Acoustic Performance and Flame Retardancy of Ammonium Polyphosphate/Diethyl Ethylphosphonate Rigid Polyurethane Foams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Rigid Polyurethane Foam
2.3. Characterization
2.3.1. Fourier Transform Infrared Spectroscopy
2.3.2. Scanning Electron Microscopy
2.3.3. Apparent Density Measurement
2.3.4. Compressive Strength
2.3.5. Acoustic Absorption Measurement
2.3.6. Thermogravimetric Analysis
2.3.7. Limited Oxygen Index (LOI)
2.3.8. Vertical Burning Test
2.3.9. Cone Calorimeter Test
3. Results and Discussion
3.1. FTIR Spectra of Samples and Flame Retardants
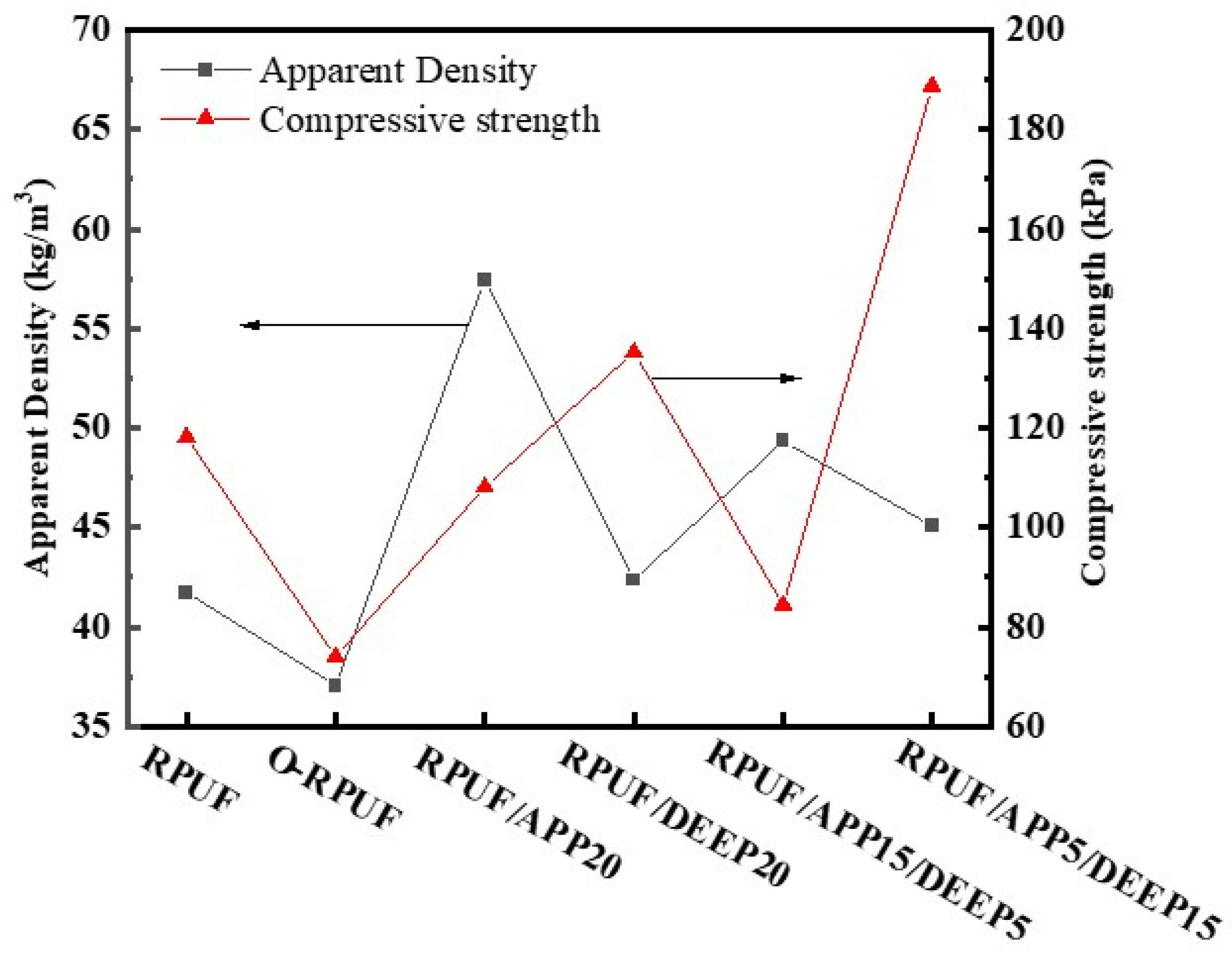
3.2. Effect of Flame Retardants on Cell Morphology, Density and Compressive Strength of Rigid Polyurethane Foam
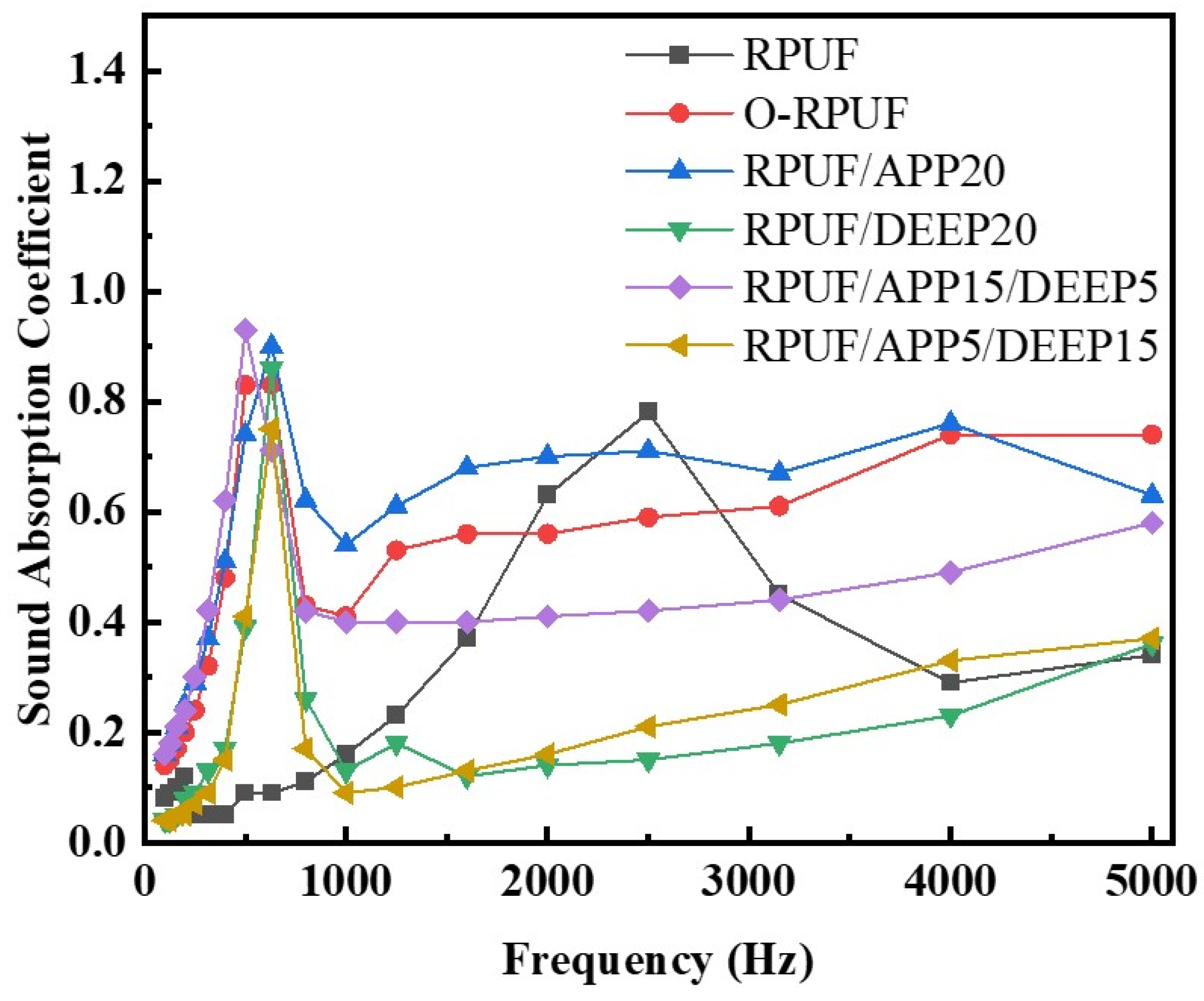
3.3. Acoustic Absorption of Rigid Polyurethane Foam
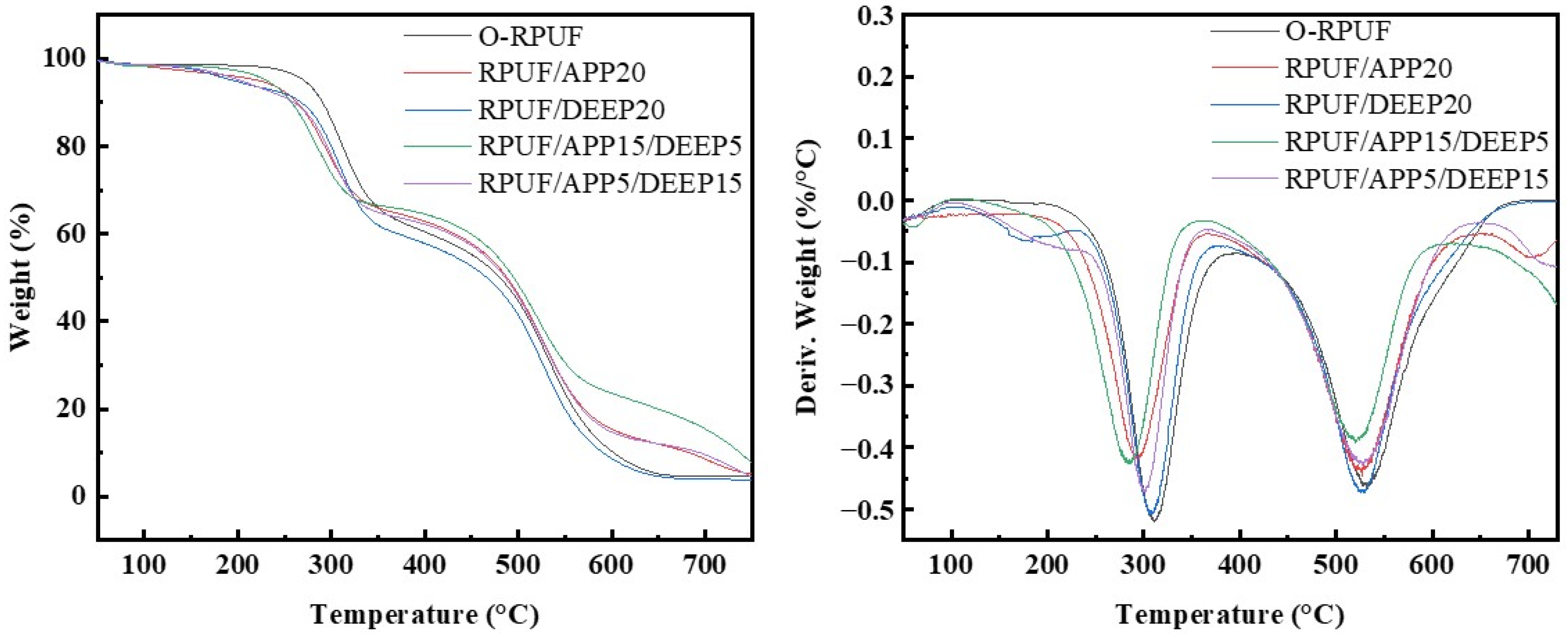
3.4. Thermal Stability of Rigid Polyurethane Foam
3.5. Flame Retardancy of Rigid Polyurethane Foam
3.5.1. Morphology of Residual Char
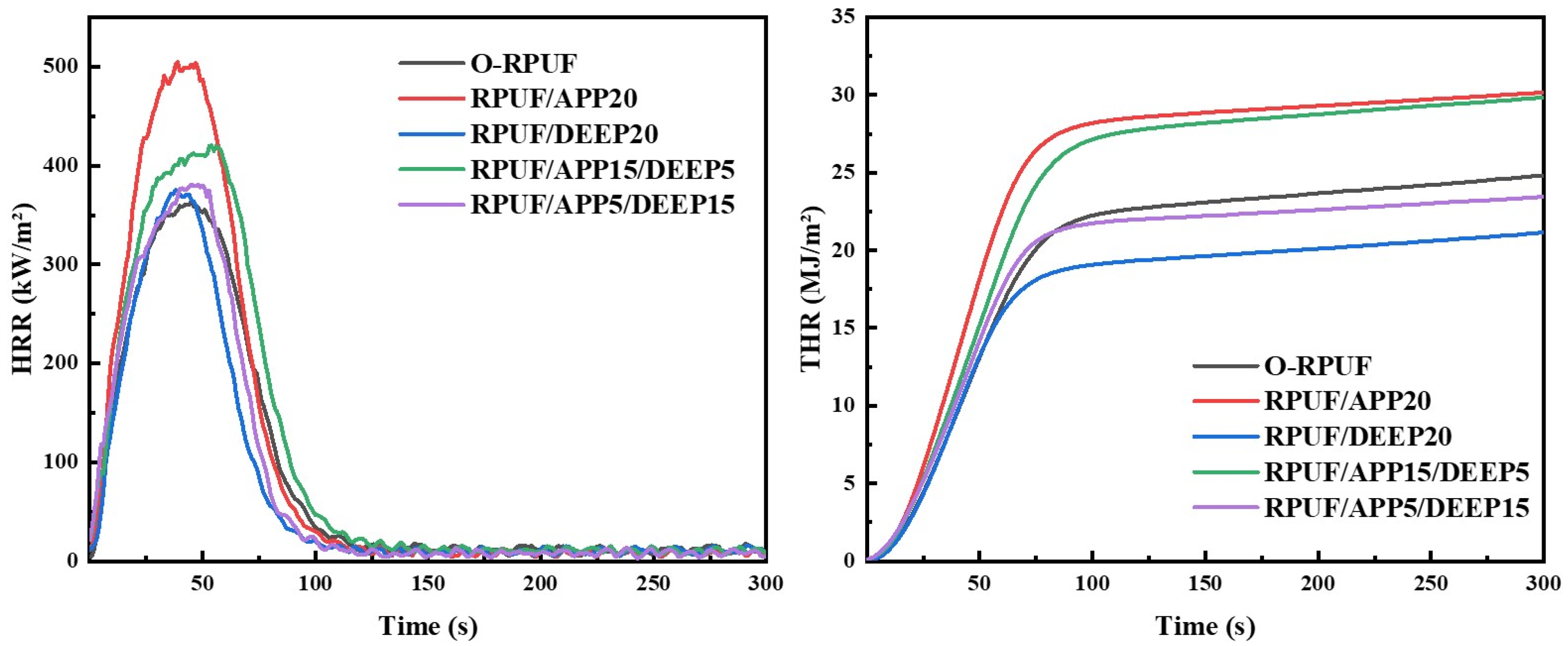
3.5.2. LOI, Vertical Burning and Cone Calorimeter Tests Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engels, H.-W.; Pirkl, H.-G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile Materials and Sustainable Problem Solvers for Today’s Challenges. Angew. Chem. Int. Ed. 2013, 52, 9422–9441. [Google Scholar] [CrossRef] [PubMed]
- Sagartzazu, X.; Hervella-Nieto, L.; Pagalday, J.M. Review in Sound Absorbing Materials. Arch. Comput. Methods Eng. 2008, 15, 311–342. [Google Scholar] [CrossRef]
- Hoang, M.T.; Bonnet, G.; Tuan Luu, H.; Perrot, C. Linear Elastic Properties Derivation from Microstructures Representative of Transport Parameters. J. Acoust. Soc. Am. 2014, 135, 3172–3185. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.L.; Koplik, J.; Dashen, R. Theory of Dynamic Permeability and Tortuosity in Fluid-Saturated Porous Media. J. Fluid Mech. 1987, 176, 379. [Google Scholar] [CrossRef]
- Champoux, Y.; Allard, J. Dynamic Tortuosity and Bulk Modulus in Air-saturated Porous Media. J. Appl. Phys. 1991, 70, 1975–1979. [Google Scholar] [CrossRef]
- Hyuk Park, J.; Suh Minn, K.; Rae Lee, H.; Hyun Yang, S.; Bin Yu, C.; Yeol Pak, S.; Sung Oh, C.; Seok Song, Y.; June Kang, Y.; Ryoun Youn, J. Cell Openness Manipulation of Low Density Polyurethane Foam for Efficient Sound Absorption. J. Sound Vib. 2017, 406, 224–236. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, S.; Zhu, W. The Effect of Pore Numbers in the Cell Walls of Soybean Oil Polyurethane Foam on Sound Absorption Performance. Appl. Acoust. 2020, 157, 107010. [Google Scholar] [CrossRef]
- Riyapan, D.; Saetung, A.; Saetung, N. A Novel Rigid PU Foam Based on Modified Used Palm Oil as Sound Absorbing Material. J. Polym. Environ. 2019, 27, 1693–1708. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, Y.; Chen, J.; Wang, D. The Effects of Various Additive Components on the Sound Absorption Performances of Polyurethane Foams. Adv. Mater. Sci. Eng. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bahrambeygi, H.; Sabetzadeh, N.; Rabbi, A.; Nasouri, K.; Shoushtari, A.M.; Babaei, M.R. Nanofibers (PU and PAN) and Nanoparticles (Nanoclay and MWNTs) Simultaneous Effects on Polyurethane Foam Sound Absorption. J. Polym. Res. 2013, 20, 72. [Google Scholar] [CrossRef]
- Tiuc, A.-E.; Vermeşan, H.; Gabor, T.; Vasile, O. Improved Sound Absorption Properties of Polyurethane Foam Mixed with Textile Waste. Energy Procedia 2016, 85, 559–565. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Jiang, Y. The Acoustic Property Study of Polyurethane Foam with Addition of Bamboo Leaves Particles. Polym. Compos. 2018, 39, 1370–1381. [Google Scholar] [CrossRef]
- Verdejo, R.; Stämpfli, R.; Alvarez-Lainez, M.; Mourad, S.; Rodriguez-Perez, M.A.; Brühwiler, P.A.; Shaffer, M. Enhanced Acoustic Damping in Flexible Polyurethane Foams Filled with Carbon Nanotubes. Compos. Sci. Technol. 2009, 69, 1564–1569. [Google Scholar] [CrossRef]
- Lee, J.; Kim, G.-H.; Ha, C.-S. Sound Absorption Properties of Polyurethane/Nano-Silica Nanocomposite Foams. J. Appl. Polym. Sci. 2012, 123, 2384–2390. [Google Scholar] [CrossRef]
- Lee, J.; Jung, I. Tuning Sound Absorbing Properties of Open Cell Polyurethane Foam by Impregnating Graphene Oxide. Appl. Acoust. 2019, 151, 10–21. [Google Scholar] [CrossRef]
- Nine, M.J.; Ayub, M.; Zander, A.C.; Tran, D.N.H.; Cazzolato, B.S.; Losic, D. Graphene Oxide-Based Lamella Network for Enhanced Sound Absorption. Adv. Funct. Mater. 2017, 27, 1703820. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Wang, Z.; Yang, Z.; Zhang, F.; You, F.; Yao, C. Structural Design and Sound Absorption Properties of Nitrile Butadiene Rubber-Polyurethane Foam Composites with Stratified Structure. Polymers 2018, 10, 946. [Google Scholar] [CrossRef] [Green Version]
- Bustamante Valencia, L.; Rogaume, T.; Guillaume, E.; Rein, G.; Torero, J.L. Analysis of Principal Gas Products during Combustion of Polyether Polyurethane Foam at Different Irradiance Levels. Fire Saf. J. 2009, 44, 933–940. [Google Scholar] [CrossRef] [Green Version]
- Nazir, R.; Gaan, S. Recent Developments in P(O/S)–N Containing Flame Retardants. J. Appl. Polym. Sci. 2020, 137, 47910. [Google Scholar] [CrossRef] [Green Version]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Chen, J.; Mishra, S.R.; Gupta, R.K. Highly Flame-Retardant Polyurethane Foam Based on Reactive Phosphorus Polyol and Limonene-Based Polyol: Research Article. J. Appl. Polym. Sci. 2018, 135, 46224. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Castor-Oil Derived Nonhalogenated Reactive Flame-Retardant-Based Polyurethane Foams with Significant Reduced Heat Release Rate. J. Appl. Polym. Sci. 2019, 136, 47276. [Google Scholar] [CrossRef]
- Zhang, K.; Hong, Y.; Wang, N.; Wang, Y. Flame Retardant Polyurethane Foam Prepared from Compatible Blends of Soybean Oil-Based Polyol and Phosphorus Containing Polyol. J. Appl. Polym. Sci. 2018, 135, 45779. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, H.; Yu, B.; Shi, Y.; Wang, W.; Song, L.; Hu, Y.; Zhang, Y. Phosphorus and Nitrogen-Containing Polyols: Synergistic Effect on the Thermal Property and Flame Retardancy of Rigid Polyurethane Foam Composites. Ind. Eng. Chem. Res. 2016, 55, 10813–10822. [Google Scholar] [CrossRef]
- Wu, N.; Niu, F.; Lang, W.; Yu, J.; Fu, G. Synthesis of Reactive Phenylphosphoryl Glycol Ether Oligomer and Improved Flame Retardancy and Mechanical Property of Modified Rigid Polyurethane Foams. Mater. Des. 2019, 181, 107929. [Google Scholar] [CrossRef]
- Yang, R.; Hu, W.; Xu, L.; Song, Y.; Li, J. Synthesis, Mechanical Properties and Fire Behaviors of Rigid Polyurethane Foam with a Reactive Flame Retardant Containing Phosphazene and Phosphate. Polym. Degrad. Stab. 2015, 122, 102–109. [Google Scholar] [CrossRef]
- Lin, Z.; Zhao, Q.; Fan, R.; Yuan, X.; Tian, F. Flame Retardancy and Thermal Properties of Rigid Polyurethane Foam Conjugated with a Phosphorus–Nitrogen Halogen-Free Intumescent Flame Retardant. J. Fire Sci. 2020, 38, 235–252. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Cheng, J.; Ma, Q.; Zhang, F.; Wang, Y.; Liu, M.; Wang, D.; Qu, W. Mechanical Property Improvement and Fire Hazard Reduction of Ammonium Polyphosphate Microencapsulated in Rigid Polyurethane Foam. J. Appl. Polym. Sci. 2020, 137, 48307. [Google Scholar] [CrossRef]
- Luo, F.; Wu, K.; Lu, M.; Nie, S.; Li, X.; Guan, X. Thermal Degradation and Flame Retardancy of Microencapsulated Ammonium Polyphosphate in Rigid Polyurethane Foam. J. Therm. Anal. Calorim. 2015, 120, 1327–1335. [Google Scholar] [CrossRef]
- Cheng, J.; Niu, S.; Ma, D.; Zhou, Y.; Zhang, F.; Qu, W.; Wang, D.; Li, S.; Zhang, X.; Chen, X. Effects of Ammonium Polyphosphate Microencapsulated on Flame Retardant and Mechanical Properties of the Rigid Polyurethane Foam. J. Appl. Polym. Sci. 2020, 137, 49591. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Qi, X.; Qian, L. The Pyrolysis Behaviors of Phosphorus-Containing Organosilicon Compound Modified APP with Different Polyether Segments and Their Flame Retardant Mechanism in Polyurethane Foam. Compos. Part B Eng. 2019, 173, 106784. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Wang, W.; Qian, L. Preparation and Characterization of Surface-Modified Ammonium Polyphosphate and Its Effect on the Flame Retardancy of Rigid Polyurethane Foam. J. Appl. Polym. Sci. 2017, 134, 45369. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.; Li, L. Flame Retardant Behavior of Ternary Synergistic Systems in Rigid Polyurethane Foams. Polymers 2019, 11, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Zheng, G.; Zhou, Y.; Hu, L.; Feng, G.; Zhang, M. Synergistic Effect of Expandable Graphite, Diethyl Ethylphosphonate and Organically-Modified Layered Double Hydroxide on Flame Retardancy and Fire Behavior of Polyisocyanurate-Polyurethane Foam Nanocomposite. Polym. Degrad. Stab. 2014, 101, 92–101. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Yang, R. Effects of Dimethyl Methylphosphonate, Aluminum Hydroxide, Ammonium Polyphosphate, and Expandable Graphite on the Flame Retardancy and Thermal Properties of Polyisocyanurate–Polyurethane Foams. Ind. Eng. Chem. Res. 2015, 54, 5876–5884. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Ye, L.; Zhang, X.-G.; Tang, P.-M.; Tang, J.-H.; Ji, X.; Li, Z.-M. Effects of Expandable Graphite and Ammonium Polyphosphate on the Flame-Retardant and Mechanical Properties of Rigid Polyurethane Foams. J. Appl. Polym. Sci. 2009, 114, 853–863. [Google Scholar] [CrossRef]
- Tarakcılar, A.R. The Effects of Intumescent Flame Retardant Including Ammonium Polyphosphate/Pentaerythritol and Fly Ash Fillers on the Physicomechanical Properties of Rigid Polyurethane Foams. J. Appl. Polym. Sci. 2011, 120, 2095–2102. [Google Scholar] [CrossRef]
- Pang, X.; Xin, Y.; Shi, X.; Xu, J. Effect of Different Size-modified Expandable Graphite and Ammonium Polyphosphate on the Flame Retardancy, Thermal Stability, Physical, and Mechanical Properties of Rigid Polyurethane Foam. Polym. Eng. Sci. 2019, 59, 1381–1394. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Wu, X. Construction of an Efficient Ternary Flame Retardant System for Rigid Polyurethane Foam Based on Bi-phase Flame Retardant Effect. Polym. Adv. Technol. 2020, 31, 3202–3210. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.; Jiang, Y.; Chen, M.; Wan, C. Density Effect on Flame Retardancy, Thermal Degradation, and Combustibility of Rigid Polyurethane Foam Modified by Expandable Graphite or Ammonium Polyphosphate. Polymers 2019, 11, 668. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Liu, D.-Y.; Liang, W.-J.; Li, F.; Wang, J.-S.; Liu, Y.-Q. Bi-Phase Flame-Retardant Actions of Water-Blown Rigid Polyurethane Foam Containing Diethyl-N,N-Bis(2-Hydroxyethyl) Phosphoramide and Expandable Graphite. J. Anal. Appl. Pyrolysis 2017, 124, 247–255. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.; Zheng, X. Research on Highly Flame-Retardant Rigid PU Foams by Combination of Nanostructured Additives and Phosphorus Flame Retardants. Polym. Degrad. Stab. 2015, 111, 142–150. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Wu, X.; Xu, B.; Qian, L. Bi-phase Flame-retardant Effect of Dimethyl Methylphosphonate and Modified Ammonium Polyphosphate on Rigid Polyurethane Foam. Polym. Adv. Technol. 2019, 30, 2721–2728. [Google Scholar] [CrossRef]
- Li, A.; Yang, D.D.; Li, H.N.; Jiang, C.L.; Liang, J.Z. Flame-Retardant and Mechanical Properties of Rigid Polyurethane Foam/MRP/Mg(OH)2 /GF/HGB Composites: Research Article. J. Appl. Polym. Sci. 2018, 135, 46551. [Google Scholar] [CrossRef]
- Qian, L.; Li, L.; Chen, Y.; Xu, B.; Qiu, Y. Quickly Self-Extinguishing Flame Retardant Behavior of Rigid Polyurethane Foams Linked with Phosphaphenanthrene Groups. Compos. Part B Eng. 2019, 175, 107186. [Google Scholar] [CrossRef]
- Samaržija-Jovanović, S.; Jovanović, V.; Konstantinović, S.; Marković, G.; Marinović-Cincović, M. Thermal Behavior of Modified Urea–Formaldehyde Resins. J. Therm. Anal. Calorim. 2011, 104, 1159–1166. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Chen, L.; Shi, H.; Hao, J. Ammonium Polyphosphate Modified with β-Cyclodextrin Crosslinking Rigid Polyurethane Foam: Enhancing Thermal Stability and Suppressing Flame Spread. Polym. Degrad. Stab. 2019, 161, 166–174. [Google Scholar] [CrossRef]
- Wang, G.; Yang, T. Preparation of Open Cell Rigid Polyurethane Foams and Modified with Organo-Kaolin. J. Cell. Plast. 2020, 56, 435–447. [Google Scholar] [CrossRef]
- Dolomanova, V.; Rauhe, J.C.M.; Jensen, L.R.; Pyrz, R.; Timmons, A.B. Mechanical Properties and Morphology of Nano-Reinforced Rigid PU Foam. J. Cell. Plast. 2011, 47, 81–93. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Kurańska, M.; Prociak, A.; Malewska, E.; Kulpa, K. Open Cell Semi-Rigid Polyurethane Foams Synthesized Using Palm Oil-Based Bio-Polyol. Ind. Crops Prod. 2017, 102, 88–96. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.B.; Naik, Y.P.; Singha, N.K. Halogen-Free Flame Retardant PUF: Effect of Melamine Compounds on Mechanical, Thermal and Flame Retardant Properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Narine, S.S.; Kong, X.; Bouzidi, L.; Sporns, P. Physical Properties of Polyurethanes Produced from Polyols from Seed Oils: II. Foams. J. Am. Oil Chem. Soc. 2007, 84, 65–72. [Google Scholar] [CrossRef]
- Yang, Z.-G.; Zhao, B.; Qin, S.-L.; Hu, Z.-F.; Jin, Z.-K.; Wang, J.-H. Study on the Mechanical Properties of Hybrid Reinforced Rigid Polyurethane Composite Foam. J. Appl. Polym. Sci. 2004, 92, 1493–1500. [Google Scholar] [CrossRef]
- Xue, B.-L.; Wen, J.-L.; Sun, R.-C. Lignin-Based Rigid Polyurethane Foam Reinforced with Pulp Fiber: Synthesis and Characterization. ACS Sustain. Chem. Eng. 2014, 2, 1474–1480. [Google Scholar] [CrossRef]
- Cui, G.; Fan, H.; Xia, W.; Ai, F.; Huang, J. Simultaneous Enhancement in Strength and Elongation of Waterborne Polyurethane and Role of Star-like Network with Lignin Core. J. Appl. Polym. Sci. 2008, 109, 56–63. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Hu, Z.; Zhu, F.; Huang, Y. Correlation between the Acoustic and Porous Cell Morphology of Polyurethane Foam: Effect of Interconnected Porosity. Mater. Des. 2012, 41, 319–325. [Google Scholar] [CrossRef]
- Gatos, K.G.; Sawanis, N.S.; Apostolov, A.A.; Thomann, R.; Karger-Kocsis, J. Nanocomposite Formation in Hydrogenated Nitrile Rubber (HNBR)/Organo-Montmorillonite as a Function of the Intercalant Type. Macromol. Mater. Eng. 2004, 289, 1079–1086. [Google Scholar] [CrossRef]
- Gao, K.; van Dommelen, J.A.W.; Geers, M.G.D. Microstructure Characterization and Homogenization of Acoustic Polyurethane Foams: Measurements and Simulations. Int. J. Solids Struct. 2016, 100–101, 536–546. [Google Scholar] [CrossRef]
- Sung, G.; Kim, J.H. Influence of Filler Surface Characteristics on Morphological, Physical, Acoustic Properties of Polyurethane Composite Foams Filled with Inorganic Fillers. Compos. Sci. Technol. 2017, 146, 147–154. [Google Scholar] [CrossRef]
- Sung, C.H.; Lee, K.S.; Lee, K.S.; Oh, S.M.; Kim, J.H.; Kim, M.S.; Jeong, H.M. Sound Damping of a Polyurethane Foam Nanocomposite. Macromol. Res. 2007, 15, 443–448. [Google Scholar] [CrossRef]
- Dhaliwal, G.S.; Anandan, S.; Chandrashekhara, K.; Dudenhoeffer, N.; Nam, P. Fabrication and Testing of Soy-Based Polyurethane Foam for Insulation and Structural Applications. J. Polym. Environ. 2019, 27, 1897–1907. [Google Scholar] [CrossRef]
- Feng, F.; Qian, L. The Flame Retardant Behaviors and Synergistic Effect of Expandable Graphite and Dimethyl Methylphosphonate in Rigid Polyurethane Foams. Polym. Compos. 2014, 35, 301–309. [Google Scholar] [CrossRef]
- Feng, J.; Ma, P.; Yang, H.; Lu, L. Understanding the Interactions of Phosphonate-Based Flame-Retarding Additives with Graphitic Anode for Lithium Ion Batteries. Electrochim. Acta 2013, 114, 688–692. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Chen, Z. Preparation and Characterization of Flame Retardant Phase Change Materials by Microencapsulated Paraffin and Diethyl Ethylphosphonate with Poly(Methacrylic Acid-Co-Ethyl Methacrylate) Shell. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Günther, M.; Lorenzetti, A.; Schartel, B. Fire Phenomena of Rigid Polyurethane Foams. Polymers 2018, 10, 1166. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, J.; Gao, M. Thermal Degradation and Flame Retardant Mechanism of the Rigid Polyurethane Foam Including Functionalized Graphene Oxide. Polymers 2019, 11, 78. [Google Scholar] [CrossRef] [Green Version]
- Duquesne, S.; Le Bras, M.; Bourbigot, S.; Delobel, R.; Camino, G.; Eling, B.; Lindsay, C.; Roels, T.; Vezin, H. Mechanism of Fire Retardancy of Polyurethanes Using Ammonium Polyphosphate. J. Appl. Polym. Sci. 2001, 82, 3262–3274. [Google Scholar] [CrossRef]

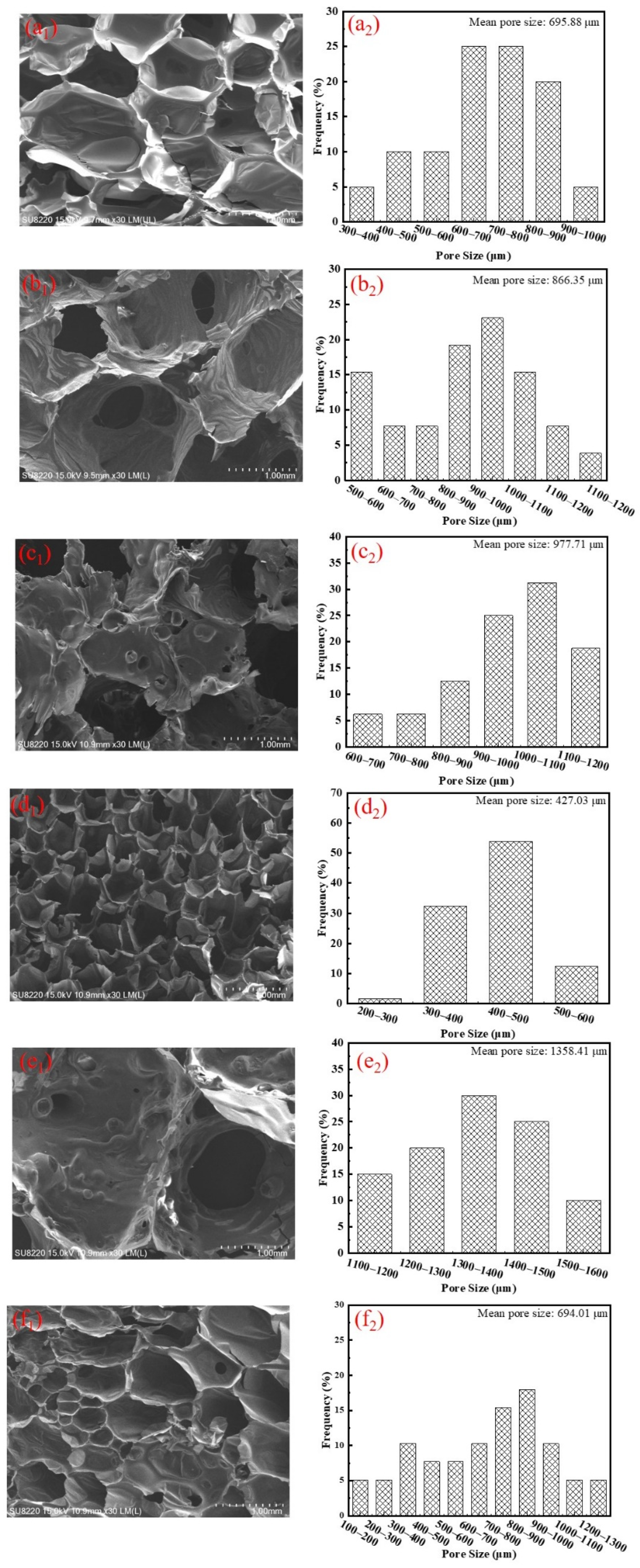

| Sample | PAPI/php a | Polyether Polyol/ php | 33LV/ php | PC-8/ php | AK-8801/ php | Ortegol-501/ php | H2O/ php | APP/ php | DEEP/ php |
|---|---|---|---|---|---|---|---|---|---|
| RPUF | 105.0 | 50.0 | 1.0 | 1.0 | 0.5 | 0 | 3.0 | 0 | 0 |
| O-RPUF | 105.0 | 50.0 | 1.0 | 1.0 | 0.5 | 2.0 | 3.0 | 0 | 0 |
| RPUF/APP20 | 105.0 | 50.0 | 1.0 | 1.0 | 0.5 | 2.0 | 3.0 | 20.0 | 0 |
| RPUF/DEEP20 | 105.0 | 50.0 | 1.0 | 1.0 | 0.5 | 2.0 | 3.0 | 0 | 20.0 |
| RPUF/APP5/DEEP15 | 105.0 | 50.0 | 1.0 | 1.0 | 0.5 | 2.0 | 3.0 | 5.0 | 15.0 |
| RPUF/APP15/DEEP5 | 105.0 | 50.0 | 1.0 | 1.0 | 0.5 | 2.0 | 3.0 | 15.0 | 5.0 |
| Sample | Apparent Density/(kg/m3) | Compressive Strength/kPa |
|---|---|---|
| RPUF | 41.74 | 118.21 |
| O-RPUF | 37.08 | 74.03 |
| RPUF/APP20 | 57.45 | 108.14 |
| RPUF/DEEP20 | 42.36 | 135.26 |
| RPUF/APP15/DEEP5 | 49.35 | 84.47 |
| RPUF/APP5/DEEP15 | 45.08 | 188.77 |
| Sample | Acoustic Absorption Coefficient | Maximum Frequency (Hz) | |
|---|---|---|---|
| Average | Maximum | ||
| RPUF | 0.232 | 0.46 | 4000 |
| O-RPUF | 0.488 | 0.83 | 500 |
| RPUF/APP20 | 0.535 | 0.90 | 630 |
| RPUF/DEEP20 | 0.170 | 0.86 | 630 |
| RPUF/APP15/DEEP5 | 0.452 | 0.93 | 500 |
| RPUF/APP5/DEEP15 | 0.18 | 0.75 | 630 |
| Sample | T5%/°C | Tmax/°C | Residual Mass/% | |
|---|---|---|---|---|
| Tmax1/°C | Tmax2/°C | |||
| O-RPUF | 270.9 | 311.8 | 532.8 | 4.52 |
| RPUF/APP20 | 222.0 | 295.6 | 527.8 | 5.11 |
| RPUF/DEEP20 | 195.4 | 309.5 | 526.3 | 3.80 |
| RPUF/APP15/DEEP5 | 230.8 | 285.5 | 523.2 | 7.64 |
| RPUF/APP5/DEEP15 | 202.4 | 299.7 | 528.1 | 4.61 |
| Sample | LOI/% | t/s | V-Rating | Dripping | Residual Mass/% |
|---|---|---|---|---|---|
| O-RPUF | 18.6 | 45.0 | - | No | 31.67 |
| RPUF/APP20 | 22.7 | 15.0 | - | No | 80.82 |
| RPUF/DEEP20 | 24.4 | 1.5 | V-0 | No | 86.58 |
| RPUF/APP15/DEEP5 | 24.3 | 3.0 | V-0 | No | 84.71 |
| RPUF/APP5/DEEP15 | 24.9 | 3 | V-0 | No | 83.33 |
| Sample | TTI/s | pHRR/kW·m−2 | THR/MJ·m−2 | TSP/m2 |
|---|---|---|---|---|
| O-RPUF | 4 | 364.37 | 24.82 | 3.85 |
| RPUF/APP20 | 5 | 504.60 | 30.17 | 7.05 |
| RPUF/DEEP20 | 4 | 375.29 | 21.15 | 5.50 |
| RPUF/APP15/DEEP5 | 8 | 420.39 | 29.84 | 7.36 |
| RPUF/APP5/DEEP15 | 7 | 380.91 | 23.43 | 6.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; LYU, X.; Huang, Z.; Yan, Y. Acoustic Performance and Flame Retardancy of Ammonium Polyphosphate/Diethyl Ethylphosphonate Rigid Polyurethane Foams. Polymers 2022, 14, 420. https://doi.org/10.3390/polym14030420
Zhang H, LYU X, Huang Z, Yan Y. Acoustic Performance and Flame Retardancy of Ammonium Polyphosphate/Diethyl Ethylphosphonate Rigid Polyurethane Foams. Polymers. 2022; 14(3):420. https://doi.org/10.3390/polym14030420
Chicago/Turabian StyleZhang, Huiping, Xiongxian LYU, Zijun Huang, and Ying Yan. 2022. "Acoustic Performance and Flame Retardancy of Ammonium Polyphosphate/Diethyl Ethylphosphonate Rigid Polyurethane Foams" Polymers 14, no. 3: 420. https://doi.org/10.3390/polym14030420
APA StyleZhang, H., LYU, X., Huang, Z., & Yan, Y. (2022). Acoustic Performance and Flame Retardancy of Ammonium Polyphosphate/Diethyl Ethylphosphonate Rigid Polyurethane Foams. Polymers, 14(3), 420. https://doi.org/10.3390/polym14030420







