 0.58 bc | 8.22 ± 0.43 b | 0.274 ± 0.02 b |
| A-2 | 14.72 ± 0.52 ab | 7.93 ± 0.19 d | 0.285 ± 0.04 b |
| D-0.5 | 11.46 ± 0.51 e | 8.68 ± 0.35 a | 0.284 ± 0.01 b |
| D-1 | 12.83 ± 0.41 d | 8.65 ± 0.12 a | 0.274 ± 0.02 b |
| D-2 | 15.25 ± 0.49 a | 8.30 ± 0.17 b | 0.213 ± 0.02 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Cao, J.; Liu, W.; Mei, J.; Xie, J. Characterization of Sodium Alginate—Locust Bean Gum Films Reinforced with Daphnetin Emulsions for the Development of Active Packaging. Polymers 2022, 14, 731. https://doi.org/10.3390/polym14040731
Cheng H, Cao J, Liu W, Mei J, Xie J. Characterization of Sodium Alginate—Locust Bean Gum Films Reinforced with Daphnetin Emulsions for the Development of Active Packaging. Polymers. 2022; 14(4):731. https://doi.org/10.3390/polym14040731
Chicago/Turabian StyleCheng, Hao, Jie Cao, Wenru Liu, Jun Mei, and Jing Xie. 2022. "Characterization of Sodium Alginate—Locust Bean Gum Films Reinforced with Daphnetin Emulsions for the Development of Active Packaging" Polymers 14, no. 4: 731. https://doi.org/10.3390/polym14040731
APA StyleCheng, H., Cao, J., Liu, W., Mei, J., & Xie, J. (2022). Characterization of Sodium Alginate—Locust Bean Gum Films Reinforced with Daphnetin Emulsions for the Development of Active Packaging. Polymers, 14(4), 731. https://doi.org/10.3390/polym14040731