Optimization and Characterization of the F-LSR Manufacturing Process Using Quaternary Ammonium Silanolate as an Initiator for Synthesizing Fluorosilicone
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
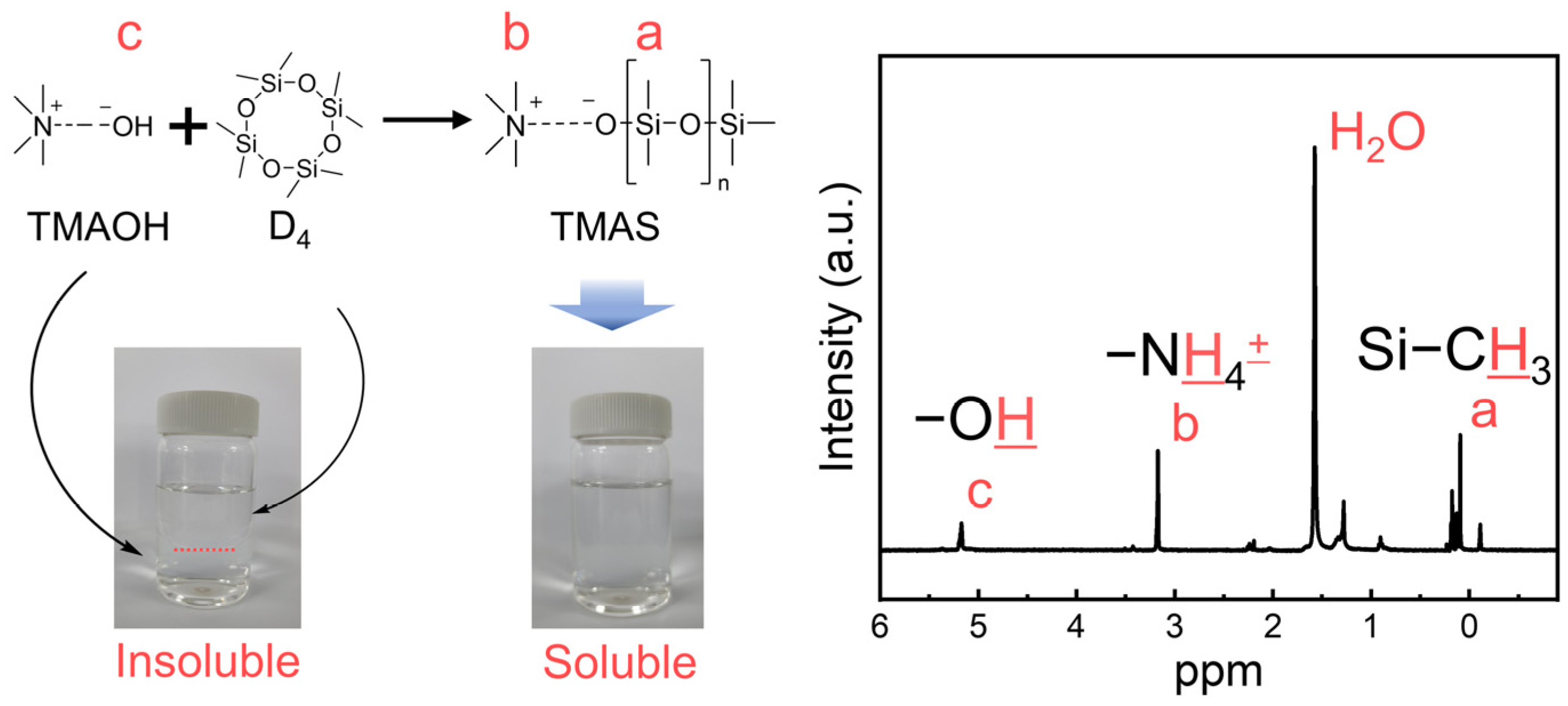
2.2. Synthesis of TMAS
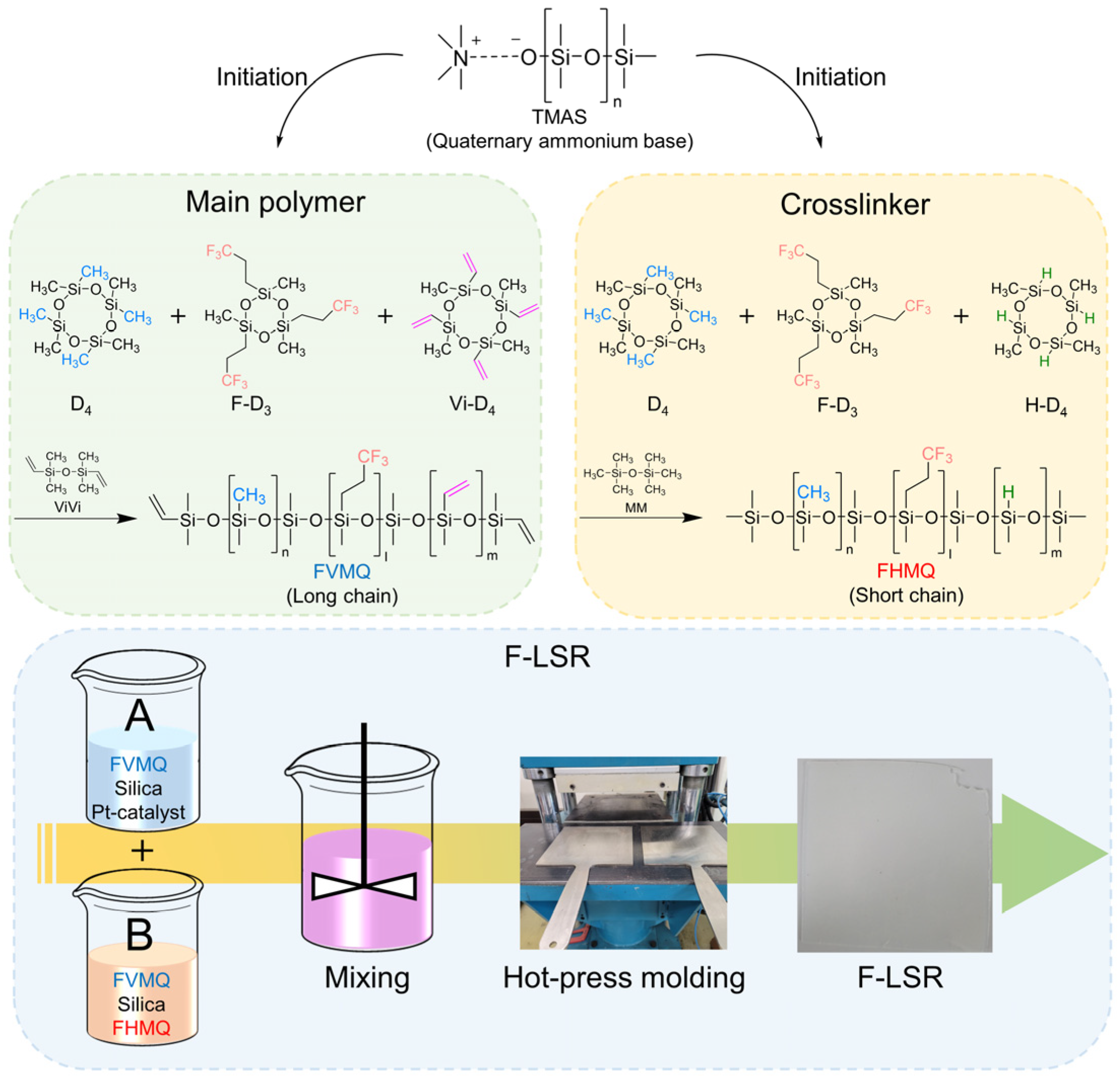
2.3. Synthesis of FVMQ
2.4. Synthesis of FHMQ
2.5. Preparation of F-LSR
2.6. Characterization
3. Results and Discussion
3.1. Synthesis of TMAS
3.2. Synthesis of FVMQ
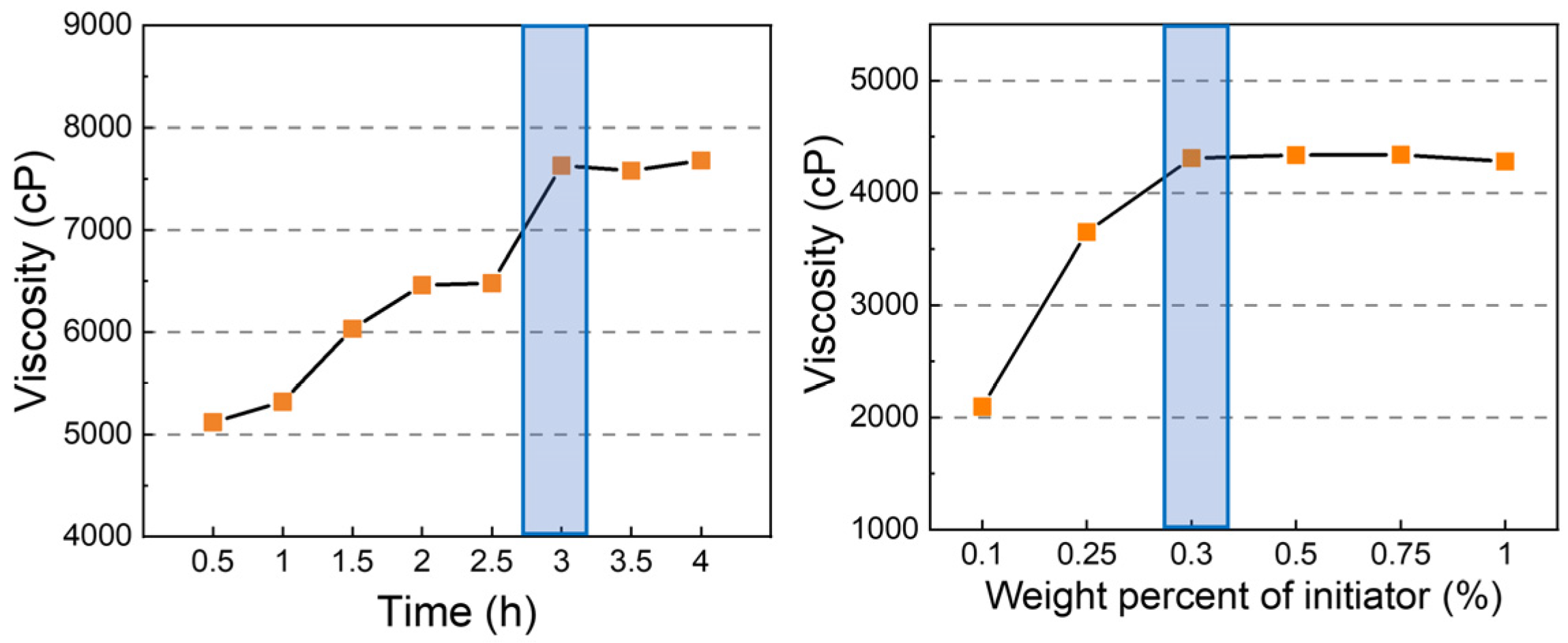
3.2.1. Optimizing the Amounts of Initiator
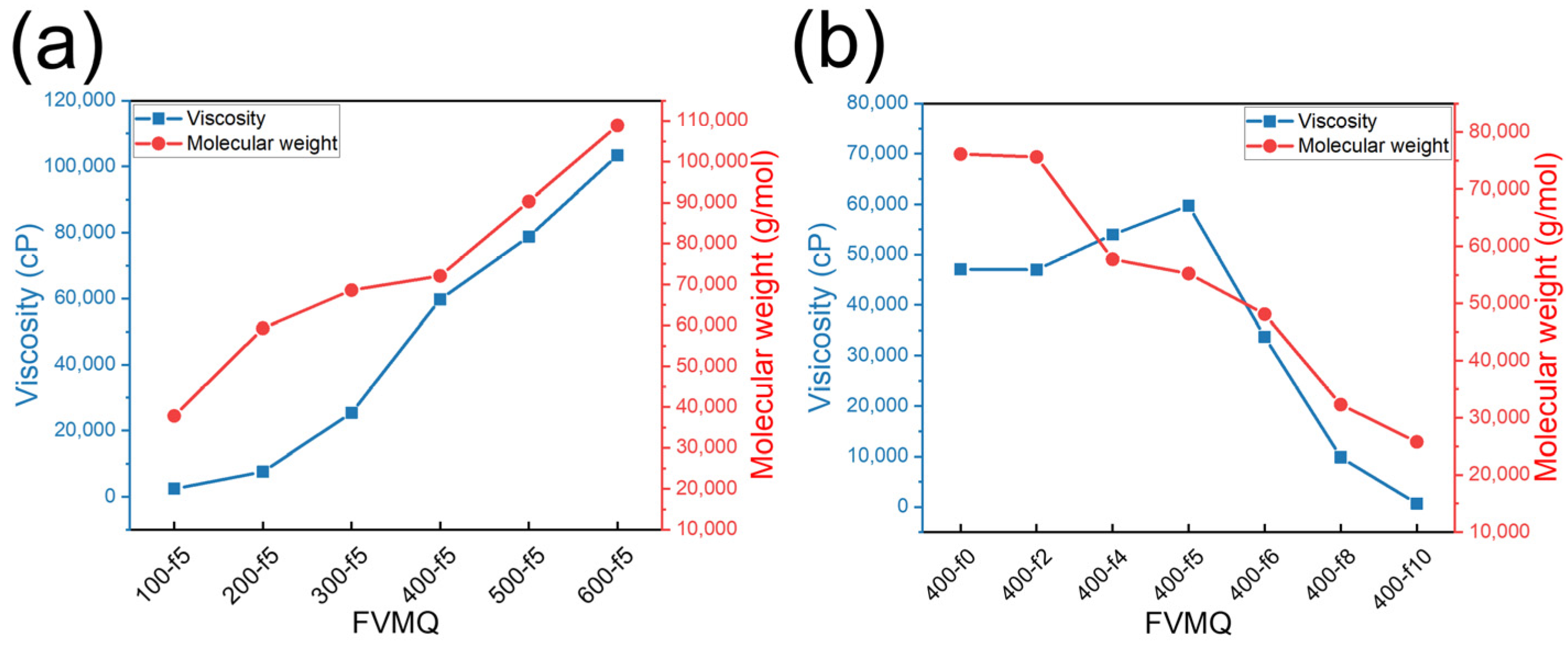
3.2.2. Viscosity and Molecular Weight of FVMQ
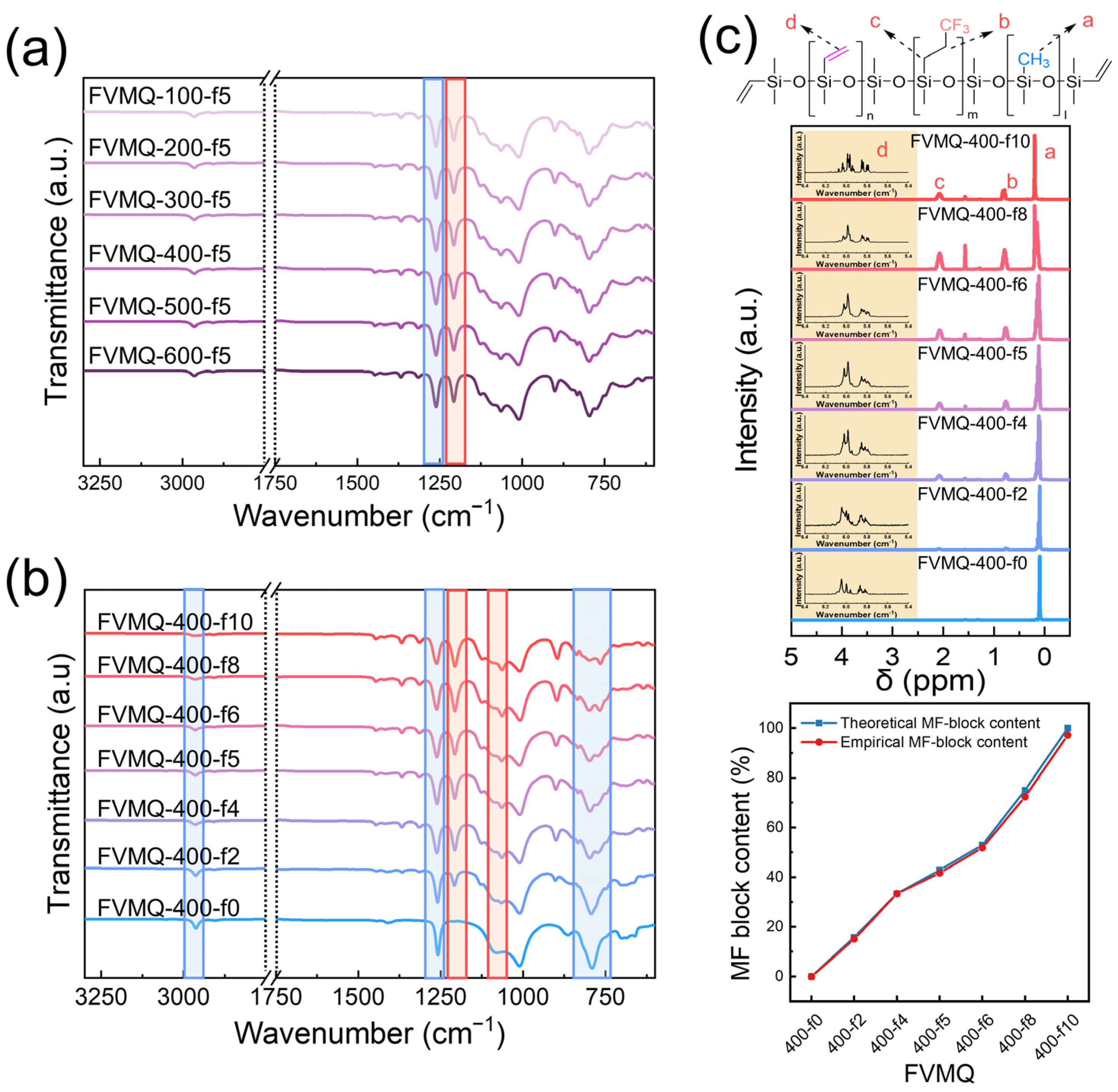
3.2.3. Characterization of the Functional Groups of FVMQ
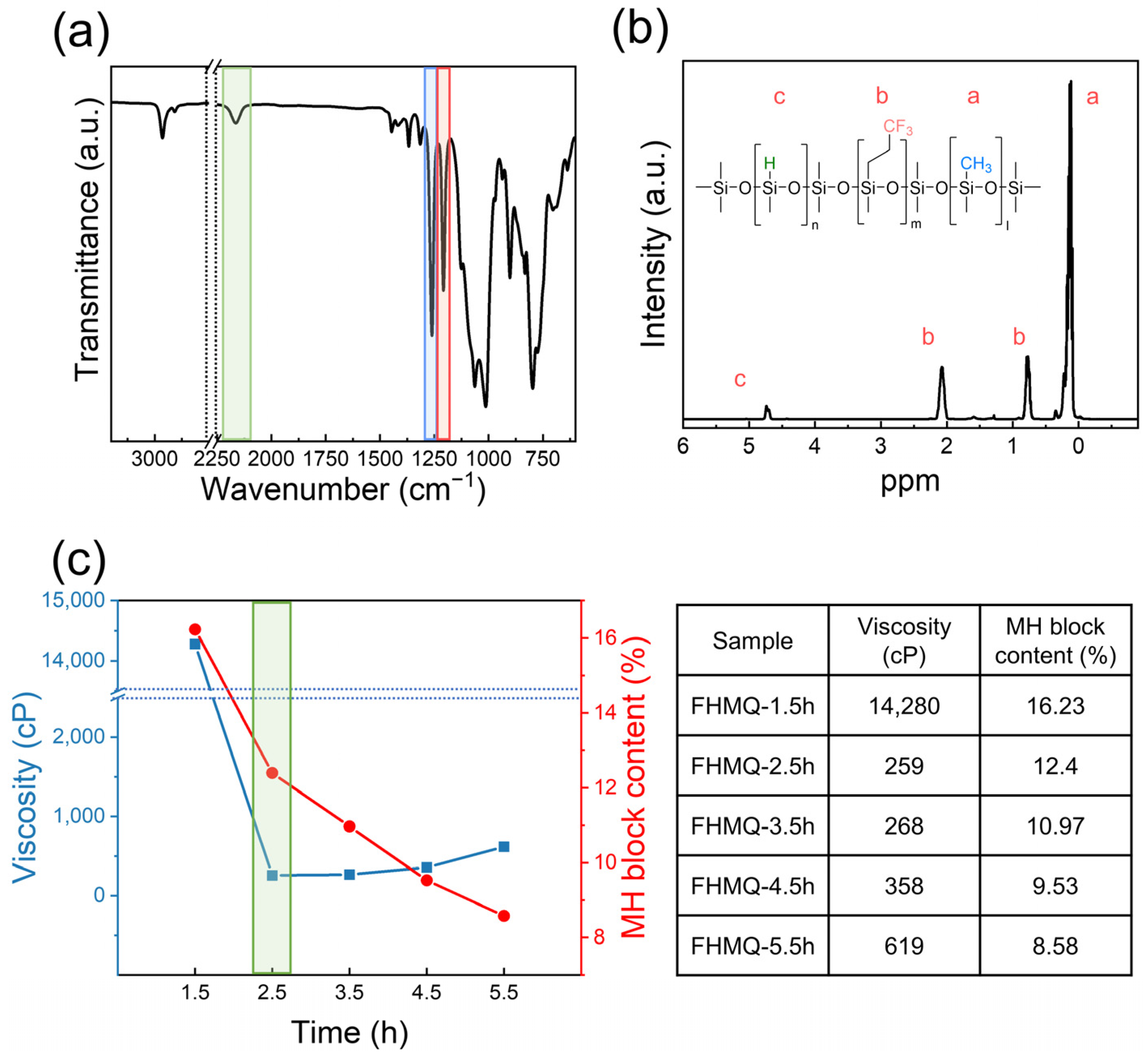
3.3. Synthesis and Characterization of FHMQ
3.4. Preparation and Characterization of F-LSR
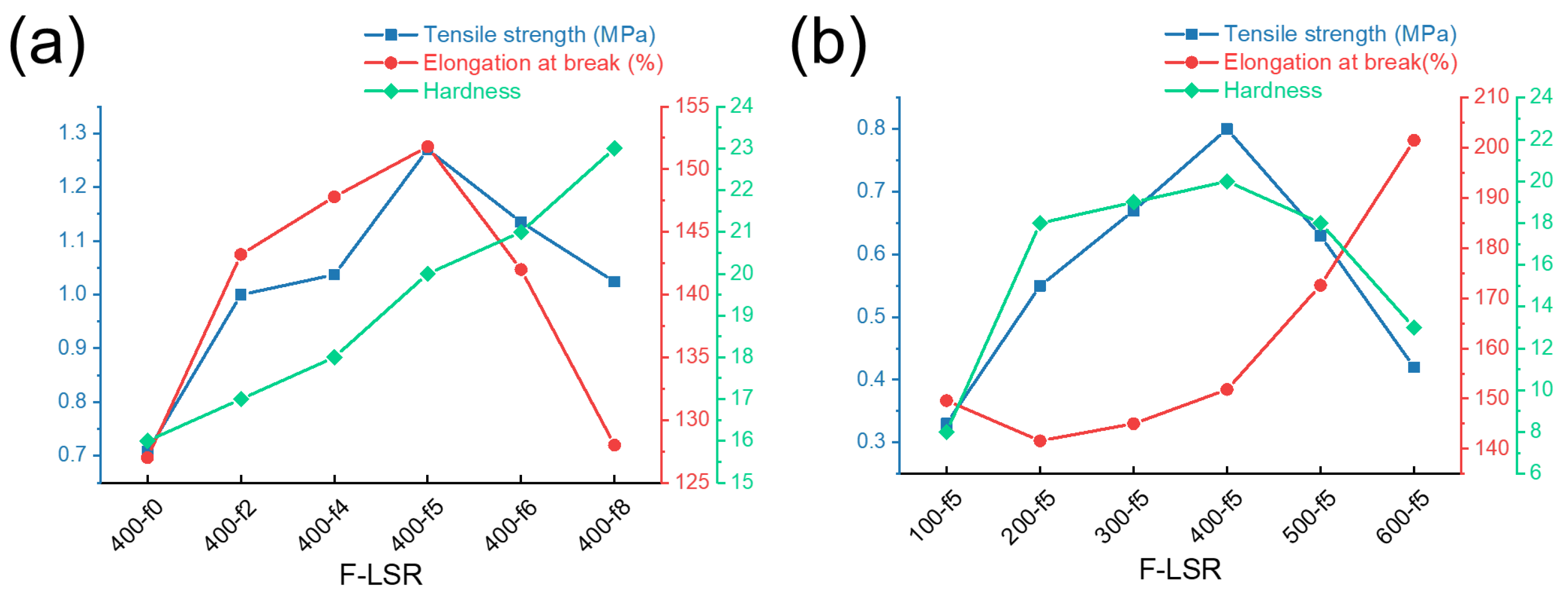
3.4.1. The Mechanical Properties of F-LSR
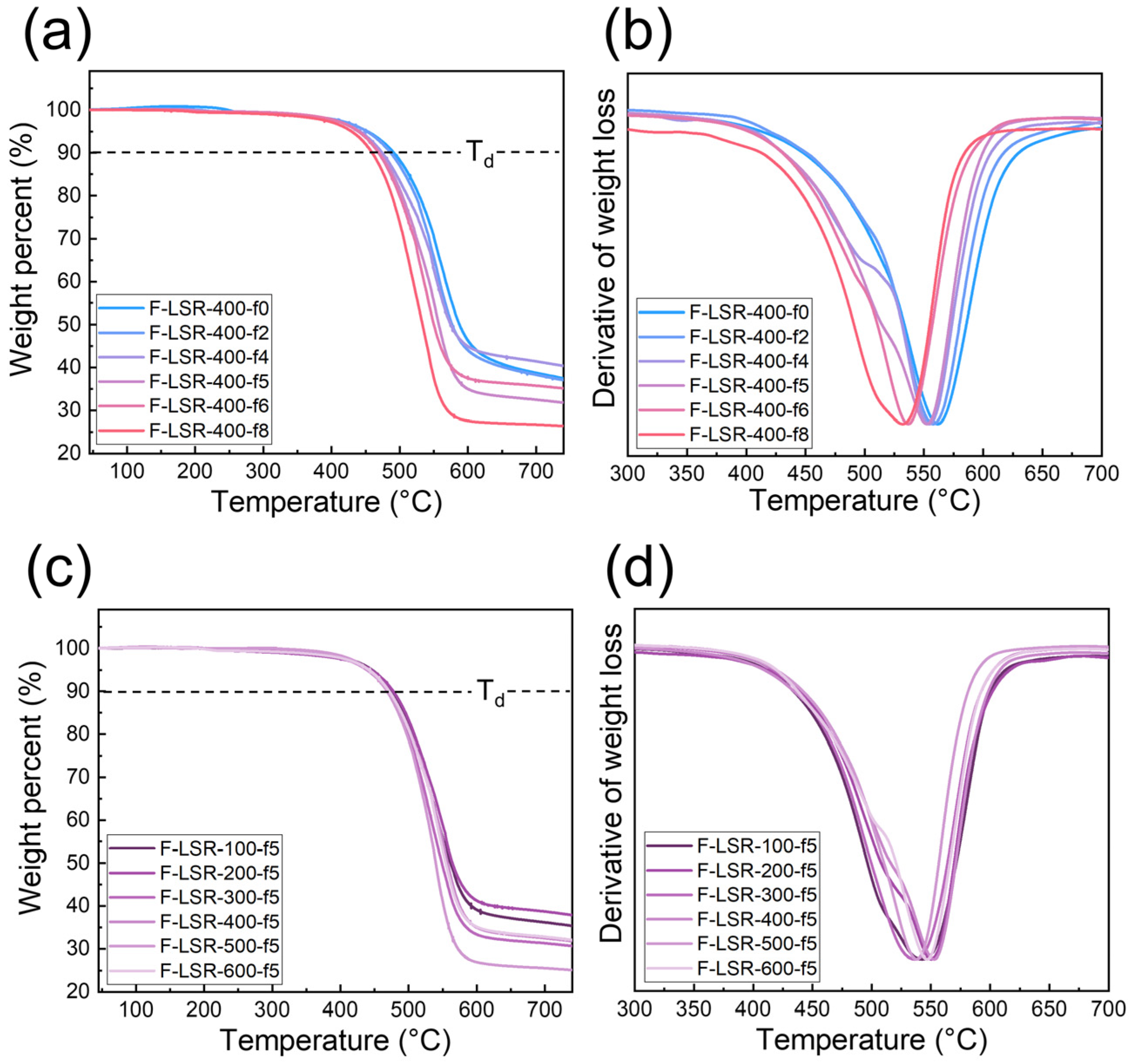
3.4.2. Heat Resistance of F-LSR
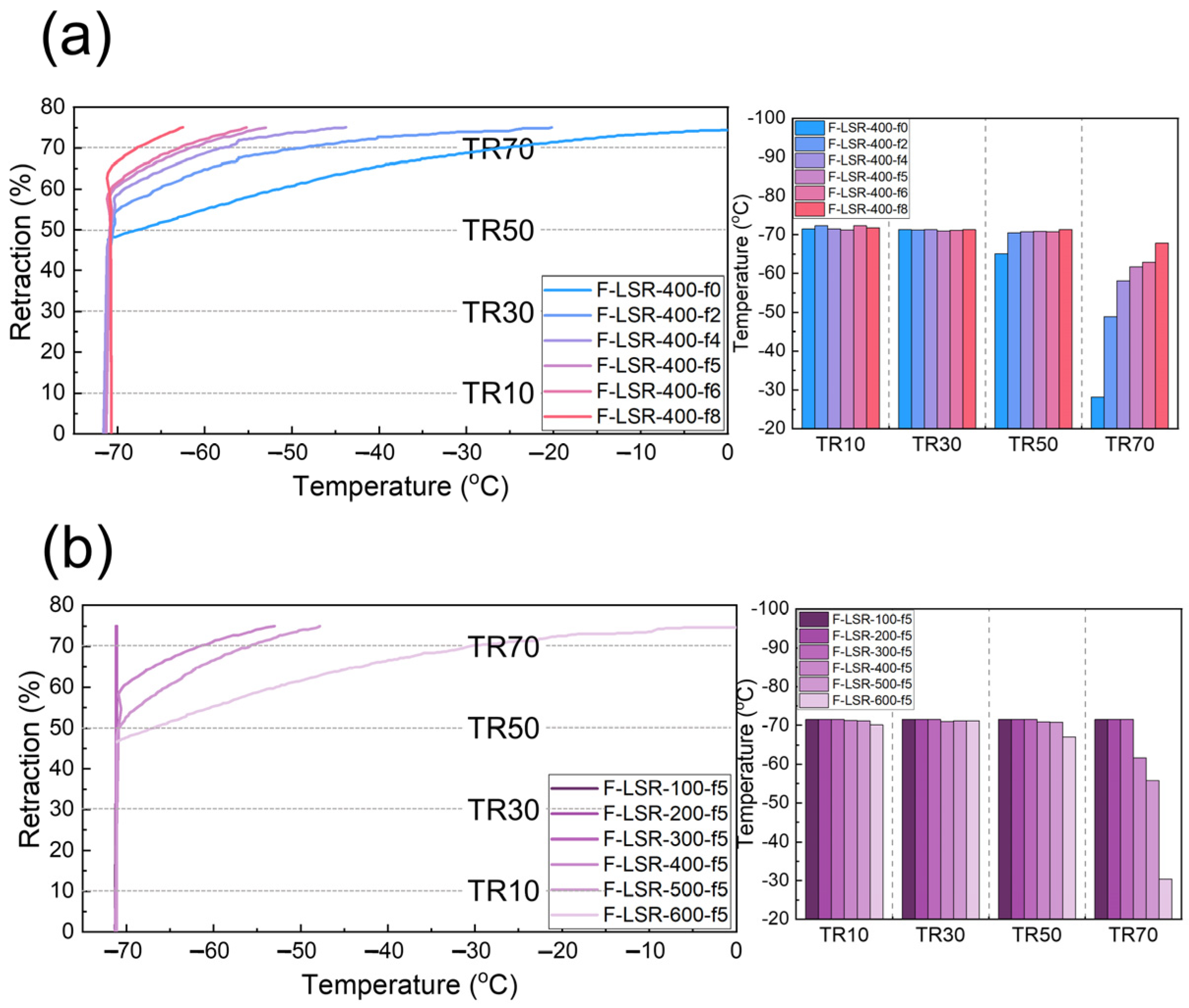
3.4.3. Thermal Properties and Elastic Retention of F-LSR at Low Temperature
4. Conclusions
- (1)
- All FVMQs were synthesized as random copolymers, and the ratios of the functional groups were almost identical to their theoretical values, with errors of less than 2% determined by 1H- and 29Si-NMR. Furthermore, GPC confirmed that the molecular weight of the FVMQs could be controlled by changing the loading of the end-blockers. The synthesized FHMQ was also successfully optimized to achieve an MH block content of more than 10% and a viscosity of less than 1000 cP;
- (2)
- The mechanical properties of all F-LSRs were confirmed: (1) the hardness of F-LSR increased as the proportion of fluorine in the chain increased; (2) at a constant fluorine ratio, elongation was proportional to the molecular length, and (3) the tensile strength was the greatest in F-LSR-400-f5;
- (3)
- In TGA, it was confirmed that the thermal stability of the synthesized F-LSR at high temperatures decreased as the fluorine group increased. Conversely, elastic retention and brittleness at low temperatures improved with a high ratio of fluorine groups and long molecular chain lengths.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of organic–inorganic hybrid materials: Prehistory, art, science, and advanced applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P. A review on silicone rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Liravi, F.; Toyserkani, E. Additive manufacturing of silicone structures: A review and prospective. Addit. Manuf. 2018, 24, 232–242. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, Y.; Meng, L.; Wang, Y.; Pang, A.; Guo, X.; Xiao, J.; Li, W. A review of poly[(3,3,3-trifluoropropyl)methylsiloxane]: Synthesis, properties and applications. Eur. Polym. J. 2022, 163, 110903. [Google Scholar] [CrossRef]
- Indulekha, K.; Behera, P.K.; Rajeev, R.; Gouri, C.; Ninan, K. Polyfluoroalkyl siloxanes with varying trifluoropropyl content: Synthesis, characterization and solvent resistance studies. J. Fluor. Chem. 2017, 200, 24–32. [Google Scholar] [CrossRef]
- Li, B.; Chen, S.; Zhang, J. Synthesis and characterization of vinyl-terminated copolysiloxanes containing 3,3,3-trifluoropropyl groups. Polym. Chem. 2012, 3, 2366–2376. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, Y.; Wei, B.; Cui, Y.; Huang, J.; Li, Y.; Meng, L.; Wang, Y. A facile preparation method of UV rapid curing fluorosilicone coatings with good hydrophobicity and excellent corrosion resistance based on thiol-ene click reaction. Prog. Org. Coat. 2023, 174, 107248. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Naderi, G.; Barmar, M. Effect of organo-clay on properties and mechanical behavior of fluorosilicone rubber. Fiber. Polym. 2014, 15, 2376–2385. [Google Scholar] [CrossRef]
- Zheng, X.; Pang, A.-m.; Wang, Y.; Wang, W.; Bai, Y. Fabrication of UV-curable fluorosilicone coatings with impressive hydrophobicity and solvent resistance. Prog. Org. Coat. 2020, 144, 105633. [Google Scholar] [CrossRef]
- Zhang, H.; Cloud, A. Research progress in calenderable fluorosilicone with excellent fuel resistance. In Proceedings of the SAMPE 2007, Baltimore, MD, USA, 3–7 June 2007; pp. 1–7. [Google Scholar]
- Cui, Y.; Jiang, W.; Li, D.; Niu, C.; Feng, S. Preparation and properties of fluorosilicone and fluorosilicone elastomer with various contents of trifluoropropyl groups. e-Polymers 2011, 11, 77. [Google Scholar] [CrossRef]
- Köhler, T.; Gutacker, A.; Mejía, E. Industrial synthesis of reactive silicones: Reaction mechanisms and processes. Org. Chem. Front. 2020, 7, 4108–4120. [Google Scholar] [CrossRef]
- Goff, J.; Sulaiman, S.; Arkles, B. Applications of hybrid polymers generated from living anionic ring opening polymerization. Molecules 2021, 26, 2755. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, J.; Huang, Y.-K.; You, Y.; Zhang, H.-Y.; Zheng, A.-N.; Xu, X.; Wei, D.-F. Synthesis of cerium-containing polymethylphenyl silicone and its antioxidant effect on fluorosilicone rubber. Chin. J. Polym. Sci. 2019, 37, 783–789. [Google Scholar] [CrossRef]
- Xu, T.; Liu, H.; Song, J.; Shang, S.; Song, Z.; Zou, K.; Yang, C. Synthesis and characterization of novel fluorosilicone rubber using imide modified vinyl-containing fluorosilicone resin as cross-linker. J. Polym. Sci. Polym. Chem. 2015, 53, 1769–1776. [Google Scholar] [CrossRef]
- Zhang, G.D.; Hu, Y.Q.; Wu, J.R.; Li, J.Y.; Lai, G.Q.; Zhong, M.Q. Improved synthesis and properties of hydroxyl-terminated liquid fluorosilicone. J. Appl. Polym. Sci. 2016, 133, 43220. [Google Scholar] [CrossRef]
- Barrère, M.; Maitre, C.; Dourges, M.; Hémery, P. Anionic polymerization of 1,3,5-tris(trifluoropropylmethyl)cyclotrisiloxane (F3) in miniemulsion. Macromolecules 2001, 34, 7276–7280. [Google Scholar] [CrossRef]
- Furukawa, Y.; Shin-Ya, S.; Miyake, H.; Kishino, H.; Yamada, M.; Kato, H.; Sato, M. Reactivity of cyclosiloxane with 3,3,4,4,5,5,6,6,6-nonafluorohexyl group and its application to fluorosilicone synthesis. J. Appl. Polym. Sci. 2001, 82, 3333–3340. [Google Scholar] [CrossRef]
- Wilczek, L.; Kennedy, J.P. Aggregation in the anionic polymerization of hexamethylcyclotrisiloxane with lithium counterion. Polym. J. 1987, 19, 531–538. [Google Scholar] [CrossRef]
- Owen, M.J. Poly[methyl(3,3,3-trifluoropropyl)siloxane]. In Handbook of Fluoropolymer Science and Technology; Wiley: Hoboken, NJ, USA, 2014; pp. 183–200. [Google Scholar]
- Ivin, K.J.; Saegusa, T. Ring-Opening Polymerization; Elsevier: Amsterdam, The Netherlands, 1984; Volume 2. [Google Scholar]
- Veith, C.A.; Cohen, R.E. Kinetic modelling and optimization of the trifluoropropylmethylsiloxane polymerization. J. Polym. Sci. Polym. Chem. 1989, 27, 1241–1258. [Google Scholar] [CrossRef]
- Fei, H.F.; Gao, X.; Han, X.; Wang, Q.; Hu, T.; Zhang, Z.; Xie, Z. Synthesis, characterization, and properties of vinyl-terminated copolysiloxanes containing trifluoropropyl and 4-trifluoromethylphenyl groups. J. Polym. Sci. Polym. Chem. 2015, 53, 1023–1031. [Google Scholar] [CrossRef]
- Bezlepkina, K.A.; Milenin, S.A.; Vasilenko, N.G.; Muzafarov, A.M. Ring-opening polymerization (ROP) and catalytic rearrangement as a way to obtain siloxane mono-and telechelics, as well as well-organized branching centers: History and prospects. Polymers 2022, 14, 2408. [Google Scholar] [CrossRef] [PubMed]
- Morariu, S.; Brunchi, C.E.; Cazacu, M.; Bercea, M. The behavior of poly(dimethylsiloxane-co-diphenylsiloxane)s in good and theta solvents. J. Chem. Eng. Data 2011, 56, 1468–1475. [Google Scholar] [CrossRef]
- Li, X.; Yu, R.; Zhao, T.; Zhang, Y.; Yang, X.; Zhao, X.; Huang, W. A self-healing polysiloxane elastomer based on siloxane equilibration synthesized through amino-ene Michael addition reaction. Eur. Polym. J. 2018, 108, 399–405. [Google Scholar] [CrossRef]
- Gelest, I.; Arkles, B.C.; Larson, G.L. Silicon Compounds: Silanes and Silicones: A Survey of Properties and Chemistry; Gelest Inc.: Morrisville, PA, USA, 2008; pp. 443–444. [Google Scholar]
- Shmeleva, O.A.; Mileshkevich, V.P. Study of the equilibrium copolymerization of dimethyl-and methyl(3,3,3-trifluoropropyl)cyclosiloxanes. Polym. Sci. USSR 1990, 32, 2112–2117. [Google Scholar] [CrossRef]
- Mazurek, P.; Vudayagiri, S.; Skov, A.L. How to tailor flexible silicone elastomers with mechanical integrity: A tutorial review. Chem. Soc. Rev. 2019, 48, 1448–1464. [Google Scholar] [CrossRef]
- Fei, H.-F.; Xie, W.; Wang, Q.; Gao, X.; Hu, T.; Zhang, Z.; Xie, Z. Controlled synthesis and characterization of poly[methyl(3,3,3-trifluoropropyl)siloxane] with selective end groups. RSC Adv. 2014, 4, 56279–56287. [Google Scholar] [CrossRef]
- Xu, T.; Liu, H.; Song, J.; Shang, S.B.; Song, Z.; Zou, K.; Yang, C. Synthesis and characterization of maleated rosin-modified fluorosilicone resin and its fluorosilicone rubber. J. Appl. Polym. Sci. 2015, 132, 41888. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Chen, P.; Zhou, X.; Zheng, A.; Guan, Y. Preparation of fluorosilicone random copolymers with properties superior to those of fluorosilicone/silicone polymer blends. J. Inorg. Organomet. Polym. Mater. 2015, 25, 1267–1276. [Google Scholar] [CrossRef]
- Kobayashi, H.; Nishiumi, W. Preparation and characterization of poly(methyl-3,3,3-trifluoropropylsiloxane-co-dimethylsiloxane). Makromol. Chem. 1993, 194, 1403–1410. [Google Scholar] [CrossRef]
- Li, Y.-F.S.; Zang, C.-G.; Zhang, Y.-L. Effect of the structure of hydrogen-containing silicone oil on the properties of silicone rubber. Mater. Chem. Phys. 2020, 248, 122734. [Google Scholar] [CrossRef]
- Chojnowski, J. Kinetically controlled siloxane ring-opening polymerization. J. Inorg. Organomet. Polym. 1991, 1, 299–323. [Google Scholar] [CrossRef]
- Lee, A.S.; Choi, S.-S.; Oh, S.Y.; Lee, H.S.; Kim, B.; Hwang, S.S.; Baek, K.-Y. Incompletely condensed POSS-based spin-on-glass networks for impeccable ultra low-k integration. J. Mater. Chem. C 2015, 3, 11605–11611. [Google Scholar] [CrossRef]
- Hu, W.-J.; Xia, Q.-Q.; Pan, H.-T.; Chen, H.-Y.; Qu, Y.-X.; Chen, Z.-Y.; Zhang, G.-D.; Zhao, L.; Gong, L.-X.; Xue, C.-G. Green and rapid preparation of fluorosilicone rubber foam materials with tunable chemical resistance for efficient oil–water separation. Polymers 2022, 14, 1628. [Google Scholar] [CrossRef]
- Fan, J.; Gong, Z.; Chen, Y. Design of strong fluorosilicone composites via thermodynamic and kinetic regulation of SiO2. Compos. Commun. 2022, 35, 101273. [Google Scholar] [CrossRef]
- Rodriguez, N.; Ruelas, S.; Forien, J.-B.; Dudukovic, N.; DeOtte, J.; Rodriguez, J.; Moran, B.; Lewicki, J.P.; Duoss, E.B.; Oakdale, J.S. 3D printing of high viscosity reinforced silicone elastomers. Polymers 2021, 13, 2239. [Google Scholar] [CrossRef]
- Eyre, D.; Van Noort, R.; Ellis, B. The rheology of silicone rubber impression materials. J. Dent. 1989, 17, 171–176. [Google Scholar] [CrossRef]
- Yan, X.; Li, M.; Zhao, M.; Zhou, H.; Wang, Y.; Ba, M. Effect of PDMS viscosity and additive amount of curing agent solution on the mechanical properties of PDMS fouling release coating. J. Phys. Conf. Ser. 2022, 2174, 012036. [Google Scholar] [CrossRef]
- Xu, Q.; Pang, M.; Zhu, L.; Zhang, Y.; Feng, S. Mechanical properties of silicone rubber composed of diverse vinyl content silicone gums blending. Mater. Des. 2010, 31, 4083–4087. [Google Scholar] [CrossRef]
- Yongha, K.; Hyeonwoo, J.; Kyoung, W.L.; Sosan, H.; Sang, E.S. Mechanical and thermal properties of environmentally benign silicone foam filled with wollastonite. Elastom. Compos. 2020, 55, 300–305. [Google Scholar]
- Kählig, H.; Zöllner, P.; Mayer-Helm, B.X. Characterization of degradation products of poly[(3,3,3-trifluoropropyl)methylsiloxane] by nuclear magnetic resonance spectroscopy, mass spectrometry and gas chromatography. Polym. Degrad. Stabil. 2009, 94, 1254–1260. [Google Scholar] [CrossRef]
- Delebecq, E.; Ganachaud, F. Looking over liquid silicone rubbers: (1) network topology vs chemical formulations. ACS Appl. Mater. Interfaces 2012, 4, 3340–3352. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, H.; Liu, S.; Jiang, Y.; Yin, Z.; Yu, B.; Ning, N.; Tian, M.; Zhang, L. Dielectric elastomer sensor with high dielectric constant and capacitive strain sensing properties by designing polar-nonpolar fluorosilicone multiblock copolymers and introducing poly(dopamine) modified CNTs. Compos. Part B Eng. 2021, 223, 109103. [Google Scholar] [CrossRef]
- Delebecq, E.; Hermeline, N.; Flers, A.; Ganachaud, F. Looking over liquid silicone rubbers: (2) mechanical properties vs. network topology. ACS Appl. Mater. Interfaces 2012, 4, 3353–3363. [Google Scholar] [CrossRef] [PubMed]
- Dascalu, M.; Dünki, S.J.; Quinsaat, J.-E.Q.; Ko, Y.S.; Opris, D.M. Synthesis of silicone elastomers containing trifluoropropyl groups and their use in dielectric elastomer transducers. RSC Adv. 2015, 5, 104516–104523. [Google Scholar] [CrossRef]
- Orrah, D.; Semlyen, J.; Ross-Murphy, S. Studies of cyclic and linear poly(dimethylsiloxanes): 27. Bulk viscosities above the critical molar mass for entanglement. Polymer 1988, 29, 1452–1454. [Google Scholar] [CrossRef]
- Roy, R.E.; Indulekha, K.; Vijayalakshmi, K.; Bhuvaneswari, S.; Soumyamol, P.; Rajeev, R. Fluorosilicone polymers with tailored mechanical, acid resistant and adhesive properties: Role of ultrasonication and functionally active single walled carbon nanotubes. Mater. Chem. Phys. 2019, 223, 523–534. [Google Scholar] [CrossRef]
- You, Y.; Chen, J.; Zheng, A.; Wei, D.; Xu, X.; Guan, Y. Effect of silanol on the thermal stability of poly[methyl(trifluoropropyl)siloxane]. J. Appl. Polym. Sci. 2020, 137, 49347. [Google Scholar] [CrossRef]
- Grunlan, M.; Mabry, J.; Weber, W. Synthesis of fluorinated copoly(carbosiloxane)s by Pt-catalyzed hydrosilylation copolymerization. Polymer 2003, 44, 981–987. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, J.W.; Choi, M.C.; Yun, Y.-M.; Jo, N.-J. Effect of phenyl vinyl methyl silicone (PVMQ) on low temperature sealing performance of fluorosilicone composites. Elastom. Compos. 2021, 56, 209–216. [Google Scholar]

| Sample | Recipe | Variable | |||||
|---|---|---|---|---|---|---|---|
| Monomer Ratio (mol%) | Initiator (wt%) | Time (h) | |||||
| D4 | F-D3 | Vi-D4 | ViVi | ||||
| FVMQ-100-f5 | 49 | 49 | 2 | 1 | 0.3 | 5 | Viscosity & molecular weight |
| FVMQ-200-f5 | 98 | 98 | 4 | 1 | |||
| FVMQ-300-f5 | 147 | 147 | 6 | 1 | |||
| FVMQ-400-f5 | 196 | 196 | 8 | 1 | |||
| FVMQ-500-f5 | 245 | 245 | 10 | 1 | |||
| FVMQ-600-f5 | 294 | 294 | 12 | 1 | |||
| FVMQ-400-f0 | 392 | 0 | 8 | 1 | 0.3 | 5 | MF-block content |
| FVMQ-400-f2 | 313.6 | 78.4 | 8 | 1 | |||
| FVMQ-400-f4 | 235.2 | 156.8 | 8 | 1 | |||
| FVMQ-400-f5 | 196 | 196 | 8 | 1 | |||
| FVMQ-400-f6 | 156.8 | 235.2 | 8 | 1 | |||
| FVMQ-400-f8 | 78.4 | 313.6 | 8 | 1 | |||
| FVMQ-400-f10 | 392 | 8 | 1 | ||||
| Sample | Viscosity (cP) | Mn (g/mol) | Mw (g/mol) | Ð |
|---|---|---|---|---|
| FVMQ-100-f5 | 2,440 | 32,078 | 37,810 | 1.17 |
| FVMQ-200-f5 | 7,568 | 55,102 | 59,278 | 1.08 |
| FVMQ-300-f5 | 25,400 | 46,564 | 68,608 | 1.47 |
| FVMQ-400-f5 | 59,760 | 55,228 | 72,089 | 1.31 |
| FVMQ-500-f5 | 78,800 | 76,439 | 90,356 | 1.18 |
| FVMQ-600-f5 | 103,400 | 85,592 | 108,909 | 1.27 |
| FVMQ-400-f0 | 47,120 | 76,121 | 89,424 | 1.08 |
| FVMQ-400-f2 | 47,040 | 75,614 | 80,934 | 1.07 |
| FVMQ-400-f4 | 54,000 | 57,738 | 74,153 | 1.28 |
| FVMQ-400-f5 | 59,760 | 55,228 | 72,089 | 1.31 |
| FVMQ-400-f6 | 33,700 | 48,098 | 70,180 | 1.50 |
| FVMQ-400-f8 | 9,840 | 32,307 | 48,904 | 1.51 |
| FVMQ-400-f10 | 670 | 25,758 | 31,009 | 1.20 |
| Sample | Theoretical Block Ratio | Empirical Block Ratio | ||||
|---|---|---|---|---|---|---|
| DM-Block | MF-Block | MV-Block | DM-Block | MF-Block | MV-Block | |
| FVMQ-100-f5 | 55.52 | 41.64 | 2.83 | 55.25 | 42.12 | 2.63 |
| FVMQ-200-f5 | 55.68 | 41.76 | 2.56 | 55.84 | 41.62 | 2.54 |
| FVMQ-300-f5 | 55.73 | 41.80 | 2.46 | 55.78 | 41.70 | 2.52 |
| FVMQ-400-f5 | 55.76 | 41.82 | 2.42 | 55.78 | 41.80 | 2.42 |
| FVMQ-500-f5 | 55.78 | 41.83 | 2.39 | 55.36 | 42.32 | 2.35 |
| FVMQ-600-f5 | 55.79 | 41.84 | 2.37 | 55.85 | 41.9 | 2.25 |
| FVMQ-400-f0 | 97.88 | 2.12 | 97.93 | 2.07 | ||
| FVMQ-400-f2 | 82.33 | 15.44 | 2.23 | 82.75 | 15.06 | 2.19 |
| FVMQ-400-f4 | 65.1 | 32.55 | 2.35 | 64.25 | 33.32 | 2.43 |
| FVMQ-400-f5 | 55.76 | 41.82 | 2.42 | 55.78 | 41.7 | 2.52 |
| FVMQ-400-f6 | 45.89 | 51.62 | 2.49 | 45.57 | 51.81 | 2.62 |
| FVMQ-400-f8 | 24.34 | 73.02 | 2.64 | 24.89 | 72.38 | 2.73 |
| FVMQ-400-f10 | 97.19 | 2.81 | 97.21 | 2.79 | ||
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Hardness | Si-H/Si-Vi |
|---|---|---|---|---|
| F-LSR-400-f0 | 0.708 | 127 | 16 | 0.599 |
| F-LSR-400-f2 | 1 | 143.2 | 17 | 0.566 |
| F-LSR-400-f4 | 1.037 | 147.8 | 18 | 0.510 |
| F-LSR-400-f5 | 1.27 | 151.8 | 20 | 0.492 |
| F-LSR-400-f6 | 1.135 | 142 | 21 | 0.473 |
| F-LSR-400-f8 | 1.024 | 128 | 23 | 0.454 |
| F-LSR-100-f5 | 0.467 | 149.6 | 8 | 0.471 |
| F-LSR-200-f5 | 0.83 | 141.6 | 18 | 0.488 |
| F-LSR-300-f5 | 1.031 | 145 | 19 | 0.492 |
| F-LSR-400-f5 | 1.27 | 151.8 | 20 | 0.512 |
| F-LSR-500-f5 | 1.075 | 172.6 | 18 | 0.528 |
| F-LSR-600-f5 | 0.652 | 201.5 | 13 | 0.551 |
| Sample | Td | Tdmax | Tg | TR10 | TR30 | TR50 | TR70 | TEm |
|---|---|---|---|---|---|---|---|---|
| F-LSR-100-f5 | 475.85 | 542.43 | −97.48 | −71.5 | −71.5 | −71.5 | −71.5 | / |
| F-LSR-200-f5 | 477.54 | 550.35 | −98.68 | −71.5 | −71.5 | −71.5 | −71.5 | / |
| F-LSR-300-f5 | 471.31 | 536.45 | −97.74 | −71.5 | −71.5 | −71.5 | −71.5 | / |
| F-LSR-400-f5 | 470.56 | 552.68 | −97.66 | −71.2 | −71.0 | −70.9 | −61.7 | / |
| F-LSR-500-f5 | 468.57 | 537.96 | −97.43 | −71.1 | −71.1 | −70.8 | −55.8 | / |
| F-LSR-600-f5 | 471.46 | 546.74 | −96.11 | −70.1 | −71.1 | −67.1 | −30.4 | / |
| F-LSR-400-f0 | 491.14 | 561.54 | −123.76 | −71.5 | −71.3 | −67.3 | −26.1 | −65.76 |
| F-LSR-400-f2 | 486.67 | 557.77 | −112.86 | −72.3 | −71.2 | −70.5 | −48.9 | / |
| F-LSR-400-f4 | 474.20 | 554.21 | −103.44 | −71.5 | −71.3 | −70.8 | −58.2 | / |
| F-LSR-400-f5 | 470.56 | 552.68 | −92.16 | −71.2 | −71.0 | −70.9 | −61.7 | / |
| F-LSR-400-f6 | 468.73 | 536.76 | −98.33 | −72.3 | −71.1 | −70.8 | −62.9 | / |
| F-LSR-400-f8 | 459.6 | 532.02 | −79.01 | −71.8 | −71.3 | −71.3 | −67.8 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, J.I.; Lee, C.S.; Jung, J.Y.; Lee, J.; Choi, J.K.; Shim, S.E.; Qian, Y. Optimization and Characterization of the F-LSR Manufacturing Process Using Quaternary Ammonium Silanolate as an Initiator for Synthesizing Fluorosilicone. Polymers 2022, 14, 5502. https://doi.org/10.3390/polym14245502
So JI, Lee CS, Jung JY, Lee J, Choi JK, Shim SE, Qian Y. Optimization and Characterization of the F-LSR Manufacturing Process Using Quaternary Ammonium Silanolate as an Initiator for Synthesizing Fluorosilicone. Polymers. 2022; 14(24):5502. https://doi.org/10.3390/polym14245502
Chicago/Turabian StyleSo, Jae Il, Chung Soo Lee, Ji Young Jung, Jaewon Lee, Jin Kyu Choi, Sang Eun Shim, and Yingjie Qian. 2022. "Optimization and Characterization of the F-LSR Manufacturing Process Using Quaternary Ammonium Silanolate as an Initiator for Synthesizing Fluorosilicone" Polymers 14, no. 24: 5502. https://doi.org/10.3390/polym14245502
APA StyleSo, J. I., Lee, C. S., Jung, J. Y., Lee, J., Choi, J. K., Shim, S. E., & Qian, Y. (2022). Optimization and Characterization of the F-LSR Manufacturing Process Using Quaternary Ammonium Silanolate as an Initiator for Synthesizing Fluorosilicone. Polymers, 14(24), 5502. https://doi.org/10.3390/polym14245502







