Fabrication of Ropivacaine/Dexamethasone-Eluting Poly(D, L-lactide-co-glycolide) Microparticles via Electrospraying Technique for Postoperational Pain Control
Abstract
:1. Introduction
2. Materials and Methods
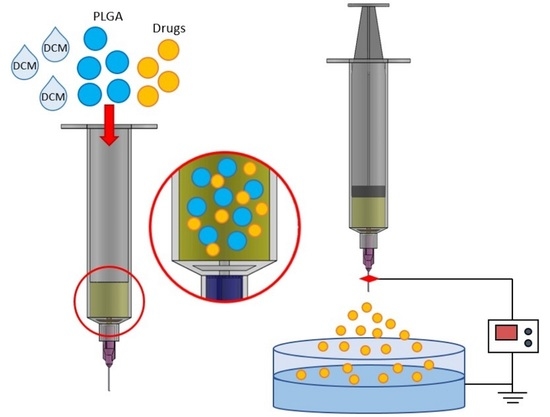
2.1. Production of Analgesics-Eluting Microparticles
2.2. Scanning Electron Microscopy (SEM)
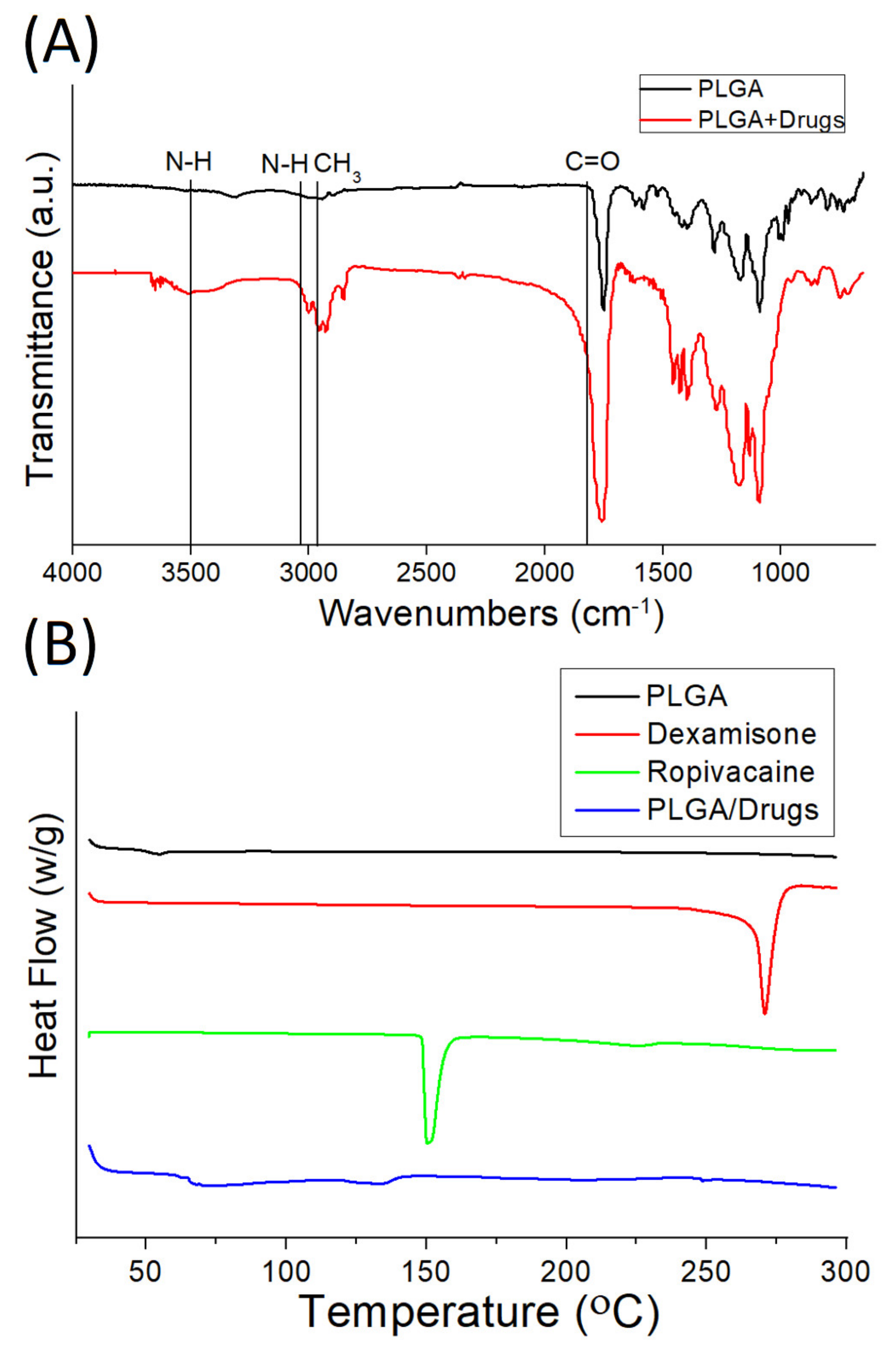
2.3. Fourier-Transform Infrared Spectrometry (FTIR)
2.4. Differential Scanning Calorimetry
2.5. Discharge of Pharmaceuticals In Vitro
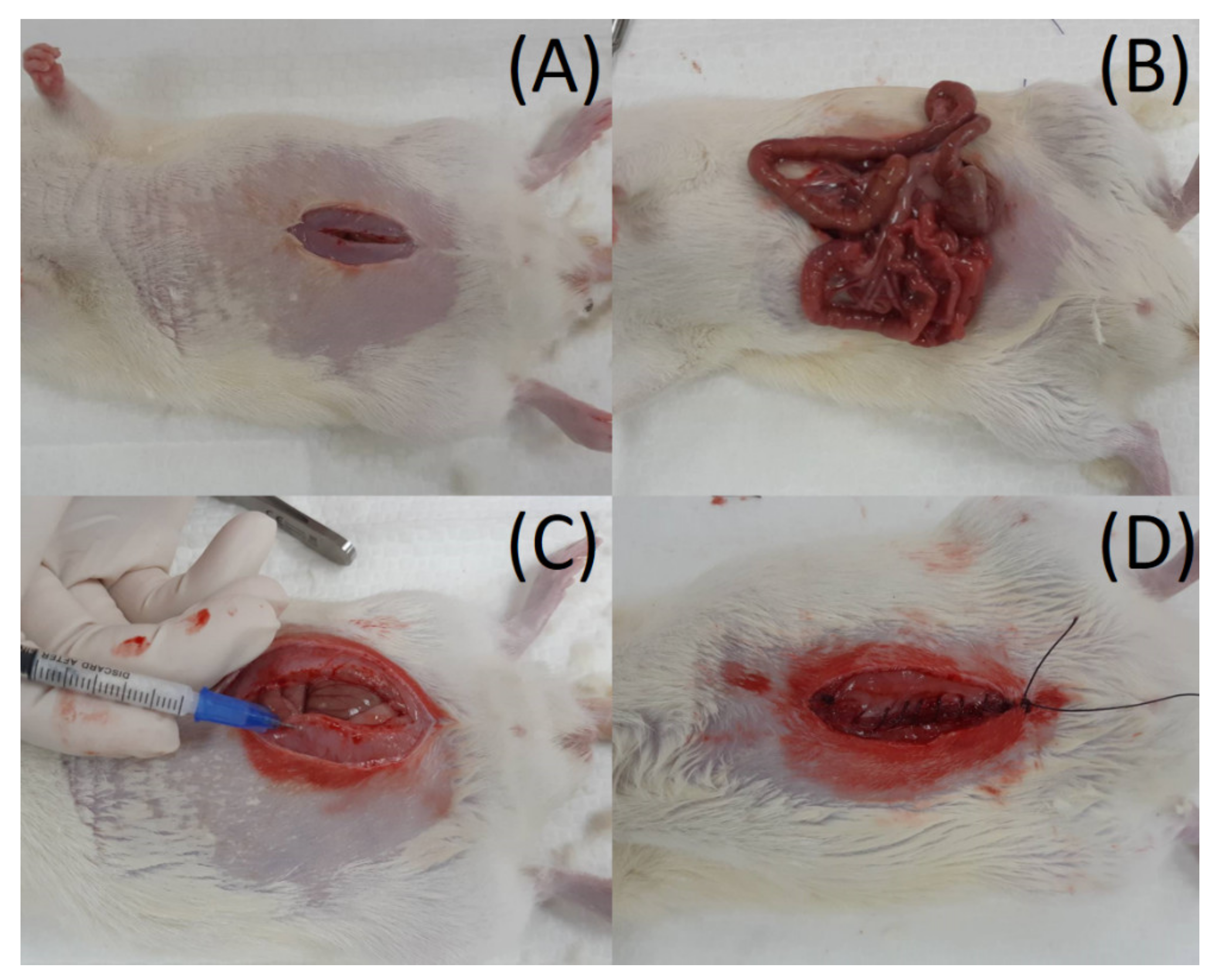
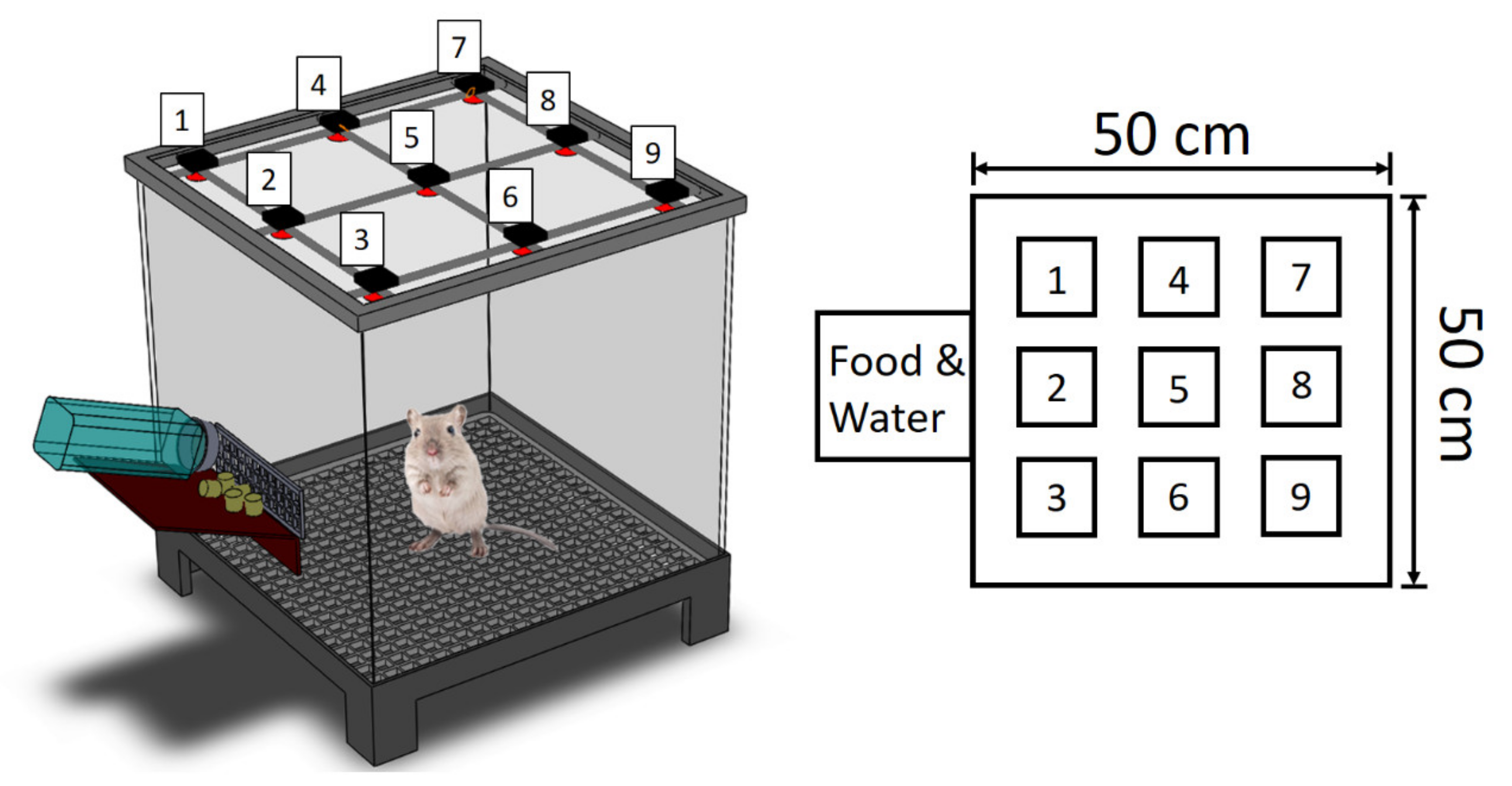
2.6. Rat Abdominal Surgery Procedure
2.7. Post-Surgery Activity Assessment
2.8. Statistics and Data Analysis
3. Results
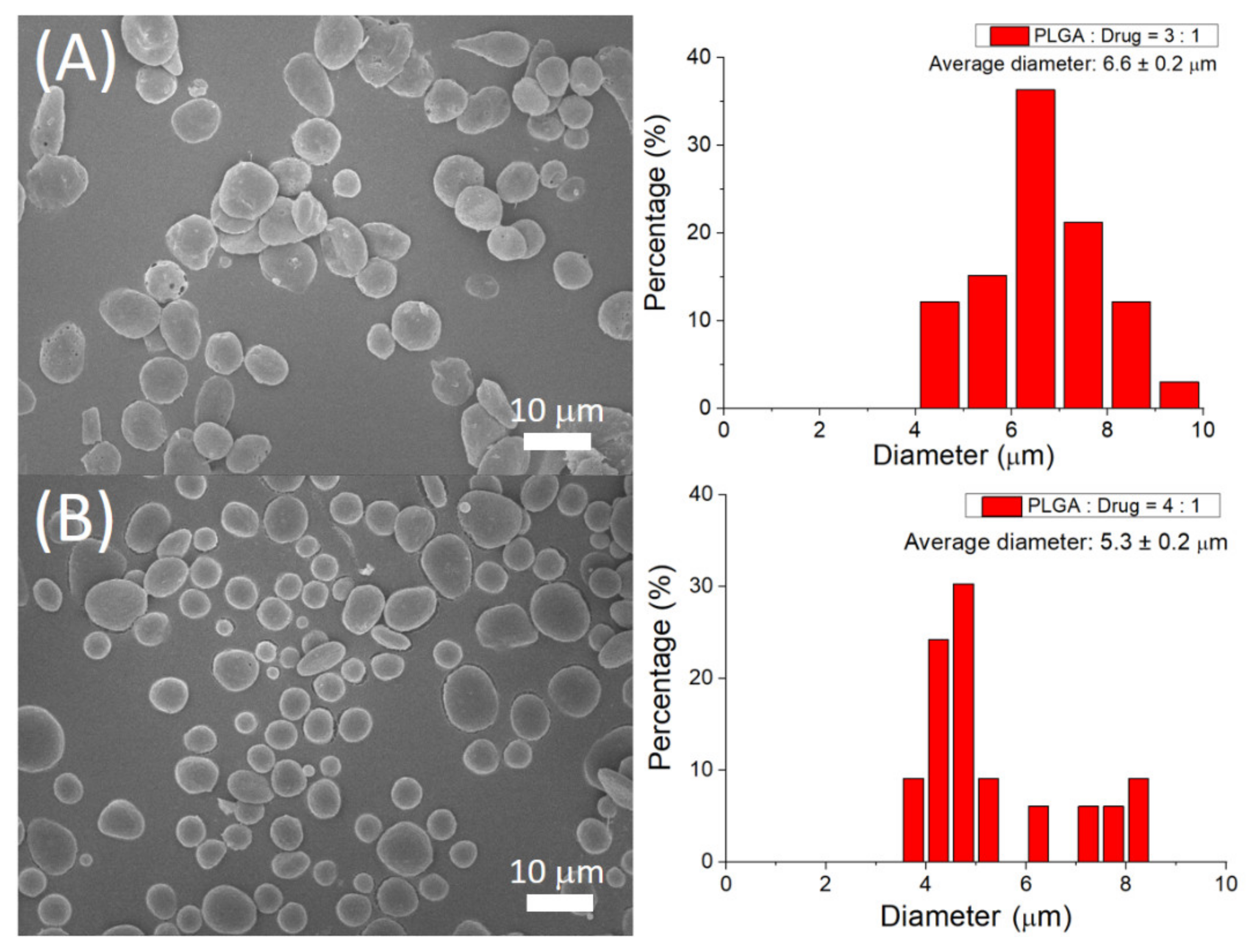
3.1. Assessment of Sprayed Microparticles
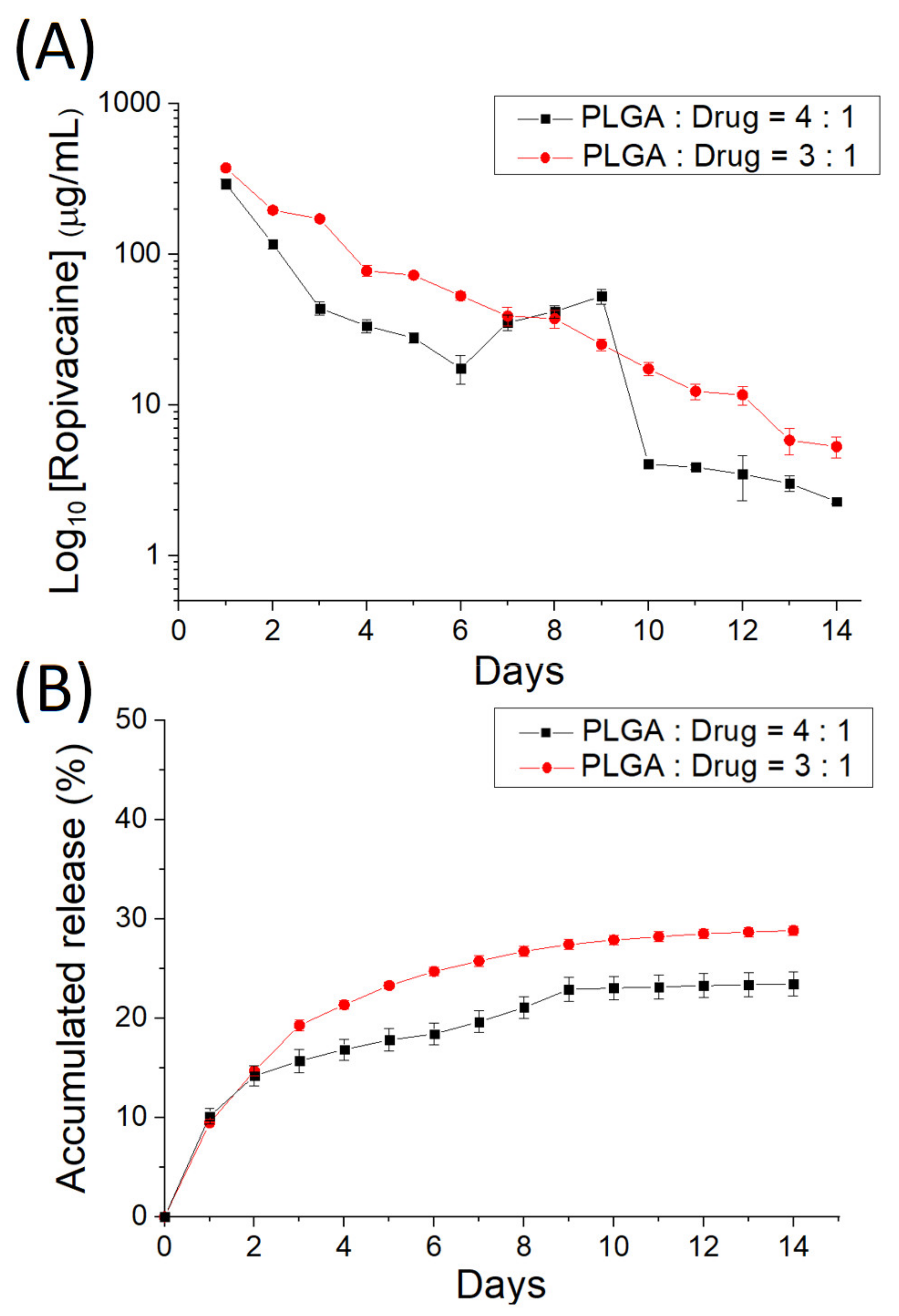
3.2. In Vitro Discharge of Analgesics-Loaded Particles
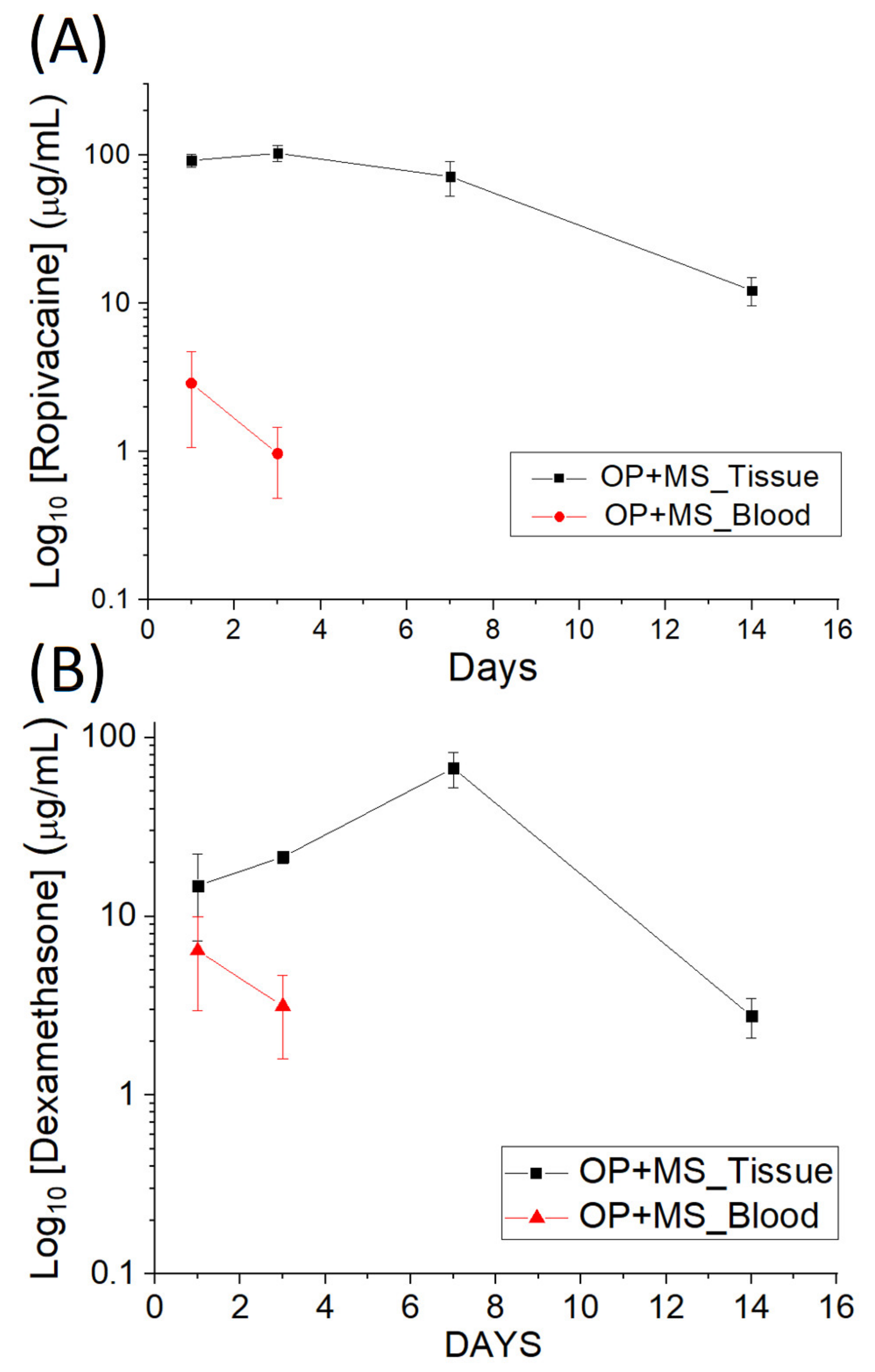
3.3. In Vivo Elution
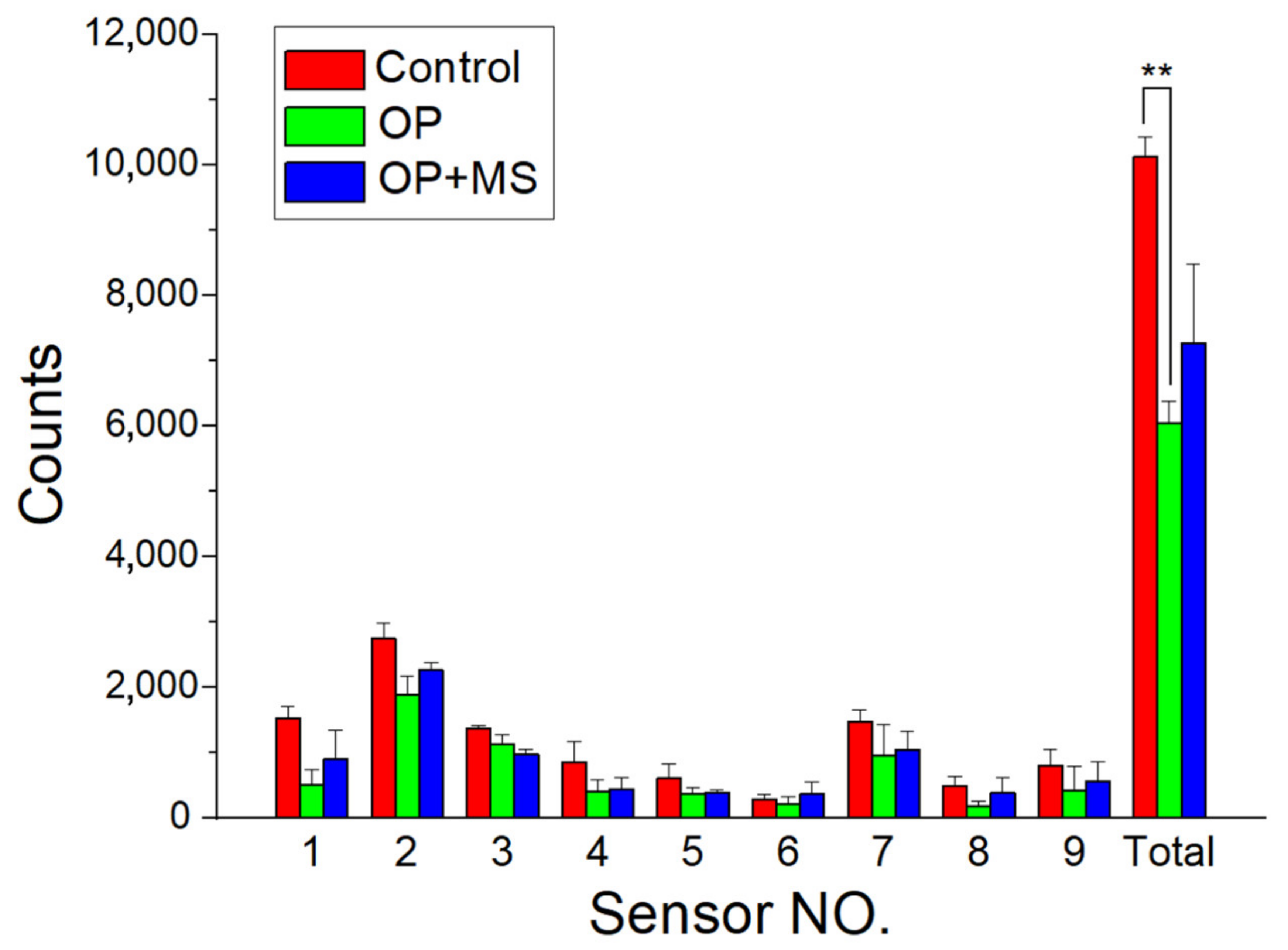
3.4. Efficacy of Release Drugs in Pain Relief
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bale, S.; Khurana, A.; Reddy, A.S.S.; Singh, M.; Godugu, C. Overview on therapeutic applications of microparticulate drug delivery systems. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 309–361. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Gaskell, S.M. Electrospray: Principles and practice. J. Mass Spectrom. 1997, 32, 677–688. [Google Scholar] [CrossRef]
- Jaworek, A. Micro- and nanoparticle production by electrospraying. Powder Technol. 2007, 176, 18–35. [Google Scholar] [CrossRef]
- Wang, J.; Jansen, J.A.; Yang, F. Electrospraying: Possibilities and challenges of engineering carriers for biomedical applications—A mini review. Front. Chem. 2019, 7, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, M.Y.; Feng, C.H.; Liu, Y.W.; Liu, S.J. An orthogonal model to study the effect of electrospraying parameters on the morphology of poly (d, l)-lactide-co-glycolide (PLGA) particles. Appl. Sci. 2019, 9, 1077. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Hsu, M.Y.; Li, M.J.; Liu, Y.W.; Liu, S.J.; Liu, C.Y.; Ito, H. Factors affecting the co-axial electrospraying of core–shell-structured poly(d, l-lactide-co-glycolide) microparticles. Japan J. Appl. Phys. 2018, 57, 116702. [Google Scholar] [CrossRef]
- Geeraerts, T.; Velly, L.; Abdennour, L.; Asehnoune, K.; Audibert, G.; Bouzat, P.; Bruder, N.; Carrillon, R.; Cottenceau, V.; Cotton, F.; et al. Management of severe traumatic brain injury (first 24 hours). Anaesth. Crit. Care Pain Med. 2018, 37, 171–186. [Google Scholar] [CrossRef]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain 2016, 17, 131–157. [Google Scholar]
- Tano, P.F.; Apiribu, F.; Tano, E.K.; Boamah Mensah, A.B.; Dzomeku, V.M.; Boateng, I. Predicting factors that determine patients’ satisfaction with post-operative pain management following abdominal surgeries at Komfo Anokye Teaching Hospital, Kumasi, Ghana. PLoS ONE 2021, 16, e0251979. [Google Scholar] [CrossRef]
- Kjølhede, P.; Bergdahl, O.; Wodlin, N.B.; Nilsson, L. Effect of intrathecal morphine and epidural analgesia on postoperative recovery after abdominal surgery for gynecologic malignancy: An open-label randomised trial. BMJ. Open 2019, 9, e024484. [Google Scholar]
- Ganapathy, S. Wound/intra-articular infiltration or peripheral nerve blocks for orthopedic joint surgery: Efficacy and safety issues. Curr. Opin. Anaesthesiol. 2012, 25, 615–620. [Google Scholar] [CrossRef]
- Kau, Y.-C.; Liao, C.-C.; Chen, Y.-C.; Liu, S.-J. Sustained release of lidocaine from solvent-free biodegradable poly[(d, l)-lactide-co-glycolide] (PLGA): In vitro and in vivo study. Materials 2014, 7, 6660–6676. [Google Scholar] [CrossRef] [Green Version]
- Durst, M.S.; Arras, M.; Palme, R.; Talbot, S.R.; Jirkof, P. Lidocaine and bupivacaine as part of multimodal pain management in a C57BL/6J laparotomy mouse model. Sci. Rep. 2021, 11, 10918. [Google Scholar] [CrossRef]
- Miller, R.D.; Pardo, M.; Stoelting, R.K. Basics of Anesthesia; Elsevier/Saunders: Philadelphia, PA, USA, 2011; p. 289. [Google Scholar]
- Cummings, K.C., 3rd; Napierkowski, D.E.; Parra-Sanchez, I.; Kurz, A.; Dalton, J.E.; Brems, J.J.; Sessler, D.I. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br. J. Anaesth. 2011, 107, 446–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huynh, T.M.; Marret, E.; Bonnet, F. Combination of dexamethasone and local anaesthetic solution in peripheral nerve blocks: A meta-analysis of randomised controlled trials. Eur. J. Anaesthesiol. 2015, 32, 751–758. [Google Scholar] [CrossRef]
- Mao, Y.; Zuo, Y.M.; Mei, B.; Chen, L.J.; Liu, X.S.; Zhang, Z.; Gu, E.W. Efficacy of perineural dexamethasone with ropivacaine in thoracic paravertebral block for postoperative analgesia in elective thoracotomy: A randomized, double-blind, placebo-controlled trial. J. Pain Res. 2018, 11, 1811–1819. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.J.; Kau, Y.C.; Wen, C.W.; Liu, K.S.; Liu, S.J. Solvent-free biodegradable scleral plugs providing sustained release of vancomycin, amikacin and dexamethasone–An in vivo study. J. Biomed. Mater. Res. A 2010, 94A, 426–432. [Google Scholar]
- Li, A.; Yang, F.; Xin, J.; Bai, X. An efficient and long-acting local anesthetic: Ropivacaine-loaded lipid-polymer hybrid nanoparticles for the control of pain. Int. J. Nanomed. 2019, 14, 913–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, M.L.; Eckert, J.; Jacobsen, H.; dos Santos, E.C.; Ignazzi, R.; de Araujo, D.R.; Bellissent-Funel, M.-C.; Natali, F.; Koza, M.M.; Matic, A.; et al. Raman and Infrared spectroscopies and X-ray diffraction data on bupivacaine and ropivacaine complexed with 2-hydroxypropyl−β−cyclodextrin. Data Brief 2017, 15, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Z.-C.; Yu, S.-H.; Chao, A.-C.; Dong, G.-C. Preparation and characterization of dexamethasone-immobilized chitosan scaffold. J. BioSci. Bioeng. 2012, 113, 654–660. [Google Scholar] [CrossRef]
- Rodrigues, L.B.; Leite, H.F.; Yoshida, M.I.; Saliba, J.B.; Cunha, A.S., Jr.; Faraco, A.A.G. In vitro release and characterization of chitosan films as dexamethasone carrier. Int. J. Pharm. 2009, 368, 1–6. [Google Scholar] [CrossRef]
- Zhai, Y.; Xu, R.; Wang, Y.; Liu, J.; Wang, Z.; Zhai, G. Ethosomes for skin delivery of ropivacaine: Preparation, characterization and ex vivo penetration properties. J. Liposome Res. 2014, 25, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Webster, T.J. Recent progress and challenges for polymeric microsphere compared to nanosphere drug release systems: Is there a real difference? Bioorg. Med. Chem. 2021, 33, 116028. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Bhatia, A.; Chopra, S.; Dua, K.; Prasher, P.; Gupta, G.; Tambuwala, M.M.; Chellappan, D.K.; Aljabali, A.A.A.; Sharma, M.; et al. Advancements on microparticles-based drug delivery systems for cancer therapy. In Advanced Drug Delivery Systems in the Management of Cancer; Academic Press: Cambridge, MA, USA, 2021; pp. 351–358. [Google Scholar]
- Jusu, S.M.; Obayemi, J.D.; Salifu, A.A.; Nwazojie, C.C.; Uzonwanne, V.; Odusanya, O.S.; Soboyejo, W.O. Drug-encapsulated blend of PLGA-PEG microspheres: In vitro and in vivo study of the effects of localized/targeted drug delivery on the treatment of triple-negative breast cancer. Sci. Rep. 2020, 10, 14188. [Google Scholar] [CrossRef] [PubMed]
- Salapa, J.; Bushman, A.; Lowe, K.; Irudayaraj, J. Nano drug delivery systems in upper gastrointestinal cancer therapy. Nano Converg. 2020, 7, 38. [Google Scholar] [CrossRef]
- Brigham, N.C.; Ji, R.-R.; Becker, M.L. Degradable polymeric vehicles for postoperative pain management. Nat. Commun. 2021, 12, 367. [Google Scholar] [CrossRef]
- Bajwa, S.J.S. Dexmedetomidine and ketamine - Comrades on an eternal journey! Indian J. Anaesth. 2021, 65 (Suppl. S1), S1–S4. [Google Scholar] [CrossRef]
- Sabuj, M.Z.R.; Islam, N. Inhaled antibiotic-loaded polymeric nanoparticles for the management of lower respiratory tract infections. Nanoscale Adv. 2021, 3, 4005–4018. [Google Scholar] [CrossRef]
- Langer, R. Drug delivery and targeting. Nature 1998, 392 (Suppl. S6679), 5–10. [Google Scholar]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.-H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.V.; Wadd, N.V.; Thorat, S.S.; Upadhye, S.S. Microspheres: A novel drug delivery system. Am. J. PharmTech Res. 2020, 10, 286–301. [Google Scholar]
- Jain, N.K. Controlled and Novel Drug Delivery; CBS Publish: Delhi, India, 2011. [Google Scholar]
- Alagusundaram, M.; Madhu, S.C.; Umashankari, K.; Attuluri, V.B.; Lavanya, C.; Ramkanth, S. Microspheres as a novel drug delivery system-A review. Int. J. ChemTech Res. 2009, 1, 526–534. [Google Scholar]
- Bhattacharjee, S. Understanding the burst release phenomenon: Toward designing effective nanoparticulate drug-delivery systems. Ther. Deliv. 2021, 12, 21–36. [Google Scholar] [CrossRef]
- Bouriche, S.; Alonso-García, A.; Cárceles-Rodríguez, C.M.; Rezgui, F.; Fernández-Varón, E. An in vivo pharmacokinetic study of metformin microparticles as an oral sustained release formulation in rabbits. BMC Veter-Res. 2021, 17, 315. [Google Scholar] [CrossRef]
- Thiele, E.L.; Nemergut, E.C. Miller’s Anesthesia, 9th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Masters, D.B.; Berde, C.B.; Dutta, S.K.; Griggs, C.T.; Hu, D.; Kupsky, W.; Langer, R. Prolonged Regional Nerve Blockade by Controlled Release of Local Anesthetic from a Biodegradable Polymer Matrix. Anesthesiology 1993, 79, 340–346. [Google Scholar] [CrossRef]
- Gan, T.J. Poorly controlled postoperative pain: Prevalence, consequences, and prevention. J. Pain Res. 2017, 10, 2287–2298. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Chen, E.; Qian, X. A narrative review on perioperative pain management strategies in enhanced recovery pathways—The past, present and future. J. Clin. Med. 2021, 10, 2568. [Google Scholar] [CrossRef]
- Gathege, D.; Abdulkarim, A.; Odaba, D.; Mugambi, S. Effectiveness of pain control of local anaesthetic wound infusion following elective midline laparotomy: A randomized trial. World J. Surg. 2021, 45, 2100–2107. [Google Scholar] [CrossRef]
- Cindea, I.; Balcan, A.; Gherghina, V.; Nicolae, G.; Samoila, B.; Costea, D. Postoperative pain management after major abdominal surgery in elderly patients. Eur. J. Anaesthesiol. 2011, 28, 221–222. [Google Scholar] [CrossRef]
- Gelman, D.; Gelmanas, A.; Urbanaitė, D.; Tamošiūnas, R.; Sadauskas, S.; Bilskienė, D.; Naudžiūnas, A.; Širvinskas, E.; Benetis, R.; Macas, A. Role of multimodal analgesia in the evolving enhanced recovery after surgery pathways. Medicina 2018, 54, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Huang, Y.-G. Role of postoperative multimodal analgesia in abdominal and pelvic enhanced recovery after surgery. Acad. Med. Sin. 2016, 38, 458–463. [Google Scholar]
- Ramakrishna, S.; Zamani, M.; Prabhakaran, M.P. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997–3017. [Google Scholar] [CrossRef] [Green Version]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) As biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.-J.; Chou, Y.-C.; Hsu, S.-C.; Lin, Y.-T.; Lu, C.-J.; Liu, S.-J. Fabrication of Ropivacaine/Dexamethasone-Eluting Poly(D, L-lactide-co-glycolide) Microparticles via Electrospraying Technique for Postoperational Pain Control. Polymers 2022, 14, 702. https://doi.org/10.3390/polym14040702
Shen S-J, Chou Y-C, Hsu S-C, Lin Y-T, Lu C-J, Liu S-J. Fabrication of Ropivacaine/Dexamethasone-Eluting Poly(D, L-lactide-co-glycolide) Microparticles via Electrospraying Technique for Postoperational Pain Control. Polymers. 2022; 14(4):702. https://doi.org/10.3390/polym14040702
Chicago/Turabian StyleShen, Shih-Jyun, Ying-Chao Chou, Shih-Chieh Hsu, Yu-Ting Lin, Chia-Jung Lu, and Shih-Jung Liu. 2022. "Fabrication of Ropivacaine/Dexamethasone-Eluting Poly(D, L-lactide-co-glycolide) Microparticles via Electrospraying Technique for Postoperational Pain Control" Polymers 14, no. 4: 702. https://doi.org/10.3390/polym14040702
APA StyleShen, S.-J., Chou, Y.-C., Hsu, S.-C., Lin, Y.-T., Lu, C.-J., & Liu, S.-J. (2022). Fabrication of Ropivacaine/Dexamethasone-Eluting Poly(D, L-lactide-co-glycolide) Microparticles via Electrospraying Technique for Postoperational Pain Control. Polymers, 14(4), 702. https://doi.org/10.3390/polym14040702