Mold and Stain Resistance of Bamboo Treated with Pyraclostrobin Fungicide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
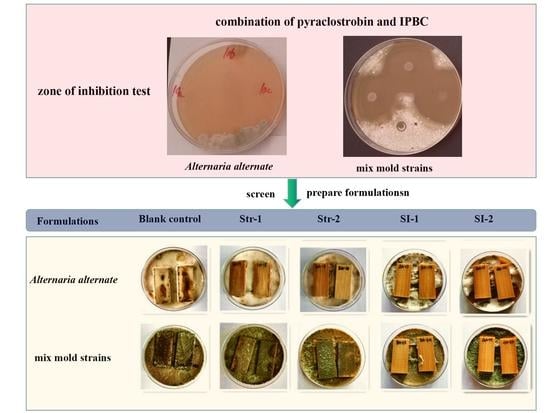
2.2.1. Zone of Inhibition Test
2.2.2. Selection of the Appropriate Ratio of Pyraclostrobin and IPBC
2.2.3. Preparation of Pyraclostrobin and Composite Formulations
2.2.4. Treatment of Bamboo with Anti-Mold Agents
2.2.5. Evaluation of Mold and Stain Resistance of Treated Bamboo
3. Results and Discussion
3.1. Preliminary Screening of Chemicals in Zone of Inhibition Test
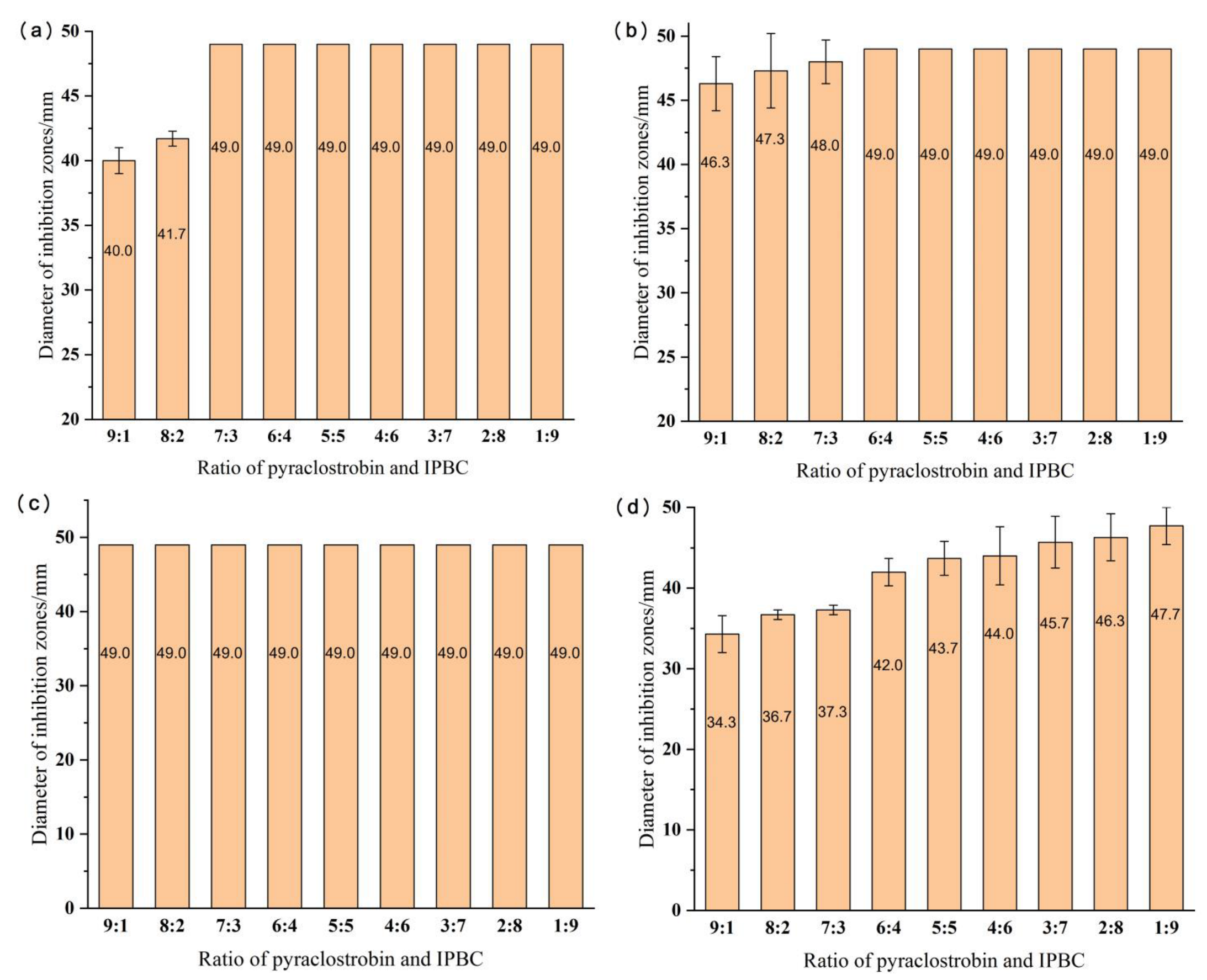
3.2. Optimization of the Ratio of Pyraclostrobin and IPBC
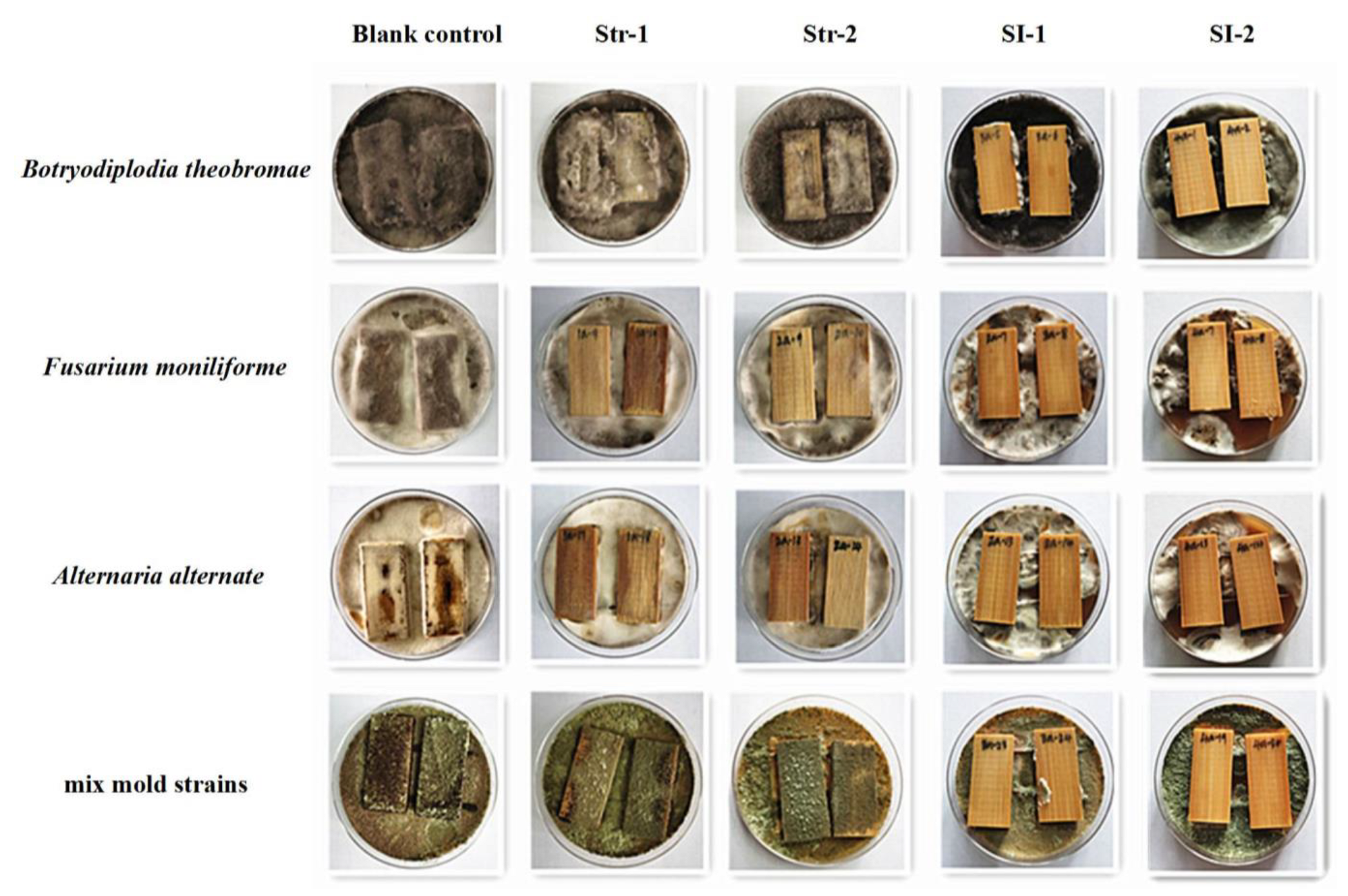
3.3. Stain and Mold Resistance of Bamboo Treated with Different Pyraclostrobin Formulations
4. Conclusions
- (1)
- Pyraclostrobin had higher antifungal activity than azoxystrobin and kresoxim-methyl. It exhibited different degrees of inhibitory activity against all stain fungi and molds in this test. In general, its activity was slightly higher against stain fungi than against mold fungi.
- (2)
- The combination of pyraclostrobin and IPBC had a good synergistic effect. It completely inhibited the three stain fungi at a concentration of 0.1% and a ratio of pyraclostrobin to IPBC of 7:3. The diameter of the inhibition zone was 37.3 mm in the test with the mixed mold strains.
- (3)
- The mold and stain resistance of bamboo treated with pyraclostrobin formulations Str-1 and Str-2 were the same, and the control efficacy increased with an increase in the concentration of the active ingredient. The composite preparations SI-1 and SI-2 had 100% control efficacy at concentrations of 0.1% against the three stain fungi and 91.8% and 100% control efficacy, respectively, against the mold strains.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.J.; Liu, M.H.; Zhai, H.B.; Wang, H.K.; Yu, Y. Preparing highly durable bamboo materials via bulk furfurylation. Constr. Build. Mater. 2020, 262, 120726. [Google Scholar] [CrossRef]
- Huang, Z.J.; Künzel, H.; Krus, M.; Zhang, W.Y. Three-dimensional tests on hygric properties of laminated bamboo and bamboo scrimber. J. Build. Eng. 2022, 56, 104712. [Google Scholar] [CrossRef]
- Zhong, Y.; Jiang, Z.; Shangguan, W.-W.; Ren, H.-Q. Design value of the compressive strength for bamboo fiber reinforced composite based on a reliability analysis. BioResources 2014, 9, 7737–7748. [Google Scholar] [CrossRef]
- Liese, W.; Kumar, S. Bamboo preservation compendium. INBAR Technol. Rep. 2003, 22, 231. [Google Scholar]
- Schmidt, O.; Wei, D.S.; Liese, W.; Wollenberg, E. Fungal degradation of bamboo samples. Holzforschung 2011, 65, 883–888. [Google Scholar] [CrossRef]
- Cheng, D.L.; Jiang, S.H.; Zhang, Q.S. Mould resistance of Moso bamboo treated by two step heat treatment with different aqueous solutions. Eur. J. Wood Prod. 2013, 71, 143–145. [Google Scholar] [CrossRef]
- Johansson, P.; Annika, E.T.; Svensson, T.; Bok, G. Laboratory study to determine the critical moisture level for mould growth on building materials. Int. Biodeter. Biodegr. 2012, 73, 23–32. [Google Scholar] [CrossRef]
- Scurlock, J.M.O.; Dayton, D.C.; Hames, B. Bamboo: An overlooked biomass resource? Biomass Bioenergy 2000, 19, 229–244. [Google Scholar] [CrossRef]
- Ma, X.X.; Jiang, M.L.; Lu, W.H.; Qin, D. Study on the biological characteristics of stain fungi and mould fungi on bamboo wood. For. Res. 2009, 22, 819–823. [Google Scholar]
- Cheng, D.L.; Jiang, S.X.; Zhang, Q.S. Effect of hydrothermal treatment with different aqueous solutions on the mold resistance of moso bamboo with chemical and FTIR analysis. BioResources 2013, 8, 371–382. [Google Scholar] [CrossRef]
- Tang, X.Y.; Guan, M.J.; Xu, X.J.; Yong, C. Effect of ultrasonic-antibacterial paraffin treatment on the antifungal performance of bamboo. J. For. Eng. 2022, 7, 45–51. [Google Scholar]
- Terzi, E.; Kartal, S.N.; Yılgör, N.; Rautkari, L.; Yoshimura, T. Role of various nano-particles in prevention of fungal decay, mold growth and termite attack in wood, and their effect on weathering properties and water repellency. Int. Biodeter. Biodegr. 2016, 107, 77–87. [Google Scholar] [CrossRef]
- Goffredo, G.B.; Citterio, B.; Biavasco, F.; Stazi, F.; Barcelli, S.; Munafò, P. Nanotechnology on wood: The effect of photocatalytic nanocoatings against Aspergillus niger. J. Cult. Herit. 2017, 27, 125–136. [Google Scholar] [CrossRef]
- Li, J.P.; Ma, R.; Wu, Z.X.; He, S.; Chen, Y.H.; Bai, R.H.; Wang, J. Visible-light-driven Ag-modified TiO2 thin films anchored on bamboo material with antifungal memory activity against Aspergillus niger. J. Fungi 2021, 7, 592. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, D.P.; Ma, D.K.; Huang, S.M. Extremely sensitive mechanochromic photonic crystals with broad tuning range of photonic bandgap and fast responsive speed for high-resolution multicolor display applications. Chem. Eng. J. 2022, 429, 132342. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, Z.; Wang, G.; Tian, G.; Wang, H.; Song, Y. Surface functionalization of bamboo with nanostructured ZnO. Wood Sci. Technol. 2012, 46, 781–790. [Google Scholar] [CrossRef]
- Jin, X.B.; Zhang, R.; Su, M.L.; Li, H.R.; Yue, X.F.; Qin, D.C.; Jiang, Z.H. Functionalization of halloysite nanotubes by enlargement and layer-by-layer assembly for controlled release of fungicide iodopropynyl butylcarbamate. RSC Adv. 2019, 9, 42062–42070. [Google Scholar] [CrossRef]
- Tumirah, K.; Salamah, S.; Rozita, A.; Salmiah, U.; Nasir, M.A.M. Determination of 2-thiocyanomethylthio-benzothiazole (TCMTB) in treated wood and wood preservative using Ultraviolet-visible spectrophotometer. Wood Sci. Technol. 2012, 46, 1021–1031. [Google Scholar] [CrossRef]
- Wang, Q.P.; Cao, J.Z.; Liu, X.E.; Yang, S.M.; Jiang, M.L. Self-healing coatings for inhibiting corrosion of ferrous metals exposed to preservative-treated bamboo. J. Wood Sci. 2020, 66, 18. [Google Scholar] [CrossRef]
- Xi, L.X.; Jiang, M.L.; Ma, X.X. Mold and stain resistance of bamboo treated with waterborne organic formulations. China Wood Ind. 2013, 27, 42–45. [Google Scholar]
- Han, L.P.; Jiang, M.L.; Zhang, J.P.; Shao, C.; Zhang, Q.S. Thermal degradation and product analysis of 3-iodo-2-propyl-butylcarbamate as a wood preservative. Polymers 2022, 14, 4531. [Google Scholar] [CrossRef] [PubMed]
- Anke, T.; Oberwinkler, F.; Steglich, W.; Schramm, G. The strobilurins-new antifungal antibiotics from the basidiomycete strobilurus tenacellus. J Antibiot. 1977, 30, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Sauter, H.; Steglich, W.; Anke, T. Strobilurins: Evolution of a new class of active substances. Angew. Chem. Int. Ed. 1999, 38, 1328–1349. [Google Scholar] [CrossRef]
- Hosokawa, N.; Momose, I.; Sekizawa, R.; Naganawa, H.; Iinuma, H.; Takeuchi, T.; Matsui, S. New strobilurins O and P from a mushroom. J. Antibiot. 2000, 53, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Sierotzki, H.; Wullschleger, J.; Gisi, U. Point mutation in cytochrome b gene conferring resistance to strobilurin fungicides in Erysiphe Graminis f. sp. tritici field isolates. Pestic. Biochem. Physiol. 2000, 68, 107–112. [Google Scholar] [CrossRef]
- Paul, M.W.; Derek, W.H. A critical evaluation of the role of alternative oxidase in the performance of strobilurin and related fungicides acting at the Qo site of Complex III. Pest Manag. Sci. 2003, 59, 499–511. [Google Scholar]
- Grossmann, N.; Retzlaff, G. Bioregulatory effects of the fungicidal strobilurin kresoxim-methyl in wheat (triticum aestivum)—grossmann. Pestic. Sci. 1997, 50, 11–20. [Google Scholar] [CrossRef]
- Zhang, J.P.; Jiang, M.L.; Ma, X.X.; Zhang, B. Wood decay performance of strobilurins preservatives. J. Beijing For. Univ. 2021, 43, 131–137. [Google Scholar]
- Jiang, M.L.; Li, X.W.; Zhang, J.P.; Ma, X.X.; Wang, Q.P.; Zhang, B. The Combination Modifier of Preservative and Mildew Resistant and Method for Treating Wood and Bamboo Materials; Nationnal Intellectual Property Administration: Beijing, China, 2018; CN 201810820686.7. [Google Scholar]
- GB/T 18261-2013; Test Method for Anti-mildew Agents in Controlling Wood Mold and Stain Fungi. China National Standardization Administration Committee: Beijing, China, 2013.
- Zhang, R.; Li, Y.; He, Y.; Qin, D. Preparation of iodopropynyl butycarbamate loaded halloysite and its anti-mildew activity. J. Mater. Res. Technol. 2020, 9, 10148–10156. [Google Scholar] [CrossRef]
- Hansen, J. IPBC-a new fungicide for wood protection. Mod. Paint. Coat. 1984, 74, 50–56. [Google Scholar]
- Kositchaiyong, A.; Rosarpitak, V.; Hamada, H.; Sombatsompop, N. Anti-fungal performance and mechanical-morphological properties of PVC and wood/PVC composites under UV-weathering aging and soil-burial exposure. Int. Biodeterior. Biodegrad. 2014, 91, 128–137. [Google Scholar] [CrossRef]

| Infection Value | Infection Area |
|---|---|
| 0 | No hyphae on surface |
| 1 | Infection area < 1/4 |
| 2 | Infection area 1/4~1/2 |
| 3 | Infection area 1/2~3/4 |
| 4 | Infection area > 3/4 |
| Chemical | Mass Concentration (%) | Diameters of Inhibition Zones/mm | ||
|---|---|---|---|---|
| Bt | Fm | Aa | ||
| Azoxystrobin | 0.05 | 0 | 0 | 0 |
| 0.1 | 0 | 10.0 ± 2.3 | 0 | |
| 0.2 | 0 | 22.2 ± 2.1 | 0 | |
| Kresoxim-methyl | 0.05 | 0 | 12.1 ± 3.0 | 25.0 ± 4.9 |
| 0.1 | 0 | 13.1 ± 1.3 | 27.1 ± 4.7 | |
| 0.2 | 0 | 19.8 ± 3.6 | 29.6 ± 4.1 | |
| Pyraclostrobin | 0.05 | 16.0 ± 2.7 | 17.7 ± 3.1 | 28.6 ± 1.5 |
| 0.1 | 20.3 ± 3.2 | 20.1 ± 1.1 | 35.9 ± 2.5 | |
| 0.2 | 24.6 ± 2.2 | 24.6 ± 3.4 | 39.3 ± 1.3 | |
| Iodopropynyl butyl carbamate (IPBC) | 0.05 | 44.7 ± 2.6 | 45.8 ± 2.4 | 45.4 ± 1.5 |
| 0.1 | 46.9 ± 2.6 | >49 | >49 | |
| 0.2 | >49 | >49 | >49 | |
| Chemical | Mass Concentration (%) | Diameters of Inhibition Zones/mm | ||
|---|---|---|---|---|
| An | Pc | Tv | ||
| Azoxystrobin | 0.05 | 0 | 0 | 0 |
| 0.1 | 0 | 0 | 0 | |
| 0.2 | 0 | 0 | 0 | |
| Kresoxim-methyl | 0.05 | 7.8 ± 0.4 | 0 | 12.4 ± 0.5 |
| 0.1 | 10.9 ± 0.5 | 0 | 14.7 ± 0.7 | |
| 0.2 | 13.5 ± 0.8 | 0 | 19.6 ± 0.8 | |
| Pyraclostrobin | 0.05 | 14.3 ± 0.7 | 15.7 ± 0.4 | 21.7 ± 1.9 |
| 0.1 | 15.9 ± 0.5 | 16.6 ± 0.5 | 22.6 ± 1.6 | |
| 0.2 | 16.0 ± 0.7 | 17.9 ± 0.4 | 26.2 ± 3.4 | |
| IPBC | 0.05 | 37.6 ± 1.6 | 35.9 ± 0.4 | 44.9 ± 3.6 |
| 0.1 | >49 | 39.1 ± 1.0 | >49 | |
| 0.2 | >49 | 41.4 ± 1.9 | >49 | |
| Chemical | Concentration (%) | Bt | Fm | Aa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R/(g·m−2) | D | E (%) | R/(g·m−2) | D | E (%) | R/(g·m−2) | D | E (%) | ||
| Str-1 | 0.1 | 0.33 | 4 | 0 | 0.32 | 0.67 | 83.3 | 0.40 | 0 | 100 |
| 0.2 | 0.65 | 4 | 0 | 0.63 | 0.33 | 91.8 | 0.80 | 0 | 100 | |
| Str-2 | 0.1 | 0.35 | 4 | 0 | 0.36 | 1.17 | 70.8 | 0.36 | 0 | 100 |
| 0.2 | 0.69 | 4 | 0 | 0.71 | 0.5 | 87.5 | 0.70 | 0 | 100 | |
| SI-1 | 0.1 | 0.41 | 0 | 100 | 0.40 | 0 | 100 | 0.39 | 0 | 100 |
| 0.2 | 0.76 | 0 | 100 | 0.79 | 0 | 100 | 0.85 | 0 | 100 | |
| SI-2 | 0.1 | 0.39 | 0 | 100 | 0.38 | 0 | 100 | 0.40 | 0 | 100 |
| 0.2 | 0.67 | 0 | 100 | 0.77 | 0 | 100 | 0.80 | 0 | 100 | |
| Chemical | Concentration (%) | Mm (Mixed Mold Strains) | ||
|---|---|---|---|---|
| R/(g·m−2) | D | E (%) | ||
| Str-1 | 0.1 | 0.39 | 4 | 0.0 |
| 0.2 | 0.73 | 4 | 0.0 | |
| Str-2 | 0.1 | 0.36 | 3.83 | 4.3 |
| 0.2 | 0.73 | 3.5 | 12.5 | |
| SI-1 | 0.1 | 0.40 | 0.33 | 91.8 |
| 0.2 | 0.77 | 0 | 100 | |
| SI-2 | 0.1 | 0.42 | 0 | 100 |
| 0.2 | 0.79 | 0 | 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Jiang, M.; Zhang, B.; Wu, Y.; Ma, X. Mold and Stain Resistance of Bamboo Treated with Pyraclostrobin Fungicide. Polymers 2022, 14, 5537. https://doi.org/10.3390/polym14245537
Zhang J, Jiang M, Zhang B, Wu Y, Ma X. Mold and Stain Resistance of Bamboo Treated with Pyraclostrobin Fungicide. Polymers. 2022; 14(24):5537. https://doi.org/10.3390/polym14245537
Chicago/Turabian StyleZhang, Jingpeng, Mingliang Jiang, Bin Zhang, Yuzhang Wu, and Xingxia Ma. 2022. "Mold and Stain Resistance of Bamboo Treated with Pyraclostrobin Fungicide" Polymers 14, no. 24: 5537. https://doi.org/10.3390/polym14245537
APA StyleZhang, J., Jiang, M., Zhang, B., Wu, Y., & Ma, X. (2022). Mold and Stain Resistance of Bamboo Treated with Pyraclostrobin Fungicide. Polymers, 14(24), 5537. https://doi.org/10.3390/polym14245537