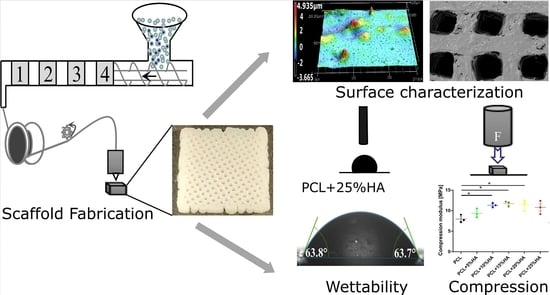
Fabrication and Characterization of PCL/HA Filament as a 3D Printing Material Using Thermal Extrusion Technology for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
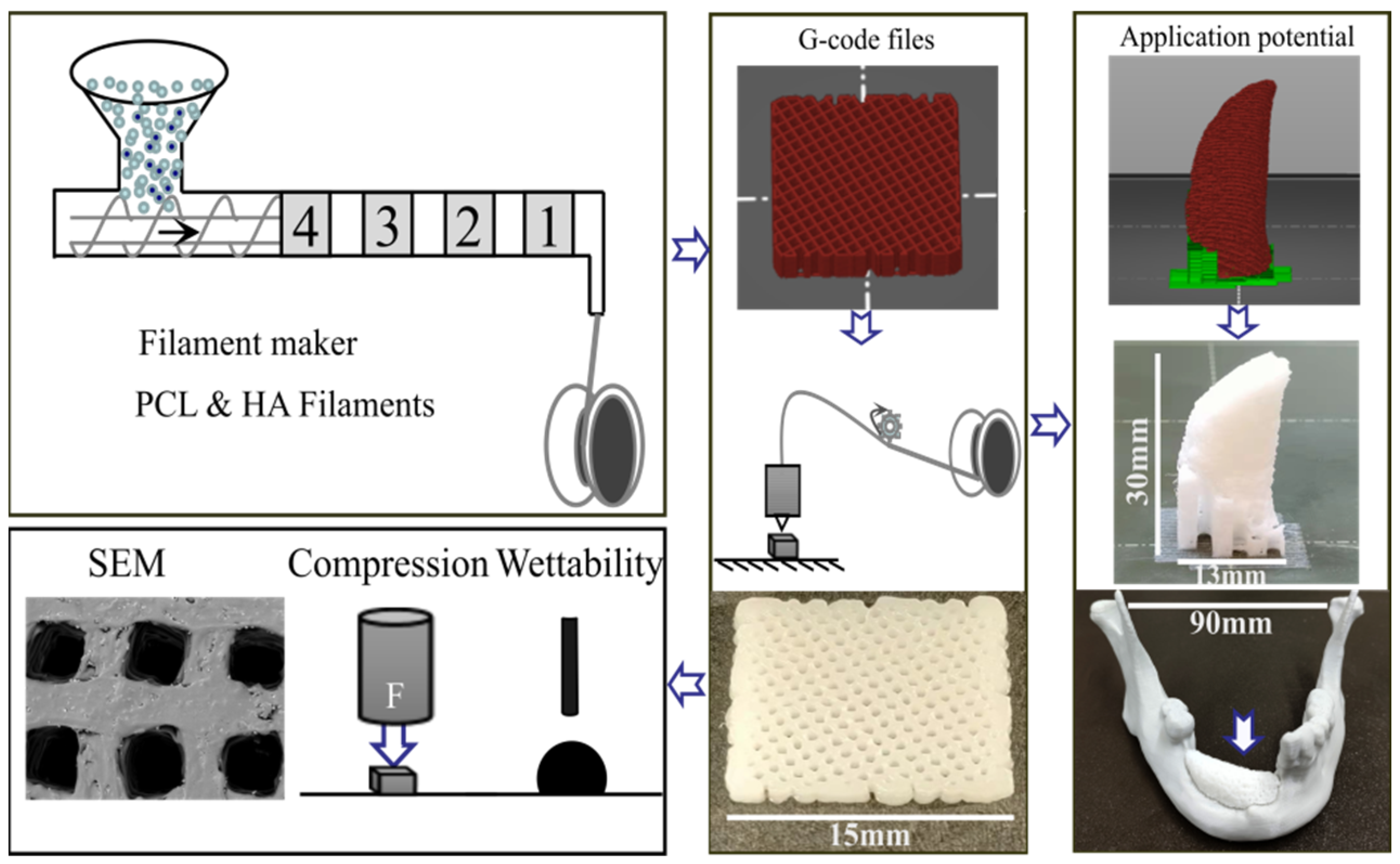
2.1. PCL and HA Composite Filament Fabrication
2.2. 3D Printing of Scaffolds
2.3. Surface Roughness and Pore Size Quantification
2.4. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy
2.5. Water Contact Angle
2.6. Mechanical Test
2.7. Statistical Analysis
3. Results
3.1. Filament Fabrication
3.2. Printing Procedure
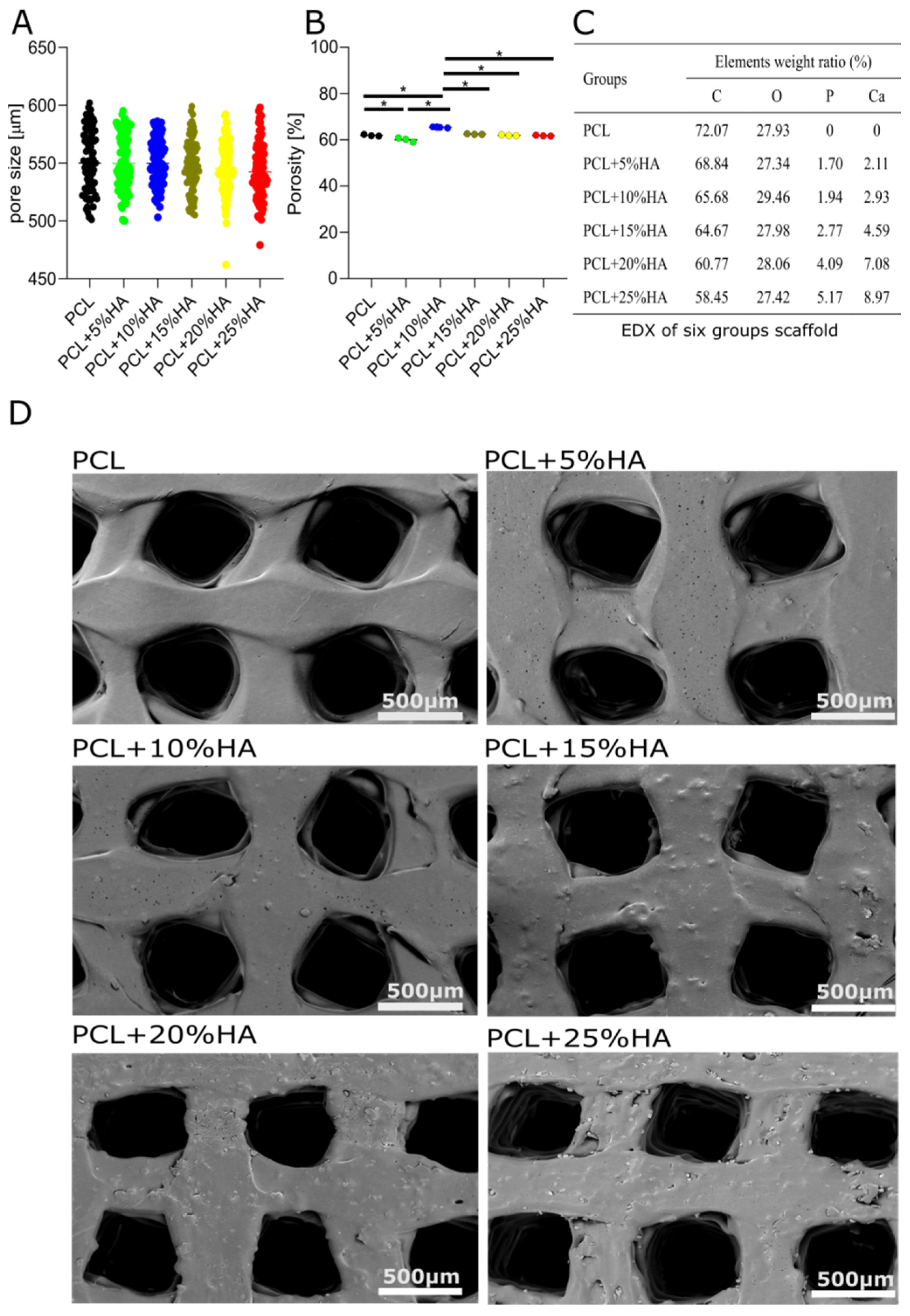
3.3. Specimen Morphological Characterization
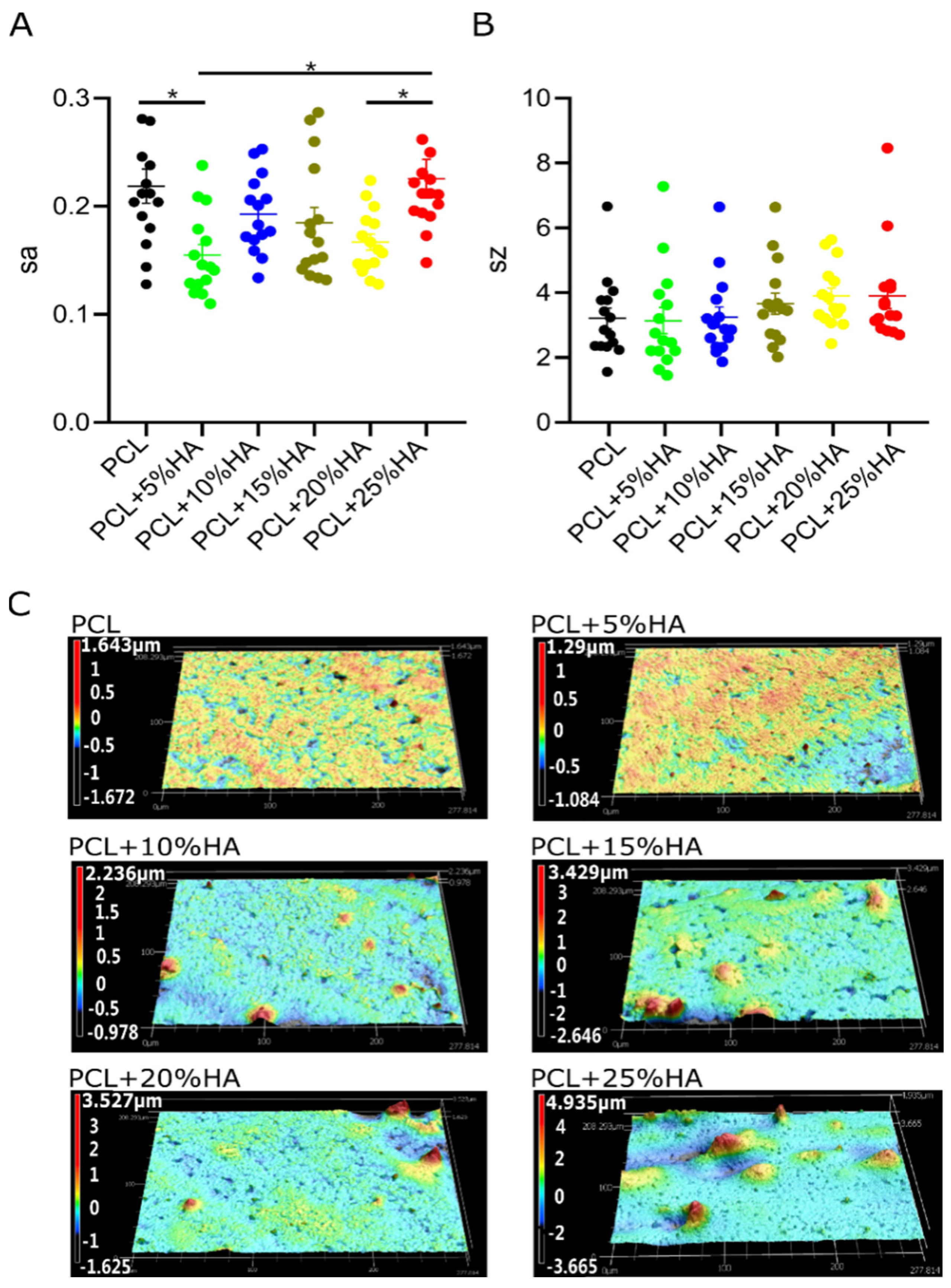
3.4. Specimen Roughness Characterization
3.5. Water Contact Angle Test
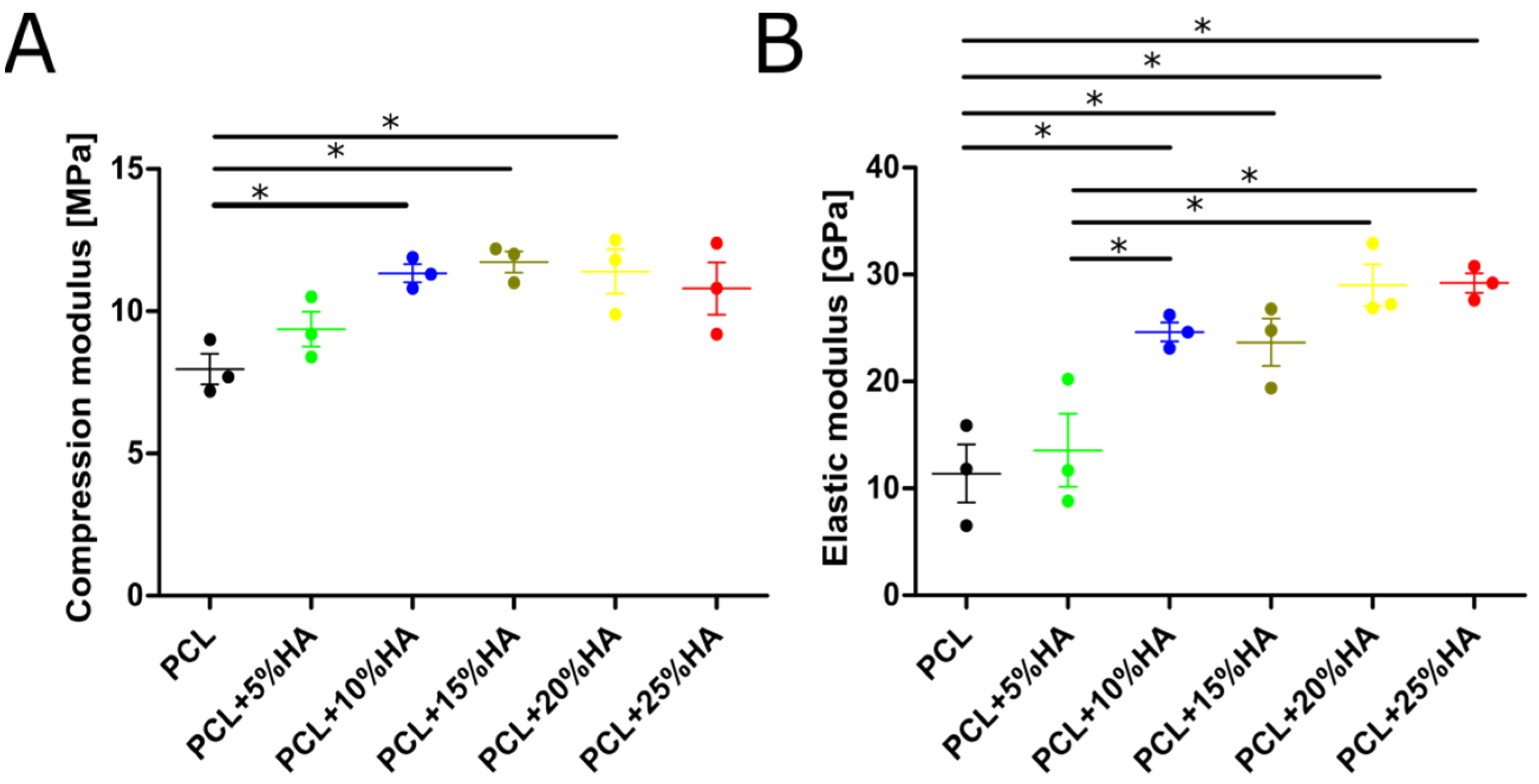
3.6. Mechanical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parthasarathy, J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann. Maxillofac. Surg. 2014, 4, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Obregon, F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Bertassoni, L. Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial Tissue Engineering. J. Dent. Res. 2015, 94, 143S–152S. [Google Scholar] [CrossRef] [PubMed]
- William, G., Jr.; Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J. Bone Joint Surg. Am. 2007, 89, 649–658. [Google Scholar]
- Mallik, S.B.; Jayashree, B.; Shenoy, R.R. Epigenetic modulation of macrophage polarization- perspectives in diabetic wounds. J. Diabetes Complicat. 2018, 32, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Han, J.; Sharma, N.; Msallem, B.; Jeong, W.; Son, J.; Kunz, C.; Kang, H.-W.; Thieringer, F.M. In vitro mechanical and biological properties of 3D Printed polymer composite and beta-tricalcium phosphate scaffold on human dental pulp stem cells. Materials 2020, 13, 3057. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Mohammadi, M. In vitro study of hydroxyapatite/polycaprolactone (HA/PCL) nanocomposite synthesized by an in situ sol–gel process. Mater. Sci. Eng. C 2013, 33, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Pramanik, K.; Jabbari, E. Osteogenic differentiation of human mesenchymal stem cells in freeze-gelled chitosan/nano β-tricalcium phosphate porous scaffolds crosslinked with genipin. Mater. Sci. Eng. C 2015, 54, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, N.; Madala, S.; Parcha, S.R.; Mallick, S.P. Osteogenic differentiation ability of human mesenchymal stem cells on Chitosan/Poly (Caprolactone)/nano beta Tricalcium Phosphate composite scaffolds. Biomed. Phys. Eng. Express 2020, 6, 015018. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Lizárraga, K.K.; Flores-Morales, C.; Del Prado-Audelo, M.; Álvarez-Pérez, M.; Piña-Barba, M.; Escobedo, C. Polycaprolactone- and polycaprolactone/ceramic-based 3D-bioplotted porous scaffolds for bone regeneration: A comparative study. Mater. Sci. Eng. C 2017, 79, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Da Silva, C.E.R.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Acutis, A.; De Maria, C.; Vozzi, G. Multimaterial and multiscale rapid prototyping of patient-specific scaffold. Adv. Sci. Technol. 2017, 100, 151–158. [Google Scholar]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachlos, E.; Czernuszka, J. Making Tissue Engineering Scaffolds Work. Review: The application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cells Mater. 2003, 5, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Luo, Y.; Cuniberti, G.; Xiao, Y.; Gelinsky, M. Three-dimensional printing of hierarchical and tough mesoporous bioactive glass scaffolds with a controllable pore architecture, excellent mechanical strength and mineralization ability. Acta Biomater. 2011, 7, 2644–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Shoma, K.; Suresh, S.; He, H.; Rajput, R.S.; Feng, Q.; Ramesh, S.; Wang, Y.; Krishnan, S.; Ostrovidov, S.; et al. 3D Printing of Micro- and Nanoscale Bone Substitutes: A Review on Technical and Translational Perspectives. Int. J. Nanomed. 2021, 16, 4289–4319. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzoli, A. Selective laser sintering in biomedical engineering. Med Biol. Eng. Comput. 2012, 51, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sharma, V. Additive manufacturing techniques for the fabrication of tissue engineering scaffolds: A review. Rapid Prototyp. J. 2021, 27, 1230–1272. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Do, T.; Tran, P. FDM-Based 3D Printing of Polymer and Associated Composite: A Review on Mechanical Properties, Defects and Treatments. Polymers 2020, 12, 1529. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tappa, K.; Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HA-PCL-Cost. Available online: https://www.sigmaaldrich.com/CH/en/search/hydroxyapatite (accessed on 21 December 2021).
- Yeong, W.Y.; Chua, C.-K.; Leong, K.F.; Chandrasekaran, M. Rapid prototyping in tissue engineering: Challenges and potential. Trends Biotechnol. 2004, 22, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Kandi, R.; Pandey, P.M.; Majood, M.; Mohanty, S. Fabrication and characterization of customized tubular scaffolds for tracheal tissue engineering by using solvent based 3D printing on predefined template. Rapid Prototyp. J. 2021, 27, 421–428. [Google Scholar] [CrossRef]
- Kim, C.; Han, K.; Lee, S.; Kim, M.; Kim, S.; Nah, J. Fabrication of Biocompatible Polycaprolactone–Hydroxyapatite Composite Filaments for the FDM 3D Printing of Bone Scaffolds. Appl. Sci. 2021, 11, 6351. [Google Scholar] [CrossRef]
- Park, A.S.; Lee, S.H.; Kim, W.D. Fabrication of porous polycaprolactone/hydroxyapatite (PCL/HA) blend scaffolds using a 3D plotting system for bone tissue engineering. Bioprocess Biosyst. Eng. 2010, 34, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Shor, L.; Gordon, J.; An, Y.; Guceri, S.; Sun, W. Precision Extruding Deposition of Polycaprolactone and Composite Polycaprolactone/Hydroxyapatite Scaffolds for Tissue Engineering. In Proceedings of the IEEE 32nd Annual Northeast Bioengineering Conference, Easton, PA, USA, 1–2 April 2006; pp. 73–74. [Google Scholar]
- Li, Y.; Yu, Z.; Ai, F.; Wu, C.; Zhou, K.; Cao, C.; Li, W. Characterization and evaluation of polycaprolactone/hydroxyapatite composite scaffolds with extra surface morphology by cryogenic printing for bone tissue engineering. Mater. Des. 2021, 205, 109712. [Google Scholar] [CrossRef]
- Naghieh, S.; Ravari, M.K.; Badrossamay, M.; Foroozmehr, E.; Kadkhodaei, M. Numerical investigation of the mechanical properties of the additive manufactured bone scaffolds fabricated by FDM: The effect of layer penetration and post-heating. J. Mech. Behav. Biomed. Mater. 2016, 59, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sa, M.-W.; Nguyen, B.-N.B.; Moriarty, R.A.; Kamalitdinov, T.; Fisher, J.P.; Kim, J.Y. Fabrication and evaluation of 3D printed BCP scaffolds reinforced with ZrO 2 for bone tissue applications. Biotechnol. Bioeng. 2018, 115, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Dezaki, M.L.; Ariffin, M.K.A.M.; Hatami, S. An overview of fused deposition modelling (FDM): Research, development and process optimisation. Rapid Prototyp. J. 2021, 27, 562–582. [Google Scholar] [CrossRef]
- Zavřel, F.; Novák, M.; Kroupová, J.; Beveridge, C.; Štěpánek, F.; Ruphuy, G. Development of hot-melt extrusion method to produce hydroxyapatite/polycaprolactone composite filaments. Adv. Eng. Mater. 2021, 2100820. [Google Scholar] [CrossRef]
- Gradwohl, M.; Chai, F.; Payen, J.; Guerreschi, P.; Marchetti, P.; Blanchemain, N. Effects of Two Melt Extrusion Based Additive Manufacturing Technologies and Common Sterilization Methods on the Properties of a Medical Grade PLGA Copolymer. Polymers 2021, 13, 572. [Google Scholar] [CrossRef]
- Manual, D.E. 3devo Filament Maker. Available online: https://3devo.com/wp-content/uploads/2017/10/NEXT-Quick-Start-Guide.pdf (accessed on 20 December 2021).
- Jin, G.; Kim, G. The effect of sinusoidal AC electric stimulation of 3D PCL/CNT and PCL/beta-TCP based bio-composites on cellular activities for bone tissue regeneration. J. Mater Chem. B 2013, 1, 1439–1452. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2018, 84, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, J.; Sandler, N. Printing and additive manufacturing. AAPS PharmSciTech 2019, 20, 261. [Google Scholar] [CrossRef] [PubMed]
- Mertz, L. Dream it, design it, print it in 3-D: What can 3-D printing do for you? IEEE Pulse 2013, 4, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.P.; Gaspar, V.M.; Mano, J.F. Decellularized Extracellular Matrix for Bioengineering Physiomimetic 3D in Vitro Tumor Models. Trends Biotechnol. 2020, 38, 1397–1414. [Google Scholar] [CrossRef] [PubMed]
- Neufurth, M.; Wang, X.; Wang, S.; Steffen, R.; Ackermann, M.; Haep, N.D.; Schröder, H.C.; Müller, W.E. 3D printing of hybrid biomaterials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone. Acta Biomater. 2017, 64, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Raeisdasteh Hokmabad, V.; Davaran, S.; Ramazani, A.; Salehi, R. Design and fabrication of porous biodegradable scaffolds: A strategy for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1797–1825. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.; D’Antò, V.; Guarino, V.; Sardella, E.; Zeppetelli, S.; Favia, P.; Ambrosio, L. Biomineralized porous composite scaffolds prepared by chemical synthesis for bone tissue regeneration. Acta Biomater. 2010, 6, 4090–4099. [Google Scholar] [CrossRef] [PubMed]
- Zimmerling, A.; Yazdanpanah, Z.; Cooper, D.M.L.; Johnston, J.D.; Chen, X. 3D printing PCL/nHA bone scaffolds: Exploring the influence of material synthesis techniques. Biomater. Res. 2021, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pu’Ad, N.M.; Haq, R.A.; Noh, H.M.; Abdullah, H.; Idris, M.; Lee, T. Review on the fabrication of fused deposition modelling (FDM) composite filament for biomedical applications. Mater. Today: Proc. 2020, 29, 228–232. [Google Scholar]
- Beatrice, C.A.G.; Shimomura, K.M.B.; Backes, E.H.; Harb, S.V.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Engineering printable composites of poly (ε-polycaprolactone)/β-tricalcium phosphate for biomedical applications. Polym. Compos. 2021, 42, 1198–1213. [Google Scholar] [CrossRef]
- Modi, Y.K.; Sahu, K.K. Process parameter optimization for porosity and compressive strength of calcium sulfate based 3D printed porous bone scaffolds. Rapid Prototyp. J. 2021, 27, 245–255. [Google Scholar] [CrossRef]
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 2006, 27, 5892–5900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vasconcellos, L.M.R.; Leite, D.O.; De Oliveira, F.N.; Carvalho, Y.R.; Cairo, C.A.A. Evaluation of bone ingrowth into porous titanium implant: Histomorphometric analysis in rabbits. Braz. Oral Res. 2010, 24, 399–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, E.M.; Oliveira, F.; Silva, R.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 1210–1219. [Google Scholar] [CrossRef]
- Murphy, C.M.; Duffy, G.P.; Schindeler, A.; O’Brien, F.J. Effect of collagen-glycosaminoglycan scaffold pore size on matrix mineralization and cellular behavior in different cell types. J. Biomed. Mater. Res. Part A 2015, 104, 291–304. [Google Scholar] [CrossRef]
- Santarella, F.; Sridharan, R.; Marinkovic, M.; Amaral, R.J.F.C.D.; Cavanagh, B.; Smith, A.; Kashpur, O.; Gerami-Naini, B.; Garlick, J.A.; O’Brien, F.J.; et al. Scaffolds functionalized with matrix from induced pluripotent stem cell fibroblasts for diabetic wound healing. Adv. Health Mater. 2020, 9, e2000307. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Cool, S. Concepts of scaffold-based tissue engineering-the rationale to use solid free-form fabrication techniques. J. Cell. Mol. Med. 2007, 11, 654–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.-H.; Heo, G.-M.; Park, H.-J.; Oh, H.-K.; Kook, M.-S. Comparative Effectiveness of Surface Functionalized Poly-ε-Caprolactone Scaffold and β-TCP Mixed PCL Scaffold for Bone Regeneration. J. Nanosci. Nanotechnol. 2020, 20, 5349–5355. [Google Scholar] [CrossRef] [PubMed]
- Rohr, N.; Balmer, M.; Jung, R.E.; Kohal, R.; Fischer, J. Influence of zirconia implant surface topography on first bone implant contact within a prospective cohort study. Clin. Implant Dent. Relat. Res. 2021, 23, 593–599. [Google Scholar] [CrossRef]
- Jiang, W.; Shi, J.; Li, W.; Sun, K. Morphology, wettability, and mechanical properties of polycaprolactone/hydroxyapatite composite scaffolds with interconnected pore structures fabricated by a mini-deposition system. Polym. Eng. Sci. 2012, 52, 2396–2402. [Google Scholar] [CrossRef]
- Jong-Woo, K.; Kwan-Ha, S.; Young-Hag, K.; Jin, H.M.; Jiyoung, M.; Hyoun-Ee, K. Production of Poly(ε-Caprolactone)/hydroxyapatite composite scaffolds with a tailored macro/micro-porous structure, high mechanical properties, and excellent bioactivity. Materials 2017, 10, 1123. [Google Scholar]
- Yeo, A.; Wong, W.J.; Khoo, H.H.; Teoh, S.H. Surface modification of PCL-TCP scaffolds improve interfacial mechanical interlock and enhance early bone formation: An in vitro and in vivo characterization. J. Biomed. Mater Res. A 2010, 92, 311–321. [Google Scholar] [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef]
- Lakatos, É.; Magyar, L.; Bojtár, I. Material properties of the mandibular trabecular bone. J. Med. Eng. 2014, 2014, 470539. [Google Scholar] [CrossRef]

| Groups | Temperature Gradients/°C | Extruder RPM & Filament (mm/s) Speed | |||
|---|---|---|---|---|---|
| T4 | T3 | T2 | T1 | ||
| PCL | 60 | 64 | 64 | 62 | 2.0 (6.7) |
| PCL + 5% HA | 61 | 66 | 66 | 67 | 2.9 (10.3) |
| PCL + 10% HA | 69 | 70 | 70 | 69 | 2.5 (9.7) |
| PCL + 15% HA | 67 | 67 | 66 | 65 | 2.4 (8.8) |
| PCL + 20% HA | 65 | 67 | 67 | 65 | 2.5 (9.7) |
| PCL + 25% HA | 61 | 66 | 67 | 66 | 2.0 (6.7) |
| Groups | Material Extrusion Printing Parameters | |||
|---|---|---|---|---|
| Nozzle (°C) | Speed (mm/s) | Heated Bed (°C) | Flow Factor (%) | |
| PCL | 174 | 100 | 30 | 95 |
| PCL + 5% HA | 175 | 100 | 30 | 95 |
| PCL + 10% HA | 175 | 110 | 30 | 95 |
| PCL + 15% HA | 185 | 100 | 30 | 95 |
| PCL + 20% HA | 198 | 120 | 30 | 100 |
| PCL + 25% HA | 205 | 110 | 30 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Tankus, E.B.; Santarella, F.; Rohr, N.; Sharma, N.; Märtin, S.; Michalscheck, M.; Maintz, M.; Cao, S.; Thieringer, F.M. Fabrication and Characterization of PCL/HA Filament as a 3D Printing Material Using Thermal Extrusion Technology for Bone Tissue Engineering. Polymers 2022, 14, 669. https://doi.org/10.3390/polym14040669
Wang F, Tankus EB, Santarella F, Rohr N, Sharma N, Märtin S, Michalscheck M, Maintz M, Cao S, Thieringer FM. Fabrication and Characterization of PCL/HA Filament as a 3D Printing Material Using Thermal Extrusion Technology for Bone Tissue Engineering. Polymers. 2022; 14(4):669. https://doi.org/10.3390/polym14040669
Chicago/Turabian StyleWang, Fengze, Esma Bahar Tankus, Francesco Santarella, Nadja Rohr, Neha Sharma, Sabrina Märtin, Mirja Michalscheck, Michaela Maintz, Shuaishuai Cao, and Florian M. Thieringer. 2022. "Fabrication and Characterization of PCL/HA Filament as a 3D Printing Material Using Thermal Extrusion Technology for Bone Tissue Engineering" Polymers 14, no. 4: 669. https://doi.org/10.3390/polym14040669
APA StyleWang, F., Tankus, E. B., Santarella, F., Rohr, N., Sharma, N., Märtin, S., Michalscheck, M., Maintz, M., Cao, S., & Thieringer, F. M. (2022). Fabrication and Characterization of PCL/HA Filament as a 3D Printing Material Using Thermal Extrusion Technology for Bone Tissue Engineering. Polymers, 14(4), 669. https://doi.org/10.3390/polym14040669