Drug Delivery from Stimuli-Responsive Poly(N-isopropylacrylamide-co-N-isopropylmethacrylamide)/Chitosan Core/Shell Nanohydrogels
Abstract
1. Introduction
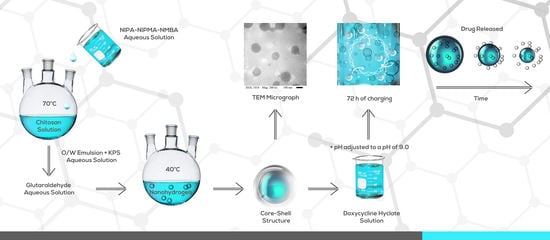
2. Materials and Methods
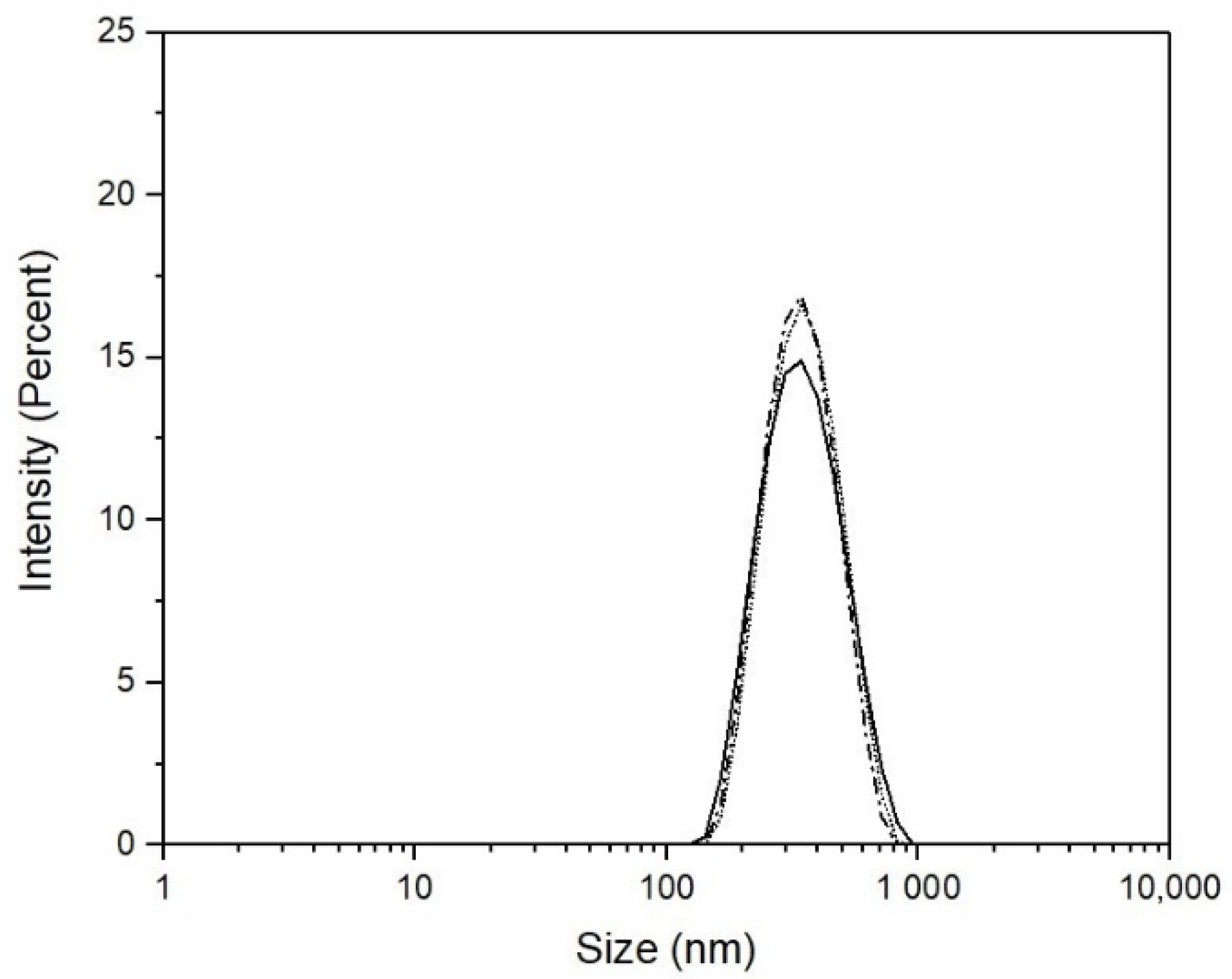
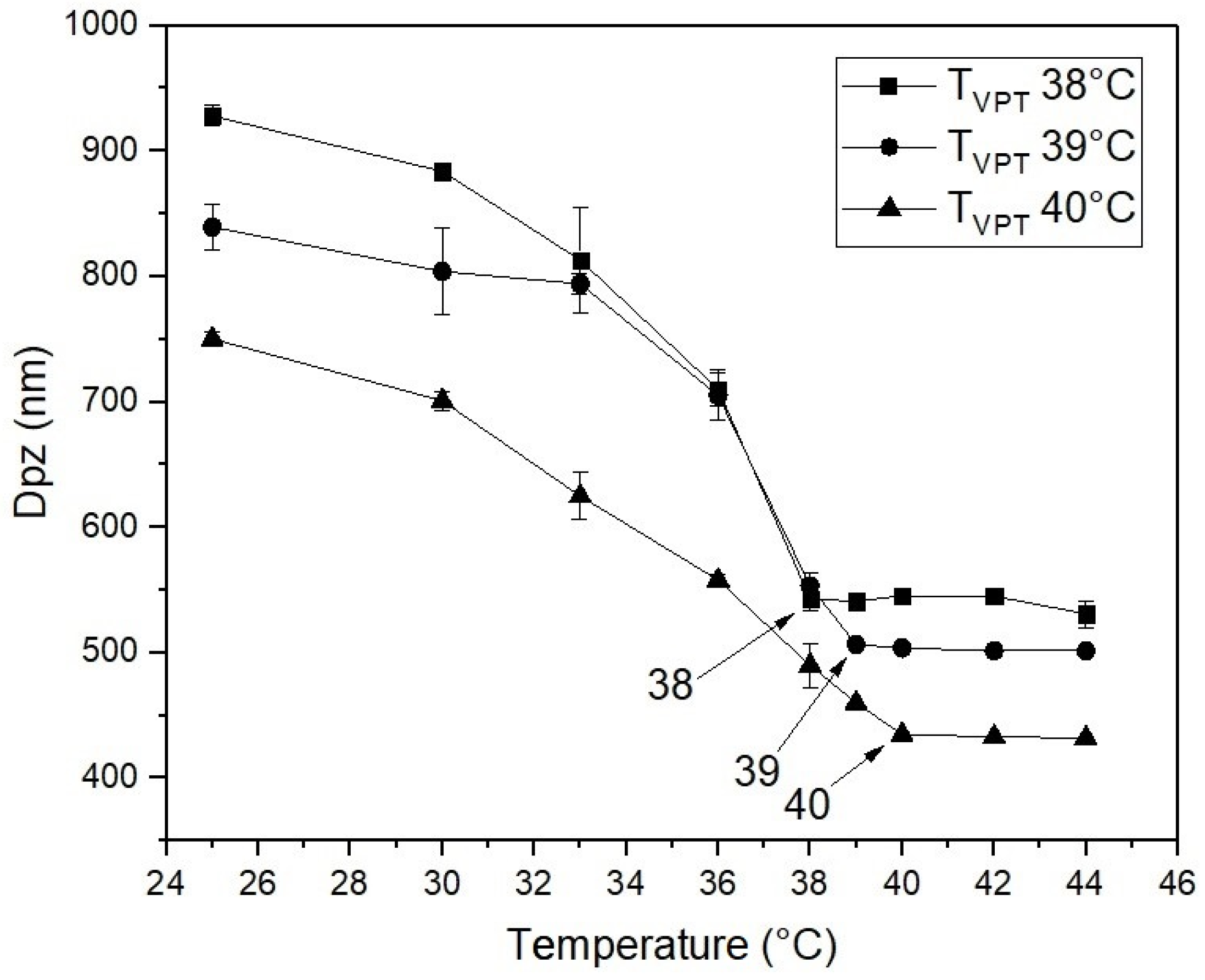
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuckling, D.; Doering, A.; Krahl, F.; Arndt, K.-F. Stimuli-Responsive Polymer Systems. Polym. Sci. Compr. Ref. 2012, 8, 377–413. [Google Scholar] [CrossRef]
- Gore, S.A.; Gholve, S.B.; Savalsure, S.M.; Ghodake, K.B.; Bhusnure, O.G.; Thakare, V.M. Smart polymer and their applications: A review. Int. J. Curr. Pharm. Rev. Res. 2017, 8, 298–310. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo-and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Javadi, A.; Ghaffari, M.; Shaoquin, G. A pH-sensitive molecularly imprinted nanospheres/hydrogel composite as coating for implantable biosensors. Biomaterials 2010, 31, 4944–4951. [Google Scholar] [CrossRef]
- Terefe, N.S.; Glagovskaia, O.; de Silva, K.; Stockmann, R. Applications of stimuli responsive polymers for sustainable ion exchange chromatography. Food Bioprod. Process. 2014, 92, 208–225. [Google Scholar] [CrossRef]
- Fernández-Barbero, A.; Suárez, I.J.; Sierra-Martín, B.; Fernández-Nieves, A.; de las Nieves, F.J.; Marquez, M.; Rubio-Retama, J.; López-Cabarcos, E. Gels and microgels for nanotechnological applications. Adv. Colloid Interface Sci. 2009, 147–148, 88–108. [Google Scholar] [CrossRef]
- Alvarez-Bautista, A.; Duarte, C.M.M.; Mendizábal, E.; Katime, I. Controlled delivery of drugs through smart pH-sensitive nanohydrogels for anti-cancer therapies: Synthesis, drug release and cellular studies. Des. Monomers Polym. 2016, 19, 319–329. [Google Scholar] [CrossRef]
- Peters, J.T.; Hutchinson, S.S.; Lizana, N.; Verma, I.; Peppas, N.A. Synthesis and characterization of poly(N-isopropyl methacrylamide) sore/shell nanogels for controlled release of chemotherapeutics. Chem. Eng. J. 2018, 340, 58–65. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1108. [Google Scholar] [CrossRef]
- Bera, R.; Dey, A.; Chakrabarty, D. Synthesis, characterization, and drug release study of acrylamide-co-itaconic acid based smart hydrogel. Polym. Eng. Sci. 2015, 55, 113–122. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, F.; Cheng, Y.; Wang, Z.; Li, Y. Stimuli-responsive polydopamine-based smart materials. Chem. Soc. Rev. 2021, 50, 8319–8343. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; MacConaghy, K.I.; Kaar, J.L.; Stoykovich, M.P. Enhance Optical Sensitivity in Thermoresponsive Photonic Crystal Hydrogels by Operating Near the Phase Transition. ACS Appl. Mater. Interfaces 2017, 9, 27927–27935. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, A.; Gimi, B.; Hu, W. Applications of semiconductor fabrication methods to nanomedicine: A review of recent inventions and techniques. Recent Pat. Nanomed. 2013, 3, 9–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, M.; Du, H.; Zhang, W.; Zhai, G. Internal stimuli-responsive nanocarriers for drug delivery: Design strategies and applications. Mater. Sci. Eng. C 2017, 71, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Cuggino, J.C.; Alvarez, I.C.I.; Strumia, M.C.; Welker, P.; Licha, K.; Steinhilbert, D.; Mutihac, R.-C.; Calderón, M. Thermosensitive nanogels based on dendritic polyglycerol and N-isopropylacrylamide for biomedical applications. Soft Matter 2011, 7, 11259–11266. [Google Scholar] [CrossRef]
- O’Neil, H.S.; Herron, C.C.; Hastings, C.L.; Deckers, R.; López-Noriega, A.; Kelly, H.M.; Hennink, W.E.; McDonnell, C.O.; O’Brien, F.J.; Ruiz-Hernández, E.; et al. A stimuli responsive liposome loaded hydrogel provides flexible on-demand release of therapeutic agents. Acta Biomater. 2016, 48, 110–119. [Google Scholar] [CrossRef]
- Chiantore, O.; Guaita, M.; Trossarelli, L. Solution properties of poly(N-isopropylacrylamide). Die Makromol. Chem. 1979, 180, 969–973. [Google Scholar] [CrossRef]
- Binkert, T.; Oberreich, J.; Meewes, M.; Nyffenegger, R.; Ricka, J. Coil-globule transition of poly(N-isopropylacrylamide): A study of segment mobility fluorescence depolarization. Macromolecules 1991, 24, 5806–5810. [Google Scholar] [CrossRef]
- Kubota, K.; Hamano, K.; Kuwahara, N.; Fujishige, S.; Ando, I. Characterization of poly(N-isopropylmethacrylamide) in wáter. Polym. J. 1990, 22, 1051–1057. [Google Scholar] [CrossRef]
- Djokpé, E.; Vogt, W. N-isopropylacrylamide and N-isopropylmethacryl-amide: Cloud points of mixtures and copolymers. Macromol. Chem. Phys. 2001, 202, 750–757. [Google Scholar] [CrossRef]
- Salmerón Sánchez, M.; Hanyková, L.; Ilavský, M.; Monleón Pradas, M. Thermal transitions of poly(N-isopropylmethacrylamide) in aqueous solutions. Polymer 2004, 45, 4087–4094. [Google Scholar] [CrossRef]
- Kokufuta, M.K.; Sato, S.; Kokufuta, E. LCST behavior of copolymers of N-isopropylacrylamide and N-isopropylmethacrylamide in water. Colloid Polym. Sci. 2012, 290, 1671–1681. [Google Scholar] [CrossRef]
- López-León, T.; Carvalho, E.L.S.; Seijo, B.; Ortega-Vinuesa, J.L.; Bastos-González, D. Physicochemical characterization of chitosan nanoparticles: Electrokinetic and stability behavior. J. Colloid Interface Sci. 2005, 283, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.R.; Elvira, C.; Gallardo, A.; Vázquez, B.; Román, J.S. Smart polymer and their applications as biomaterials. In Topics in Tissue Engineering; Ashammakhi, N., Reis, R., Chiellini, E., Eds.; University of Oulu: Oulu, Finland, 2007; Volume 3, pp. 1–27. [Google Scholar]
- Huang, C.-H.; Wang, C.-F.; Don, T.-M.; Chiu, W.-Y. Preparation of pH- and thermo-sensitive chitosan-PNIPAAm core-shell nanoparticles and evaluation as drug carriers. Cellulose 2013, 20, 1791–1805. [Google Scholar] [CrossRef]
- Alvarado, A.G.; Ortega, A.; Pérez-Carrillo, L.A.; Ceja, I.; Arellano, M.; López, R.G.; Puig, J.E. Synthesis, characterization, and drug delivery from pH- and thermoresponsive poly(N-isopropylacrylamide)/chitosan core/shell nanocomposites made by semicontinuous heterophase polymerization. J. Nanomater. 2017, 2017, 6796412. [Google Scholar] [CrossRef]
- Fox, T.G. Influence of diluent and copolymer composition on the glass temperature of a polymer system. Bull. Am. Phys. Soc. 1956, 1, 123–125. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Ríos-Donato, N.; Peña-Flores, A.M.; Katime, I.; Leyva-Ramos, R.; Mendizábal, E. Kinetics and thermodynamics of adsorption of red dye 40 from acidic aqueous solutions onto a novel chitosan sulfate. Afinidad LXXIV 2017, 74, 214–220. [Google Scholar]
- Singhvi, G.; Singh, M. Review: In-vitro drug release characterization models. Int. J. Pharm. Stud. Res. 2011, 2, 77–84. [Google Scholar]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanism of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Gouda, R.; Baishya, H.; Qing, Z. Applications of mathematical models in drug release kinetics of carbidopa and levodopa ER tablets. J. Dev. Drugs 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Lindner, W.D.; Lippold, B.C. Drug release from hydrocolloid embeddings with high or low susceptibility to hydrodynamics stress. Pharm. Res. 1995, 12, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.O.; Sousa, J.J.S.; Pais, A.A.C.C.; Formosinho, S.J. Comparison of dissolution profiles of ibuprofen pellets. J. Control. Release 2003, 89, 199–212. [Google Scholar] [CrossRef]
- Ramteke, K.H.; Dighe, P.A.; Kharat, A.R.; Patil, S.V. Mathematical models of drug dissolution: A review. Sch. Acad. J. Pharm. 2014, 3, 388–396. [Google Scholar] [CrossRef]
- Balleño, J.A.; Mendizábal-Ruiz, A.P.; Saade, H.; Díaz de León-Gómez, R.; Mendizábal, E.; Rios-Donato, N.; López, R.G. Ibuprofen release from poly(ethyl cyanoacrylate) nanoparticles prepared by semicontinuous heterophase polymerization. Int. J. Polym. Sci. 2018, 2018, 4527203. [Google Scholar] [CrossRef]
- Serrano-Medina, A.; Cornejo-Bravo, J.M.; Licea-Claveríe, A. Synthesis of pH and temperature sensitive, core-shell nano/microgels, by one pot, soap-free emulsion polymerization. J. Colloid Interface Sci. 2012, 369, 82–90. [Google Scholar] [CrossRef]
- Fundueanu, G.; Constantin, M.; Bucatariu, S.; Ascenzi, P. Poly(N-isopropylacrylamide-co-N-isopropylmethacrylamide) thermo-responsive microgels as self-regulated drug delivery system. Macromol. Chem. Phys. 2016, 271, 2525–2533. [Google Scholar] [CrossRef]
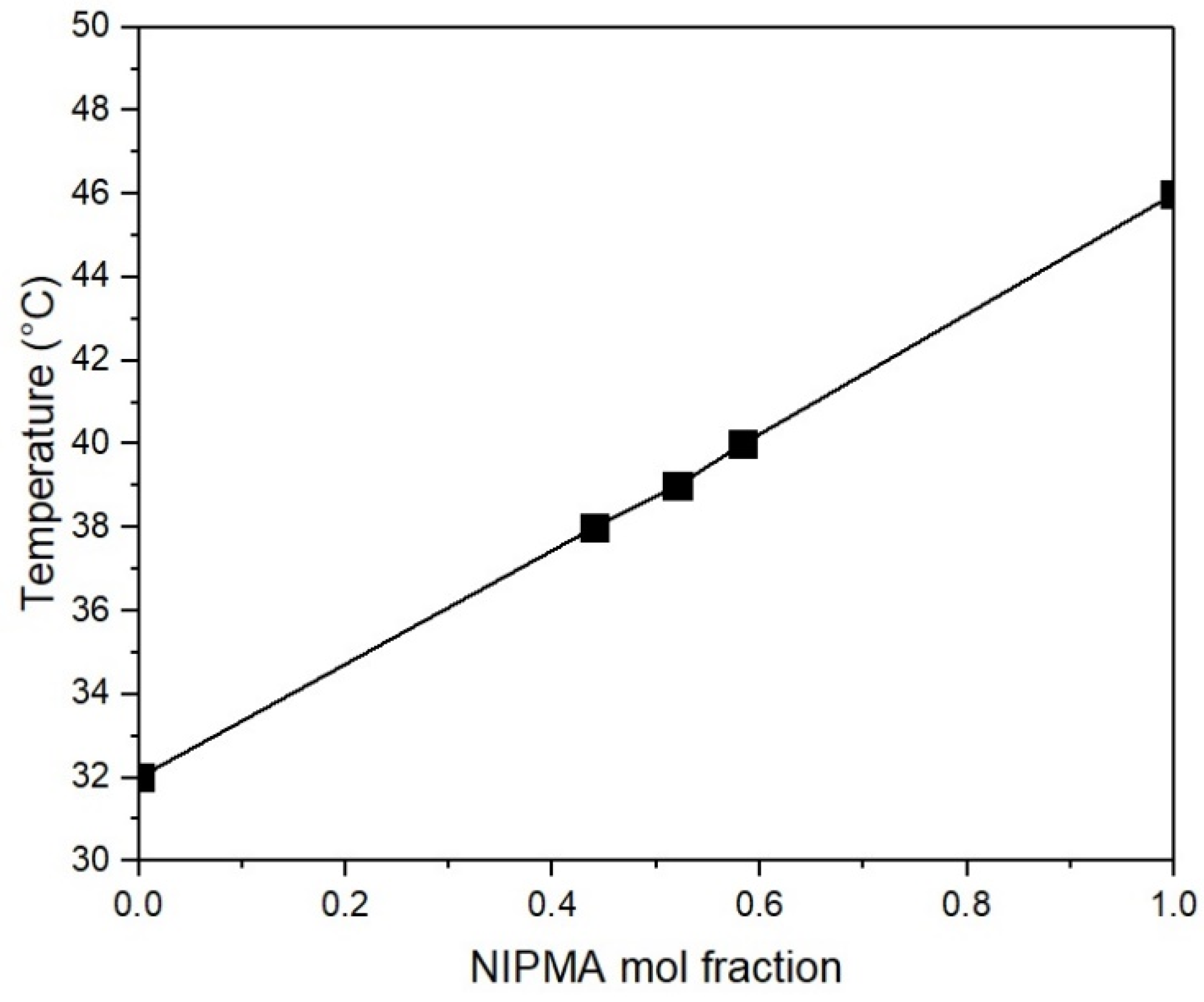


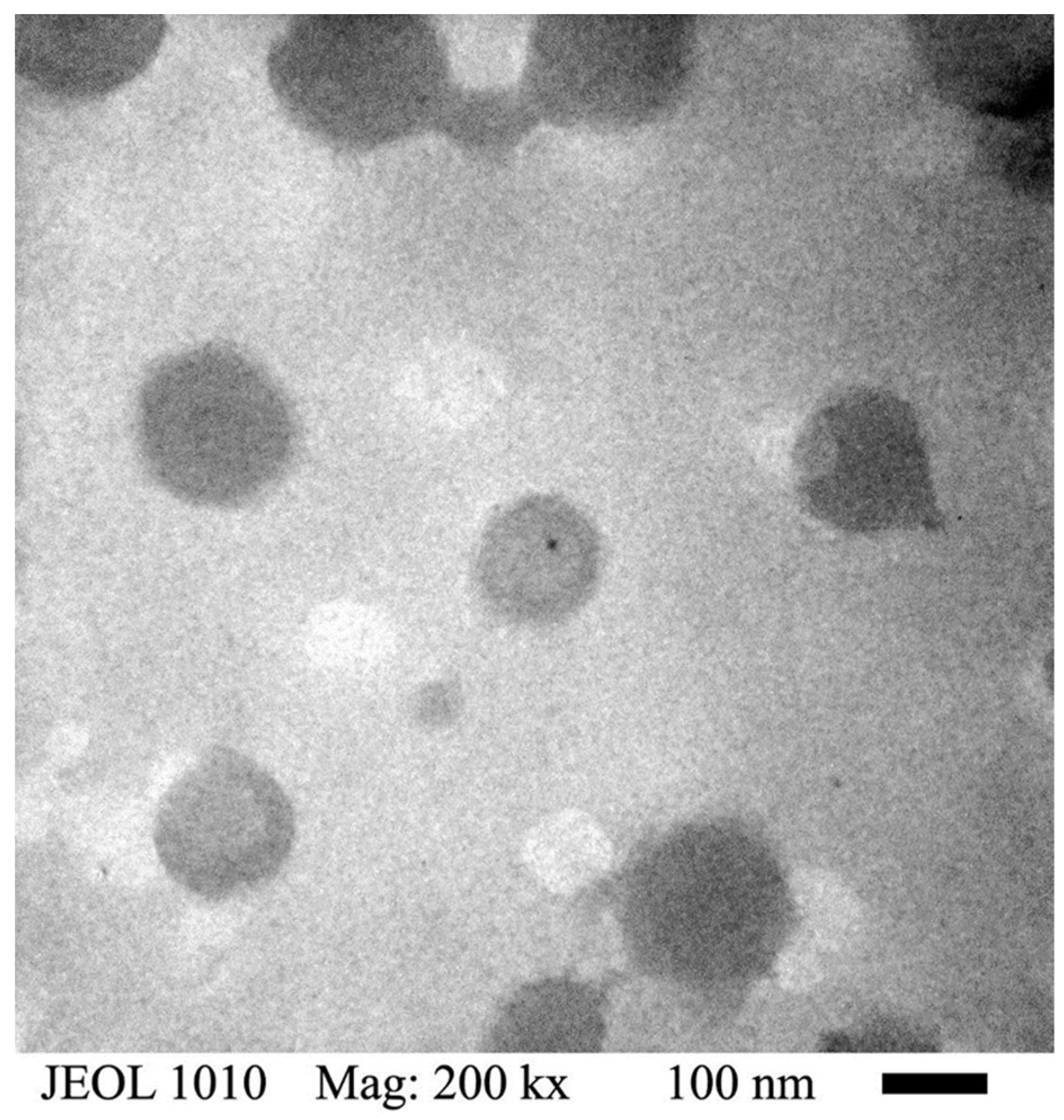

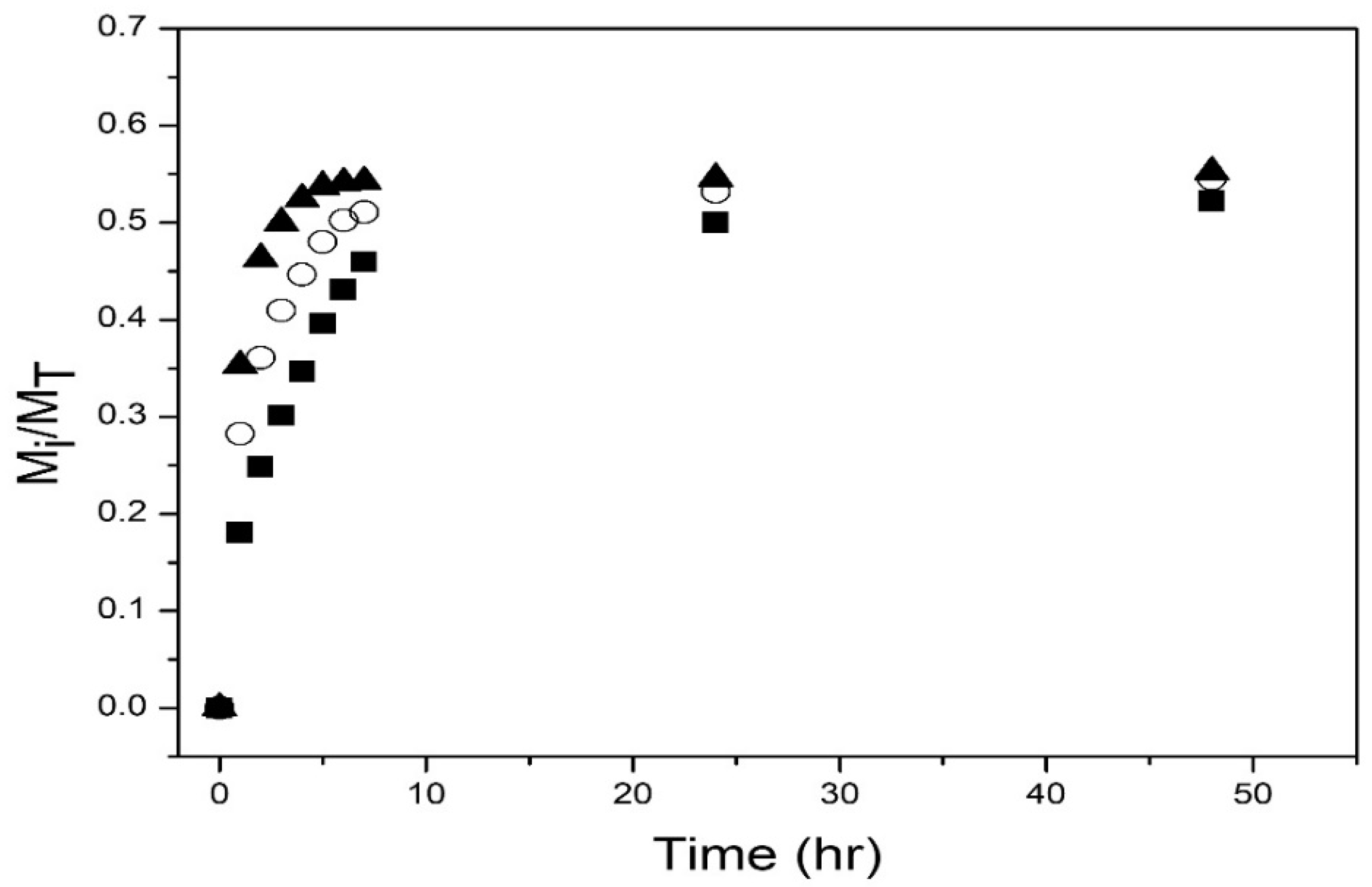


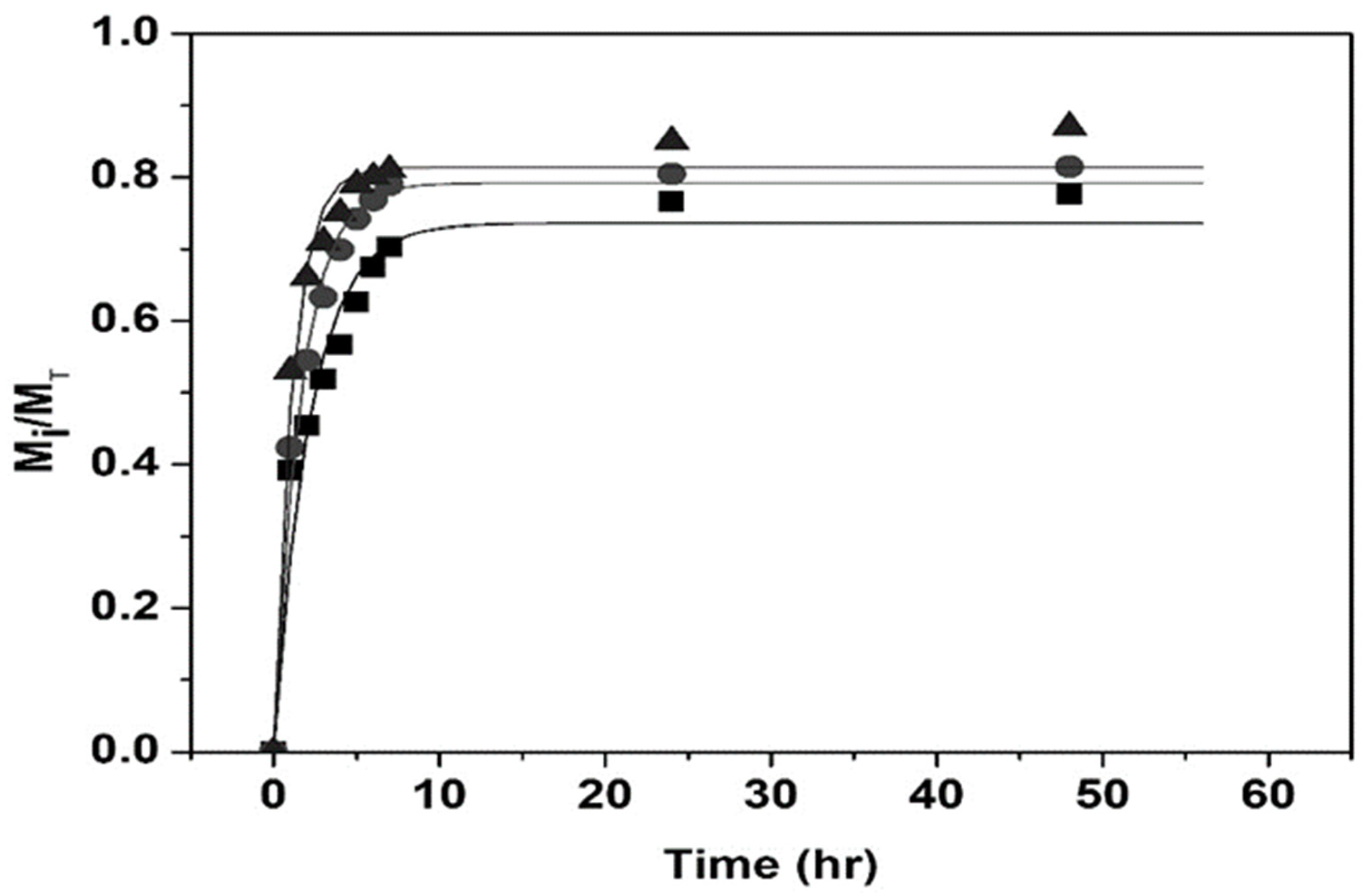

| TVPTc (°C) | NIPA Moles | NIPMA Moles | NIPMA/NIPA Ratio | NMBA Moles |
|---|---|---|---|---|
| 38 | 0.014 | 0.011 | 0.785 | 0.12 |
| 39 | 0.012 | 0.013 | 1.083 | 0.12 |
| 40 | 0.010 | 0.014 | 1.400 | 0.12 |
| TVPT (°C) | Sample | Average Size (nm) | Conversion |
|---|---|---|---|
| 38 | 1 | 296.5 | 96.1 |
| 2 | 312.9 | ||
| 39 | 1 | 300.8 | 97.7 |
| 2 | 315.3 | ||
| 40 | 1 | 300.1 | 96.4 |
| 2 | 316.2 |
| TVPT of the Nanohydrogels (°C) | |||
|---|---|---|---|
| TVPT | 38 | 39 | 40 |
| % of drug content | 5.00 | 5.20 | 5.41 |
| pH = 2.0 | pH = 7.4 | ||||||
|---|---|---|---|---|---|---|---|
| Model | K | n | R2 | b | k | n | R2 |
| 0.632 | 0.098 | 0.9647 | 0.444 | 0.0742 | 0.943 | ||
| 0.632 | 0.098 | 0.9647 | −0.001 | 0.445 | 0.7407 | 0.943 | |
| 16.9 | 7.009 | 0.7872 | 26.16 | 4.242 | 0.00 | ||
| 0.814 | 0.887 | 0.9801 | 0.541 | 0.998 | 0.988 | ||
| 0.028 | 0.005 | 0.033 | 0.008 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-García, A.; Martínez-Bernal, B.G.; Ceja, I.; Mendizábal, E.; Puig-Arévalo, J.E.; Pérez-Carrillo, L.A. Drug Delivery from Stimuli-Responsive Poly(N-isopropylacrylamide-co-N-isopropylmethacrylamide)/Chitosan Core/Shell Nanohydrogels. Polymers 2022, 14, 522. https://doi.org/10.3390/polym14030522
Ortega-García A, Martínez-Bernal BG, Ceja I, Mendizábal E, Puig-Arévalo JE, Pérez-Carrillo LA. Drug Delivery from Stimuli-Responsive Poly(N-isopropylacrylamide-co-N-isopropylmethacrylamide)/Chitosan Core/Shell Nanohydrogels. Polymers. 2022; 14(3):522. https://doi.org/10.3390/polym14030522
Chicago/Turabian StyleOrtega-García, Andrés, Bryan Giovanny Martínez-Bernal, Israel Ceja, Eduardo Mendizábal, Jorge Emilio Puig-Arévalo, and Lourdes Adriana Pérez-Carrillo. 2022. "Drug Delivery from Stimuli-Responsive Poly(N-isopropylacrylamide-co-N-isopropylmethacrylamide)/Chitosan Core/Shell Nanohydrogels" Polymers 14, no. 3: 522. https://doi.org/10.3390/polym14030522
APA StyleOrtega-García, A., Martínez-Bernal, B. G., Ceja, I., Mendizábal, E., Puig-Arévalo, J. E., & Pérez-Carrillo, L. A. (2022). Drug Delivery from Stimuli-Responsive Poly(N-isopropylacrylamide-co-N-isopropylmethacrylamide)/Chitosan Core/Shell Nanohydrogels. Polymers, 14(3), 522. https://doi.org/10.3390/polym14030522