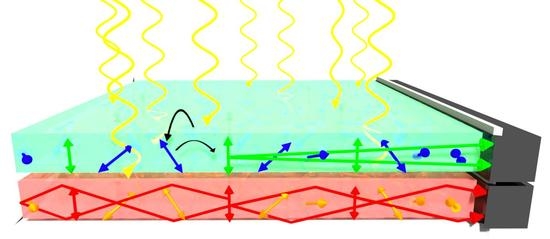
A Refined Prediction Parameter for Molecular Alignability in Stretched Polymers and a New Light-Harvesting Material for AlGaAs Photovoltaics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation for Screening the Alignability of Dyes Containing Larger π-Systems
2.2. Sample Preparation of the Red FunDiLight LSC
2.3. Fluorescence Spectroscopy to Dertermine the Alignability
2.4. Three-Dimensional Single-Molecule Orientation Microscopy
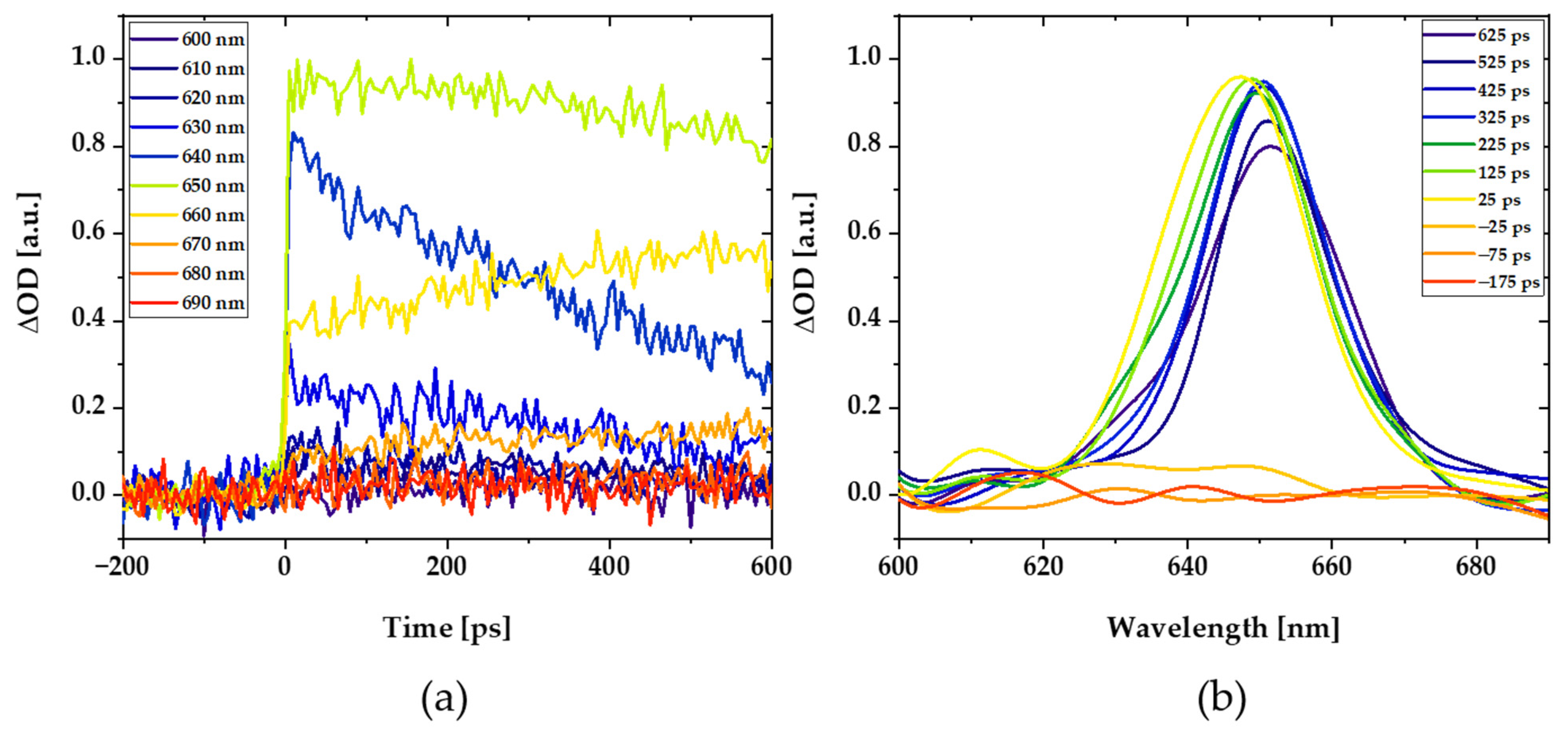
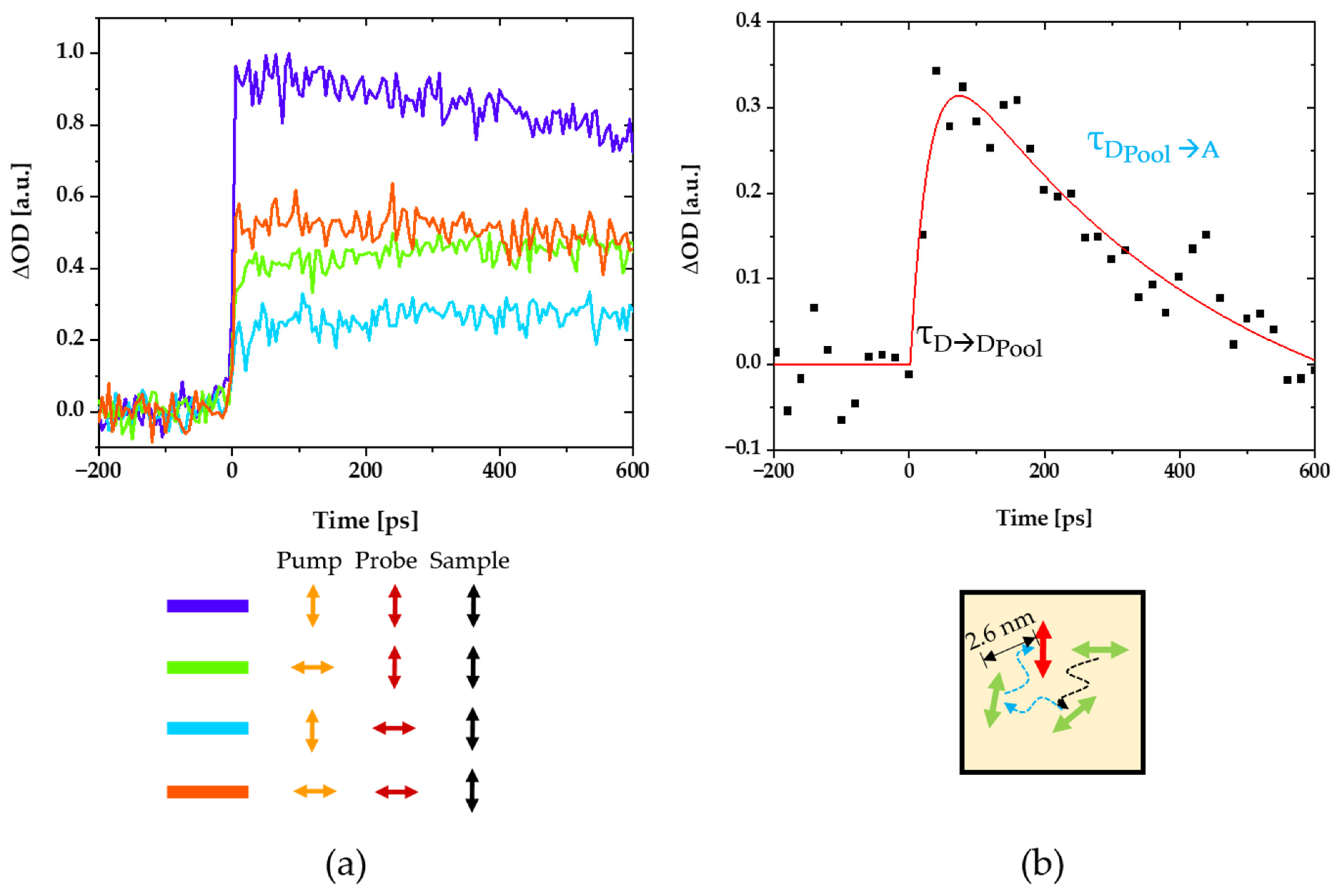
2.5. Pump-Probe Spectroscopy
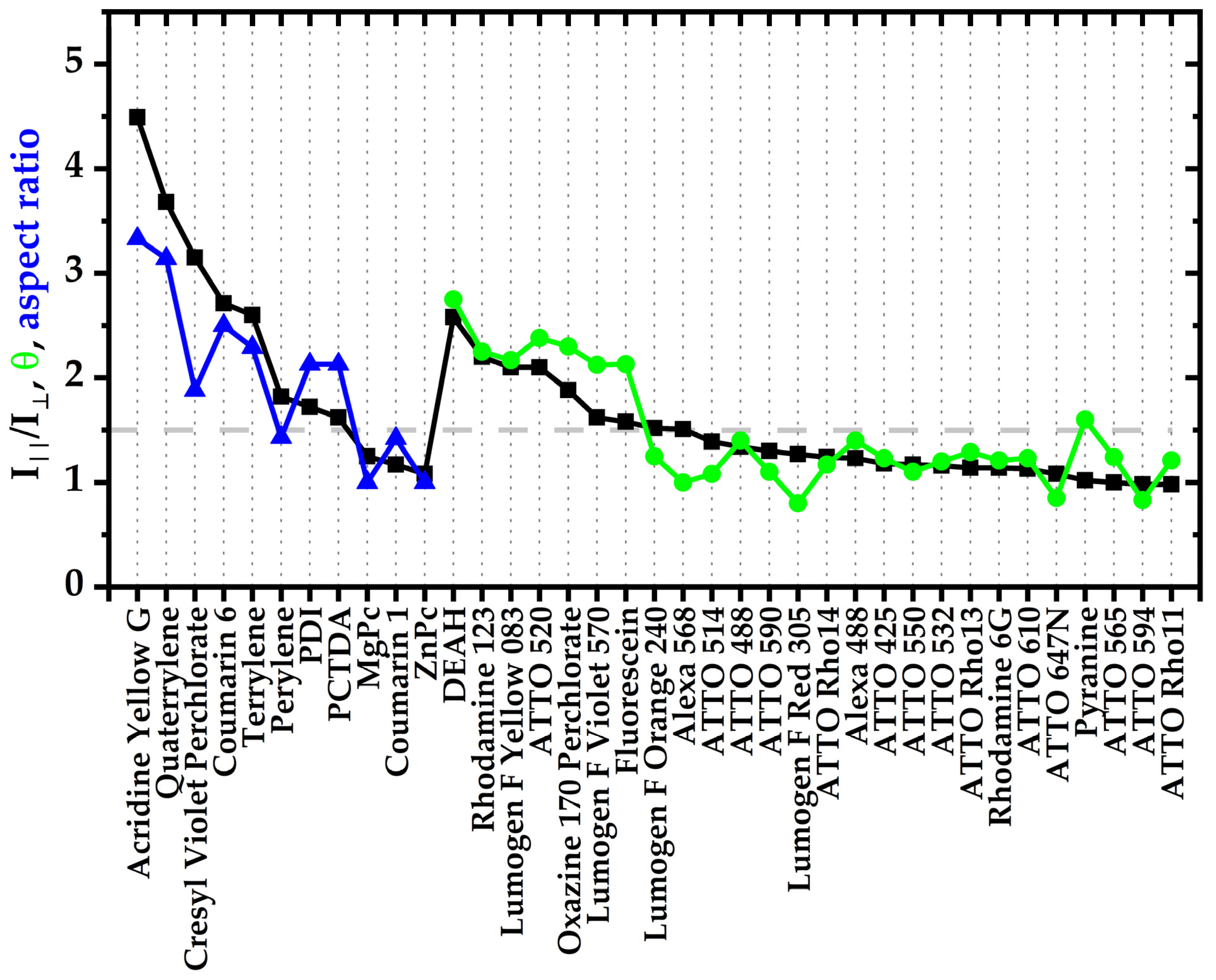
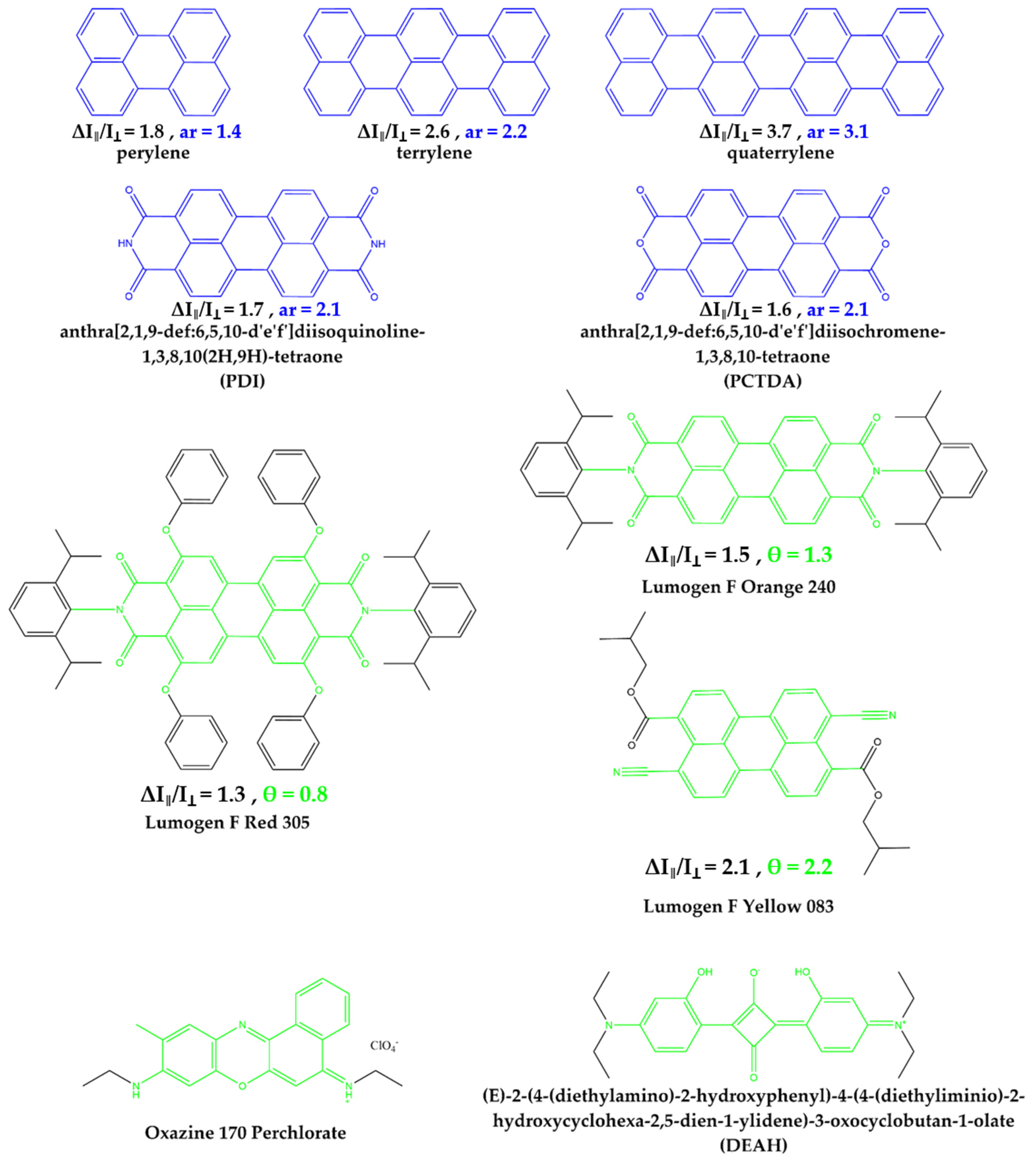
3. Results
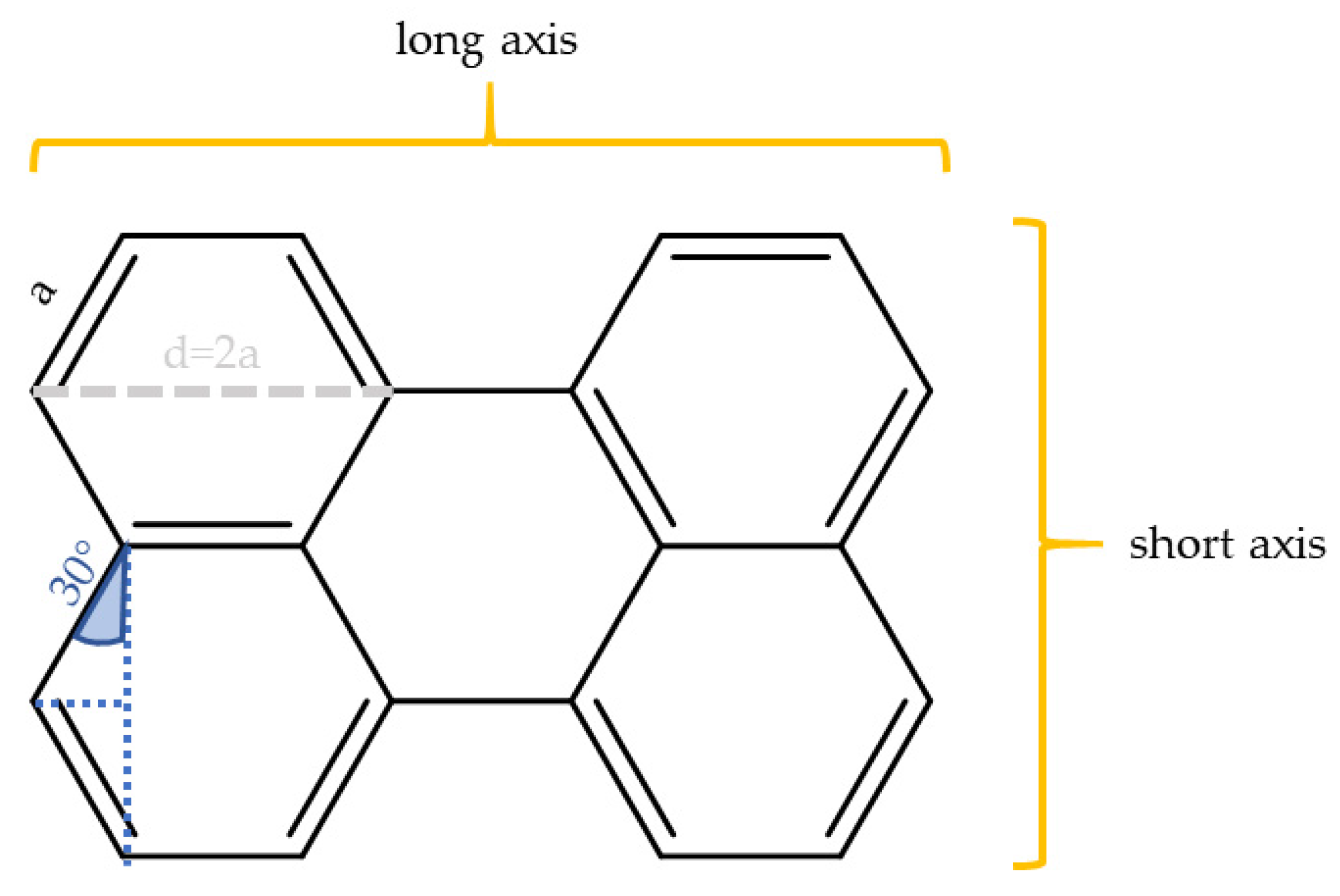
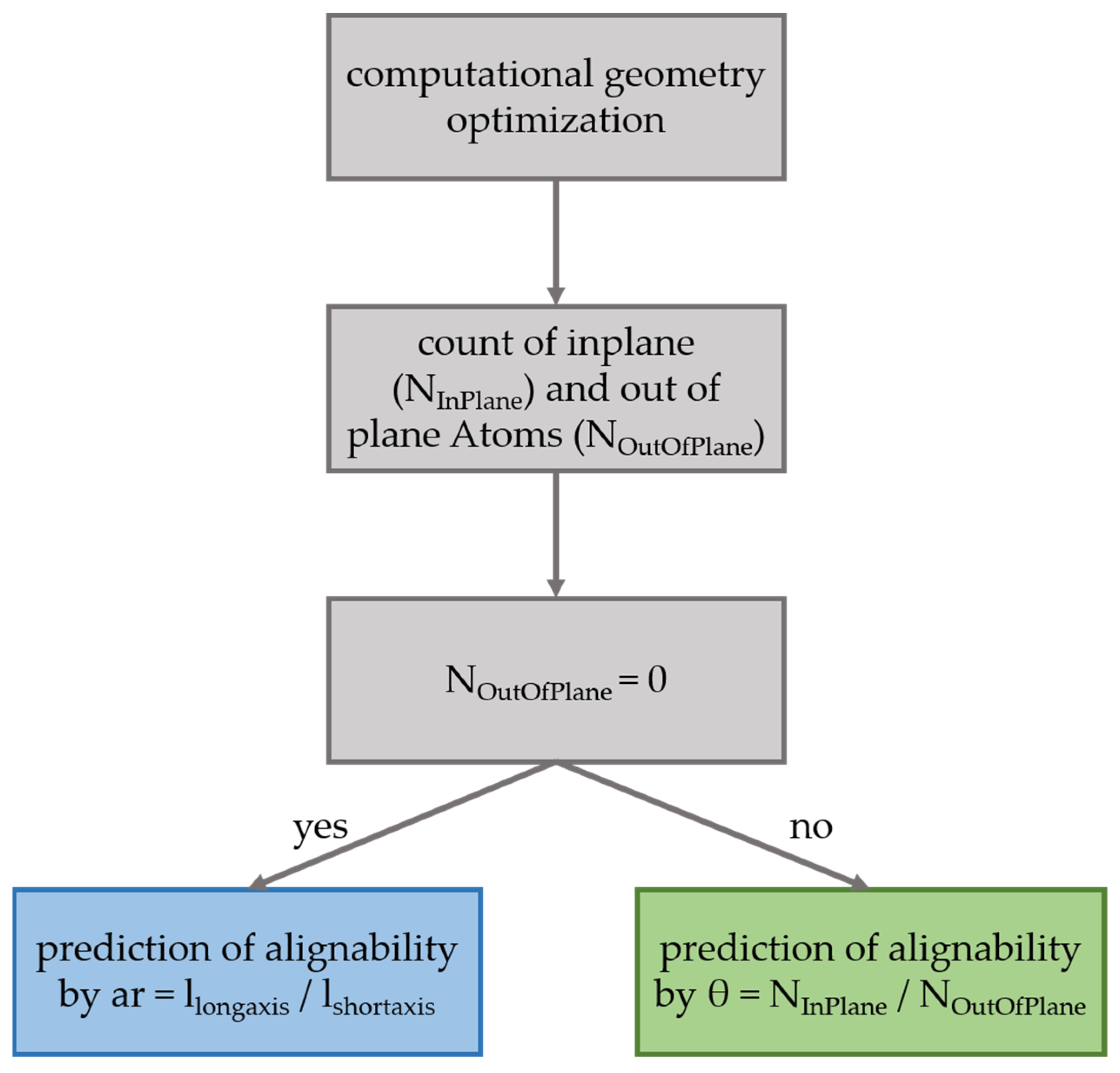
3.1. An Improved Prediction Parameter and Scheme for the Alignability of Molecules in Stretched Poylmers
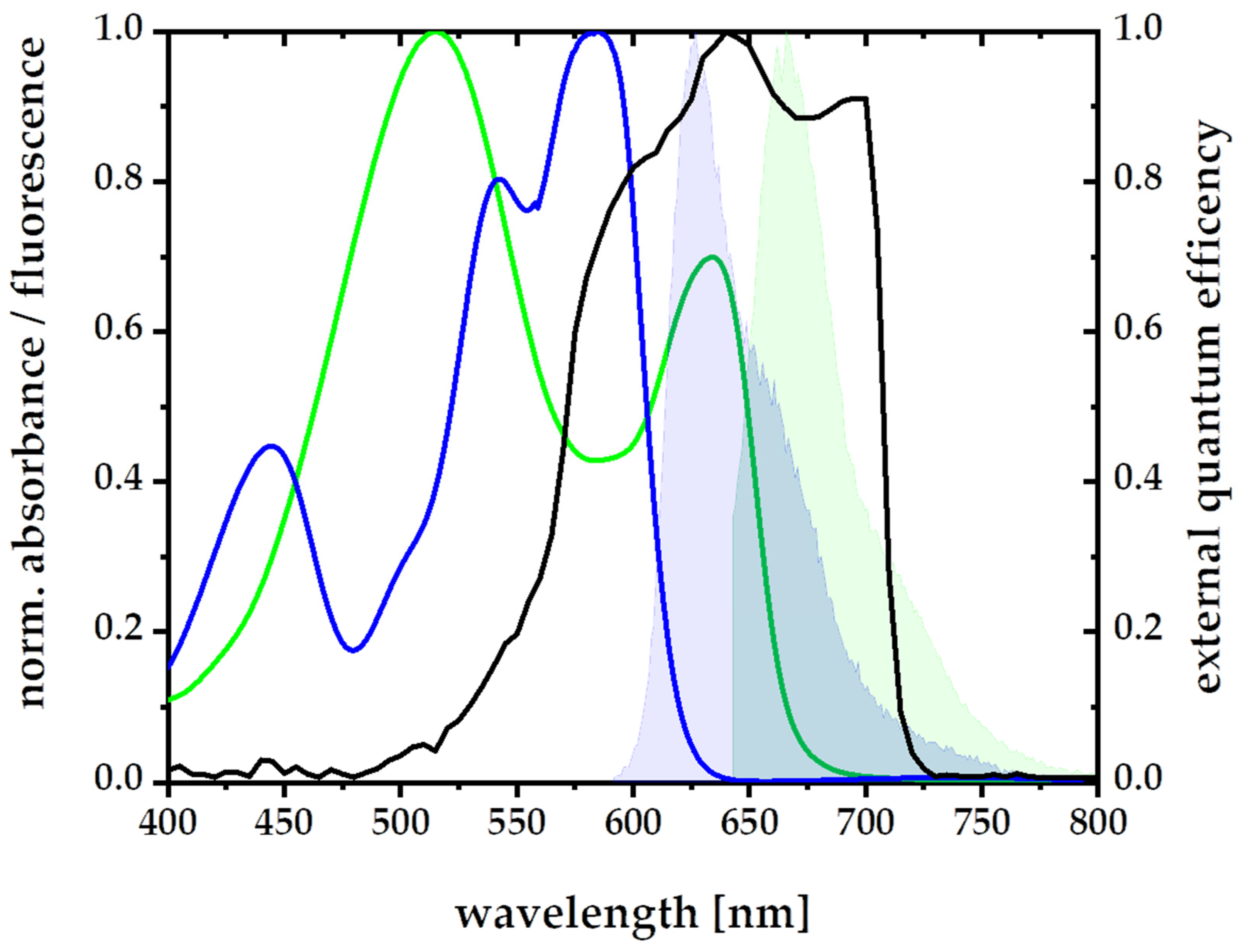
3.2. A New Light-Harvesting Solar Concentrator for the AlGaAs Layer of High-Efficiency Photovoltaics
4. Discussion
5. Conclusions and Perspective

6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Zdanowicz, T.; Rodziewicz, T.; Zabkowska-Waclawek, M. Theoretical analysis of the optimum energy band gap of semiconductors for fabrication of solar cells for applications in higher latitudes locations. Sol. Energy Mater. Sol. Cells 2005, 87, 757–769. [Google Scholar] [CrossRef]
- Geisz, J.F.; France, R.M.; Schulte, K.L.; Steiner, M.A.; Norman, A.G.; Guthrey, H.L.; Young, M.R.; Song, T.; Moriarty, T. Six-junction III–V solar cells with 47.1% conversion efficiency under 143 Suns concentration. Nat. Energy 2020, 5, 326–335. [Google Scholar] [CrossRef]
- Dimroth, F.; Grave, M.; Beutel, P.; Fiedeler, U.; Karcher, C.; Tibbits, T.N.D.; Oliva, E.; Siefer, G.; Schachtner, M.; Wekkeli, A.; et al. Wafer bonded four-junction GaInP/GaAs//GaInAsP/GaInAs concentrator solar cells with 44.7% efficiency. Prog. Photovolt. Res. Appl. 2014, 22, 277–282. [Google Scholar] [CrossRef]
- Dimroth, F.; Tibbits, T.N.D.; Niemeyer, M.; Predan, F.; Beutel, P.; Karcher, C.; Oliva, E.; Siefer, G.; Lackner, D.; Fus-Kailuweit, P.; et al. Four-junction wafer-bonded concentrator solar cells. IEEE J. Photovolt. 2016, 6, 343–349. [Google Scholar] [CrossRef]
- Batchelder, J.S. The Luminescent Solar Concentrator. Ph.D. Thesis, California Institute of Technology, Pasadena, CA, USA, 4 August 1981. [Google Scholar]
- Fang, L.; Parel, T.S.; Danos, L.; Markvart, T. Photon reabsorption in fluorescent solar collectors. J. Appl. Phys. 2012, 111, 076104. [Google Scholar] [CrossRef] [Green Version]
- Olson, R.W.; Loring, R.F.; Fayer, M.D. Luminescent solar concentrators and the reabsorption problem. Appl. Opt. 1981, 20, 2934–2940. [Google Scholar] [CrossRef]
- Ten Kate, O.M.; Hooning, K.M.; van der Kolk, E. Quantifying self-absorption losses in luminescent solar concentrators. Appl. Opt. 2014, 53, 5238–5245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batchelder, J.S.; Zewail, A.H.; Cole, T. Luminescent solar concentrators. 1: Theory of operation and techniques for performance evaluation. Appl. Opt. 1979, 18, 3090–3110. [Google Scholar] [CrossRef]
- McDowall, S.; Butler, T.; Bain, E.; Scharnhorst, K.; Patrick, D. Comprehensive analysis of escape-cone losses from luminescent waveguides. Appl. Opt. 2013, 52, 1230–1239. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Gao, C.; Soleimaninejad, H.; White, J.M.; Smith, T.A.; Jones, D.J.; Ghiggino, K.P.; Wong, W.W.H. Highly efficient luminescent solar concentrators by selective alignment of donor–emitter fluorophores. Chem. Mater. 2019, 31, 3001–3008. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.-S.; Cotlet, M. Efficient light harvesting biotic–abiotic nanohybrid system incorporating atomically thin van der Waals transition metal dichalcogenides. ACS Photonics 2019, 6, 1451–1457. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.-S.; Cotlet, M. Light-induced interfacial phenomena in atomically thin 2D van der Waals material hybrids and heterojunctions. ACS Energy Lett. 2019, 4, 2323–2335. [Google Scholar] [CrossRef] [Green Version]
- Mazzaro, R.; Vomiero, A. The renaissance of luminescent solar concentrators: The role of inorganic nanomaterials. Adv. Energy Mater. 2018, 8, 1801903. [Google Scholar] [CrossRef]
- Im, S.W.; Ha, H.; Yang, W.; Jang, J.H.; Kang, B.; Seo, D.H.; Seo, J.; Nam, K.T. Light polarization dependency existing in the biological photosystem and possible implications for artificial antenna systems. Photosynth. Res. 2020, 143, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Sriramdas, R.; Kumar, P.; Sanghadasa, M.; Kang, M.G.; Priya, S. Maximizing power generation from ambient stray magnetic fields around smart infrastructures enabling self-powered wireless devices. Energy Environ. Sci. 2020, 13, 1462–1472. [Google Scholar] [CrossRef]
- Kumari, B.; Singh, A.; Jana, P.; Radhakrishna, M.; Kanvah, S. White light emission in water through admixtures of donor–π–acceptor siblings: Experiment and simulation. New J. Chem. 2019, 43, 11701–11709. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, Z.; Guan, P.; Wu, X.; Chen, Y.-C. Lasing-encoded microsensor driven by interfacial cavity resonance energy transfer. Adv. Opt. Mater. 2020, 8, 1901596. [Google Scholar] [CrossRef]
- Scholes, G.D.; Fleming, G.R.; Olaya-Castro, A.; van Grondelle, R. Lessons from nature about solar light harvesting. Nat. Chem. 2011, 3, 763–774. [Google Scholar] [CrossRef]
- Verbunt, P.P.C.; Kaiser, A.; Hermans, K.; Bastiaansen, C.W.M.; Broer, D.J.; Debije, M.G. Controlling Light Emission in Luminescent Solar Concentrators Through Use of Dye Molecules Aligned in a Planar Manner by Liquid Crystals. Adv. Funct. Mater. 2009, 19, 2714–2719. [Google Scholar] [CrossRef]
- Minkowski, C.; Calzaferri, G. Förster-Type Energy Transfer along a Specified Axis. Angew. Chem. 2005, 117, 5459–5463. [Google Scholar] [CrossRef]
- Pieper, A.; Hohgardt, M.; Willich, M.; Gacek, D.A.; Hafi, N.; Pfennig, D.; Albrecht, A.; Walla, P.J. Biomimetic light-harvesting funnels for re-directioning of diffuse light. Nat. Commun. 2018, 9, 666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willich, M.M.; Wegener, L.; Vornweg, J.; Hohgardt, M.; Nowak, J.; Wolter, M.; Jacob, C.R.; Walla, P.J. A new ultrafast energy funneling material harvests three times more diffusive solar energy for GaInP photovoltaics. Proc. Natl. Acad. Sci. USA 2020, 117, 32929–32938. [Google Scholar] [CrossRef]
- Wilson, L.; Richards, B. Measurement method for photoluminescent quantum yields of fluorescent organic dyes in polymethyl methacrylate for luminescent solar concentrators. Appl. Opt. 2009, 48, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, A.; Pfennig, D.; Nowak, J.; Grunwald, M.; Walla, P.J. On the efficiency limits of artificial and ultrafast light-funnels. Nano Sel. 2020, 1, 525–538. [Google Scholar] [CrossRef]
- Law, K.-Y. Squaraine Chemistry. Effects of Structural Changes on the Absorption and Multiple Fluorescence Emission of Bis[4-(dimethylamino)phenyl]squaraine and Its Derivates. J. Phys. Chem. 1987, 91, 5184–5193. [Google Scholar] [CrossRef]
- Sens, R.; Drexhage, K.H. Fluorescence quantum yield of oxazine and carbazine laser dyes. J. Lumin. 1918, 24–25, 709–712. [Google Scholar] [CrossRef]
- Green, A.P.; Buckley, A.R. Solid state concentration quenching of organic fluorophores in PMMA. Phys. Chem. Chem. Phys. 2015, 17, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Walla, P.J. Modern Biophysical Chemistry Detection and Analysis of Biomolecules, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014. [Google Scholar]
- Thuillier, G.; Hersé, M.; Foujols, T.; Peetermans, W.; Gillotay, D.; Simon, P.C.; Mandel, H. The solar spectral irradiance from 200 to 2400 nm as measured by the solspec spectrometer from the atlas and eureka missions. Solar Phys. 2003, 214, 1–22. [Google Scholar] [CrossRef]
- Honsberg, C.B.; Bowden, S.G. Photovoltaics Education Website. 2019. Available online: www.pveducation.org (accessed on 21 December 2021).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohgardt, M.; Gädeke, F.E.; Wegener, L.; Walla, P.J. A Refined Prediction Parameter for Molecular Alignability in Stretched Polymers and a New Light-Harvesting Material for AlGaAs Photovoltaics. Polymers 2022, 14, 532. https://doi.org/10.3390/polym14030532
Hohgardt M, Gädeke FE, Wegener L, Walla PJ. A Refined Prediction Parameter for Molecular Alignability in Stretched Polymers and a New Light-Harvesting Material for AlGaAs Photovoltaics. Polymers. 2022; 14(3):532. https://doi.org/10.3390/polym14030532
Chicago/Turabian StyleHohgardt, Manuel, Franka Elisabeth Gädeke, Lucas Wegener, and Peter Jomo Walla. 2022. "A Refined Prediction Parameter for Molecular Alignability in Stretched Polymers and a New Light-Harvesting Material for AlGaAs Photovoltaics" Polymers 14, no. 3: 532. https://doi.org/10.3390/polym14030532
APA StyleHohgardt, M., Gädeke, F. E., Wegener, L., & Walla, P. J. (2022). A Refined Prediction Parameter for Molecular Alignability in Stretched Polymers and a New Light-Harvesting Material for AlGaAs Photovoltaics. Polymers, 14(3), 532. https://doi.org/10.3390/polym14030532