Effects of Different Delignification and Drying Methods on Fiber Properties of Moso Bamboo
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Delignification Treatment
2.3. Drying Treatment
2.4. Characterization
3. Results and Discussion
3.1. Microstructure
3.2. Chemical Functional Groups
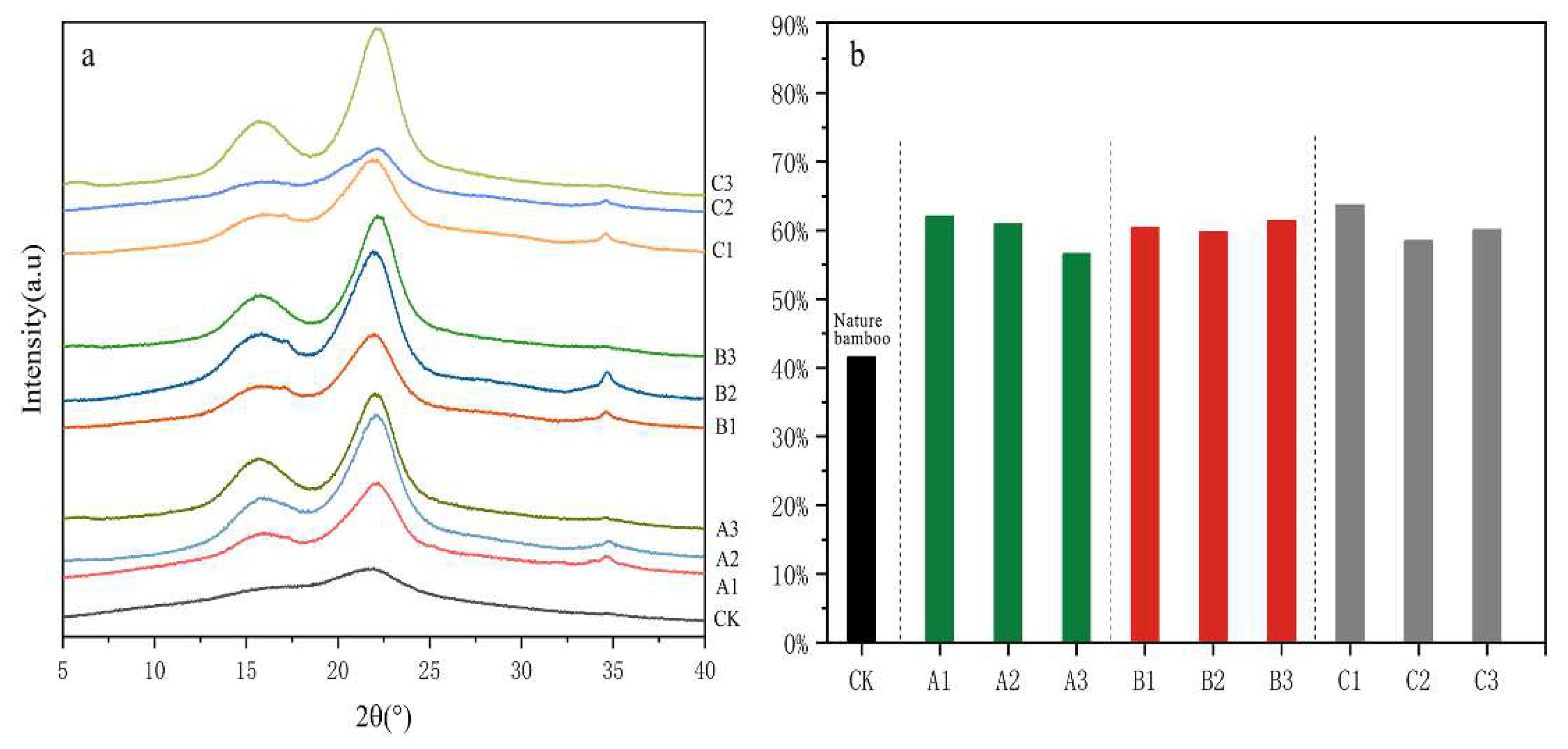
3.3. Crystalline Structure
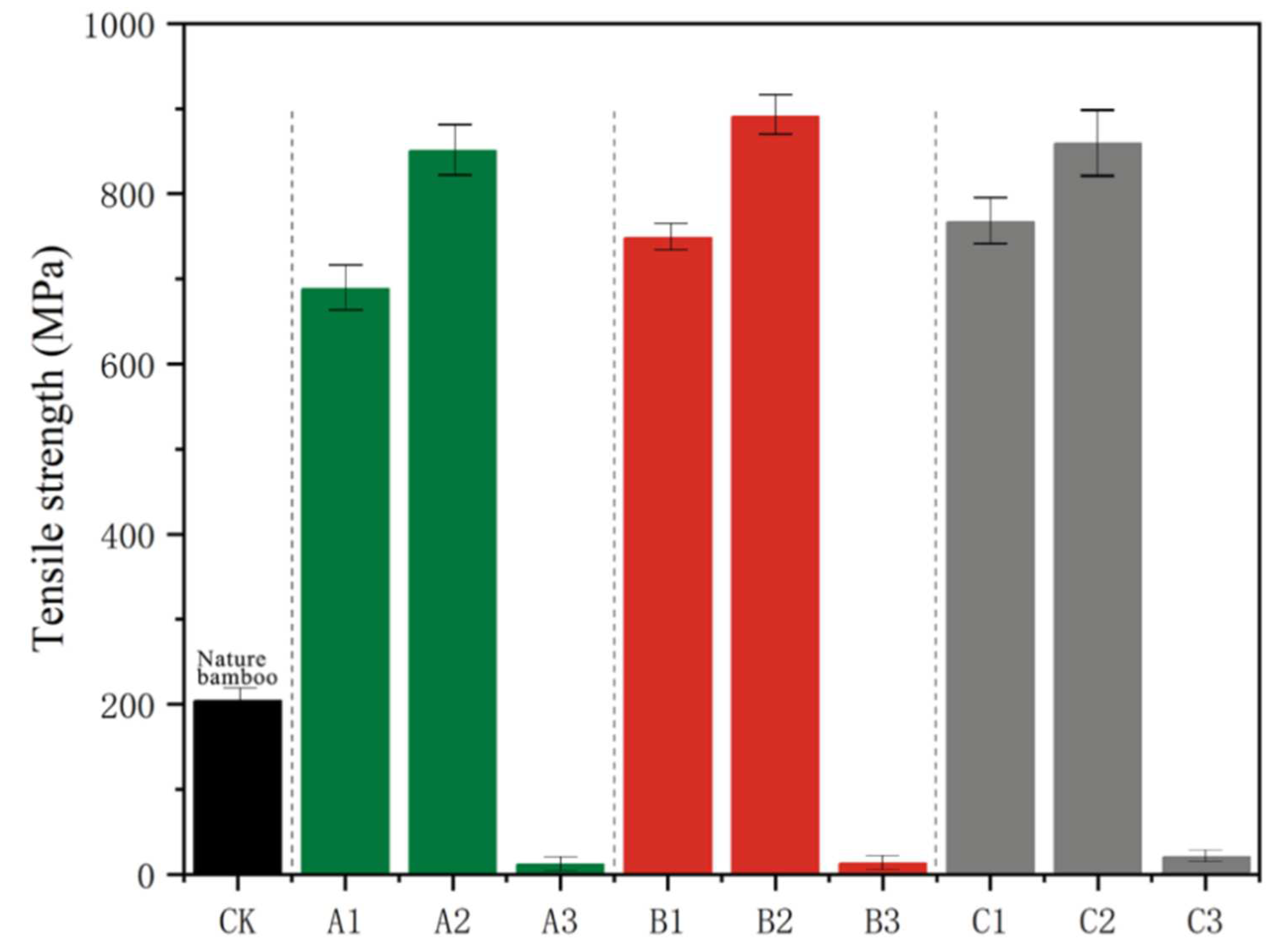
3.4. Mechanical Properties
3.5. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, D.J.; Park, S.H.; Ryu, G.S.; Koh, K.T. Comparative Flexural Behavior of Hybrid Ultra High Performance Fiber Reinforced Concrete with Different Macro Fibers. Constr. Build. Mater. 2011, 25, 4144–4155. [Google Scholar] [CrossRef]
- Ellringmann, T.; Wilms, C.; Warnecke, M.; Seide, G.; Gries, T. Carbon Fiber Production Costing: A Modular Approach. Text. Res. J. 2016, 86, 178–190. [Google Scholar] [CrossRef]
- Liu, D.; Song, J.; Anderson, D.P.; Chang, P.R.; Hua, Y. Bamboo Fiber and its Reinforced Composites: Structure and Properties. Cellulose 2012, 19, 1449–1480. [Google Scholar] [CrossRef]
- Li, X.G.; Zheng, X.; Wu, Y.Q. Study on Technology of Bamboo Processing. Adv. Mater. Res. 2012, 557, 2454–2457. [Google Scholar] [CrossRef]
- Mi, R.; Chen, C.; Keplinger, T.; Pei, Y.; He, S.; Liu, D.; Li, J.; Dai, J.; Hitz, E.; Yang, B. Scalable Aesthetic Transparent Wood for Energy Efficient Buildings. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Chen, G.; Wu, Z.; Shen, Z.; Li, H.-Y.; Li, J.; Lü, B.; Song, G.; Gong, X.; Qin, M.; Yao, C.-L. Scalable, Strong and Water-Stable Wood-Derived Bioplastic. Chem. Eng. J. 2022, 439, 135680. [Google Scholar] [CrossRef]
- Banu Jamaldheen, S.; Kurade, M.B.; Basak, B.; Yoo, C.G.; Oh, K.K.; Jeon, B.; Kim, T.H. A Review on Physico-Chemical Delignification as a Pretreatment of Lignocellulosic Biomass for Enhanced Bioconversion. Bioresour. Technol. 2022, 346, 126591. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Q.; Yu, S.; Yan, M.; Berglund, L. Optically Transparent Wood from a Nanoporous Cellulosic Template: Combining Functional and Structural Performance. Biomacromolecules 2016, 17, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, C.; Xie, H.; Yao, Y.; Zhang, X.; Brozena, A.; Li, J.; Ding, Y.; Zhao, X.; Hong, M. Sustainable High-Strength Macrofibres Extracted from Natural Bamboo. Nat. Sustain. 2022, 5, 235–244. [Google Scholar] [CrossRef]
- Salmén, L.; Stevanic, J.S. Effect of Drying Conditions on Cellulose Microfibril Aggregation and “Hornification”. Cellulose 2018, 25, 6333–6344. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Shi, J.; Zhang, W.; Wang, H. Effect of Alkali Treatment on Microstructure and Thermal Stability of Parenchyma Cell Compared with Bamboo Fiber. Ind. Crops Prod. 2021, 164, 113380. [Google Scholar] [CrossRef]
- Yu, H.; Gui, C.; Ji, Y.; Li, X.; Rao, F.; Huan, W.; Li, L. Changes in Chemical and Thermal Properties of Bamboo after Delignification Treatment. Polymers 2022, 14, 2573. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhang, S.; Wang, C.; Wu, Y. Effect of delignification treatment on fiber morphology and chemical composition of Phyllostachys pubescens. World Bamboo Ratt. 2021, 19, 34–38. (In Chinese) [Google Scholar]
- Yuan, Z.; Kapu, N.S.; Beatson, R.; Chang, X.F.; Martinez, D.M. Effect of Alkaline Pre-Extraction of Hemicelluloses and Silica On Kraft Pulping of Bamboo (Neosinocalamus Affinis Keng). Ind. Crops Prod. 2016, 91, 66–75. [Google Scholar] [CrossRef]
- Beluns, S.; Platnieks, O.; Gaidukovs, S.; Starkova, O.; Sabalina, A.; Grase, L.; Thakur, V.K.; Gaidukova, G. Lignin and Xylan as Interface Engineering Additives for Improved Environmental Durability of Sustainable Cellulose Nanopapers. Int. J. Mol. Sci. 2021, 22, 12939. [Google Scholar] [CrossRef]
- Toba, K.; Nakai, T.; Shirai, T.; Yamamoto, H. Changes in the Cellulose Crystallinity of Moso Bamboo Cell Walls During the Growth Process by X-ray Diffraction Techniques. J. Wood Sci. 2015, 61, 517–524. [Google Scholar] [CrossRef]
- Beluns, S.; Gaidukovs, S.; Platnieks, O.; Gaidukova, G.; Mierina, I.; Grase, L.; Starkova, O.; Brazdausks, P.; Thakur, V.K. From Wood and Hemp Biomass Wastes to Sustainable Nanocellulose Foams. Ind. Crops Prod. 2021, 170, 113780. [Google Scholar] [CrossRef]
- Tian, G. Study on the Main Factors Affecting the Mechanical Properties of Bamboo Fiber. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2015. (In Chinese). [Google Scholar]
- Jiang, Z.; Wang, H.; Tian, G.; Liu, X.; Yu, Y. Sensitivity of Several Selected Mechanical Properties of Moso Bamboo to Moisture Content Change Under the Fibre Saturation Point. BioResources 2012, 7, 5048–5058. [Google Scholar] [CrossRef]
- Zhang, S. Study on the Effect of Chemical Composition on the Mechanical Properties of Wood Cell Wall. PhD Thesis, Chinese Academy of Forestry, Beijing, China, 2011. (In Chinese). [Google Scholar]
- Sergejs, B.; Sergejs, G.; Oskars, P.; Anda, B.; Gerda, G.; Liga, G.; Martins, N.; Andrejs, K.; Kumar, T.V. Clean manufacturing of cellulose nanopapers by incorporating lignin and xylan as sustainable additives. Carbohydr. Polym. Technol. Appl. 2022, 3, 100207. [Google Scholar]
- Biswas, S.; Rahaman, T.; Gupta, P.; Mitra, R.; Dutta, S.; Kharlyngdoh, E.; Guha, S.; Ganguly, J.; Pal, A.; Das, M. Cellulose and Lignin Profiling in Seven, Economically Important Bamboo Species of India by Anatomical, Biochemical, FTIR Spectroscopy and Thermogravimetric Analysis. Biomass Bioenergy 2022, 158, 106362. [Google Scholar] [CrossRef]
- Xiaofeng, Z.; Hanxiao, H.; Yan, Q.; Hankun, W.; Xingong, L. A Comparison Study on the Characteristics of Nanofibrils Isolated from Fibers and Parenchyma Cells in Bamboo. Materials 2020, 13, 237. [Google Scholar]
- Zhang, N.; Tao, P.; Lu, Y.; Nie, S. Effect of lignin on the thermal stability of cellulose nanofibrils produced from bagasse pulp. Cellulose 2019, 26, 13–14. [Google Scholar] [CrossRef]



| Wave Number (cm−1) | Functional Group | Assignment |
|---|---|---|
| 1737 | C=O | non-conjugated C=O (xylan) in hemicellulose |
| 1602 | C=C | C=C unsaturated bonds, aromatic skeleton vibration in lignin |
| 1511 | C=C | aromatic skeleton vibration in lignin |
| 1459 | C–O, C–H | asymmetric bending in CH3 (lignin) |
| 1423 | CH2 | aromatic skeleton vibration (lignin) and plane C–H deformation (cellulose) |
| 1376 | C–H | C–H deformation in cellulose and hemicellulose |
| 1324 | O–H | phenol group (cellulose) |
| 1243 | C–O | clove ring and C–O stretching in lignin and xylan |
| 1158 | C–O–C | C–O–C vibrations in cellulose and hemicellulose |
| 1035 | C–O, C–H | C–O stretching in cellulose and C–H stretching of hemicellulose in lignin |
| 895 | C–H | C–H deformation in cellulose |
| 831 | C–H | C–H vibrations in Guaiac-based derivatives |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Wang, W.; Zhang, Y.; Wang, X.; Wang, X.; Shi, J. Effects of Different Delignification and Drying Methods on Fiber Properties of Moso Bamboo. Polymers 2022, 14, 5464. https://doi.org/10.3390/polym14245464
Bai Y, Wang W, Zhang Y, Wang X, Wang X, Shi J. Effects of Different Delignification and Drying Methods on Fiber Properties of Moso Bamboo. Polymers. 2022; 14(24):5464. https://doi.org/10.3390/polym14245464
Chicago/Turabian StyleBai, Yifeng, Wenqing Wang, Yongyue Zhang, Xiangwei Wang, Xinzhou Wang, and Jiangtao Shi. 2022. "Effects of Different Delignification and Drying Methods on Fiber Properties of Moso Bamboo" Polymers 14, no. 24: 5464. https://doi.org/10.3390/polym14245464
APA StyleBai, Y., Wang, W., Zhang, Y., Wang, X., Wang, X., & Shi, J. (2022). Effects of Different Delignification and Drying Methods on Fiber Properties of Moso Bamboo. Polymers, 14(24), 5464. https://doi.org/10.3390/polym14245464









