Fabrication and Characterization of Hydrogen Peroxide and Thymol-Loaded PVA/PVP Hydrogel Coatings as a Novel Anti-Mold Surface for Hay Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
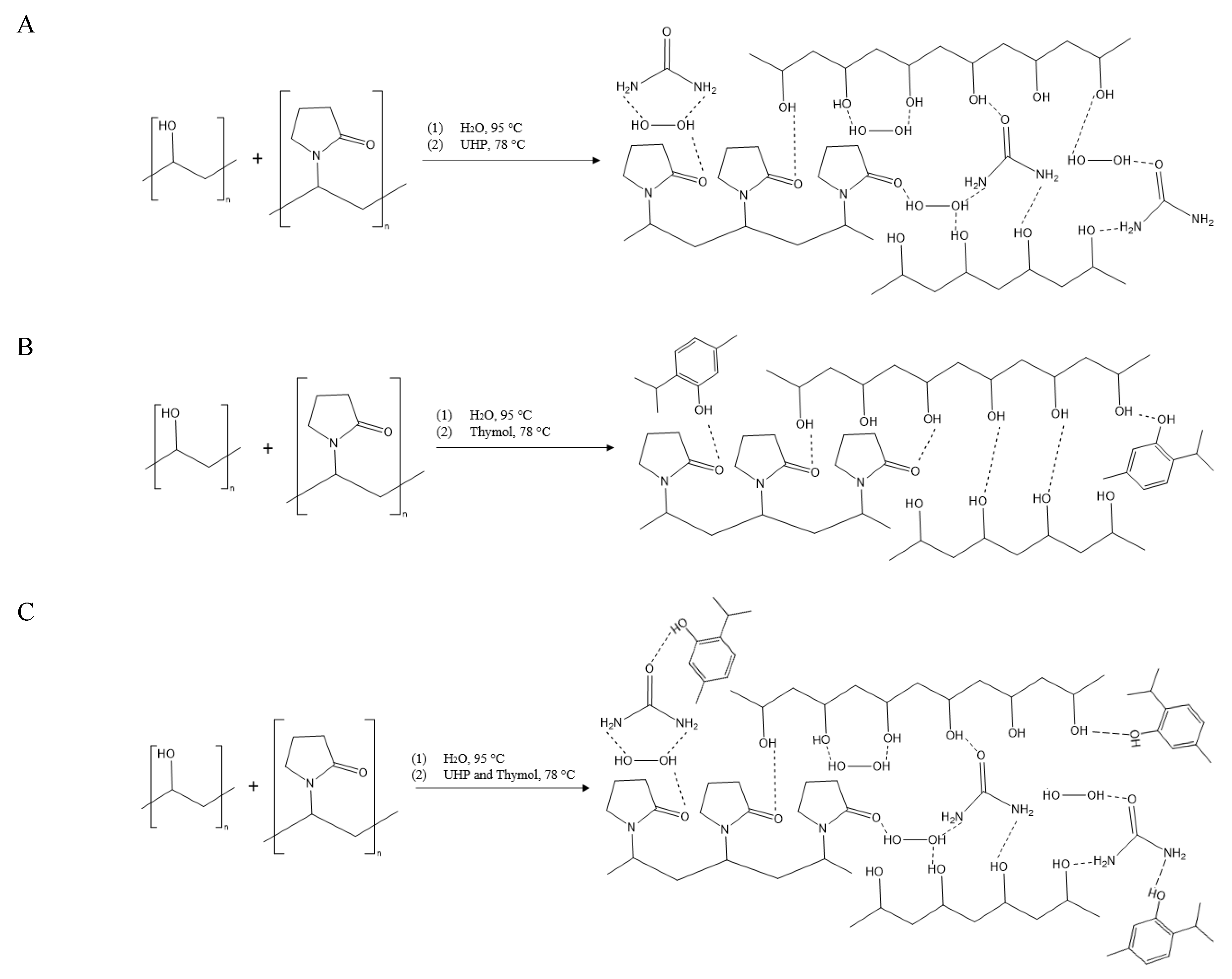
2.2. Fabrication of PVA/PVP Hydrogels
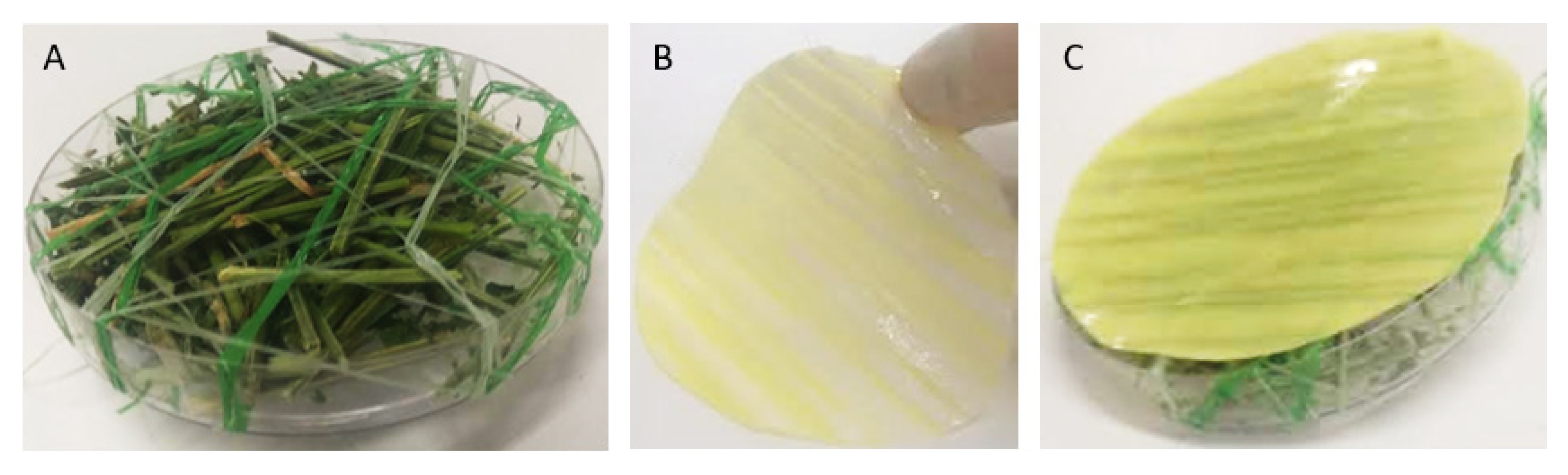
2.3. Hydrogel Gelation and Coating Process
2.4. Characterization
2.4.1. Hydrogel Coating Molecular Characterization
2.4.2. Determination of Thymol Content in Hydrogel Coatings
PVA/PVP/Thymol Hydrogel Coating
PVA/PVP/HP-Thymol Hydrogel Coating
2.4.3. Determination of HP Content in Hydrogel Coatings
PVA/PVP/HP Hydrogel Coating
PVA/PVP/HP-Thymol Hydrogel Coating
2.4.4. Release Rate System
Thymol Release Rate
HP Release Rate
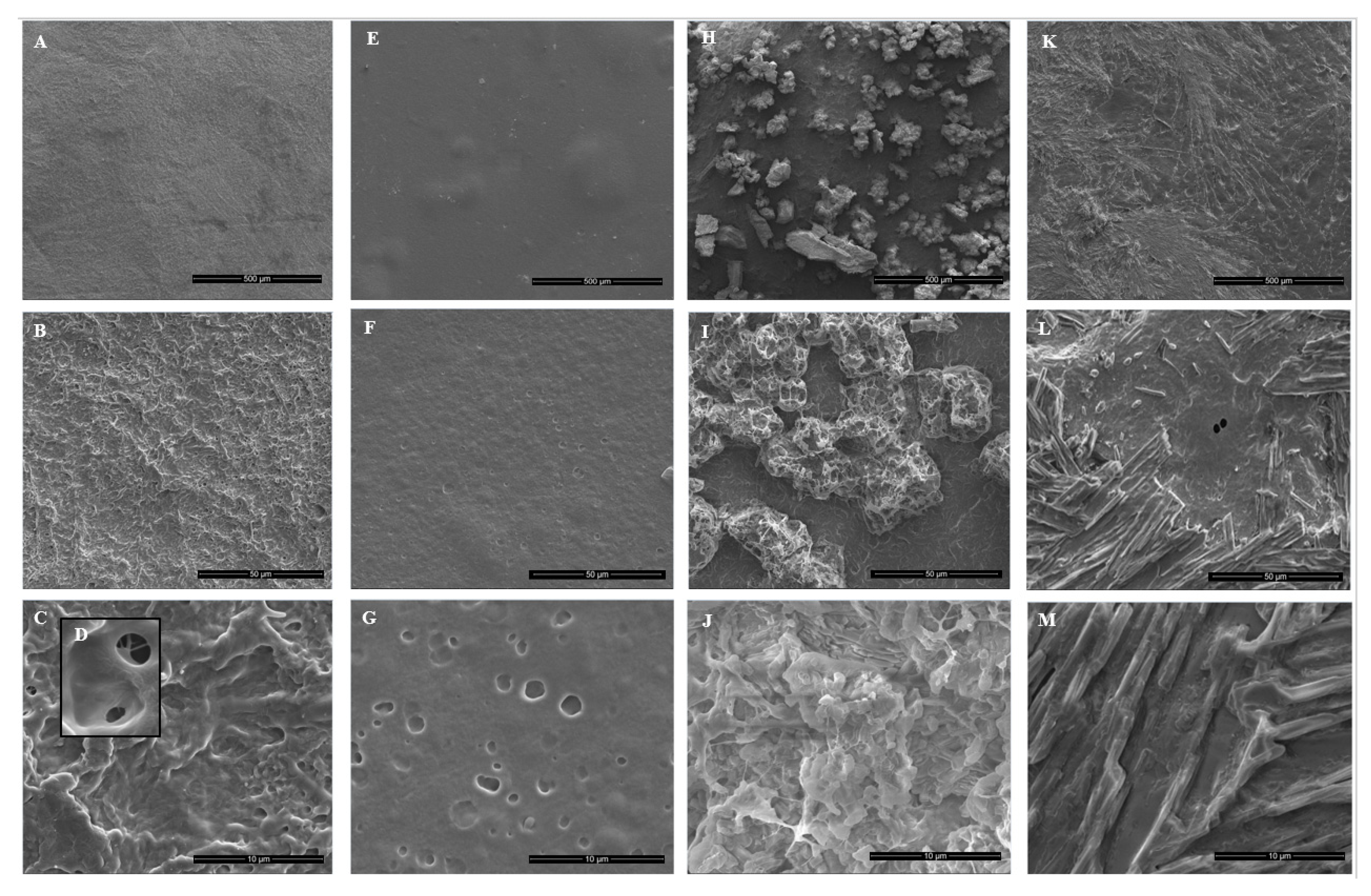
2.4.5. Morphological Characterization
2.4.6. Surface Roughness
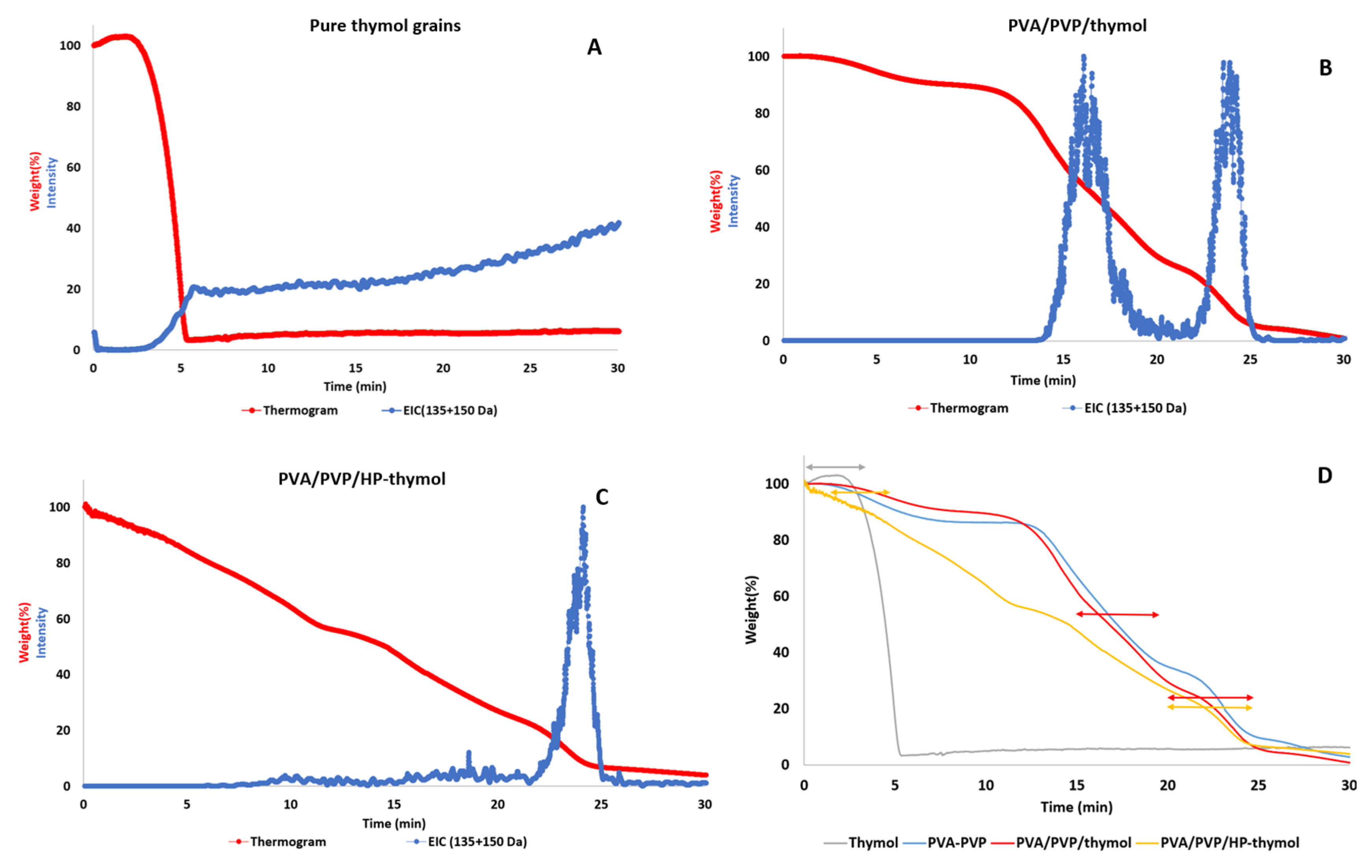
2.4.7. Thymol Thermal Stability in PVA/PVP Thymol and HP-Thymol Hydrogel Coatings
2.4.8. HP Thermal Stability in PVA/PVP/HP Hydrogel Coating and Water
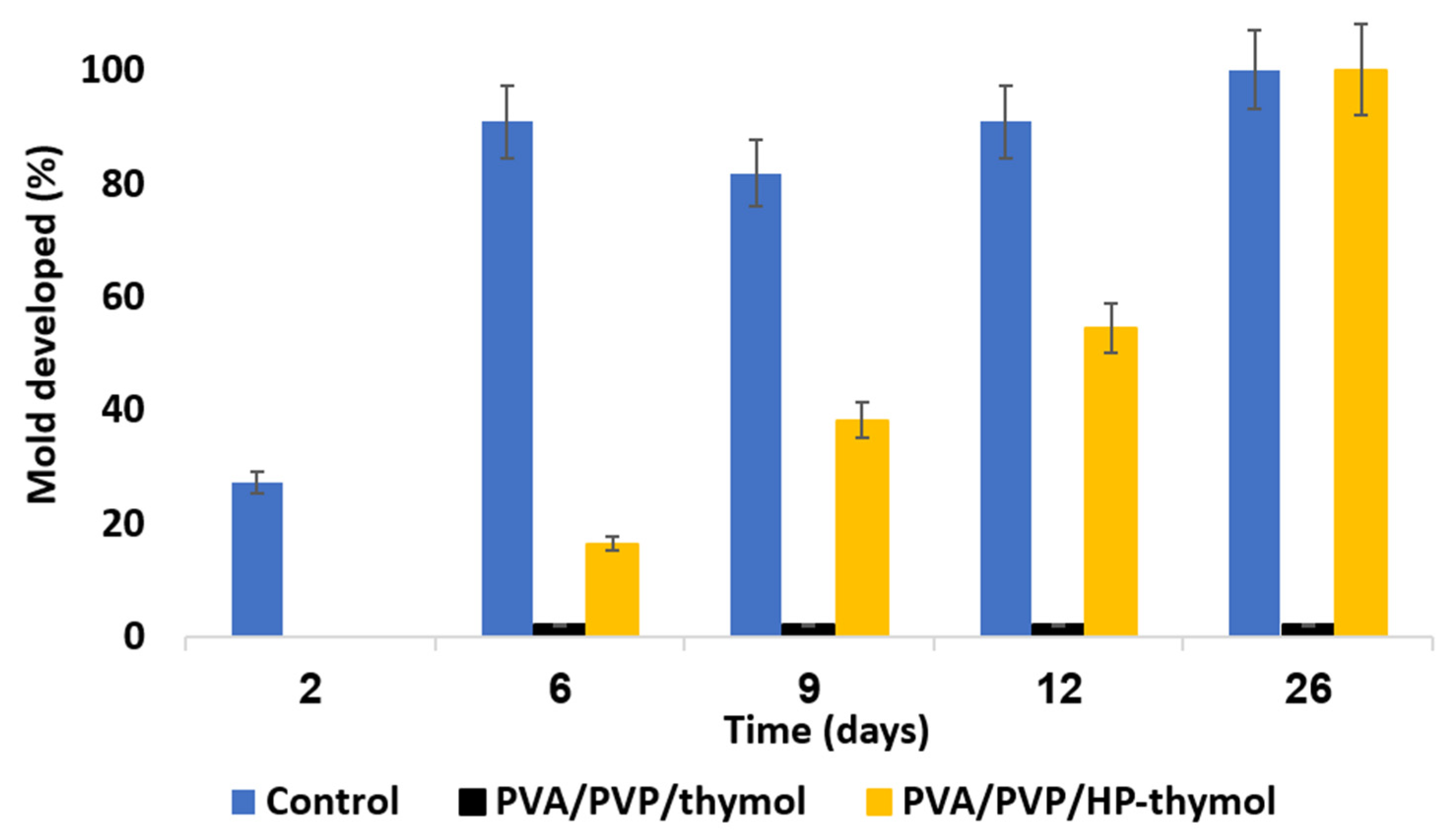
2.4.9. Determination of Anti-Mold Properties
3. Results and Discussion
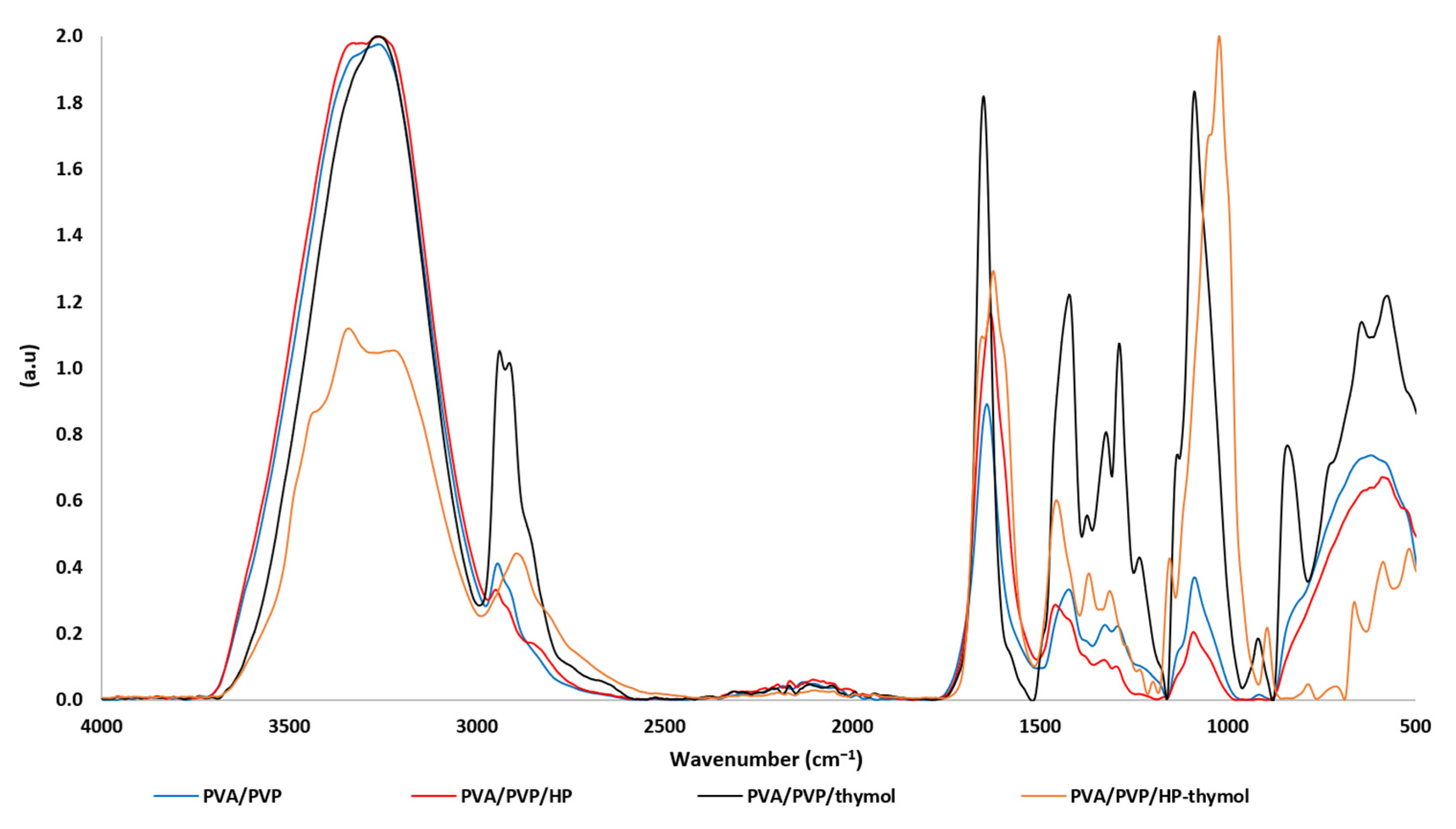
3.1. FTIR/ATR Spectra of PVA/PVP Hydrogels
3.2. Entrapped HP and/or Thymol Content
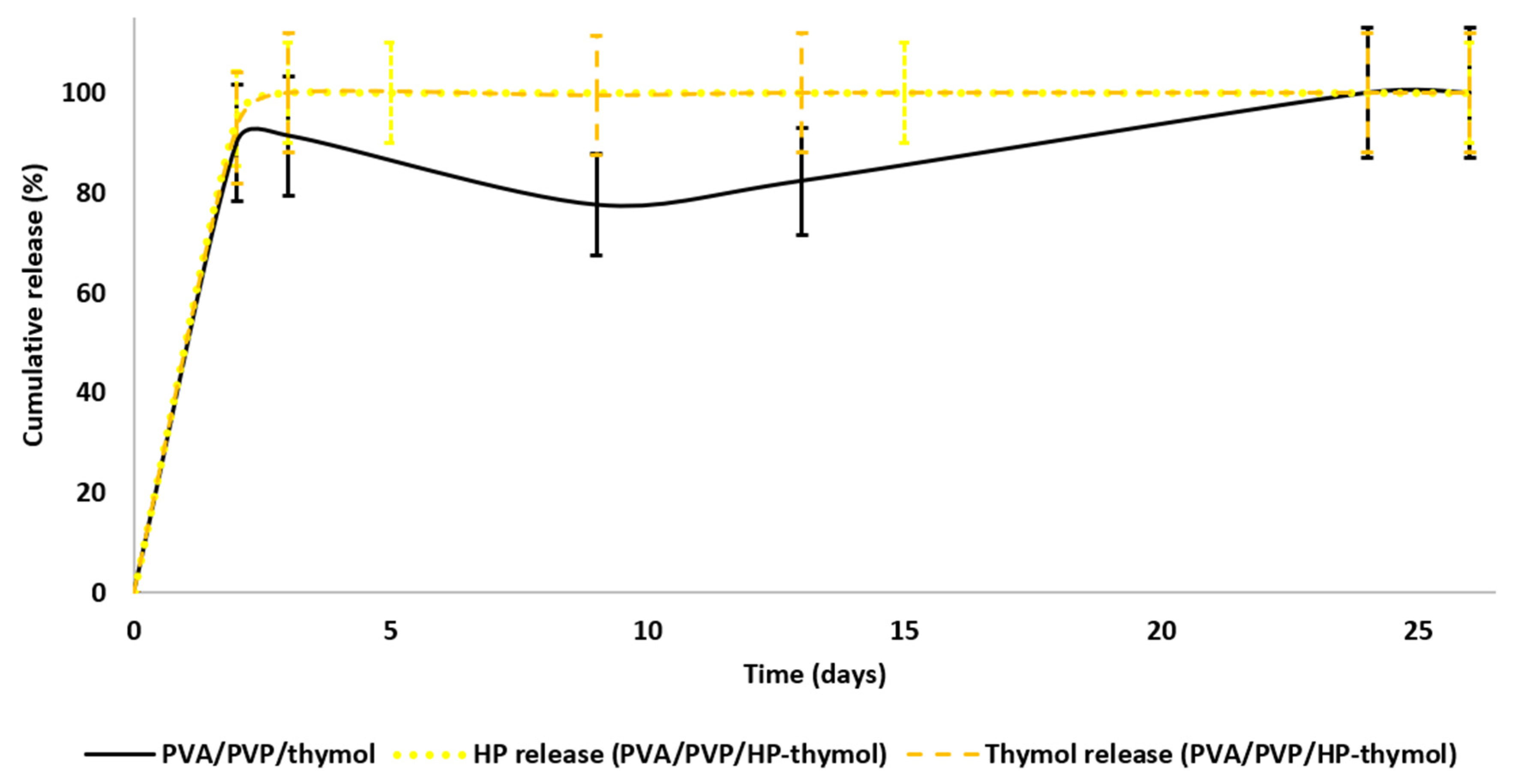
3.3. HP and Thymol Cumulative Release Rates from Loaded PVA/PVP Hydrogel Coatings
3.4. Effect of HP and/or Thymol on PVA/PVP Hydrogel Coating Morphology
3.5. Effect of HP and/or Thymol on Hydrogel Coating Roughness
3.6. Hydrogel Entrapping Effect on Thymol Thermal Stability
3.7. PVA/PVP Hydrogel Entrapping Effect on HP Thermal Stability
3.8. Anti-Mold Activity of HP and/or Thymol Loaded Hydrogel Coatings
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komarek, A.M.; Dunston, S.; Enahoro, D.; Godfray, H.C.J.; Herrero, M.; Mason-D’Croz, D.; Rich, K.M.; Scarborough, P.; Springmann, M.; Sulser, T.B.; et al. Income, Consumer Preferences, and the Future of Livestock-Derived Food Demand. Glob. Environ. Change 2021, 70, 102343. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.L.; Thomas, U.C. Innovative Techniques in Fodder Production-a Review. Forage Res. 2019, 44, 217–223. [Google Scholar]
- Pepys, J.; Jenkins, P.A.; Festenstein, G.N.; Lacey, M.E.; Gregory, P.H.; Skinner, F.A. Farmer’s Lung Actinomycetes as a Source of “Farmer’s Lung Hay” Antigen. Lancet 1963, 282, 607–611. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of Mycotoxins on Humans and Animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Bradford, K.J.; Dahal, P.; van Asbrouck, J.; Kunusoth, K.; Bello, P.; Thompson, J.; Wu, F. The Dry Chain: Reducing Postharvest Losses and Improving Food Safety in Humid Climates. Trends Food Sci. Technol. 2018, 71, 84–93. [Google Scholar] [CrossRef]
- Duchaine, C.; Lavoie, M.C.; Cormier, Y. Effects of a Bacterial Hay Preservative (Pediococcus Pentosaceus) on Hay under Experimental Storage Conditions. Appl. Environ. Microbiol. 1995, 61, 4240–4243. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; de Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol Bioactivity: A Review Focusing on Practical Applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- de Castro, R.D.; de Souza, T.M.P.A.; Bezerra, L.M.D.; Ferreira, G.L.S.; de Brito Costa, E.M.M.; Cavalcanti, A.L. Antifungal Activity and Mode of Action of Thymol and Its Synergism with Nystatin against Candida Species Involved with Infections in the Oral Cavity: An in Vitro Study. BMC Complement. Altern. Med. 2015, 15, 417. [Google Scholar] [CrossRef]
- Kure, C.F.; Langsrud, S.; Møretrø, T. Efficient Reduction of Food Related Mould Spores on Surfaces by Hydrogen Peroxide Mist. Foods 2021, 10, 55. [Google Scholar] [CrossRef]
- McDonnell, G. The Use of Hydrogen Peroxide for Disinfection and Sterilization Applications. PATAI’S Chem. Funct. Groups 2014, 1, 1–34. [Google Scholar] [CrossRef]
- Zhang, S.; Zhai, Y.; Zhang, Z. Study on Medical Polyvinyl Alcohol (PVA)/Polyvinyl Pyrrolidone (PVP) Hydrogel Burns Dressing. In Proceedings of the 2011 International Conference on Electronic and Mechanical Engineering and Information Technology, EMEIT 2011, Harbin, China, 12–14 August 2011; Volume 9, pp. 4485–4488. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of Poly (Vinyl Alcohol) Based Materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Franco, P.; de Marco, I. The Use of Poly(N-Vinyl Pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Tabacco, E.; Bisaglia, C.; Revello-Chion, A.; Borreani, G. Assessing the Effect of Securing Bales with Either Polyethylene Film or Netting on the Fermentation Profiles, Fungal Load, and Plastic Consumption in Baled Silage of Grass-Legume Mixtures. Appl. Eng. Agric. 2013, 29, 795–804. [Google Scholar] [CrossRef]
- Mandras, N.; Nostro, A.; Roana, J.; Scalas, D.; Banche, G.; Ghisetti, V.; Del Re, S.; Fucale, G.; Cuffini, A.M.; Tullio, V. Liquid and Vapour-Phase Antifungal Activities of Essential Oils against Candida Albicans and Non-Albicans Candida. BMC Complement. Altern. Med. 2016, 16, 330. [Google Scholar] [CrossRef]
- Tatlisu, N.B.; Yilmaz, M.T.; Arici, M. Fabrication and Characterization of Thymol-Loaded Nanofiber Mats as a Novel Antimould Surface Material for Coating Cheese Surface. Food Packag Shelf Life 2019, 21, 100347. [Google Scholar] [CrossRef]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative Study of Encapsulated Peppermint and Green Tea Essential Oils in Chitosan Nanoparticles: Encapsulation, Thermal Stability, in-Vitro Release, Antioxidant and Antibacterial Activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation, Characterization and in Vitro Release Study of Carvacrol-Loaded Chitosan Nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, D.; Wadsworth, L.C. Corona Treatment of Polyolefin Films—A Review. Adv. Polym. Technol. 1999, 18, 171–180. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Q.; Wadsworth, L.C. Mechanism of Corona Treatment on Polyolefin Films. Polym. Eng. Sci. 1998, 38, 965–970. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, M.; Yao, L.; Lai, W.Y. Mayer Rod-Coated Organic Light-Emitting Devices: Binary Solvent Inks, Film Topography Optimization, and Large-Area Fabrication. Adv. Eng. Mater. 2022, 24, 2101558. [Google Scholar] [CrossRef]
- Kenawy, E.R.; El-Newehy, M.H.; Al-Deyab, S.S. Controlled Release of Atenolol from Freeze/Thawed Poly (Vinyl Alcohol) Hydrogel. J. Saudi Chem. Soc. 2010, 14, 237–240. [Google Scholar] [CrossRef]
- Reichert, J.S.; McNeight, S.A.; Rudel, H.W. Determination of Hydrogen Peroxide and Some Related Peroxygen Compounds. Ind. Eng. Chem. Anal. Ed. 1939, 11, 194–197. [Google Scholar] [CrossRef]
- Malka, E.; Dombrovsky, A.; Margel, S. Preparation and Characterization of a Novel PVA/PVP Hydrogel Containing Entrapped Hydrogen Peroxide for Agricultural Applications. ACS Agric. Sci. Technol. 2022, 2, 430–436. [Google Scholar] [CrossRef]
- Deveci, H. B13-Factors Affecting Decomposition of Hydrogen Peroxide. In Proceedings of the IMPS 2010 XIIth International Mineral Processing Symposium, Cappadocia, Turkey, 6–8 October 2010; pp. 609–616. [Google Scholar] [CrossRef]
- Valderrama, A.C.S.; Rojas De, G.C. Traceability of Active Compounds of Essential Oils in Antimicrobial Food Packaging Using a Chemometric Method by ATR-FTIR. Am. J. Analyt. Chem. 2017, 8, 726–741. [Google Scholar] [CrossRef]
- Zamani, Z.; Alipour, D.; Moghimi, H.R.; Mortazavi, S.A.R.; Saffaryc, M. Development and Evaluation of Thymol Microparticles Using Cellulose Derivatives as Controlled Release Dosage Form. Iran. J. Pharm. Res. 2015, 14, 1031–1040. [Google Scholar]
- Kumari, S.; Kumaraswamy, R.V.; Choudhary, R.C.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Thymol Nanoemulsion Exhibits Potential Antibacterial Activity against Bacterial Pustule Disease and Growth Promotory Effect on Soybean. Sci. Rep. 2018, 8, 6650. [Google Scholar] [CrossRef]
- Voraberger, H.; Ribitsch, V.; Janotta, M.; Mizaikoff, B. Application of Mid-Infrared Spectroscopy: Measuring Hydrogen Peroxide Concentrations in Bleaching Baths. Appl. Spectrosc. 2003, 57, 574–579. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; López-Jiménez, A.J.; García Martínez, J.C.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Thymoquinone-Loaded Chitosan Nanoparticles as Natural Preservative Agent in Cosmetic Products. Int. J. Mol. Sci. 2022, 23, 898. [Google Scholar] [CrossRef]
- Agarwal, S.; Srivastava, R.; Mishra, N. An Overview of Therapeutic Potential of Thymoquinone. Int. J. Pharm. Sci. Res. 2019, 10, 3532–3539. [Google Scholar] [CrossRef]
- Pędziwiatr, P.; Mikołajczyk, F.; Zawadzki, D.; Mikołajczyk, K.; Pędziwiatr, A.B.P.; Agnieszka, B. Decomposition of hydrogen peroxide—Kinetics and review of chosen catalysts. Acta Innov. 2018, 26, 45–52. [Google Scholar] [CrossRef]
- Heaney, H. Oxidation Reactions Using Magnesium Monoperphthalate and Urea Hydrogen Peroxide. Aldrichim. Acta 1993, 26, 35–136. [Google Scholar]
- Yang, H.; Dongpeng, Y.; Dekun, M.; Shaoming, H. extremely sensitive mechanochromic photonic crystals with broad tuning range of photonic bandgap and fast responsive speed for high-resolution multicolor display applications. Chem. Eng. J. 2022, 429, 2–14. [Google Scholar] [CrossRef]
- Yang, H.; Boru, W.; Dongpeng, Y.; Dekun, M.; Shaoming, H. Chameleon-Inspired Brilliant and Sensitive Mechano-Chromic Photonic Skins for Self-Reporting the Strains of Earthworms. Appl. Mater. Interfaces 2022, 14, 11672–11680. [Google Scholar] [CrossRef]
- Yang, H.; Dongpeng, Y.; Dekun, M.; Shaoming, H. Liquid, Transparent, and Antideformable Thermochromic Photonic Crystals for Displays. Adv. Opt. Mater. 2022, 10, 2200769. [Google Scholar] [CrossRef]
- Yang, D.; Hu, Y.; Ma, D.; Ge, J.; Huang, S. Reconfigurable Mechanochromic Patterns into ChameleonInspired Photonic Papers. Research 2022, 2022, 9838071. [Google Scholar] [CrossRef]
- Jaswinder, S. Role of Earthworm in Sustainable Agriculture. In Sustainable Food Systems from Agriculture to Industry; Academic Press: Amritsar, India, 2018; pp. 83–122. [Google Scholar] [CrossRef]




| Initial Concentration | 2.5 w% Thymol, HP | 0.63 w% Thymol, HP |
|---|---|---|
| Thymol (w%) | 5.9 ± 0.5 | 0.95 ± 0.04 |
| HP (w%) | 5.6 ± 0.5 | 0.66 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malka, E.; Caspi, A.; Cohen, R.; Margel, S. Fabrication and Characterization of Hydrogen Peroxide and Thymol-Loaded PVA/PVP Hydrogel Coatings as a Novel Anti-Mold Surface for Hay Protection. Polymers 2022, 14, 5518. https://doi.org/10.3390/polym14245518
Malka E, Caspi A, Cohen R, Margel S. Fabrication and Characterization of Hydrogen Peroxide and Thymol-Loaded PVA/PVP Hydrogel Coatings as a Novel Anti-Mold Surface for Hay Protection. Polymers. 2022; 14(24):5518. https://doi.org/10.3390/polym14245518
Chicago/Turabian StyleMalka, Eyal, Ayelet Caspi, Reut Cohen, and Shlomo Margel. 2022. "Fabrication and Characterization of Hydrogen Peroxide and Thymol-Loaded PVA/PVP Hydrogel Coatings as a Novel Anti-Mold Surface for Hay Protection" Polymers 14, no. 24: 5518. https://doi.org/10.3390/polym14245518
APA StyleMalka, E., Caspi, A., Cohen, R., & Margel, S. (2022). Fabrication and Characterization of Hydrogen Peroxide and Thymol-Loaded PVA/PVP Hydrogel Coatings as a Novel Anti-Mold Surface for Hay Protection. Polymers, 14(24), 5518. https://doi.org/10.3390/polym14245518





