Luminescent Coatings Based on (3-Aminopropyl)triethoxysilane and Europium Complex β-Diketophosphazene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Compound 1
2.3. Synthesis of Compound 2
2.4. Synthesis of Compound 3
2.5. Coating Preparation for Testing Adhesion, Hydrophobicity and Luminescence Properties
2.6. Preparation of Samples for IR Spectroscopy, TGA and DSC
2.7. Synthesis of Compound 4
2.8. Methods
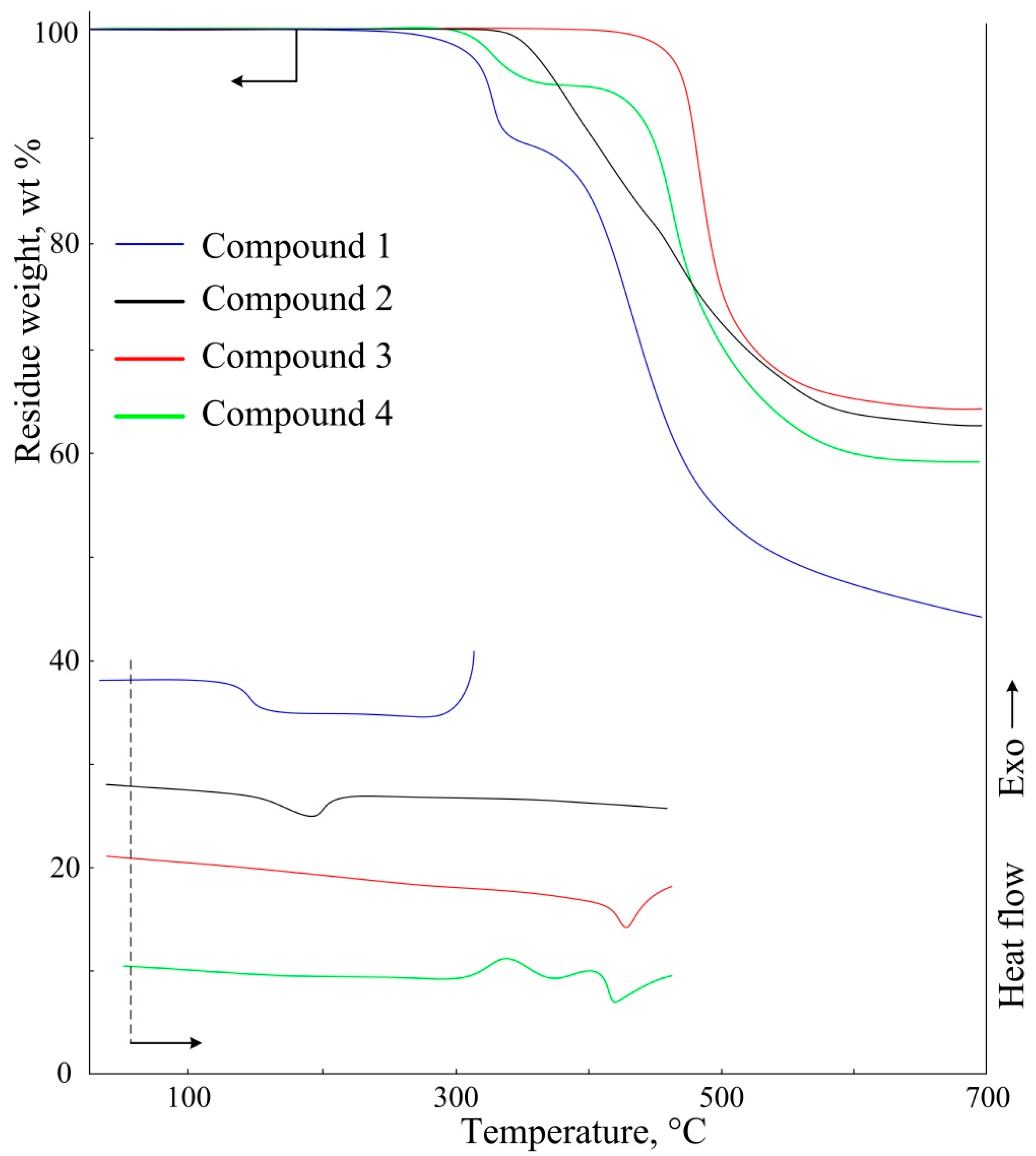
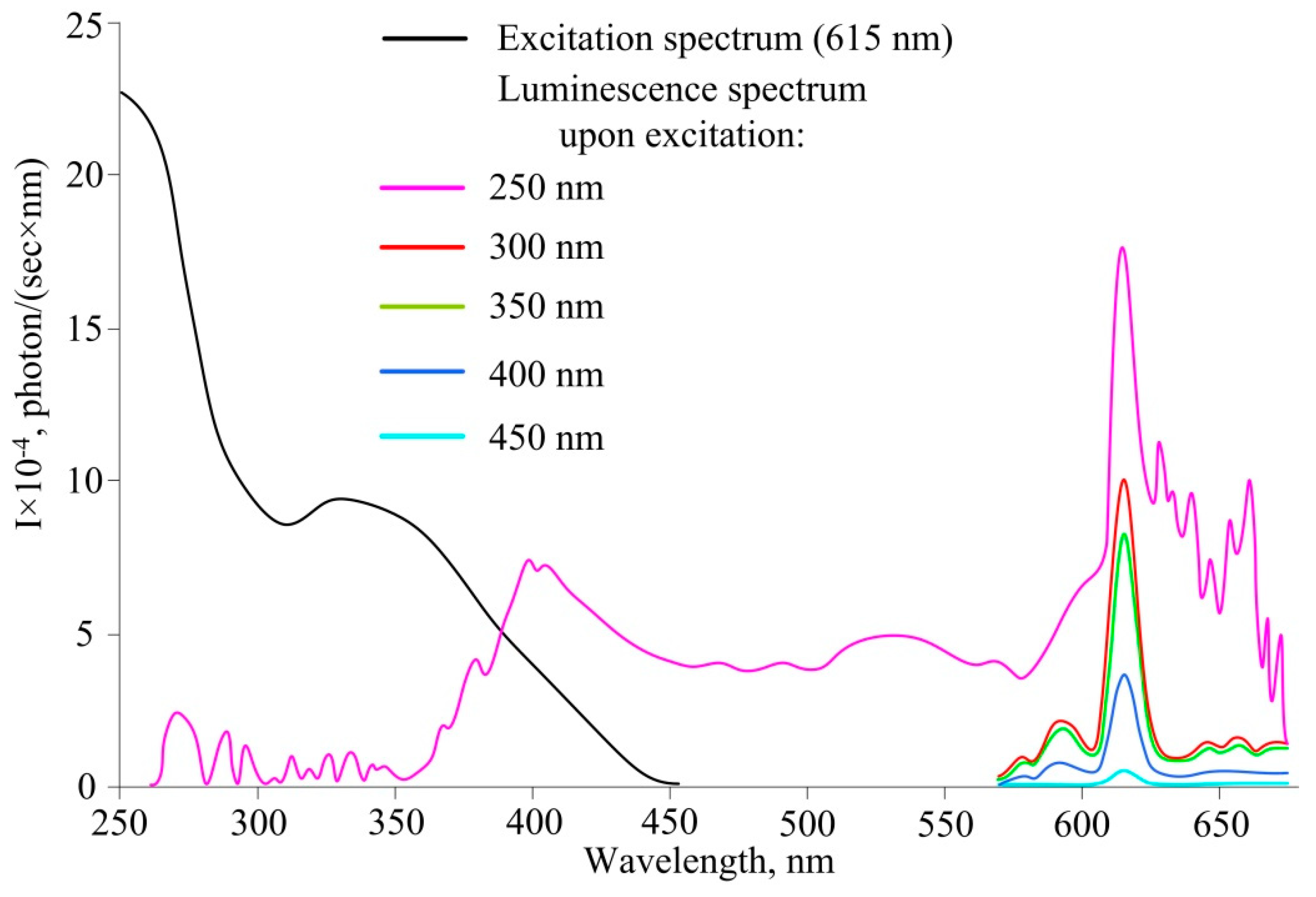
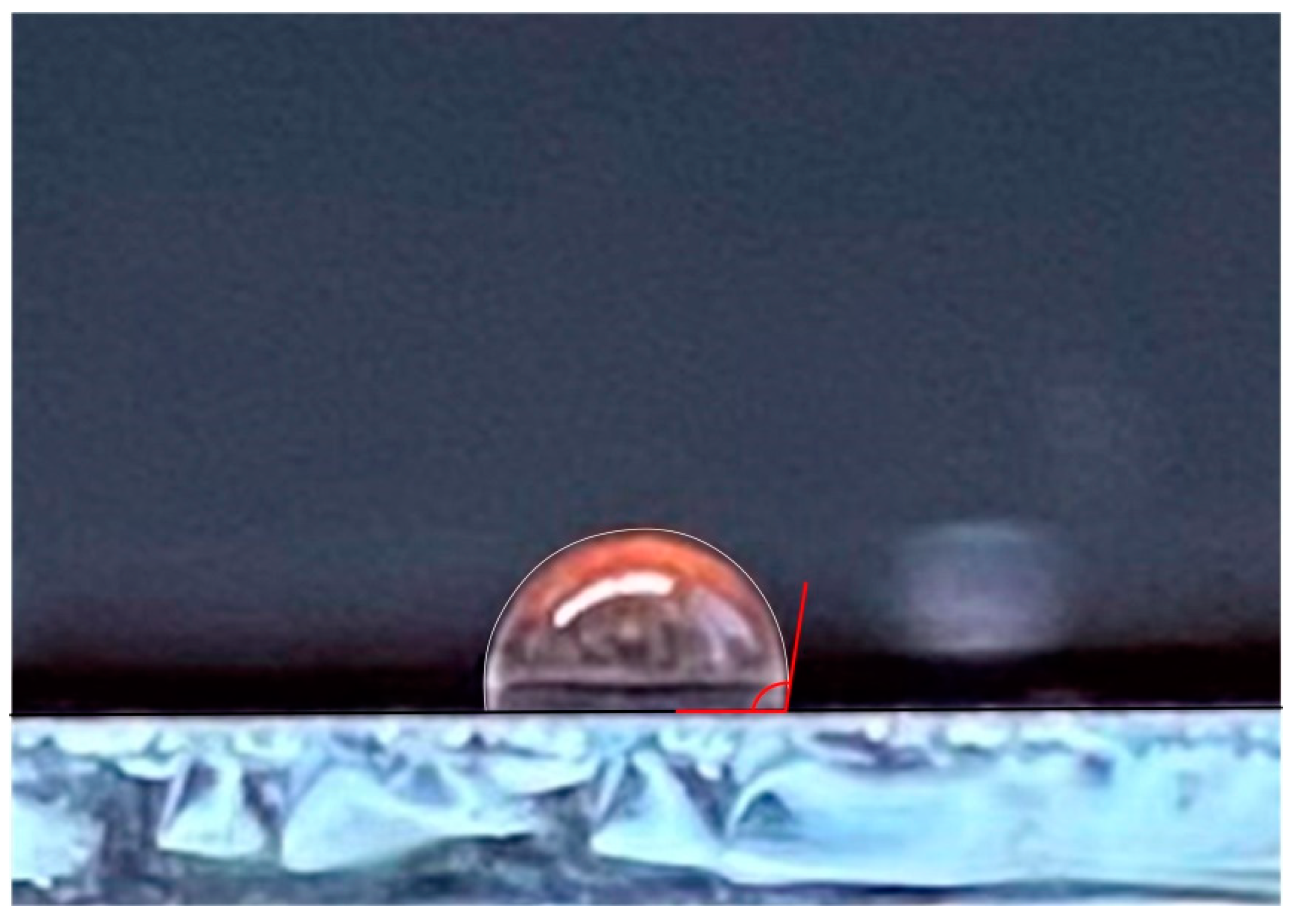
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Chen, K.; Qu, Y.; Li, K.; Zhang, Y.; Fu, E. Hairy Fluorescent Nanospheres Based on Polyelectrolyte Brush for Highly Sensitive Determination of Cu(II). Polymers 2020, 12, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazin, I.; Rossegger, E.; Guedes de la Cruz, G.; Griesser, T.; Schlögl, S. Recent Advances in Functional Polymers Containing Coumarin Chromophores. Polymers 2021, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Simon, T.; Diaconu, O.; Focsan, M.; Vulpoi, A.; Botiz, I.; Craciun, A.-M. Pluronic stabilized conjugated polymer nanoparticles for NIR fluorescence imaging and dual phototherapy applications. J. Mol. Struct. 2021, 1243, 130931. [Google Scholar] [CrossRef]
- Oliveira, N.A.; Airton, B.G., Jr.; Shinohara, M.M.G.; Lima, A.M.S.; Pires, A.M. The influence of the complexing agent on the luminescence of multicolor-emitting Y2O3:Eu3+, Er3+, Yb3+ phosphors obtained by the Pechini’s method. Mater. Chem. Phys. 2020, 257, 123840. [Google Scholar] [CrossRef]
- Mariselvam, K.; Liu, J.; Chen, H. Photoluminescence and gamma ray shielding properties of novel Dy3+: GSBS glass for solid state W-LEDs and radiation applications. Mater. Chem. Phys. 2021, 263, 124421. [Google Scholar] [CrossRef]
- Min, B.H.; Jung, K.Y. Luminescence improvement of (Ti, Si)O2:Eu3+/Li+ spherical particles for anti-counterfeiting application. Mater. Chem. Phys. 2021, 267, 124612. [Google Scholar] [CrossRef]
- Suarasan, S.; Tira, C.; Rusu, M.M.; Craciun, A.-M.; Focsan, M. Controlled fluorescence manipulation by core-shell multilayer of spherical gold nanoparticles: Theoretical and experimental evaluation. J. Mol. Struct. 2021, 1244, 130950. [Google Scholar] [CrossRef]
- Xu, J.; Jia, L.; Ma, Y.; Liu, X.; Tian, H.; Liu, W.; Tang, Y. Novel lanthanide hybrid functional materials for high performance luminescence application: The relationship between structures and photophysical behaviors. Mater. Chem. Phys. 2012, 136, 112. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Wang, S.-D.; Qi, H.; Wang, W.-J.; Hao, X.-X. Two new Zn2+/Cd2+ Metal-Organic Frameworks (MOFs) constructed from asymmetrical tricarboxylic acid ligands. J. Mol. Struct. 2020, 1205, 127620. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Wang, S.-D.; Xu, L.; Wang, S.-Y.; Zhang, J.-M. Two Cu(II) coordination polymers assembled by 5-(3,4-dicarboxylphenoxy) nicotic acid: Synthesis, crystal structure and photoluminescence property. J. Mol. Struct. 2021, 1233, 130099. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Su, H.; Zheng, X.; Liu, W.-B.; He, Y.; Fei, N.-N.; Qiao, R.; Ren, Y.-L.; Niu, C.-Y. A new A3B zinc(II)-porphyrin ligand and its ruthenium(II) complex: Synthesis, photophysical properties and photocatalytic applications. J. Mol. Struct. 2021, 1237, 130358. [Google Scholar] [CrossRef]
- Jachak, M.; Khopkar, S.; Patel, K.; Patil, Y.; Shankarling, G. Synthesis of Novel d-π-A chromophores: Effect of structural manipulations on photophysical properties, viscosity and DFT study. J. Mol. Struct. 2021, 1233, 130086. [Google Scholar] [CrossRef]
- Maouche, R.; Belaid, S.; Benmerad, B.; Bouacida, S.; Daiguebonne, C.; Suffren, Y.; Guillou, O. A new praseodymium-based coordination polymers with 1,10-phenantroline and glutarate ligands: Synthesis, crystal structure and luminescent properties. J. Mol. Struct. 2021, 1225, 129164. [Google Scholar] [CrossRef]
- Wang, H.; Niu, L.; Li, G.; Zhang, W.; Chen, X. Structures and luminescent properties of two Tb(III) coordination polymers with aromatic dicarboxylates. J. Mol. Struct. 2020, 1211, 128081. [Google Scholar] [CrossRef]
- Santos, G.C.; de Oliveira, C.A.F.; da Silva, F.F.; Alves, S., Jr. Photophysical studies of coordination polymers and composites based on heterometallic lanthanide succinate. J. Mol. Struct. 2020, 1207, 127829. [Google Scholar] [CrossRef]
- Fang, M.; Fu, L.; Correia, S.F.H.; Ferreira, R.A.S.; Carlos, L.D. Highly Efficient Luminescent Polycarboxylate Lanthanide Complexes Incorporated into Di-Ureasils by an In-Situ Sol-Gel Process. Polymers 2018, 10, 434. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Fu, Z.; Zhao, H.; Liang, R.; Wang, C.; Wang, D.; Li, J. Preparation of PVA Fluorescent Gel and Luminescence of Europium Sensitized by Terbium (III). Polymers 2020, 12, 893. [Google Scholar] [CrossRef] [Green Version]
- Baojiao, G.; Zongwen, Q.; Tao, C. Structure and photoluminescence property of complexes of aromatic carboxylic acid-functionalized polysulfone with Eu(III) and Tb(III). Mat. Chem. Phys. 2014, 143, 1119. [Google Scholar] [CrossRef]
- Enculescu, M.; Preda, N.; Matei, E.; Enculescu, I. Luminescent micro- and nanofibers based on novel europium phthalate complex. Mat. Chem. Phys. 2012, 136, 51. [Google Scholar] [CrossRef]
- Bai, J.; Gu, H.; Hou, Y.; Wang, S. Luminescence properties and molecular mechanics calculation of bis-β-diketonate Eu3+ complex/polymer hybrid fibers. Opt. Mater. 2018, 79, 310. [Google Scholar] [CrossRef]
- Vovna, V.I.; Chekh, A.S.; Korochentsev, V.V.; Tikhonov, S.A.; Samoilov, I.S. Photoelectron spectra and electronic structure of Zn(II)bis-β-diketonates with aromatic substituents by the PES, DFT, and OVGF methods. J. Mol. Struct. 2020, 1223, 128815. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, P.; Li, H.F.; Sun, W.B.; Gao, T.; Yan, P.F. Structural effects on the photophysical properties of mono-β-diketonate and bis-β-diketonate EuIII complexes. Phys. Chem. Chem. Phys. 2015, 17, 16136. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.P.; Martín-Ramos, P.; Coya, C.; Álvarez, A.L.; Pereira, L.C.; Díaz, R.; Martín-Gil, J.; Ramos Silva, M. Lanthanide tetrakis-β-diketonate dimers for solution-processed OLEDs. Mat. Chem. Phys. 2014, 147, 1157. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Narayanan, R.S. Phosphazenes. Organophosphorus Chem. 2016, 45, 375. [Google Scholar] [CrossRef] [Green Version]
- Allcock, H.R.; Brennan, D.J.; Whittle, R.R. The synthesis and structure of triphenylsiloxycyclotriphosphazenes. Heteroat. Chem. 1996, 7, 67. [Google Scholar] [CrossRef]
- Ardıç, A.H.; Meydan, G.Ö.; Yunus, Z.; Ferda, H.; Ünügür, Ç.S.; Ayhan, B.; Adem, K.; Serkan, Y. Synthesis and proton conductivity of azole-substituted cyclic and polymeric phosphazenes. Polymer 2013, 54, 2250. [Google Scholar] [CrossRef]
- Görgülü, A.O.; Koran, K.; Özen, F.; Tekin, S.; Sandal, S. Synthesis, structural characterization and anti-carcinogenic activity of new cyclotriphosphazenes containing dioxybiphenyl and chalcone groups. J. Mol. Struct. 2015, 1087, 1–10. [Google Scholar] [CrossRef]
- Gorlov, M.; Bredov, N.; Esin, A.; Sirotin, I.; Soldatov, M.; Oberemok, V.; Kireev, V.V. Novel Approach for the Synthesis of Chlorophosphazene Cycles with a Defined Size via Controlled Cyclization of Linear Oligodichlorophosphazenes [Cl(PCl2=N)n–PCl3]+[PCl6]−. Int. J. Mol. Sci. 2021, 22, 5958. [Google Scholar] [CrossRef]
- Koran, K.; Arif, B.; Ali, D.; Dere, A.; Özen, F.; Al-Sehemi, A.G.; Al-Ghamdi, A.; Görgülü, A.O.; Yakuphanoğlu, F. Investigation of electrical properties of organophosphazene layer based photodiode. Chem. Phys. 2020, 538, 110897. [Google Scholar] [CrossRef]
- Xue, R.; Gou, H.; Zheng, Y.-P.; Liu, Y.-S.; Rao, H.-H.; Zhao, G.-H. A novel microporous phosphazene-triazinyl based covalent organic framework: Preparation, characterization and fluorescence properties. Optik 2021, 242, 167184. [Google Scholar] [CrossRef]
- Nikovskii, I.A.; Chistyakov, E.M.; Tupikov, A.S. Phosphazene-containing ligands and complexes on their base. Russ. J. Gen. Chem. 2018, 88, 474. [Google Scholar] [CrossRef]
- Inoue, K.; Sasaki, Y.; Itaya, T.; Tanigaki, T. Polymerization of styrenes with pendant aminophosphazenes and fluorescence behaviors of their Eu3+ complexes. Eur. Polym. J. 1997, 33, 841. [Google Scholar] [CrossRef]
- Koran, K.; Özen, F.; Biryan, F.; Demirelli, K.; Görgülü, A.O. Eu+3-doped chalcone substituted cyclotriphosphazenes: Synthesis, characterizations, thermal and dielectrical properties. Inorg. Chim. Acta 2016, 450, 162. [Google Scholar] [CrossRef]
- Channa, I.A.; Shah, A.A.; Rizwan, M.; Makhdoom, M.A.; Chandio, A.D.; Shar, M.A.; Mahmood, A. Process Parameter Optimization of a Polymer Derived Ceramic Coatings for Producing Ultra-High Gas Barrier. Materials 2021, 14, 7000. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, E.M.; Tupikov, A.S.; Buzin, M.I.; Borisov, R.S.; Kireev, V.V. Preparation of films based on β-diketophosphazene and different amines and study their properties. Mat. Chem. Phys. 2019, 223, 353. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslennikova, V.V.; Filatov, S.N.; Orlov, A.V.; Surin, N.M.; Svidchenko, E.A.; Chistyakov, E.M. Luminescent Coatings Based on (3-Aminopropyl)triethoxysilane and Europium Complex β-Diketophosphazene. Polymers 2022, 14, 728. https://doi.org/10.3390/polym14040728
Maslennikova VV, Filatov SN, Orlov AV, Surin NM, Svidchenko EA, Chistyakov EM. Luminescent Coatings Based on (3-Aminopropyl)triethoxysilane and Europium Complex β-Diketophosphazene. Polymers. 2022; 14(4):728. https://doi.org/10.3390/polym14040728
Chicago/Turabian StyleMaslennikova, Violetta V., Sergey N. Filatov, Alexey V. Orlov, Nikolay M. Surin, Evgeniya A. Svidchenko, and Evgeniy M. Chistyakov. 2022. "Luminescent Coatings Based on (3-Aminopropyl)triethoxysilane and Europium Complex β-Diketophosphazene" Polymers 14, no. 4: 728. https://doi.org/10.3390/polym14040728
APA StyleMaslennikova, V. V., Filatov, S. N., Orlov, A. V., Surin, N. M., Svidchenko, E. A., & Chistyakov, E. M. (2022). Luminescent Coatings Based on (3-Aminopropyl)triethoxysilane and Europium Complex β-Diketophosphazene. Polymers, 14(4), 728. https://doi.org/10.3390/polym14040728