The Effects of Electron Beam Irradiation on the Morphological and Physicochemical Properties of Magnesium-Doped Hydroxyapatite/Chitosan Composite Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Magnesium-Doped Hydroxyapatite/Chitosan Composite Powders
2.3. Synthesis of Magnesium-Doped Hydroxyapatite/Chitosan Composites Layers
2.4. Electron Beam Irradiation of Magnesium Doped Hydroxyapatite/Chitosan Composite Layers
2.5. Characterization Methods
3. Results and Discussions
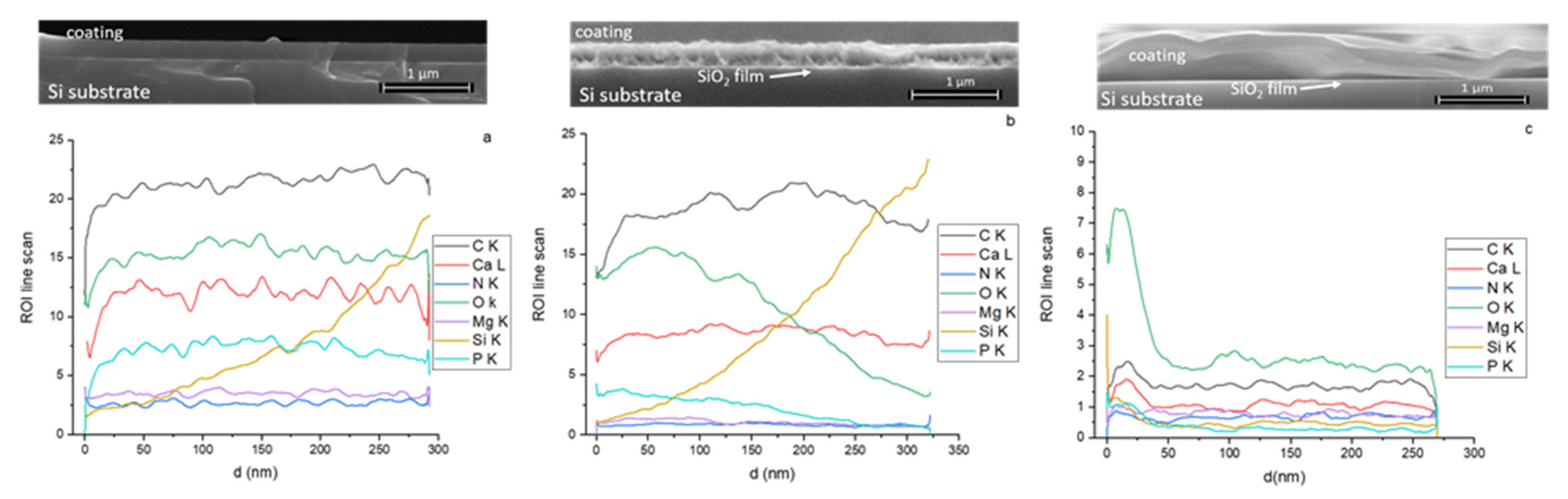
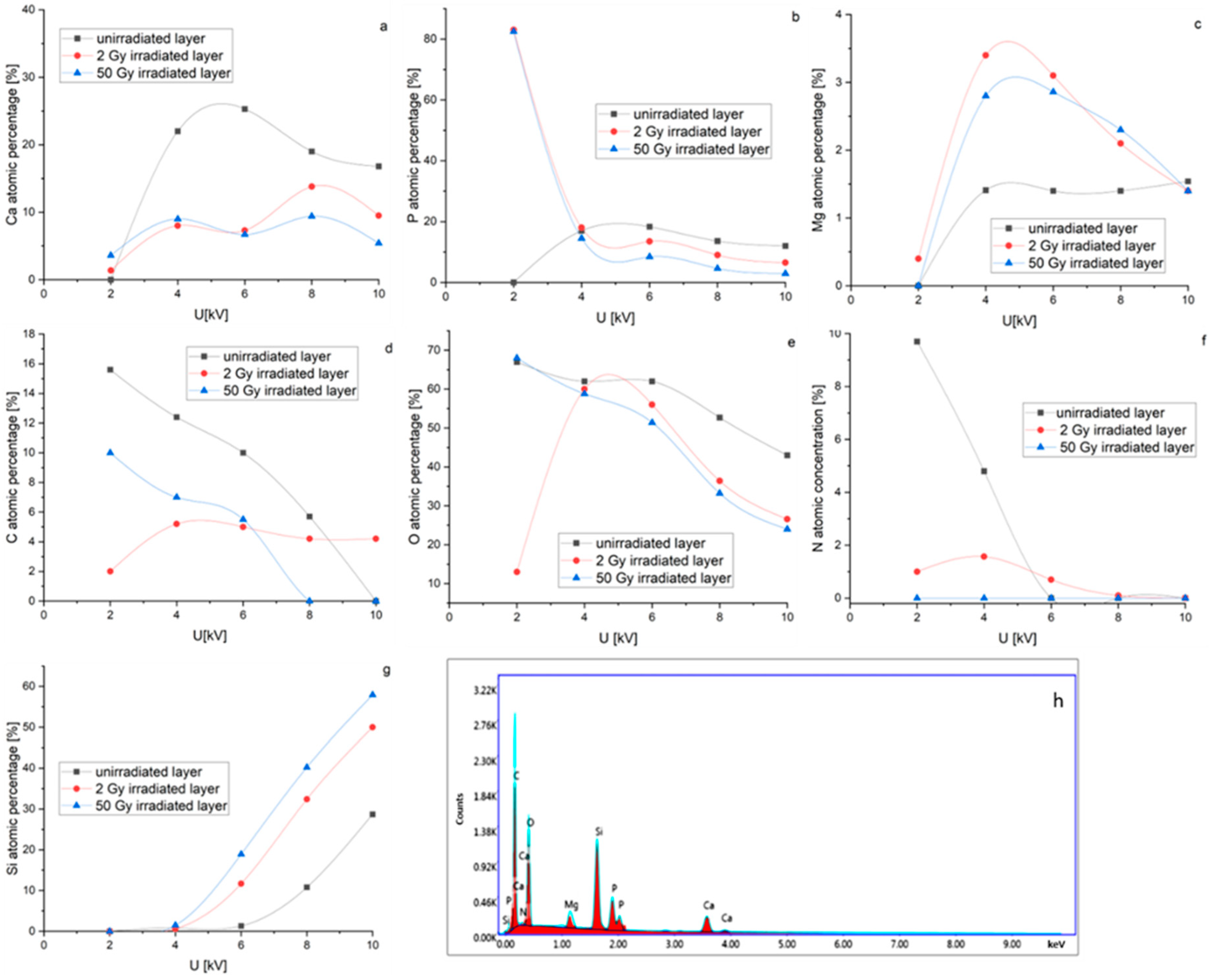
3.1. SEM and EDS Analysis of Magnesium Doped Hydroxyapatite/Chitosan Composite Layers
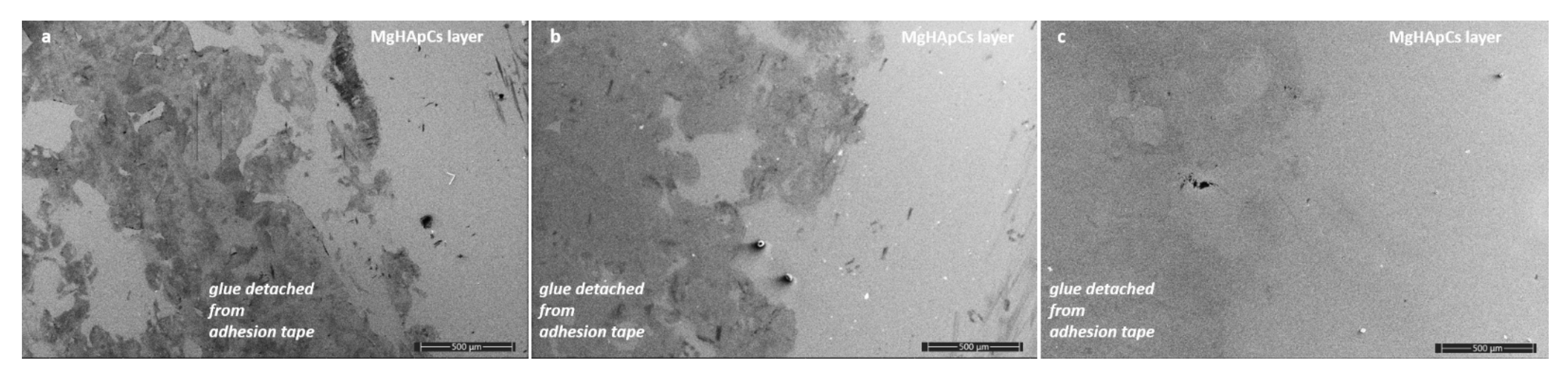
3.2. SEM Analysis of Magnesium Doped Hydroxyapatite/Chitosan Composite Layers Adhesion by Tape Test
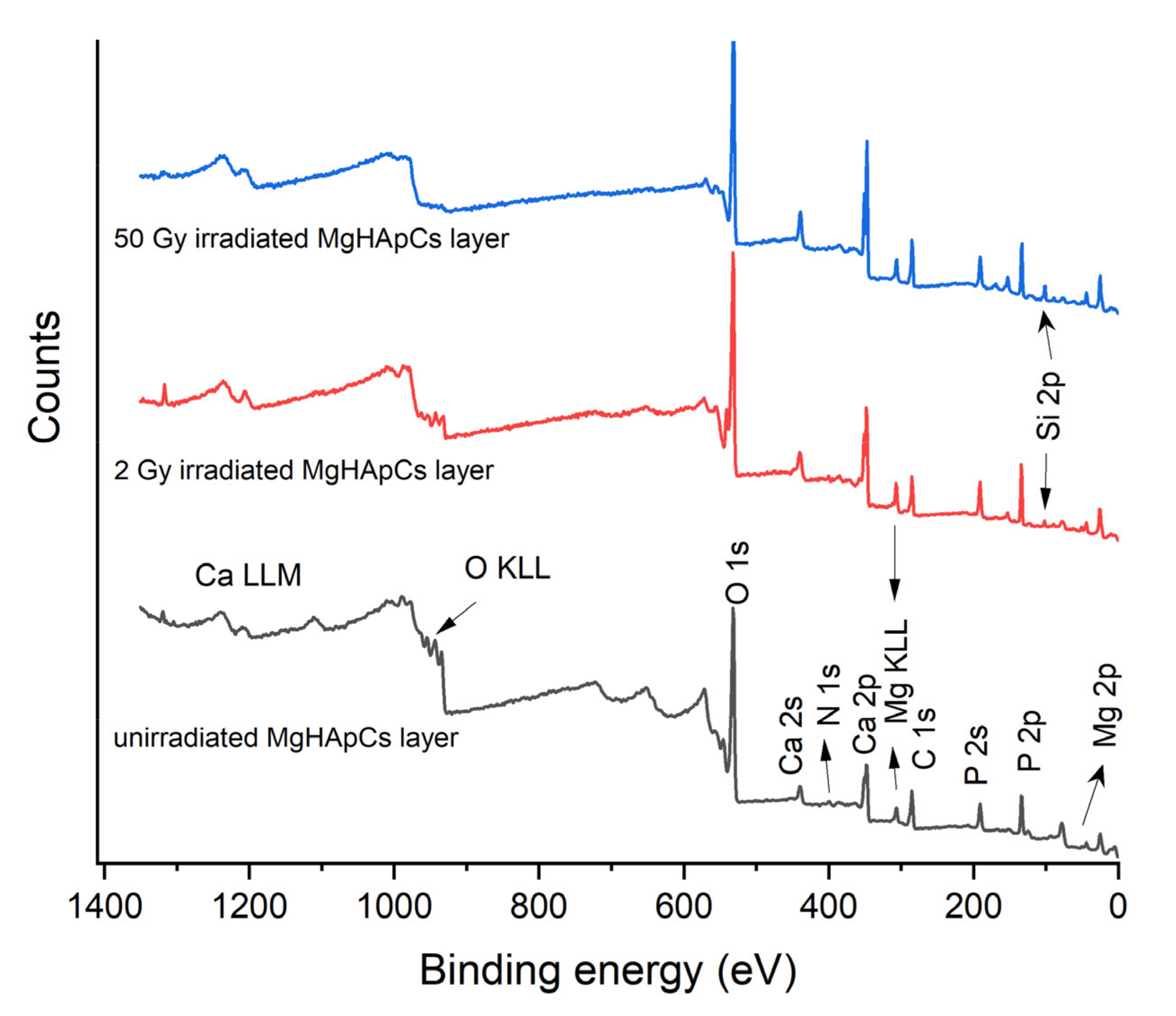
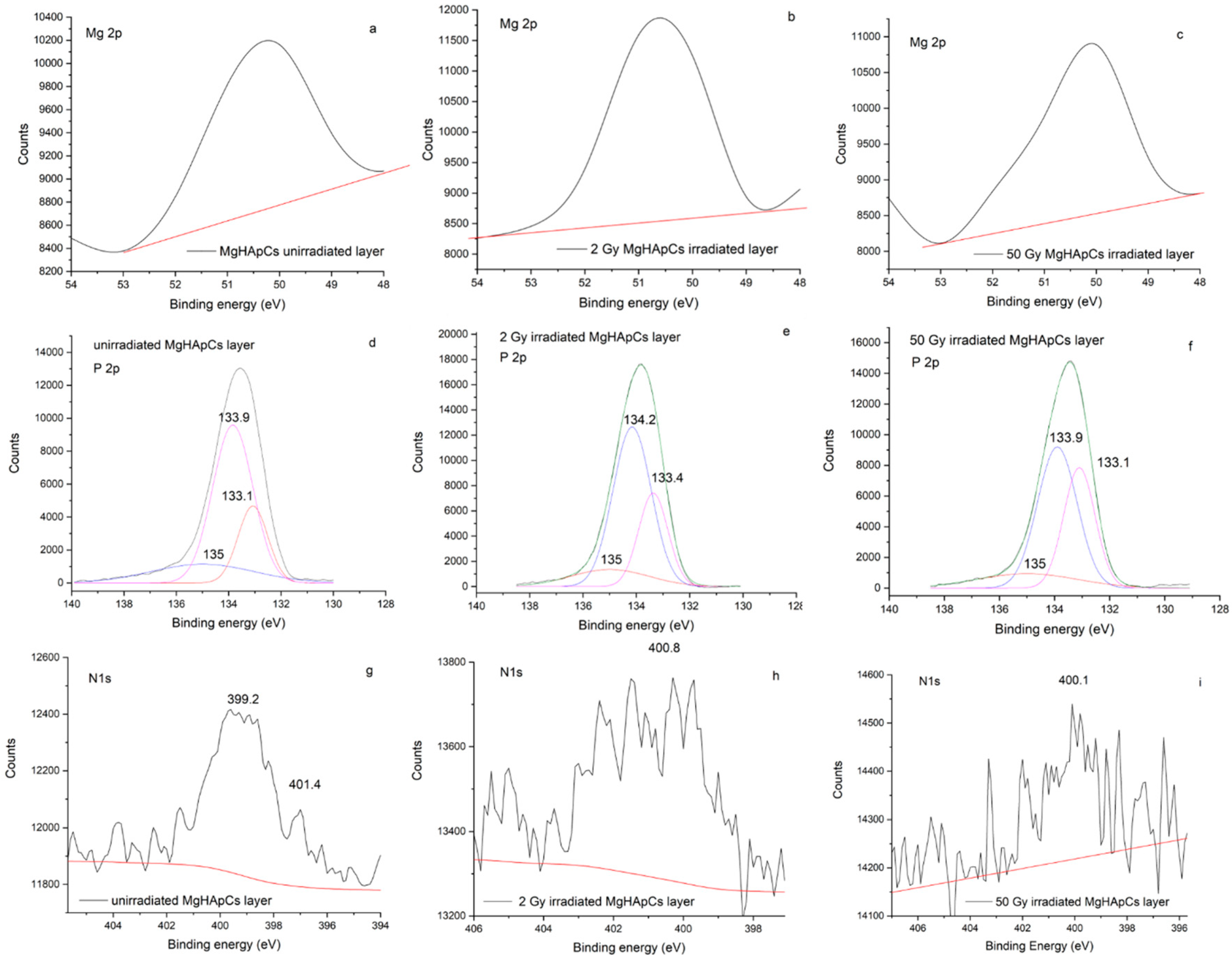
3.3. XPS Analysis of Magnesium Doped Hydroxyapatite/Chitosan Composite Layers
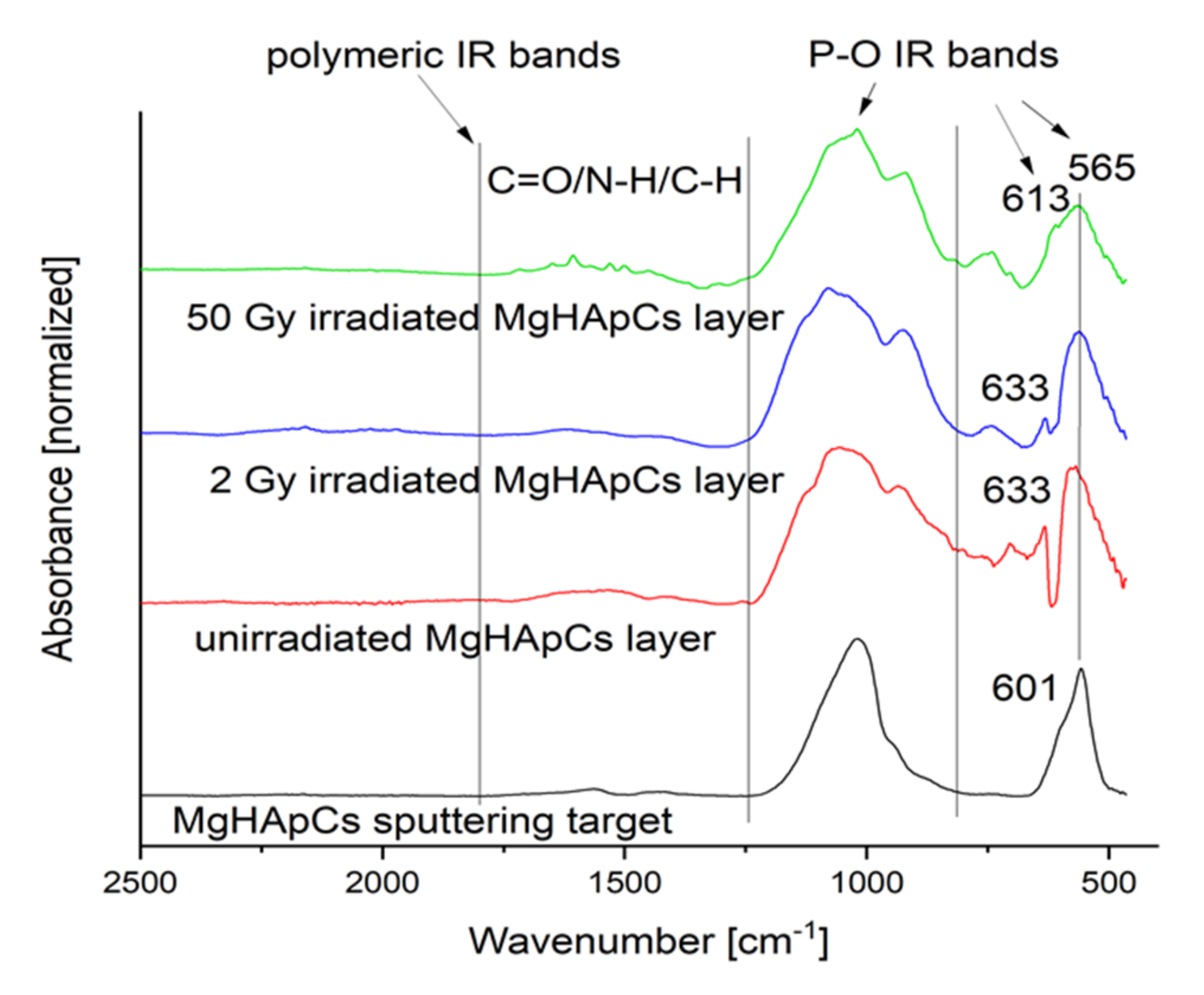
3.4. Fourier Transform Infrared Spectroscopy Analyses of Magnesium-Doped Hydroxyapatite/Chitosan Composite Coatings
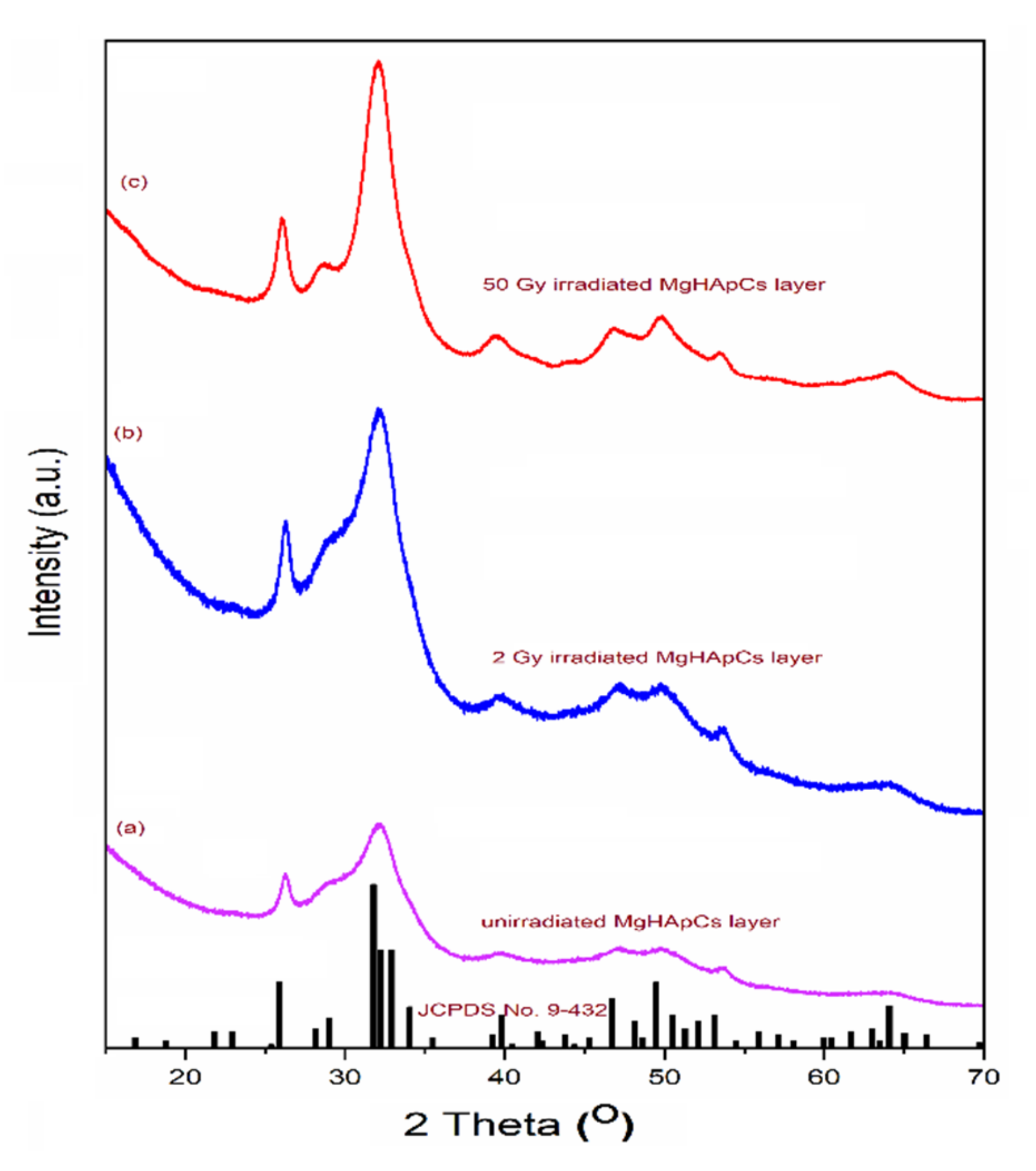
3.5. X-ray Diffraction Analyses of Magnesium-Doped Hydroxyapatite/Chitosan Composite Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Wu, Y.; Zhao, X.; Wang, Z. Fabrication and applications of bioactive chitosan-based organic-inorganic hybrid materials: A review. Carbohydr. Polym. 2021, 267, 118179. [Google Scholar] [CrossRef] [PubMed]
- Pighinelli, L.; Kucharska, M. Chitosan-hydroxyapatite composites. Carbohydr. Polym. 2013, 93, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.; Vladescu, A.; Surmeneva, M.; Ivanova, A.; Braic, M.; Grubova, I.; Cotrut, C.M. Radio Frequency Magnetron Sputter Deposition as a Tool for Surface Modification of Medical Implants chapter 12. In Modern Technologies for Creating the Thin-Film Systems and Coatings; Nikitenkov, N.N., Ed.; IntechOpen: London, UK, 2017; pp. 213–248. [Google Scholar] [CrossRef] [Green Version]
- Boudemagh, D.; Venturini, P.; Fleutot, S.; Cleymand, F. Elaboration of hydroxyapatite nanoparticles and chitosan/hydroxyapatite composites: A present status. Polym. Bull. 2019, 76, 2621–2653. [Google Scholar] [CrossRef]
- Goreninskii, S.I.; Bogomolova, N.N.; Malchikhina, A.I.; Golovkin, A.S.; Bolbasov, E.N.; Safronova, T.V.; Putlyaev, V.I.; Tverdokhlebov, S.I. Biological Effect of the Surface Modification of the Fibrous Poly(L-lactic acid) Scaffolds by Radio Frequency Magnetron Sputtering of Different Calcium-Phosphate Targets. Bionanoscience 2017, 7, 50–57. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Singh, H.; Batra, U. Magnesium doped hydroxyapatite: Synthesis, characterization and bioactivity evaluation. In Biomaterials Science: Processing, Properties, and Applications V; Narayan, R., Bose, S., Bandyopadhyay, A., Eds.; The American Ceramic Society: Pittsburgh, PA, USA, 2015. [Google Scholar]
- Jenifer, A.; Senthilarasan, K.; Arumugam, S.; Sivaprakash, P.; Sagadevan, S.; Sakthivel, P. Investigation on antibacterial and hemolytic properties of magnesium-doped hydroxyapatite nanocomposite. Chem. Phys. Lett. 2021, 771, 138539. [Google Scholar] [CrossRef]
- Adzila, S.; Ramesh, S.; Sopyan, I.; Hamdi, M. Magnesium Doped Hydroxyapatite through Mechanochemical Synthesis. Adv. Mater. Res. 2015, 1087, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Liang, C.; Huang, N.; Zhou, Z.; Duan, F.; Wang, L. Morphology and composition of coatings based on a hydroxyapatite-chitosan-RuCl3 system on AZ91D prepared by pulsed electrochemical deposition. J. Alloys Compd. 2016, 656, 961–971. [Google Scholar] [CrossRef]
- Sutha, S.; Kavitha, K.; Karunakaran, G.; Rajendran, V. In-vitro bioactivity, biocorrosion and antibacterial activity of silicon integrated hydroxyapatite/chitosan composite coating on 316 L stainless steel implants. Mater. Sci. Eng. C 2013, 33, 4046–4054. [Google Scholar] [CrossRef]
- Song, L.; Gan, L.; Xiao, Y.F.; Wu, Y.; Wu, F.; Gu, Z.W. Antibacterial hydroxyapatite/chitosan complex coatings with superior osteoblastic cell response. Mater. Lett. 2011, 65, 974–977. [Google Scholar] [CrossRef]
- Dreghici, D.B.; Butoi, B.; Predoi, D.; Iconaru, S.L.; Stoican, O.; Groza, A. Chitosan–Hydroxyapatite Composite Layers Generated in Radio Frequency Magnetron Sputtering Discharge: From Plasma to Structural and Morphological Analysis of Layers. Polymers 2020, 12, 3065. [Google Scholar] [CrossRef] [PubMed]
- Groza, A.; Dreghici, D.B.; Ganciu, M. Calcium Phosphate Layers Deposited on Thermal Sensitive Polymer Substrates in Radio Frequency Magnetron Plasma Discharge. Coatings 2019, 9, 709. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Groza, A.; Gaiaschi, S.; Rokosz, K.; Raaen, S.; Negrila, C.C.; Prodan, A.; Costescu, A.; et al. Development of Cerium-Doped Hydroxyapatite Coatings with Antimicrobial Properties for Biomedical Applications. Coatings 2020, 10, 516. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Groza, A.; Gaiaschi, S.; Rokosz, K.; Raaen, S.; Ciobanu, S.C.; Chapon, P.; Predoi, D. Antimicrobial Properties of Samarium Doped Hydroxyapatite Suspensions and Coatings. Coatings 2020, 10, 1124. [Google Scholar] [CrossRef]
- NCCN. Guidelines Version 2. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 8 December 2021).
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.-W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [Green Version]
- Vishwanathan, N.; Bhaskar, N.; Supe, S.S.; Swamy, K.; Ravichandran, R.; Kannan, V.; Ramesh, C. Role of electron beam treatment in postoperative management of carcinoma of the breast. Indian J. Cancer 1998, 35, 1–9. [Google Scholar] [PubMed]
- Antebia, U.; Mathor, M.B.; Silva, A.F.D.; Guimarães, R.P.; Honda, E.K. Effects of ionizing radiation on proteins in lyophilized or frozen demineralized human bone. Revista Brasileira de Ortopedia 2016, 51, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Henrique, P.; Limirio, P.H.J.O.; Soares, P.B.F.; Emi, E.T.P.; Lopes, C.D.C.A.; Rocha, F.S.; Batista, J.D.; Rabelo, G.D.; Dechichi, P. Ionizing radiation and bone quality: Time dependent effects. Radiat. Oncol. 2019, 14, 15. [Google Scholar] [CrossRef]
- Ng, H.-M.; Bee, S.-T.; Sin, L.T.; Ratnam, C.T.; Rahmat, A.R. Effect of electron beam irradiation sterilization on biomedical polylactic acid composite filled with Scomberomorus Guttatus-derived hydroxyapatite. Compos. Part B Eng. 2019, 176, 107273. [Google Scholar] [CrossRef]
- Singh, R.; Singh, D.; Singh, A. Radiation sterilization of tissue allografts: A review. World J. Radiol. 2016, 8, 355–369. [Google Scholar] [CrossRef]
- Groza, A.; Iconaru, S.L.; Jiga, G.; Chapon, P.; Gaiaschi, S.; Verga, N.; Beuran, M.; Prodan, A.M.; Matei, M.; Marinescu, S.A.; et al. The Effect of the Ionizing Radiation on Hydroxyapatite–Polydimethylsiloxane Layers. Polym. Eng. Sci. 2019, 59, 2406–2412. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Popa, C.L.; Motelica-Heino, M.; Predoi, D. Evaluation of samarium doped hydroxyapatite, ceramics for medical application: Antimicrobial activity. J. Nanomater. 2015, 2015, 849216. [Google Scholar] [CrossRef] [Green Version]
- ASTM D3359-09; Standard Test Methods for Measuring Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2009. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/D3359-09.htm (accessed on 29 October 2019).
- Groza, A.; Surmeian, A. Characterization of the oxides present in a polydimethylsiloxane layer obtained by polymerization of its liquid precursor in corona discharge. J. Nanomater. 2015, 2015, 204296. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Predoi, D.; Buton, N.; Magier, C.; Stan, G. Dextran-thyme coated magnesium doped hydroxyapatite layers on glass substrate. Coatings 2020, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Avram, A.M.; Bunea, A.C.; Obreja, C.; Avram, M.; Bita, B.; Parvulescu, C.; Popescu, M.; Neculoiu, D. Fabrication of Thin Dielectric Membranes for Microwave Applications. Dig. J. Nanomater. Biostructures 2014, 9, 475–481. [Google Scholar]
- Live Wt% Line Scan across the Cutting Edge of a Razor Blade Sample—Experimental Brief, EDAX Insight, 2020, Volume 18, Issue 4. Available online: https://info.em-ametek.com/acton/attachment/11413/f-ab030958-a14d-4d31-bf21-ad6161d5df49/1/-/-/-/-/Insight_Vol19_No4_FL1.pdf (accessed on 8 December 2021).
- Optimizing Spatial Resolution for EDS Analysis, EDAXInsight, Application Note. 2020. Available online: https://www.edax.com/-/media/ametekedax/files/resources/tips_tricks/optimizingspatialresolutionforedsanalysis.pdf (accessed on 8 December 2021).
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Motelica-Heino, M.; Buton, N.; Megier, C. Obtaining and Characterizing Thin Layers of Magnesium Doped Hydroxyapatite by Dip Coating Procedure. Coatings 2020, 10, 510. [Google Scholar] [CrossRef]
- Felker, D.L.; Sherwood, P.M.A. Magnesium Phosphate (Mg3(PO4)2) by XPS. Surf. Sci. Spectra 2002, 9, 83. [Google Scholar] [CrossRef]
- López, E.O.; Mello, A.; Sendão, H.; Costa, L.T.; Rossi, A.L.; Ospina, R.O.; Borghi, F.F.; Silva Filho, J.G.; Rossi, A.M. Growth of Crystalline Hydroxyapatite Thin Films at Room Temperature by Tuning the Energy of the RF-Magnetron Sputtering Plasma. ACS Appl. Mater. Interfaces 2013, 5, 9435–9445. [Google Scholar] [CrossRef]
- Maachou, H.; Genet, M.J.; Aliouche, D.; Dupont-Gillain, C.C.; Rouxhet, P.G. XPS analysis of chitosan–hydroxyapatite biomaterials: From elements to compounds. Surf. Interface Anal. 2013, 45, 1088–1097. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Bala, Y.; Farlay, D.; Delmas, P.D.; Meunier, P.J.; Boivin, G. Time sequence of secondary mineralization and microhardness in cortical and cancellous bone from ewes. Bone 2010, 46, 1204–1212. [Google Scholar] [CrossRef]
- Popa, C.L.; Groza, A.; Chapon, P.; Ciobanu, C.S.; Ghita, R.V.; Trusca, R.; Ganciu, M.; Predoi, D. Physicochemical analysis of the polydimethylsiloxane interlayer influence on a hydroxyapatite doped with silver coating. J. Nanomater. 2015, 2015, 250617. [Google Scholar] [CrossRef] [Green Version]

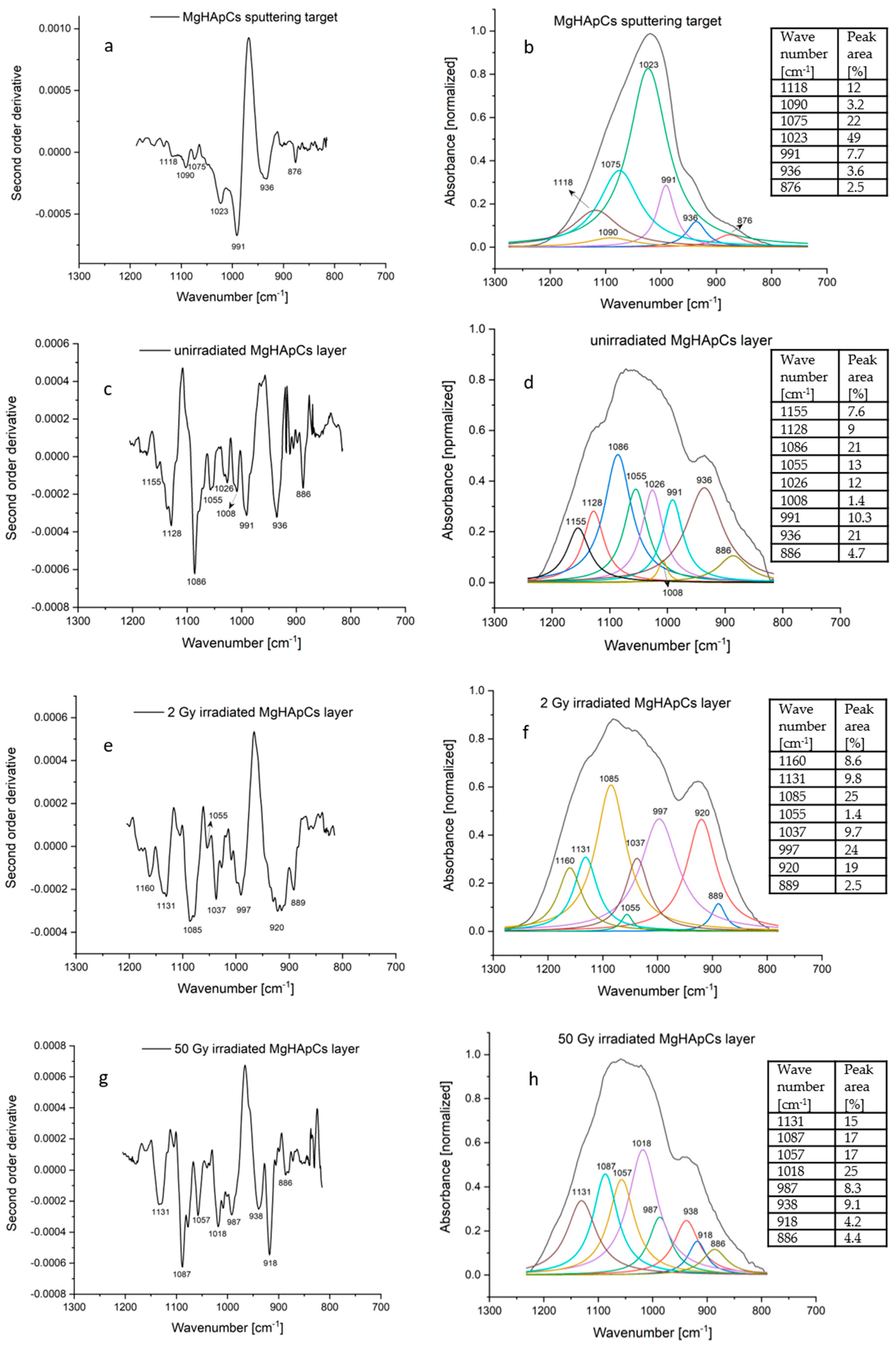
| MgHApCs Sputtering Target | Unirradiated MgHApCs Layer | 2 Gy Irradiated MgHApCs Layer | 50 Gy Irradiated MgHApCs Layer | ||||
|---|---|---|---|---|---|---|---|
| Wave- Number [cm−1] | IR Band Assignment | Wave-Number [cm−1] | IR Band Assignment | Wave-Number [cm−1] | IR Band Assignment | Wave- Number [cm−1] | IR band Assignment |
| 1118 | P-O vibration in non-apatite phosphate structure | 1155, 1128 | P-O vibration in non-apatite phosphate structure | 1160, 1131 | P-O vibration in non-apatite phosphate structure | 1131 | P-O vibration in non-apatite phosphate structure |
| 1090, 1075 1023, 936 | P-O asymmetric and symmetric stretching vibrations in [PO4]3− groups of the apatite structure | 1086, 1055, 1026, 936 | P-O asymmetric and symmetric stretching vibrations in [PO4]3−groups of the apatite structure | 1085, 1055, 1037 | P-O asymmetric and symmetric stretching vibrations in [PO4]3−groups of the apatite structure | 1087, 1057, 1018, 938 | P-O asymmetric and symmetric stretching vibrations in [PO4]3−groups of the apatite structure |
| 991 | C-H/C-O vibrations in chitosan | 1008, 991 | C-H/C-O vibrations in chitosan | 997 | C-H/C-O vibrations in chitosan | 987 | C-H/C-O vibrations in chitosan |
| 920 | Si-OH vibrations | 918 | Si-OH vibrations | ||||
| 876 | C-O vibrations in CO32−/ P-O vibrations in HPO4− | 886 | C-O vibrations in CO32−/ P-O vibrations in HPO4 | 889 | C-O vibrations in CO32−/ P-O vibrations in HPO4− | 886 | C-O vibrations in CO32−/ P-O vibrations in HPO4− |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bita, B.; Stancu, E.; Stroe, D.; Dumitrache, M.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, D.; Groza, A. The Effects of Electron Beam Irradiation on the Morphological and Physicochemical Properties of Magnesium-Doped Hydroxyapatite/Chitosan Composite Coatings. Polymers 2022, 14, 582. https://doi.org/10.3390/polym14030582
Bita B, Stancu E, Stroe D, Dumitrache M, Ciobanu SC, Iconaru SL, Predoi D, Groza A. The Effects of Electron Beam Irradiation on the Morphological and Physicochemical Properties of Magnesium-Doped Hydroxyapatite/Chitosan Composite Coatings. Polymers. 2022; 14(3):582. https://doi.org/10.3390/polym14030582
Chicago/Turabian StyleBita, Bogdan, Elena Stancu, Daniela Stroe, Mirabela Dumitrache, Steluta Carmen Ciobanu, Simona Liliana Iconaru, Daniela Predoi, and Andreea Groza. 2022. "The Effects of Electron Beam Irradiation on the Morphological and Physicochemical Properties of Magnesium-Doped Hydroxyapatite/Chitosan Composite Coatings" Polymers 14, no. 3: 582. https://doi.org/10.3390/polym14030582
APA StyleBita, B., Stancu, E., Stroe, D., Dumitrache, M., Ciobanu, S. C., Iconaru, S. L., Predoi, D., & Groza, A. (2022). The Effects of Electron Beam Irradiation on the Morphological and Physicochemical Properties of Magnesium-Doped Hydroxyapatite/Chitosan Composite Coatings. Polymers, 14(3), 582. https://doi.org/10.3390/polym14030582









