1. Introduction
Nafion
TM (C
7HF
13O
5S × C
2F
4) consists of perfluoro-vinyl ether groups terminated with sulfonic groups on a tetrafluoroethylene (Teflon) backbone. Teflon is very hydrophobic, while the sulfonic groups are essentially hydrophilic. While swelling in an aqueous media, a nanostructure consisting of cylindrical reverse micelles appears. Water-filled channels of 2–3 nm diameter form within the Nafion membrane; see [
1] for more details. The polymer Nafion is widely studied in various fields, such as physics, chemistry, and hydrogen energetics (see, e.g., references [
2,
3,
4,
5,
6], related to the articles issued in 2021). The majority of techniques, applied for Nafion studies, are focused on the study of the polymer bulk properties. At the same time, water adjacent to the swollen polymer surface has also been explored, see recent works [
7,
8,
9,
10], and the monograph [
11] describes experiments, in which a Nafion membrane is immersed in an aqueous suspension of colloidal microspheres. It transpired that the microspheres are repelled from the membrane up to a distance of several hundreds of microns. The area, from which the colloidal microspheres are effectively pushed out, has been termed the “exclusion zone” (EZ). EZ phenomena may have important engineering applications in water filtration, reducing biofouling [
12] and microfluidics [
13]. EZ phenomena also have an obvious importance to understanding biological systems and resolving outstanding questions regarding “biological water” [
14]. In accordance with the model developed in monograph [
11], Nafion’s surface imparts a quasicrystalline structure on a macroscopic scale to adjacent water layers; in monograph [
11] (see also numerous references therein), this effect was called the formation of the “fourth phase” of water.
The width of the exclusion zone is approximately 300 µm. This is astonishing and not explicable by standard physical chemistry theories. Some physical mechanisms that might be responsible for the EZ formation have been discussed in a recent review [
15]. As was shown in our recent work [
16], when Nafion swells in water, polymer fibers unwind into the water bulk, but do not completely detach from the membrane surface, thus forming a stiff brush-like structure. Thus, according to our model, the EZ is not a special phase of water, and colloidal microspheres are pushed out of the area adjacent to the membrane due to the unwinding of the polymer fibers. It is very important that the effect of unwinding depends on the deuterium content in a liquid sample; for more detail see [
16]. In fact, for ordinary natural water (the deuterium content is 157 ± 1 ppm, see [
17]), the EZ size amounts to several hundreds of microns due to unwinding, while for the so-termed deuterium depleted water (DDW, the deuterium content is ≤1 ppm), the effect of unwinding is missing, and the EZ size is zero. Bearing in mind that the Nafion membrane is “decorated” with unwound polymer fibers, we see a clear analogy with human cell membrane. Indeed, the structure of the channels in the bulk of the polymer is similar to the lipid bilayer, while the unwound fibers are similar to the glycocalyx (extracellular matrix) and endothelial surface layer of vascular tissue of humans; see, for example, [
18]. This is a matter of utmost importance to physiology; see [
19]. Developing the analogy between the human cell membrane and the Nafion membrane, we note that there are a number of organic compounds that stimulate the permeability of various ions through the cation channels of the cell membrane. We can say that the Nafion polymer membrane is similar to synthetic biological polymer membranes; see the recent review [
20]. In this regard, the alkaloid piperine (C
17H
19NO
3), which stimulates the conductivity of calcium [
21] and potassium [
22] ions in human cell channels, is of particular interest to study. Indeed, because the Nafion membrane contains negatively charged channels that are the conductors of protons, it is very important to study the interaction of piperine with Nafion.
One of the goals of this work is to clarify the mechanisms of interaction of cell membranes with non-ionizing low-frequency electromagnetic radiation. This interaction underlies the treatment of various diseases with a low-frequency electromagnetic field; see [
23,
24,
25,
26,
27,
28,
29,
30]. The cited monographs and reviews confirm the effectiveness of this technique. These works were written mainly by physicians who use this technique in their medical practice; the physical nature of the low-frequency effect on living tissue is usually not discussed. Within the framework of the analogy between the cell membrane and the Nafion membrane, it is of interest to study the features of the interaction of Nafion with low-frequency electromagnetic radiation.
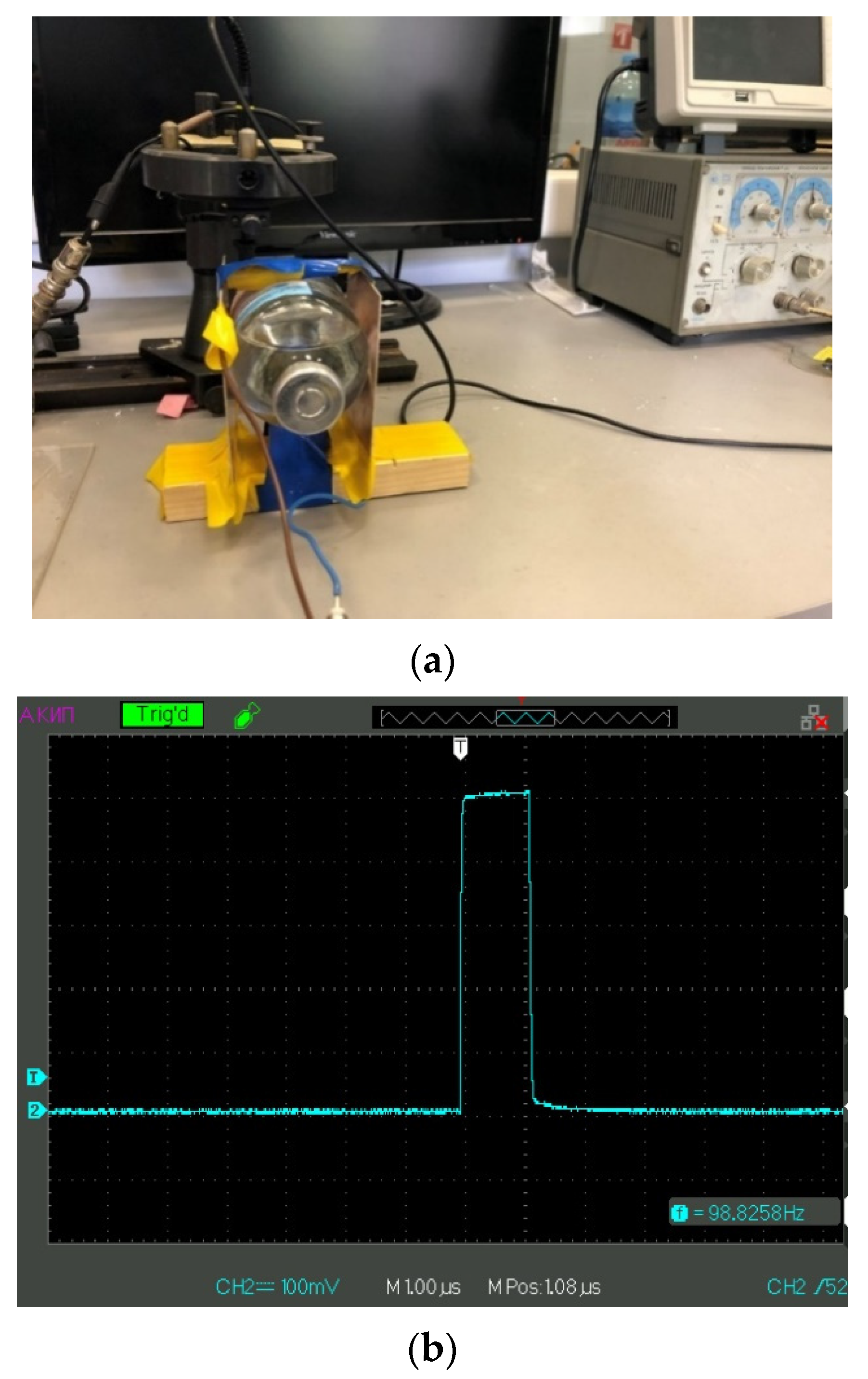
We, in our group, performed a number of experiments on the effect of low-frequency electromagnetic irradiation of isotonic solutions, in which the Nafion membrane was then soaked; see [
31]. As shown in [
31], the characteristic time of swelling of polymer membrane is different for irradiated/non-irradiated (reference) liquid samples. The time interval between electromagnetic treatment and measurements of the irradiation effects was about 20 min. The purpose of this study is to clarify the mechanisms of interaction of a polymer membrane with isotonic solutions with low-frequency electromagnetic radiation, which can be used in medical practice. In this work, we performed a number of experiments with photo-luminescent spectroscopy, where we studied in detail the range of repetition rates of electrical pulses from 5 Hz to 500 kHz with an interval of 5 Hz; if at certain frequencies any features were found in the dynamics of polymer swelling (see below), the corresponding frequency interval was passed with a step of 1 Hz. In some experiments we covered our experimental setup with aluminum foil. Furthermore, we investigated liquid samples with different isotopic compositions. This allowed us to detect a number of isolated frequencies of electromagnetic processing, at which the luminescence intensity, usually described by a regular function of time, exhibits a sporadic temporal behavior. The novelty of our work consists of the study of the spectral characteristics of the stochastic dynamics of luminescence and a qualitative theoretical model underlying the stochastic regimes, explaining the differences in the swelling kinetics of polymer in natural water and DDW. The results can be used to clarify the mechanisms of interaction of the polymer membrane with isotonic solutions with different isotopic compositions in the field of external low-frequency irradiation, which can be applied in medical practice.
3. Experimental Results
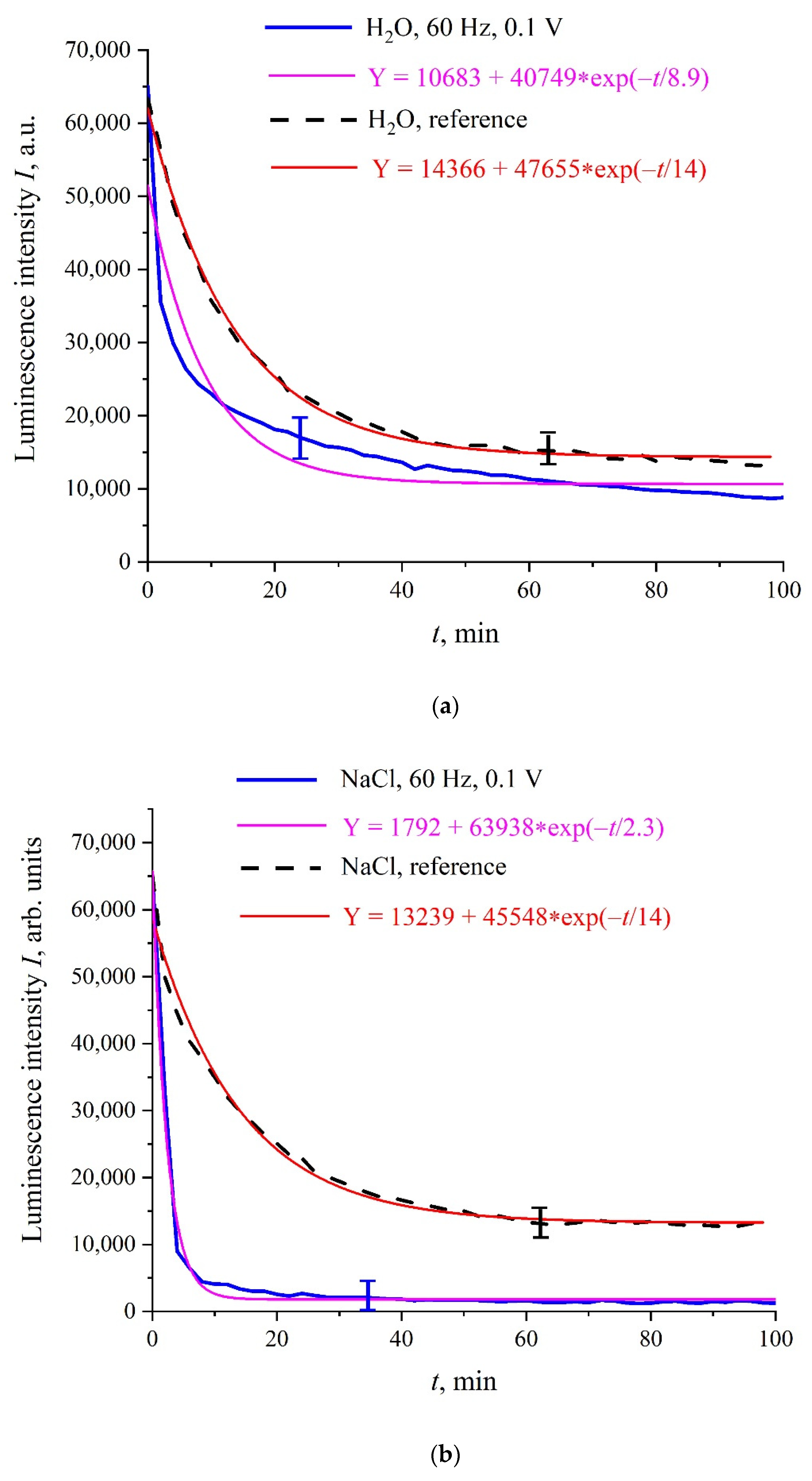
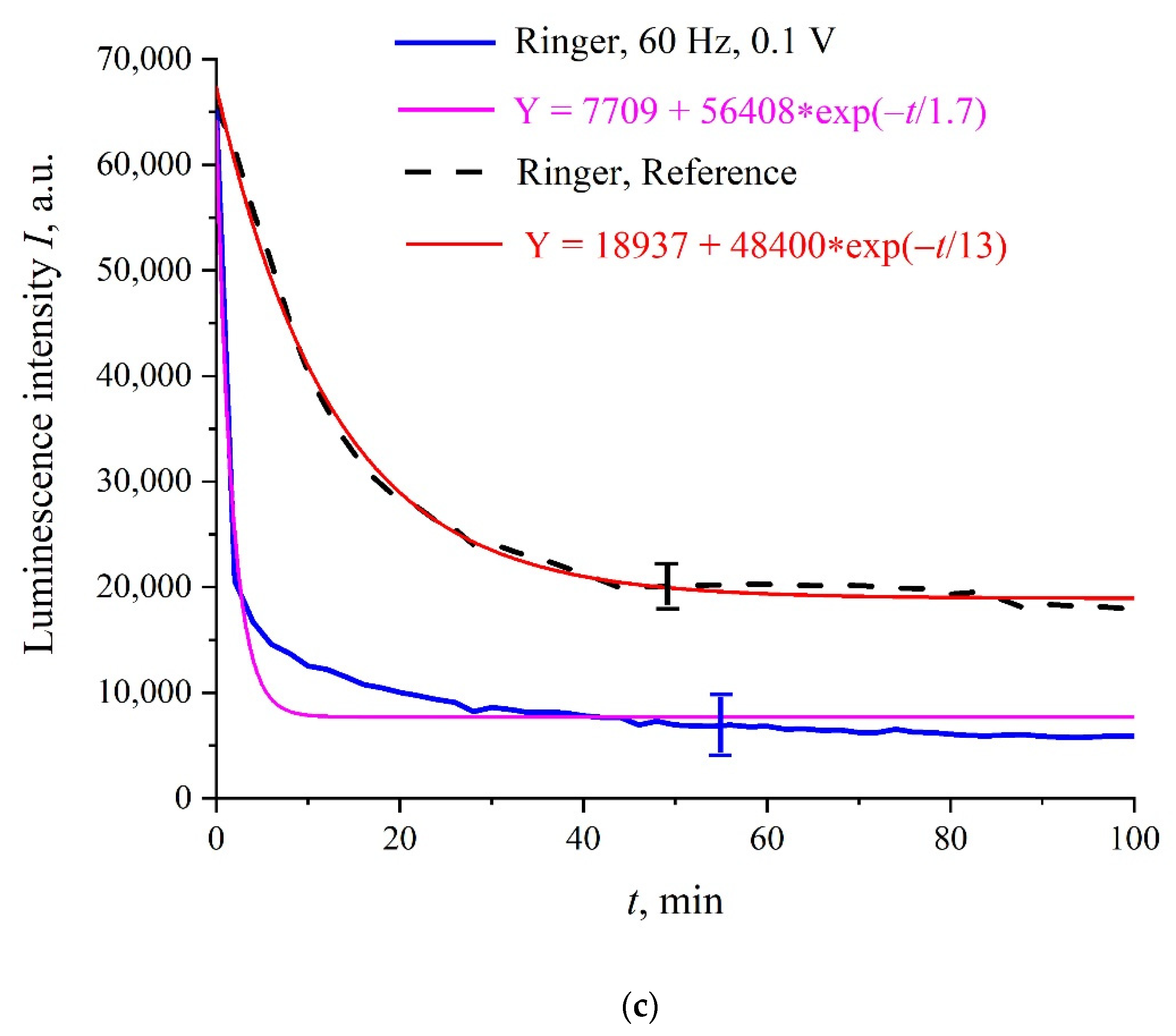
Figure 3a–c shows the typical dependences of
I at its spectral maximum (λ = 460 nm) vs. swelling time,
t, in Milli-Q water, isotonic NaCl, and Ringer solutions; for definiteness, here we present the dependences for 60 Hz pulse repetition rate. Reference curves for untreated (reference) samples are also shown. The experimental points correspond to averaging over five successive measurements. Confidence intervals are indicated in all graphs. All obtained dependences are well approximated by a single exponential function with characteristic decay times,
τ; the corresponding formulas are given in the insets and highlighted with different colors. As follows from the graphs, the reference dependences are described by approximately the same exponents: Y = 14,366 + 47,655⋅exp(−
t/14) for water, Y = 13,239 + 45,548⋅exp(−
t/14) for NaCl solution, and Y = 18,937 + 48,400⋅exp(−
t/13) for Ringer’s solution, i.e., the ionic additives would hardly influence the Nafion swelling dynamic. In this particular case, the luminescence intensity in all treated samples is lower than the intensity in the reference samples; note that for some pulse repetition rates the situation changes, i.e., the luminescence intensity in the treated samples can exceed the reference dependence or coincide with the reference curve to a good accuracy. The main feature of these results are as follows: the luminescence intensity
I(
t) after treatment is described by a single exponential function, and the results obtained have fairly good reproducibility. Similar results (at other frequencies) were presented in [
31], so this will not be discussed in detail here.
The exponential dependence of
I(
t) means that the luminescence cross section,
σlum, is constant. Indeed, assuming that the volume number density,
nNaf, of the luminescence centers (sulfonic groups in our case) obeyed Equation (1), i.e.,
nNaf in the near-surface polymer layer decreases due to penetrating of water molecules into this layer, we can write:
where
τ is the characteristic time of swelling. Thus, we obtain:
Because the I(t) is proportional to nNaf (see Equation (1)), it should exponentially decay.
We see that the processing of liquid samples with electrical pulses does not lead to significant changes in the kinetics of the swelling of the polymer; the exponential behavior of
I(
t) remains after treatment. It transpired, however, that the addition of some organic compounds to the liquid samples under study can lead to a violation of the exponential behavior of
I(
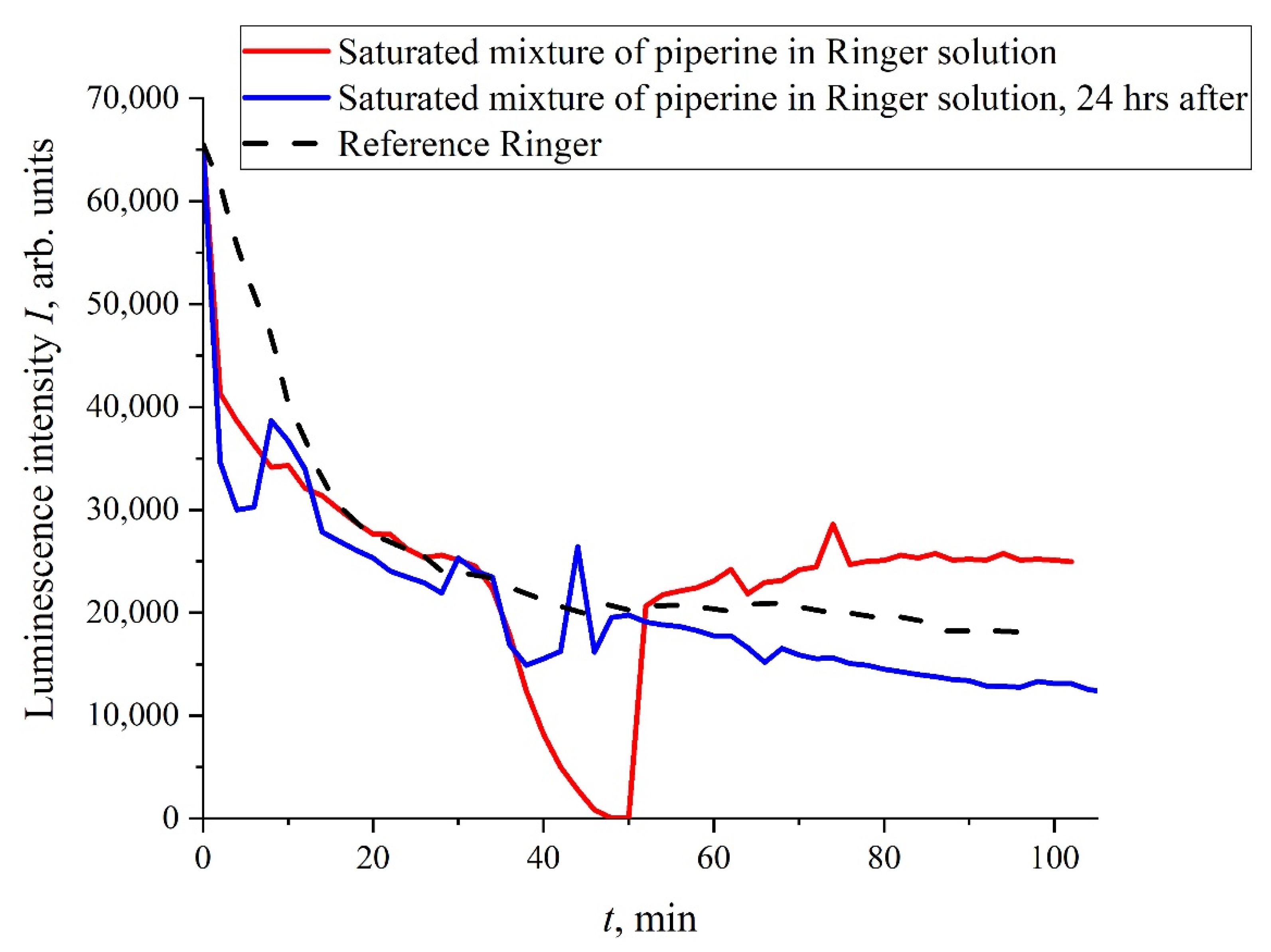
t), and this does not require treatment with electric pulses. In
Figure 4 we exhibit the dependence of
I(
t) for the mixture “Ringer’s solution-piperine” with a piperine concentration of 40 mg/L (saturated mixture). We see that, in this case, the dependence of
I(
t) is rather random; the results of more detailed studies of mixtures of piperine in water and isotonic solutions, including the treatment of liquid samples with electric pulses, will be published elsewhere. It is important for us that a stochastic character in the behavior of
I(
t) can arise, for example, at the adding of certain organic compounds to the test liquids.
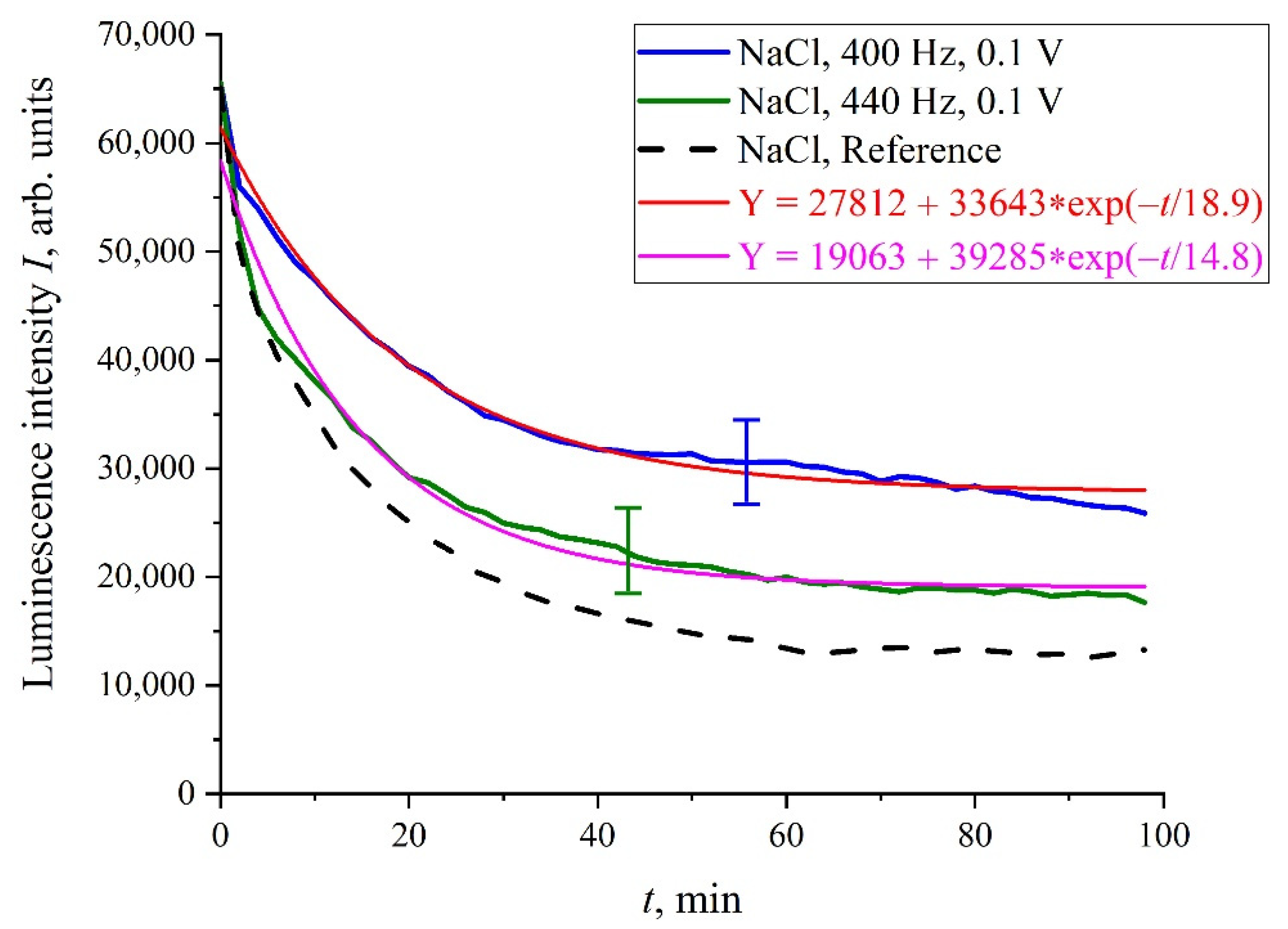
We will further consider only NaCl isotonic solutions. In
Figure 5 we show the luminescence intensity,
I(
t), dependences at the spectral maximum for 400 and 440 Hz pulse repetition rates. In contrast to the situation depicted in
Figure 4, the graphs in
Figure 5 are quite close to the reference curves and are described by decaying exponential functions, which are well reproduced.
However, there exist a number of pulse repetition frequencies for which the treatment by electrical pulses leads to irregular temporal behavior of
I(
t), that is, the effect of treatment is similar to the addition of piperine to a liquid sample. We found the following peculiar isolated frequencies: 82.5, 174, and 417 Hz. The values of these frequencies obey the approximate ratio 1:2:5. Perhaps there exist some other special frequencies, but we have not found them yet. We also do not know the physical mechanism behind the effects, connected with these frequencies. Hereinafter, we will, for definiteness, restrict ourselves with the case of 417 Hz repetition frequency and isotonic NaCl solution. In
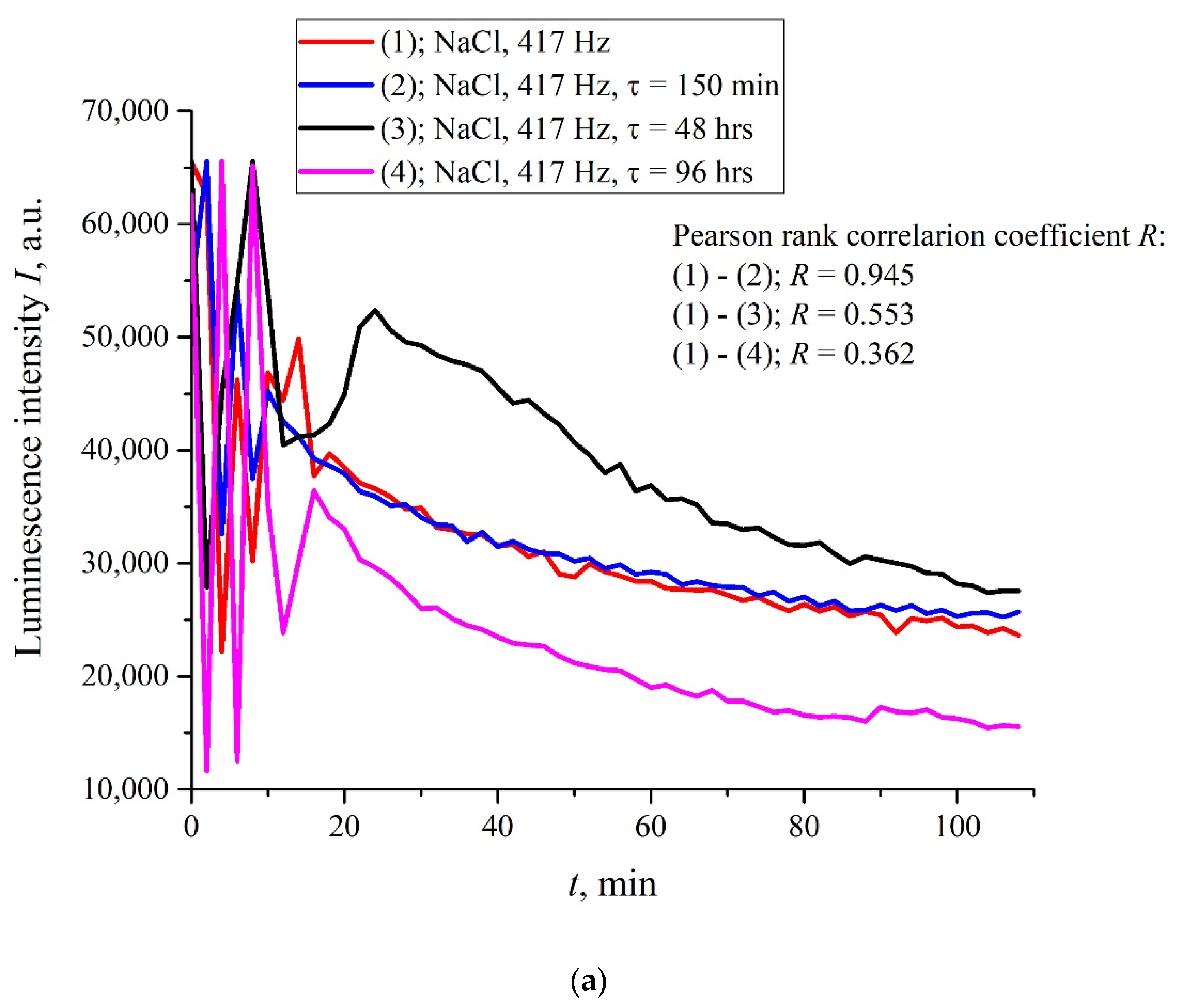
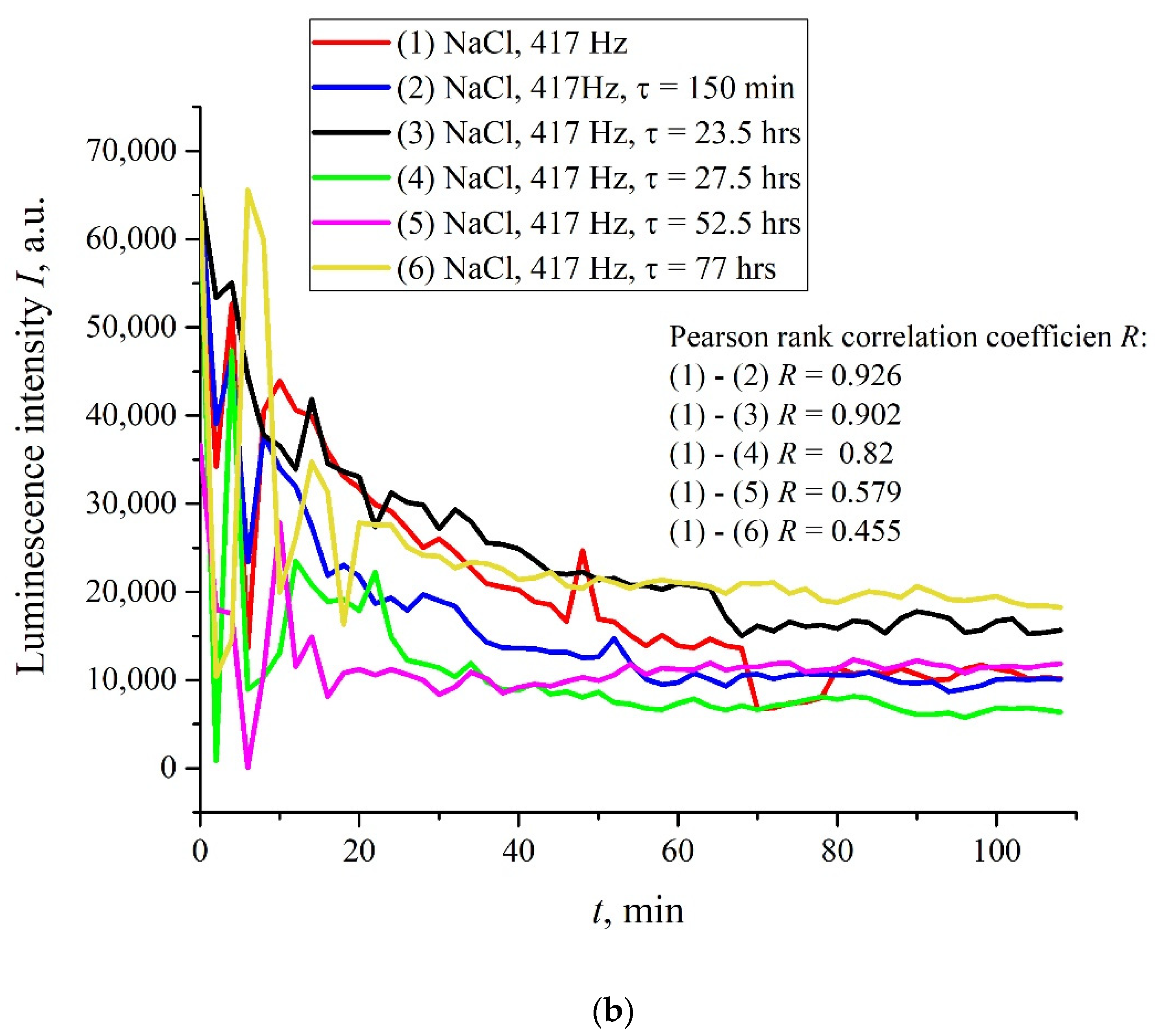
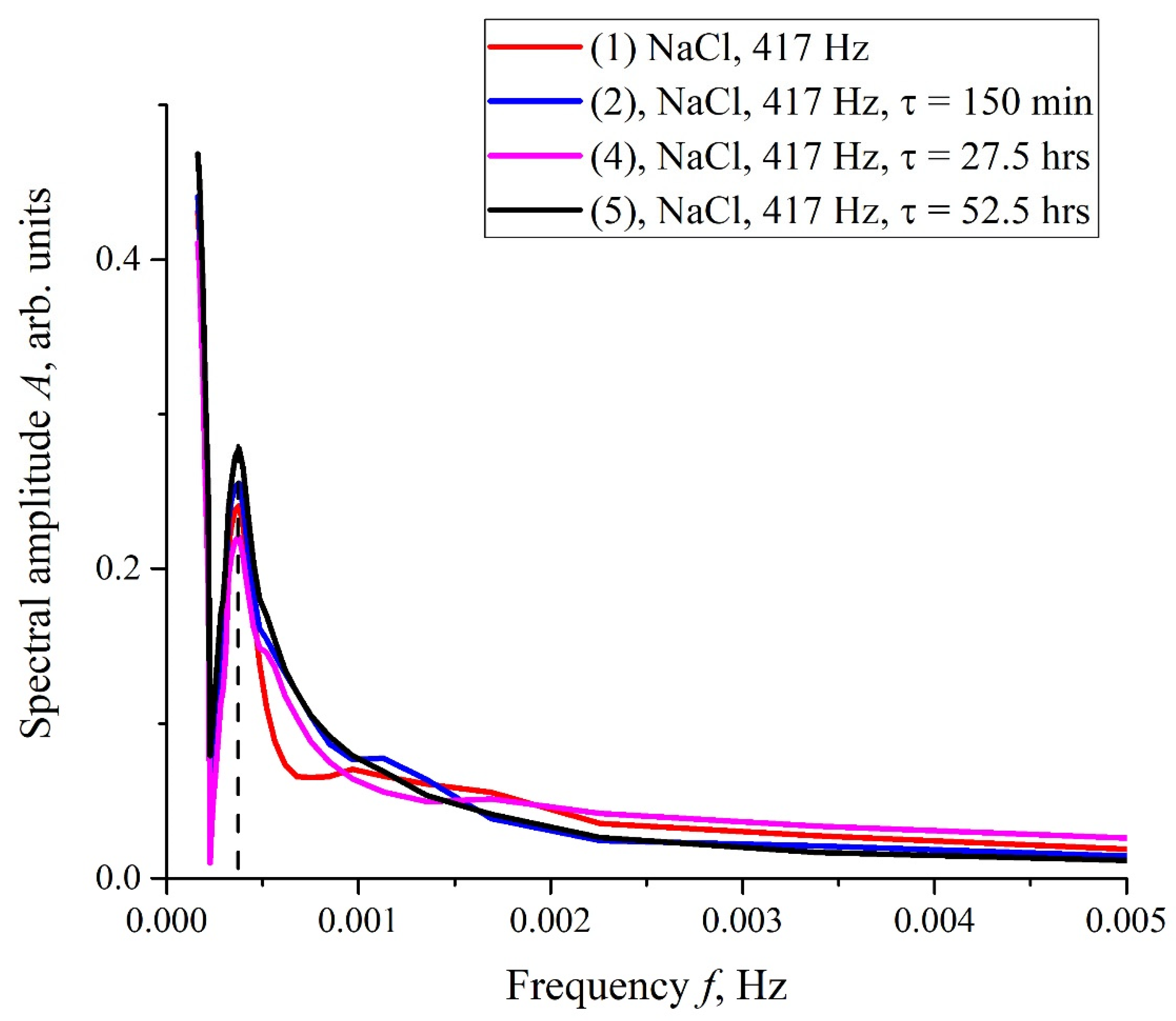
Figure 6a,b (cf. graphs in
Figure 5 for 400 and 440 Hz frequencies) we demonstrate the dependencies in several successive experiments performed two weeks apart in different laboratories; the distance between these laboratories is approximately 20 km. The intervals between the start of measurements for each experiment are denoted by the letter
τ. Obviously, there is no good reproducibility from measurement to measurement, so the confidence intervals are not shown. We also give the results of calculations of Pearson rank correlation coefficient, R, between the graphs.
The results of spectral and correlation processing of the experimental curves presented in
Figure 6 are shown in
Figure 7,
Figure 8 and
Figure 9. In
Figure 7, we exhibit the dependences,
A(
f), which is the Fourier transform of
I(
t) shown in
Figure 6b for various
τ; these dependences were obtained by using the Morlet wavelet transform; see [
35]. In our particular case, the Morlet wavelet is given by the formula
Here,
A(
f) is the spectral amplitude at frequency
f. It is seen that there exists a spectral maximum at a frequency of ~4 × 10
−4 Hz on all curves. It is also seen that at the frequency
f → 0 the dependence
A(
f) diverges, which is related to the specifics of the wavelet transforms using the Morlet wavelet at ultralow frequencies (see [
35]), i.e., the growth of the function
A(
f) at low frequencies can be ignored. In further analysis, a segment of
A(
f) with an isolated spectral maximum was allocated from the dependences
A(
f), and for this segment the spectral density,
A2(
f), was found, which, in turn, was approximated by Lorentzian:
Here
C and
B are the constants, f
0 is the central (resonant) frequency, and
τcorr is the correlation time of random process with the Lorentzian spectral line; the Lorentzian contour width is Δ
f~(
τcorr)
−1, see [
36]. In
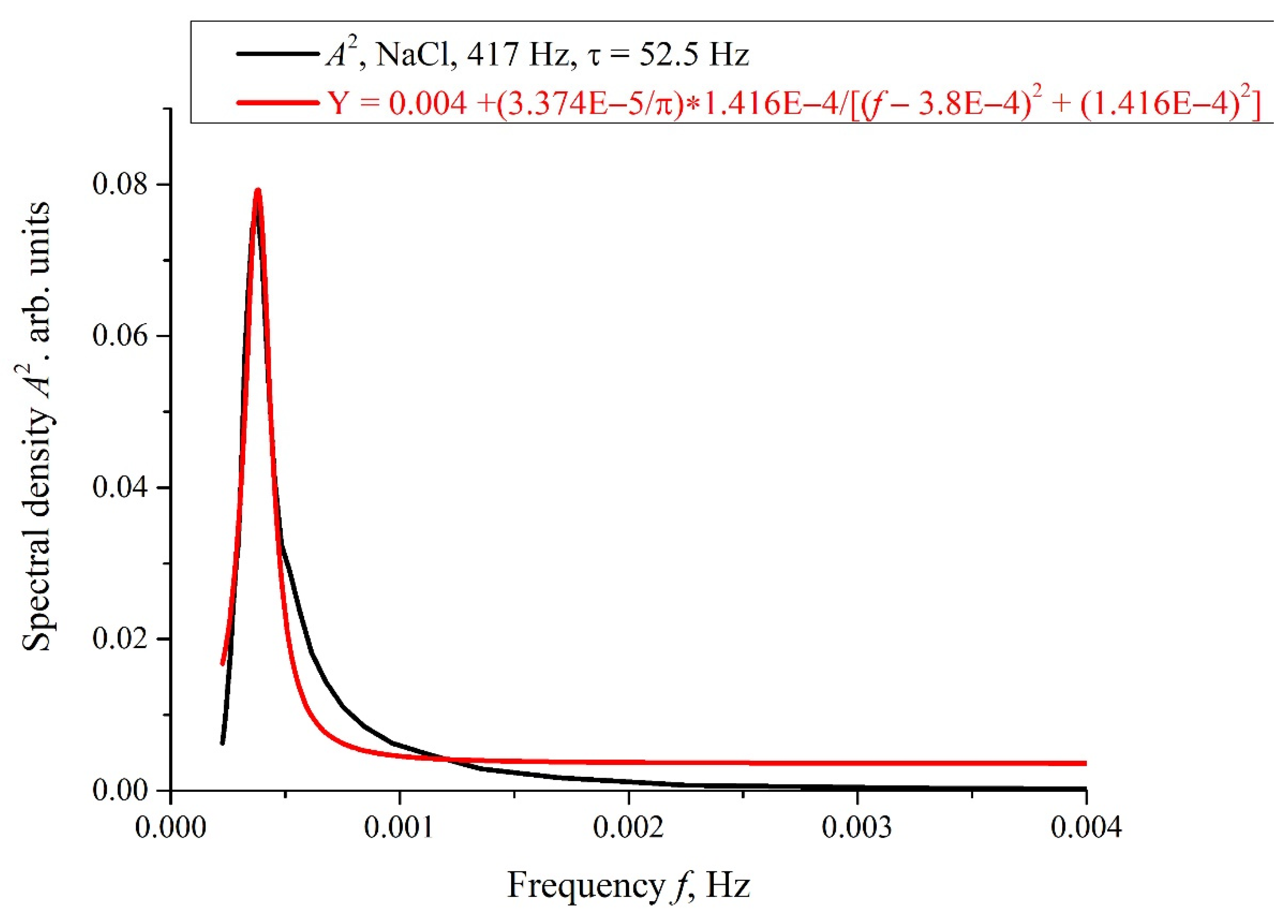
Figure 8, we show the dependence,
A2(
f), for the curve,
A(
f), shown in
Figure 7 for
τ = 52.5 h. In
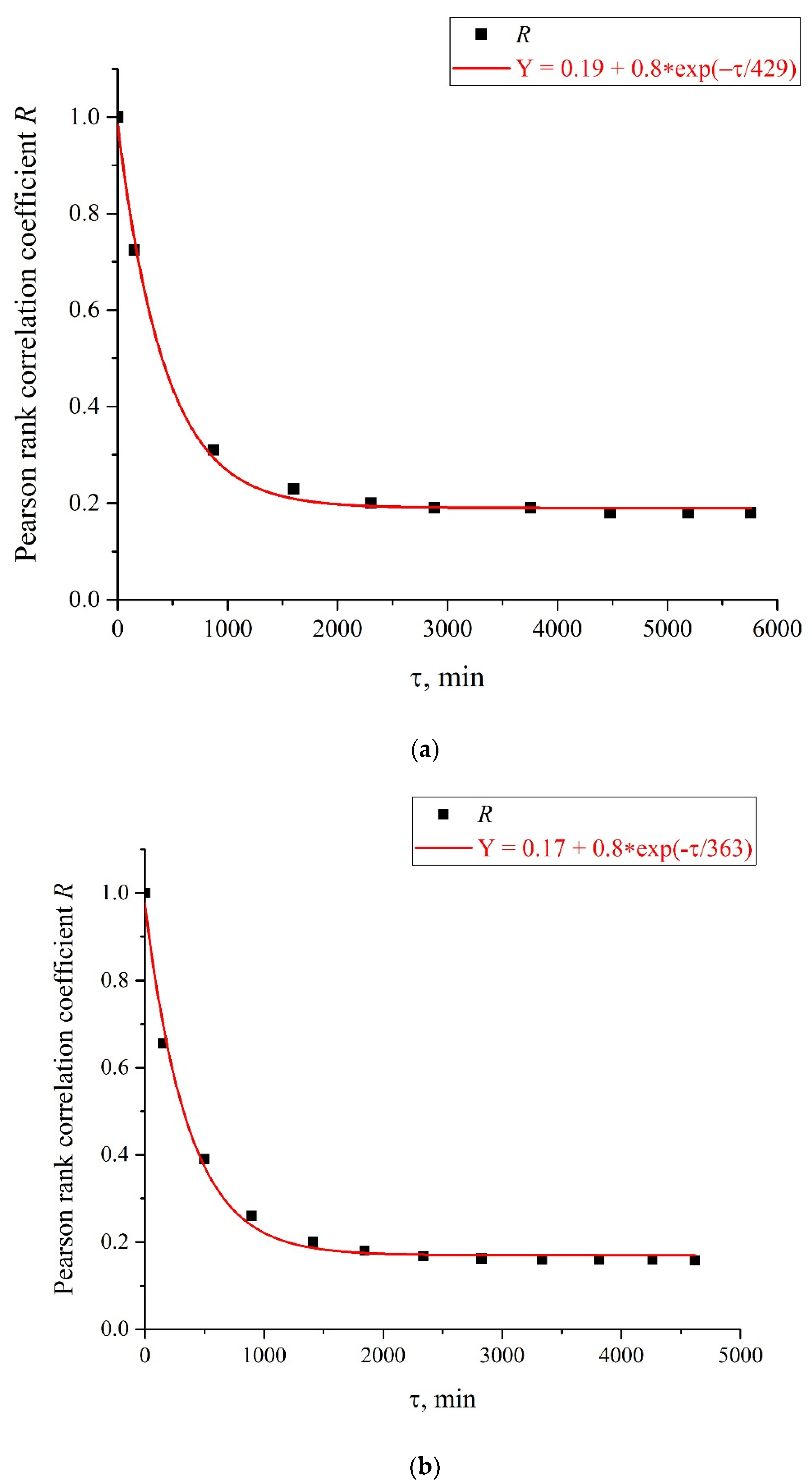
Figure 9a,b, we exhibit the dependences of the Pearson rank correlation coefficient,
R, vs. time,
τ. A detailed description of the methods for calculating the Pearson rank correlation coefficient can be found in the hyperlink [
37]. In our case, Pearson’s correlation coefficient, when applied to a sample, is described by the formula:
where
n is sample size and
xi and
yi are the individual sample points indexed with
i,
(the sample mean; analogously for
). In Formula (5), the set of values
xi corresponds to the first measurement of
I(
t) in
Figure 6 (indicated by the symbol (1) on the graphs), and the set of values
yi corresponds to the measurement of
I(
t) after a time,
τ. As follows from Equation (5),
R(
τ = 0) = 1.
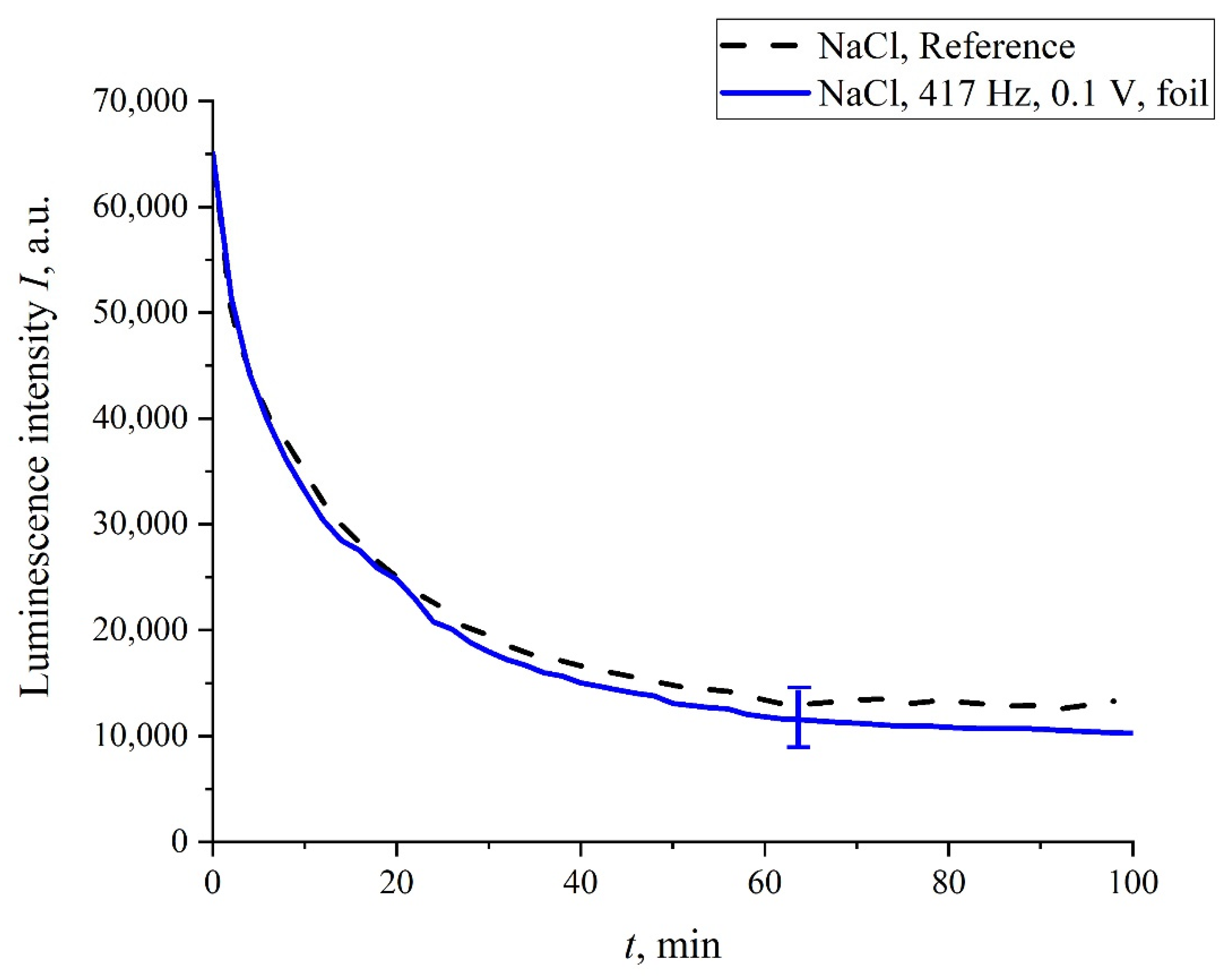
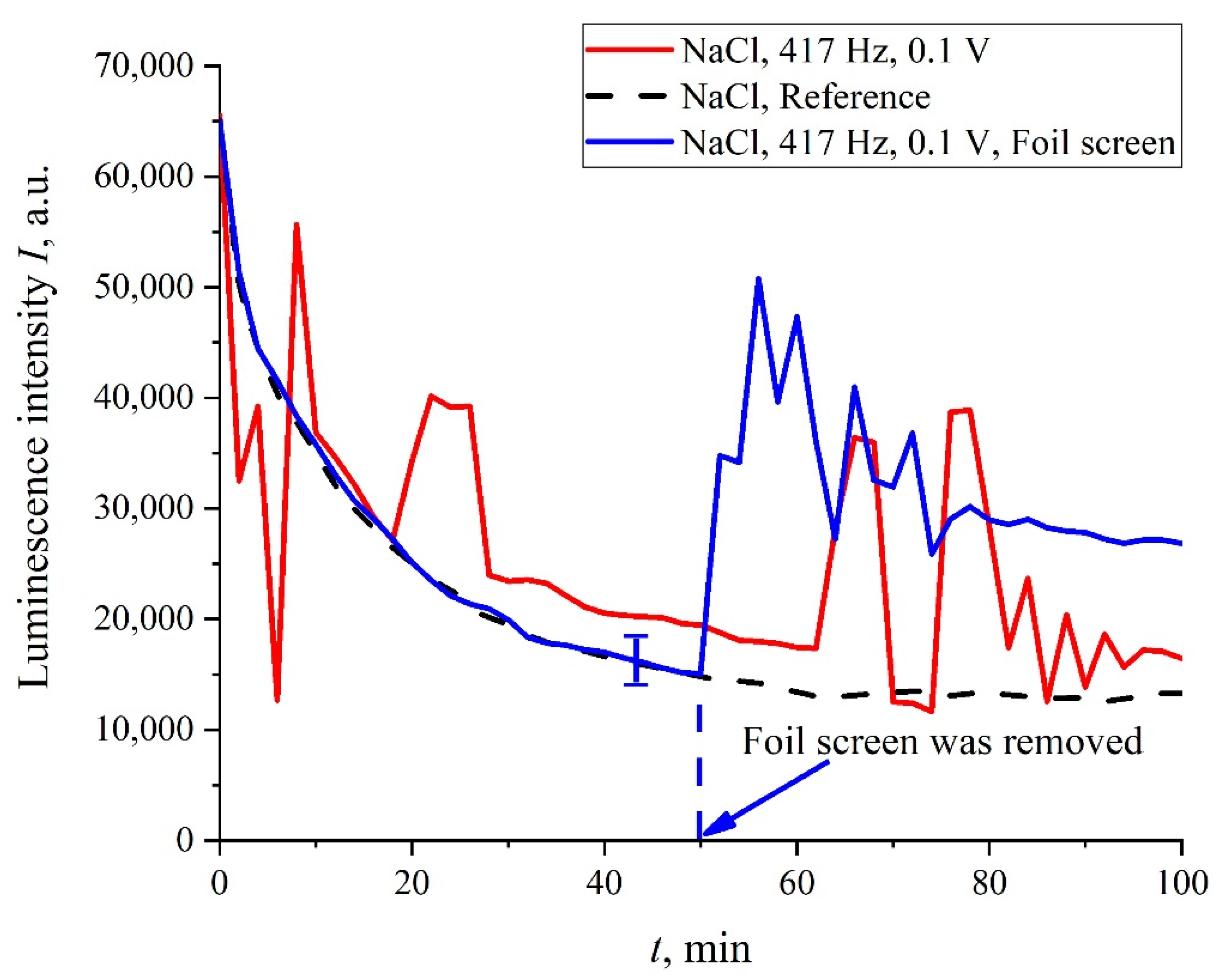
In addition, in some experiments we wrapped the flask containing the liquid sample with 10 μm-thick aluminum foil, and the sample was treated with electric pulses at 417 Hz repetition frequency. The results of this experiment are shown in
Figure 10. It is seen that, in this case, no stochastic behavior was observed, and the luminescence intensity,
I(
t), coincides with a good accuracy with the reference curve. This is quite expected: the treatment effect in this case is absent due to the screening of the liquid sample.
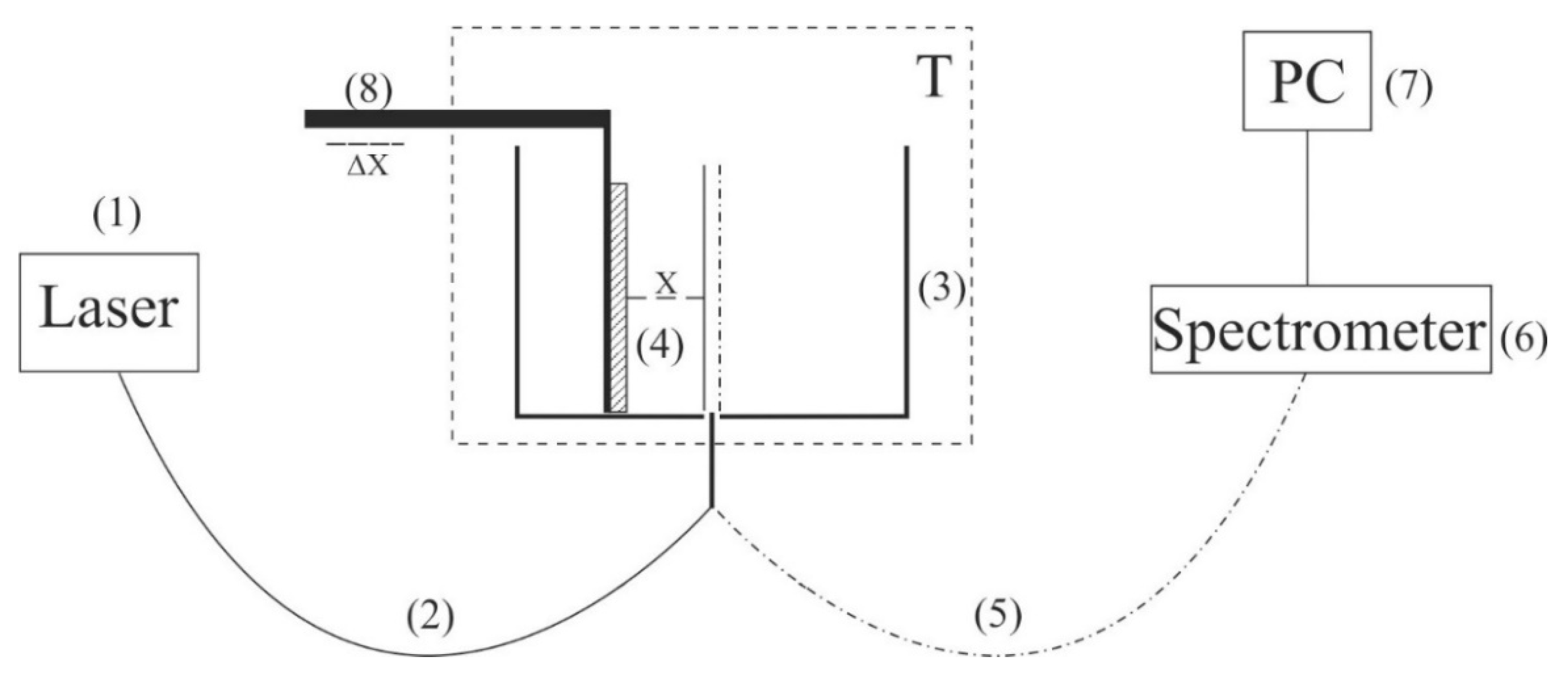
In addition, in some experiments we covered the photoluminescence setup (see
Figure 2) with 10 μm-thick aluminum foil; in these experiments, we also studied the luminescence from Nafion, swollen in isotonic solution of NaCl treated with electric pulses at 417 Hz frequency. In
Figure 11, we present two graphs obtained during the same experiment: without the foil screen and with the screen. We can see that if the setup is screened, the stochastic behavior is absent, and the luminescence intensity,
I(
t), is close to the reference curve. However, as the screen was removed, stochastic jumps in the luminescence signal appeared once again. This behavior can be explained by the effect of an external random force of an electromagnetic nature, which is manifested during the luminescent spectroscopy experiment.
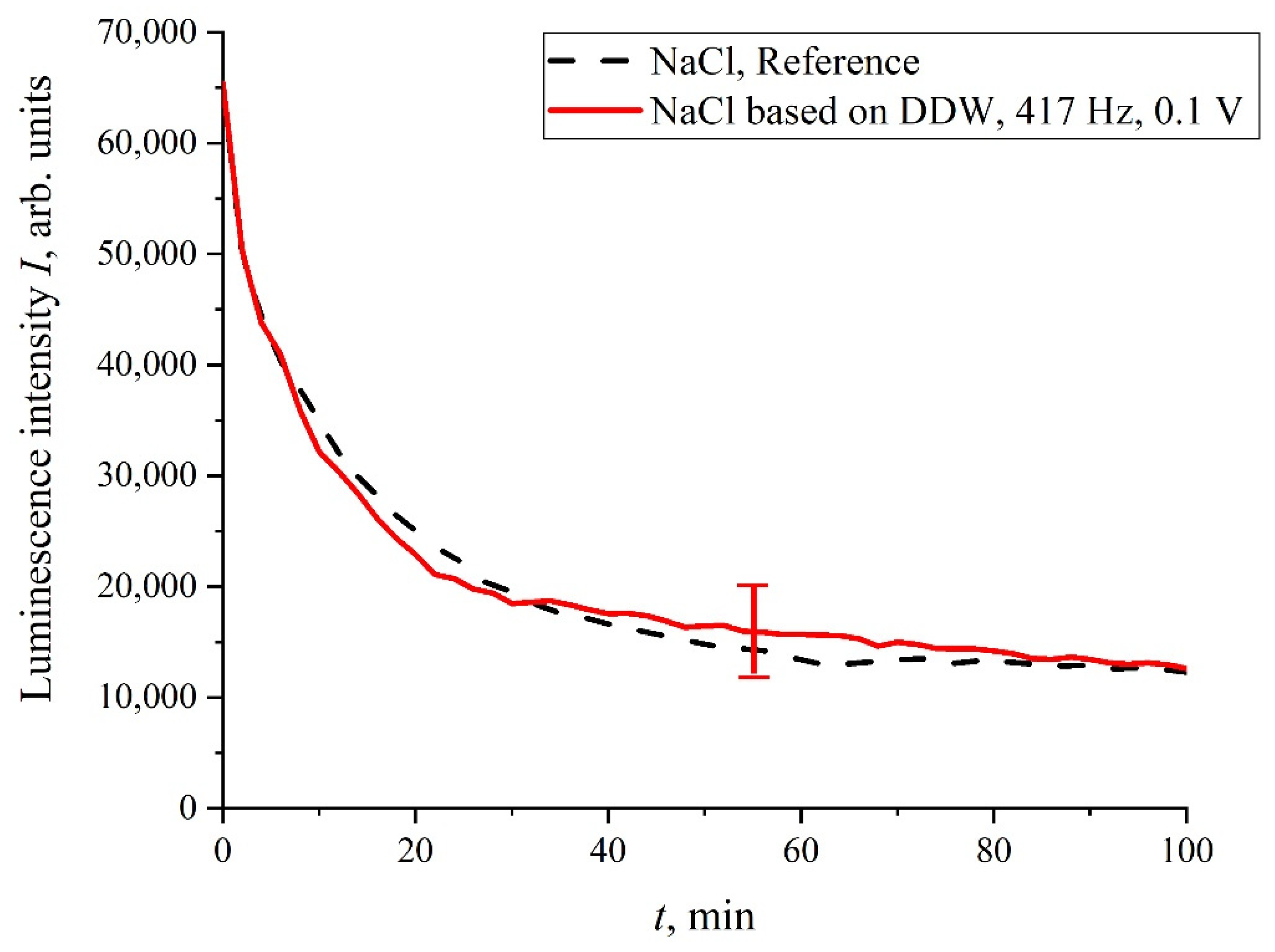
As was mentioned in the Introduction Section, in the process of swelling, the polymer fibers are effectively unwound into the bulk of liquid, but do not completely detach from the surface, i.e., a rigid brush-like structure is formed; the length of the area, occupied with unwound fibers, amounts to hundreds of microns. As shown in [
16], the unwinding effect depends on the deuterium content in a liquid sample. In fact, this effect is absent in the so-termed deuterium depleted water (DDW, the content of deuterium is ≤1 ppm). Thus, it is necessary to perform the luminescence experiment with the DDW-based liquid sample. In
Figure 12, we show the luminescence intensity,
I(
t), in isotonic NaCl DDW-based solution. It is seen that no stochastic oscillations are observed, i.e., the luminescence intensity,
I(
t), coincides with a good accuracy with the reference curve.
4. Discussion
The stochastic behavior of the intensity,
I(
t), shown in the graphs of
Figure 6 can be explained by the effects associated with an external low-frequency electromagnetic field. This follows from the fact that the random character of
I(
t) disappears when the experimental setup is screened with aluminum foil (see
Figure 11), but it appears once again when the screen is removed. As follows from the graph in
Figure 8, the spectral density of this electromagnetic wave is centered at the frequency
f0 = 3.8 × 10
−4 Hz, and the spectral line width at the half-height level is Δ
f = 1.416 × 10
−4 Hz (see the inset in
Figure 8). The value of Δ
f is consistent with the dependences of the correlation coefficient
R(
τ); see
Figure 9a,b. As is seen in
Figure 9a,b, the experimental points fit well the decaying exponential function
R(
τ) ~ exp(−
τ/
τcorr), where
τcorr is the decay time (correlation time). As follows from the graphs in
Figure 9, it does not matter from what moment of time we started the measurements of
I(
t). In addition, the results obtained are indifferent to the spatial position of the experimental setup. Thus, we are apparently dealing with stationary and spatially homogeneous external electromagnetic waves. As follows from the Wiener–Khinchin theorem [
36], the dependence
R(
τ) ~ exp(−
τ/
τcorr) corresponds to the spectral density of a random process, described by Lorentzian contour of width Δ
f ~ (
τcorr)
−1. In the case of pattern, exhibited in
Figure 9a, we have
τcorr = 25,740 s and Δ
f ≈ 0.4 × 10
−4 Hz. In the case of pattern in
Figure 9b, we have
τcorr = 21,780 s and Δ
f ≈ 0.5 × 10
−4 Hz. Thus, the values of Δf obtained on the basis of the data in the graphs of
Figure 8 and
Figure 9 are of the same order of magnitude. We still do not know why this stochastic behavior reveals only at certain frequencies of processing liquid samples.
In our opinion, an external source of electromagnetic radiation, due to which the effects described above arise, can be associated with the pulsations of distant space objects. In this regard, it is necessary to mention the recently published article [
38] devoted to the discovery of an unknown phenomenon that generates a giant energy outburst with a regular time interval of 18.18 min. This cosmic phenomenon is unlike anything scientists have encountered before. Researchers believe that the mysterious source of flashes is a neutron star or a white dwarf with a super-powerful magnetic field. This object is possibly rotating, emitting a beam of radiation, which becomes a very bright radio source within 30–60 s. The object itself is located at a distance of about four thousand light years from Earth. As shown in [
37], this object can be a magnetar with a very long period and an extremely strong magnetic field. This object is likely to effectively convert magnetic energy into radio waves. The frequencies of radio waves emitted during flashes of a minute duration lie in the range of 80–220 MHz, and this radiation is linearly polarized.
These flashes occur with a frequency of 1/18.18 min
−1 = 9 × 10
−4 Hz ≈ 2
f0. Therefore, we cannot assert that the source of the radio waves leading to the stochastic behavior of
I(
t) corresponds to that described in [
38]. It is very important for us that such sources really do exist. Therefore, without loss of generality, we will assume that we are dealing with pulses of a linearly polarized electromagnetic wave with a frequency of 100 MHz; the repetition rate of these pulses is
f0 = 3.8 × 10
−4 Hz.
Let us first show that there is no absorption of electromagnetic wave in water at the frequency
ω = 100 MHz. As is known [
39], the interaction of an external electromagnetic wave with medium is described using the complex permittivity
ε =
ε′ −
iε″, where the real part,
ε′, describes the “ability” of the medium to be polarized by the external field, and the imaginary part,
ε″, describes the energy loss associated with absorption and conversion to heat. For the coefficients
ε′ and
ε″, we have:
where
τ is the time of rotational diffusion of water molecules,
εS is static permittivity (for water εS = 81), ε∞ is the dielectric permittivity in the optical range (for water ε∞ = 1.77), r is molecular radius (for water r = 1.38 Å), and η is the dynamic viscosity (for water η = 8.9 × 10−4 Pa·s). After the substitutions for water under normal conditions, we obtain τ ≈ 8.27 ps. This implies the estimate ωτ ≈ 8.3 × 10−4, that is, ε″ << ε′ ≈ εS, and absorption at this frequency can be neglected.
As follows from
Figure 6 and
Figure 12, for the stochastic oscillations to occur, it is necessary that, during the swelling of the membrane, the polymer fibers must be unwound into the bulk of the liquid. We also recall that experiments on photoluminescent spectroscopy begin approximately 20 min after the end of the liquid treatment with electrical pulses. Below we present a qualitative model that describes the appearance of stochastic oscillations, taking into account the effects of long-term relaxation of liquid samples, as well as taking into account the unwinding of polymer fibers.
As shown in our recent work [
40], if a liquid is saturated with a dissolved gas (for example, atmospheric air) and has an ionic component, then gas nanobubbles spontaneously arise in it. These nanobubbles are stabilized due to the anion’s adsorption on the inner (from the side of gas phase) surface of the nanobubbles. Such nanobubbles were termed bubstons (abbreviation of bubble, stabilized by ions). When considering the stabilization of bubstons due to ionic adsorption, it is necessary to take into account that negatively charged (due to the adsorption of anions) gas cores are always surrounded by a spherically symmetric diffusion cloud of counterions (Debye screening). When bubstons move in a viscous liquid, the peripheral layers of this cloud are effectively “washed away”, and the bubstons are negatively charged. Indeed, during electrophoresis the bubbles move to the anode, see [
41,
42,
43]; regarding the methods for measuring the electrophoretic mobility of particles, see also [
44,
45,
46].
Note that in aqueous salt solutions with a sufficiently high ionic concentration, individual bubstons are capable of coagulating to one another with the formation of micron-sized bubston clusters. As shown in [
47], the bubstons, moving in viscous electrolytes solutions, acquire opposite signs, which leads to their coagulation due to centrally symmetric Coulomb attractive force:
Here,
Q is the bubston charge, which can be estimated based on the experiments with dynamic light scattering results and ζ-potential measurements; see [
40]. We get
Q~10
−17 C, i.e., the bubston charge is approximately a hundred elementary charges. The other parameters in Equation (9) are the following:
r is the bubston cluster radius (~1 μm) and
q is the effective charge of the center, which attracts a bubston in the coagulation process. Thus, we obtain an estimate
F′ . Here we do not know the exact value of the charge,
q; in Equation (9) we assume
q~−
Q. However, because the cluster is an aggregate of dimers consisting of particles, having the opposite sign, the charge,
q, should be calculated as a sum of the terms of the alternating series. Thus, most likely, |
q|
<< |Q|, i.e.,
F′ << 10
−14 N. It is clear that, due to the interaction (9) a spherically symmetric bubston cluster should form. Experimental studies of bubston clusters in aqueous solutions of NaCl are presented in [
48]; transpired that the cluster phase is manifested in experiments at ion concentrations >0.1 M. It is obvious that isotonic NaCl and Ringer solutions meet this condition, while in deionized water the concentration of ions is less, and the cluster phase is absent. Indeed, in accordance with our measurements, the pH value in deionized water is 5.7, i.e., the ion content is 10
−6 M, which is not sufficient for the coagulation of bubstons, and the clusters are not manifested in experiments with dynamic light scattering in water.
The characteristic lifetime of the bubstons/bubston clusters was measured in [
40]. In this experiment, the distribution of sizes of the scatterers in 1 M NaCl solution was measured, and then the liquid sample was settled for 6 months under stationary conditions in hermetically sealed (without access of atmospheric air) cell of 2-cm height. It transpired that the micron-sized scatterers (bubston clusters) disappear after this long-term settling, while submicron-sized scatterers (individual bubstons) remain in the liquid (“survive”). The disappearance of large scatterers is obviously due to their floating up, following by destroying at the liquid interface, while individual bubstons have a neutral buoyancy, i.e., they do not float up. Thus, we can argue that bubstons are an equilibrium phase of an aqueous electrolyte solution under normal conditions, and the bubston clusters are a long-lived phase.
Let us now assume that the uniform electric field of a flat capacitor, used for the treatment of the liquid samples, has a strength of
E ~ 1 V/m (the voltage was ~0.1 V). Thus, a force
F = QE~10
−17 N arises in a flat capacitor, which is superimposed on the spherically symmetric Coulomb force (9). Assuming
F′~10
−14 N, we have an
F/F′~10
−3, but this is apparently underestimated, see above. Assuming
F′ ≈
F, we find that the force,
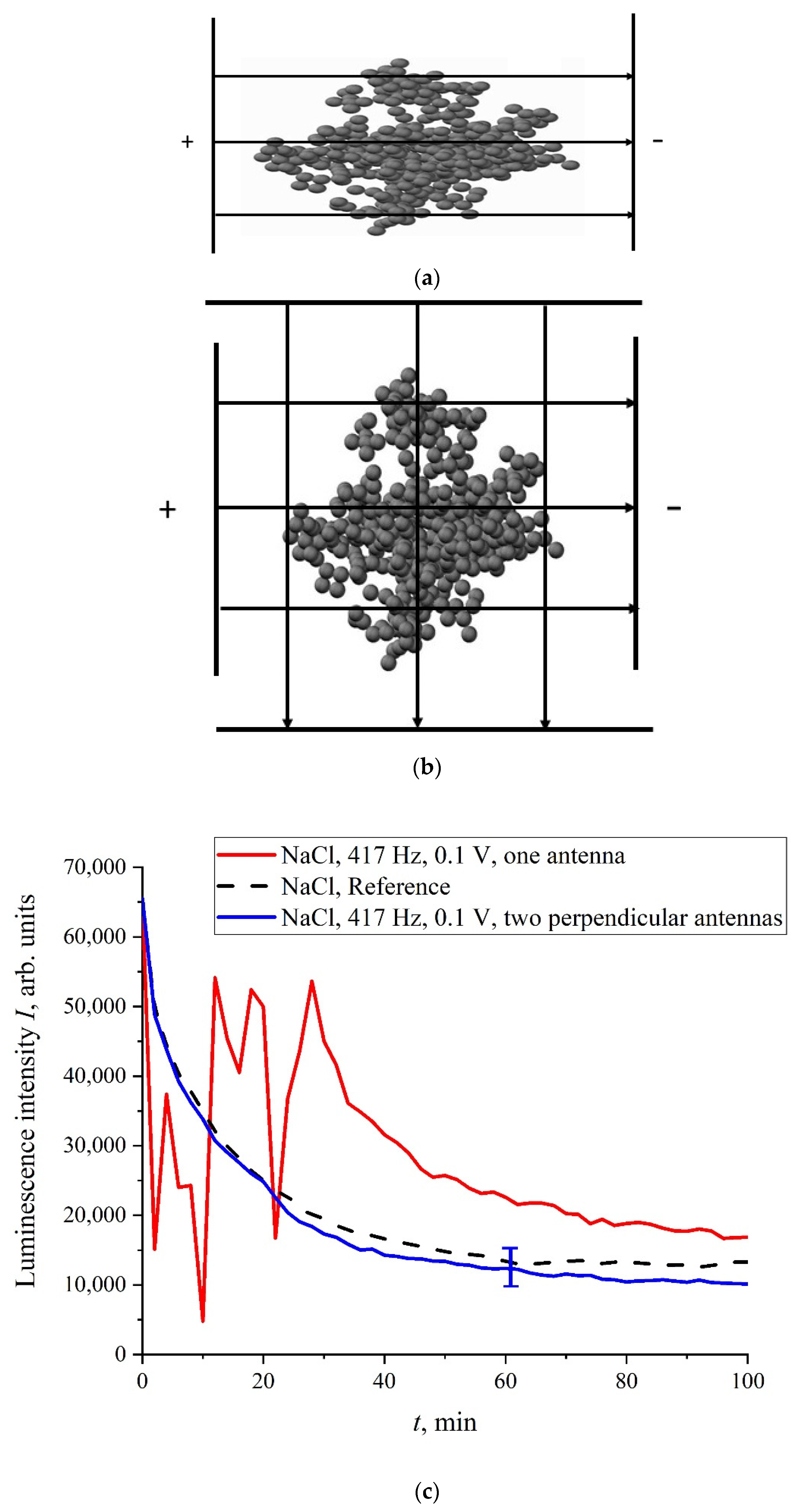
F, generated inside a plane capacitor, can violate the bubston cluster’s spherical symmetry, and the cluster becomes slightly anisotropic. This was indirectly confirmed in the experiment with the NaCl solution treatment at a frequency of 417 Hz; in this case, one flat capacitor (see
Figure 13a) or two flat capacitors, installed normally with each other (
Figure 13b), were used. The dependence,
I(
t), is shown in
Figure 13c. As follows from the graphs in
Figure 13c, when using one flat capacitor, the luminescence intensity,
I(
t), behaves stochastically (red curve), while processing with two mutually perpendicular capacitors (blue curve), the run of
I(
t) is close to the reference dependence.
Obviously, when we are dealing with two perpendicular capacitors, the external electric field does not have a fixed axis, i.e., this field does not change the bubston cluster sphericity. In this case, the luminescence intensity is close to the reference dependence, which is fairly well reproduced. If the processing takes place in the field of one flat capacitor, then the behavior of I(t) is random. Thus, for the memory effect it is necessary to change the cluster geometry: the cluster must be slightly anisotropic, and these anisotropic properties should persist for a rather long time. Indeed, any physical mechanisms for the relaxation of an anisotropic cluster to its spherical symmetry are evidently absent.
Let us imagine that external linearly polarized radiation at a frequency of 100 MHz is scattered by bubston clusters. If the clusters are spherically symmetric, then the scattered radiation will remain linearly polarized. However, if the clusters are anisotropic, the scattered radiation will be depolarized, see, for example, [
49]. Of course, it should be kept in mind that the scattering cross section,
σsca, in this case will be very small, because
σsca ~ 1/λ
4 [
39] and λ ~ 1 m. Scattered radiation will interact with charged polymer fibers unwound into the bulk of the liquid. We do not know the mass and charge of these fibers, so we do not present formulas that describe the dynamics of these fibers in the field of the incident wave. However, in accordance with the literature data, radiation at frequencies of this range is used in medical practice (in particular, for the treatment of oncological diseases), and this radiation is related not only to electromagnetic waves, but also to acoustic waves; see [
50,
51]. Based on these indirect data, we can argue that the polymer fibers unwound into the liquid bulk will oscillate at frequency of 100 MHz. In the case of linearly polarized radiation, these oscillations will occur in one plane, but in the case of depolarized radiation, the oscillations will happen in different planes.
As follows from Equation (1), if the luminescence cross section,
σlum, does not change upon swelling, then the luminescence intensity,
I(
t), decreases exponentially. Thus, the stochastic behavior can be explained by random oscillations of
σlum. To explain this effect on a qualitative level, it is necessary to use the model of non-radiative energy transfer from a donor of luminescence to an acceptor of luminescence; see monograph [
52]. Let us imagine that there exists a luminescence center on the membrane surface, which we will call a donor of luminescence. Let us further imagine that at a certain distance,
R, from the donor there exists another particle (acceptor of luminescence), whose absorption spectrum coincides with the absorption spectrum of the donor. Then, at a certain
R the process of resonant energy transfer from the donor luminescent level to the acceptor level is possible. An electron from an acceptor level passes into the ground state of an acceptor, which can be accompanied by a photon (luminescence) emission, but a non-radiative transition is also possible. In this case, the luminescence from the donor is quenched. If we consider protein membranes, then donors and acceptors, as a rule, are the same groups of proteins, but in the case of acceptors these groups are slightly changed; see [
52]. Because in our case the luminescence centers are sulfonic groups, we can assume that the acceptor is a slightly modified sulfonic group, which is not active with respect to the luminescence. The efficiency,
S, of energy transfer from a donor to an acceptor is given as:
Here,
R0 = 30–60 Å is the so-called Forster parameter; see [
52]. Thus, the value of
S varies as
R−6, i.e., it is a very steep function. Note that if the donor and acceptor are rigidly fixed on the membrane surface, then the distance,
R, between them is always fixed, i.e., the luminescence cross section,
σlum, does not change upon swelling. However, in our case, the donor and acceptor are located on the polymer fibers unwound in the liquid bulk, i.e., their spatial position can change due to some external electromagnetic forces (remember that sulfonic groups are charged). If these forces change the distance,
R, then, according to Equation (10), the value of
S can be either ~1, and the luminescence stops (the effect of quenching), or
S << 1, and in this case there is no energy transfer, and the luminescence is quite intense. Thus, the only mechanism in our experiments, due to which the luminescence can disappear/reappear in NaCl solutions based upon natural water, is changing the distance,
R, which leads to the oscillations of
σlum. It seems obvious that when the polymer fibers, unwound into the liquid bulk, are driven by linearly polarized radiation, these fibers will vibrate in the single plane. In this case the distance,
R, between the donor and the acceptor does not change, that is, the value of
σlum remains constant. At the same time, if these fibers are swayed by depolarized radiation, that is, there exist polarizations in different planes, then the distance,
R, will change randomly, and stochastic switching of luminescence quenching/excitation modes is possible. Within the framework of this model, the appearance of stochastic phenomena in our experiments is qualitatively explained. The question of why the addition of piperine also leads to the stochastization in the behavior of
I(
t) remains unclear.
We can assume that random oscillations of
I(
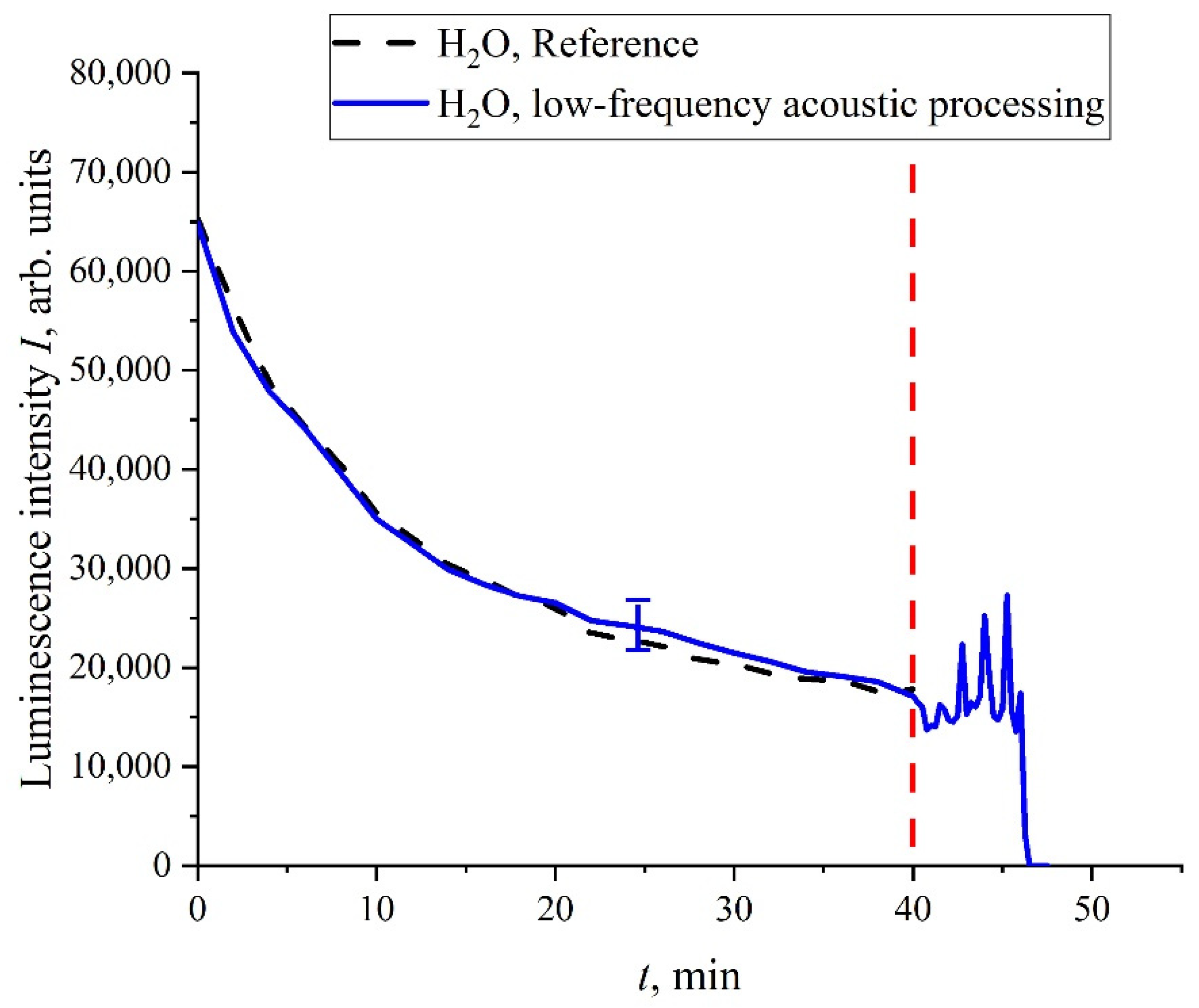
t) can also arise under mechanical disturbances of the unwound polymer fibers. To verify this, the following experiment was performed. We studied doubly distilled water where the bubstons content is very low, and the cluster phase is practically absent; the liquid samples were not processed with electric pulses. A capillary of 0.5 mm thickness was inserted into the cell (not shown in
Figure 2), where the Nafion membrane was soaked. After 40 min of soaking, atmospheric air was pumped through this capillary into the cell. This was accompanied by the generation of bubbles that floated up to the surface of water and generated shear capillary waves at the surface. In
Figure 14, we show the dependence of
I(
t). It is seen that, prior to the pumping of bubbles, the curve,
I(
t), coincides to a good accuracy with the reference dependence. As soon as the shear capillary wave was excited, the oscillations of
I(
t) started; at a certain moment of time, the luminescence signal dropped to zero. It is very important that, in this experiment, the Nafion membrane was entirely submerged in the bulk of water. As is known, shear sound waves are very strongly attenuated in the liquid bulk; see [
53]. Nevertheless, the shear wave generation at the surface was accompanied by the oscillations of
I(
t) in the liquid bulk. This wave should be very weak in the bulk, but nonetheless it can change the distance,
R, between the donor and the acceptor on the unwound polymer fibers. We can argue that Nafion is a highly sensitive polymer matrix: very weak external force, which can change the distance,
R, and can lead to a noticeable change in the behavior of
I(
t). Therefore, depolarized scattered radiation leads to stochastization effects despite a very small scattering cross section,
σsca.