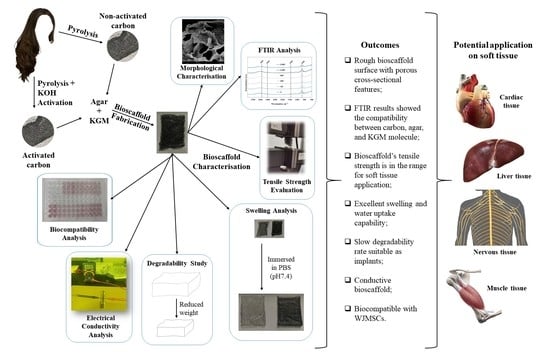
Carbonised Human Hair Incorporated in Agar/KGM Bioscaffold for Tissue Engineering Application: Fabrication and Characterisation
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Hair-Derived Carbon
2.1.1. Synthesis of Non-Activated Carbon
2.1.2. Synthesis of Activated Carbon
2.1.3. Characterisation of Carbon
2.2. Fabrication of Bioscaffold
2.3. Characterisation of Bioscaffold
2.3.1. Morphological Analysis
2.3.2. FTIR Analysis
2.3.3. Swelling and Water Uptake Analysis
2.3.4. Degradation Analysis
2.3.5. Tensile Test
2.3.6. Electrical Conductivity Analysis
2.4. Cell Culture
2.4.1. Preparation of WJMSCs
2.4.2. Characterisation of WJMSCs
2.4.3. Biocompatibility Analysis of Bioscaffold
2.5. Statistical Analysis
3. Results and Discussion
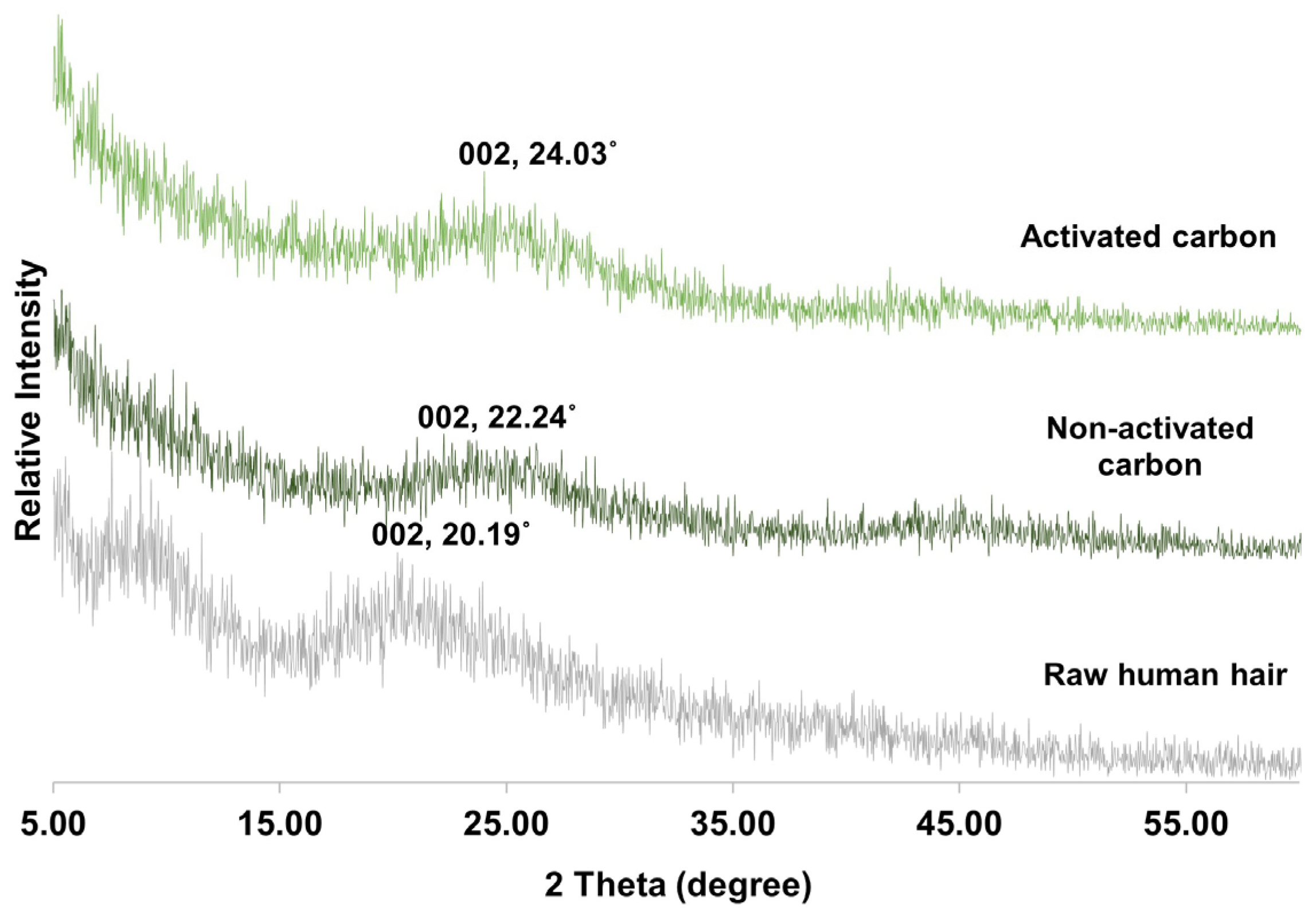
3.1. Carbon-Derived Human Hair
3.2. Fabrication and Characterisation of Carbon-Based Bioscaffold
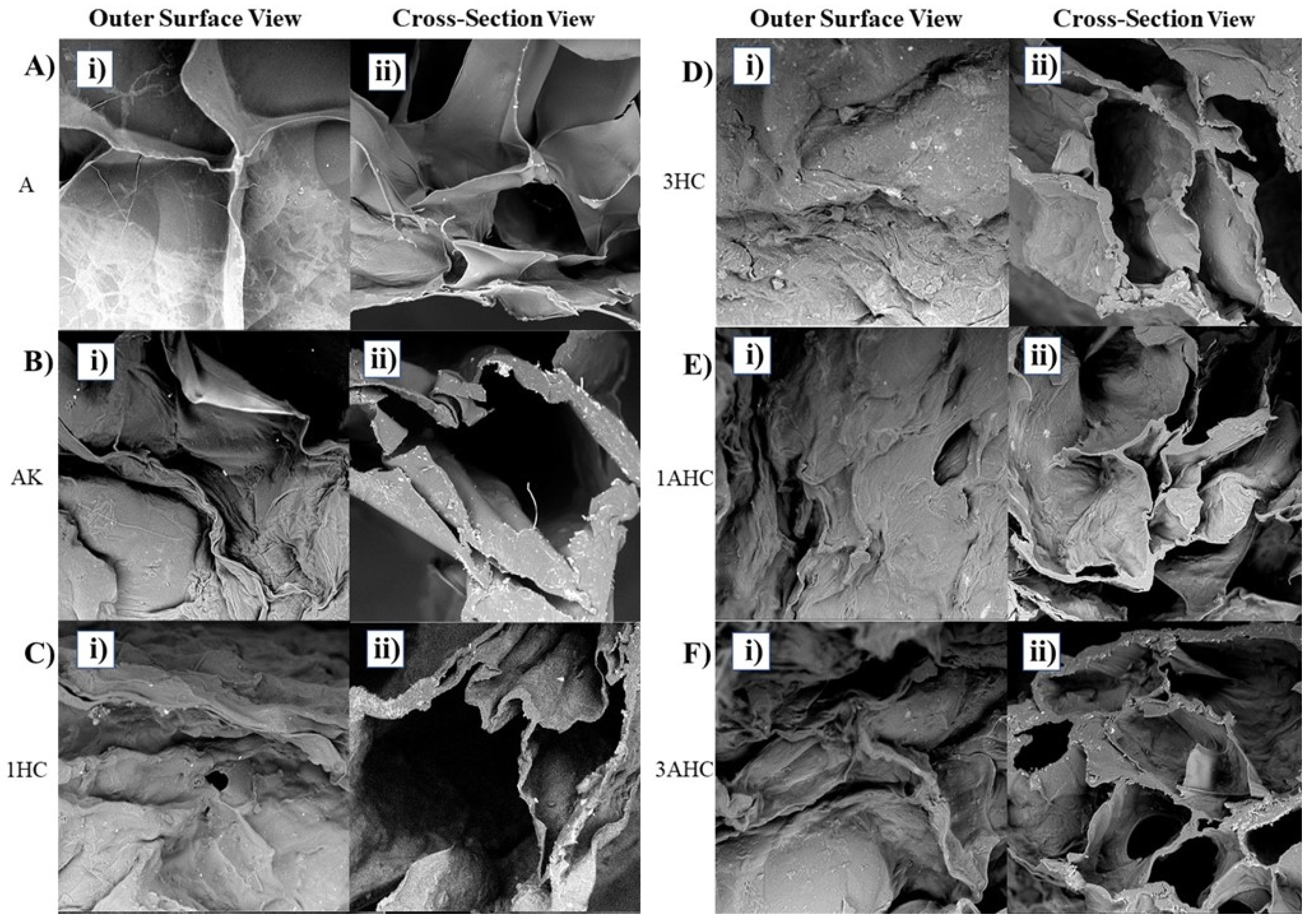
3.2.1. Morphological Analysis of Bioscaffold
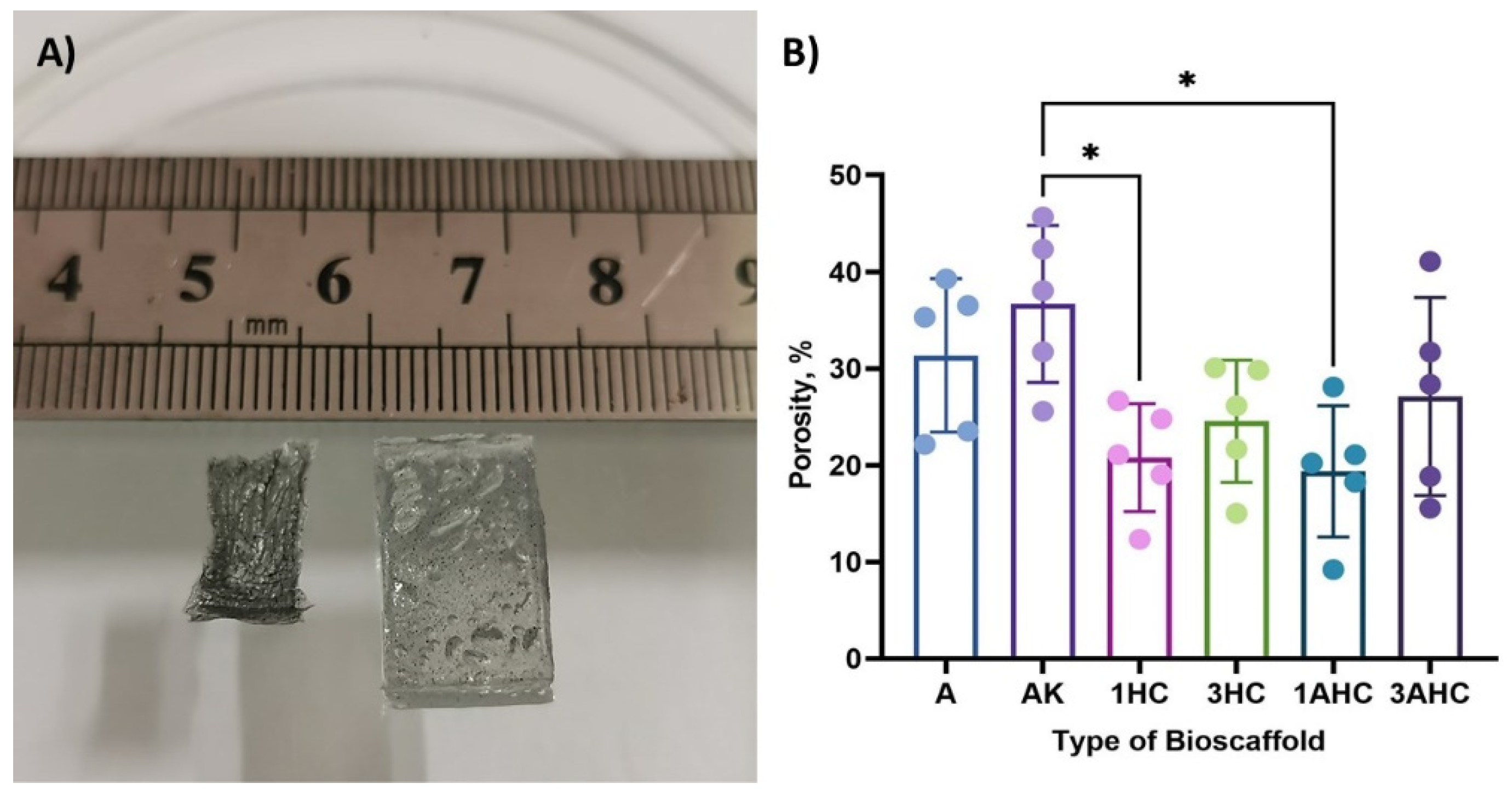
3.2.2. Porosity Analysis of Bioscaffold
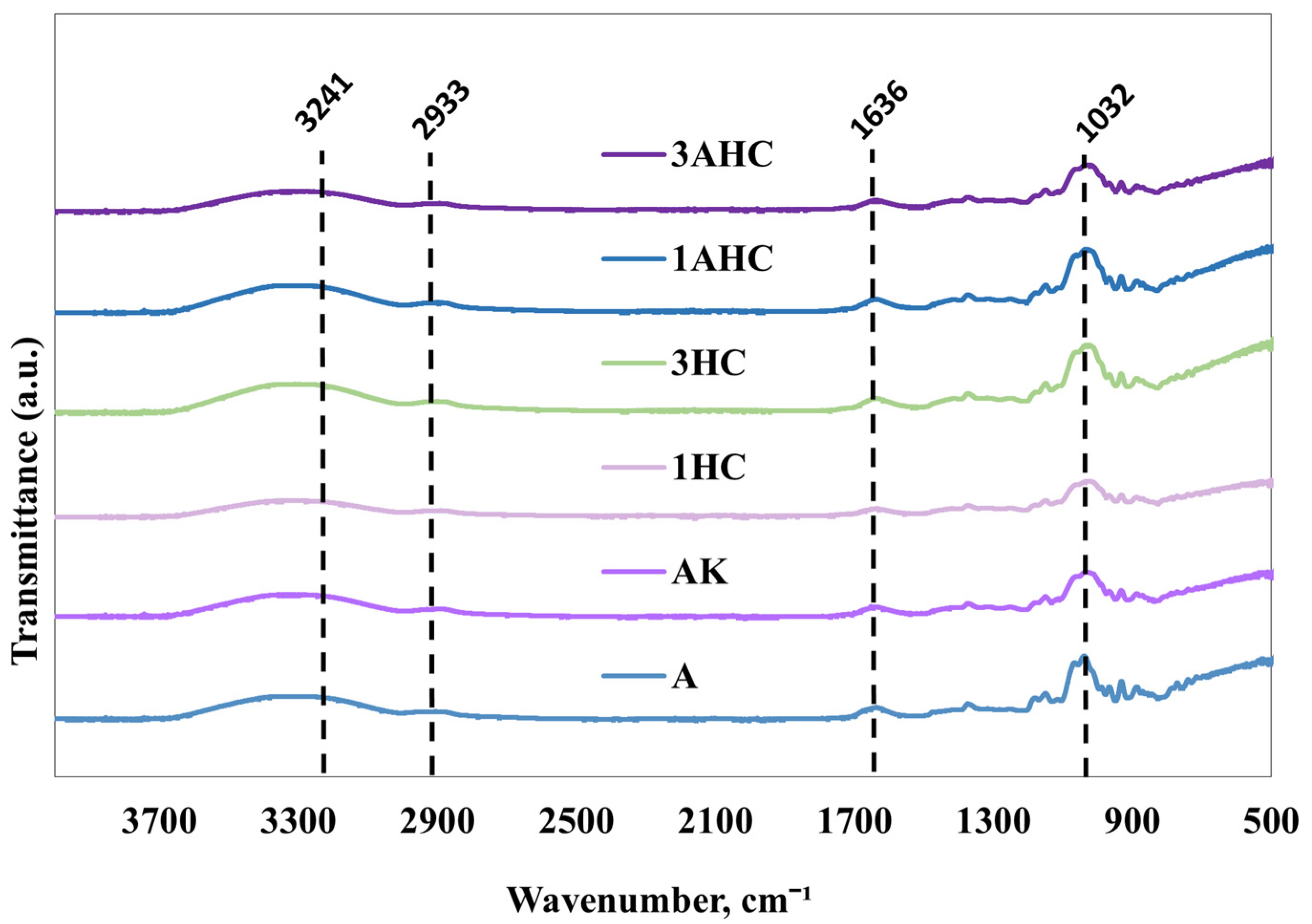
3.2.3. Fourier Transform Infrared Analysis
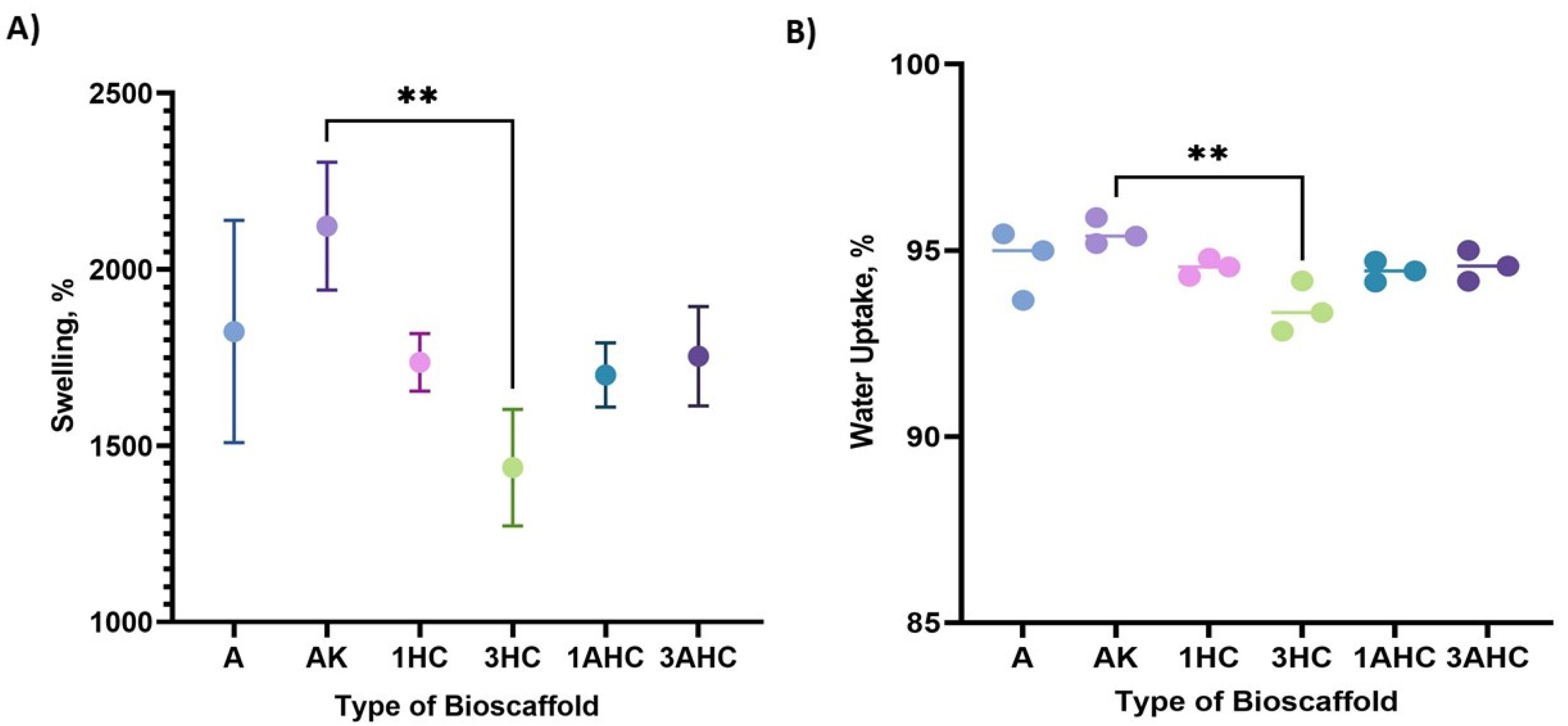
3.2.4. Swelling Ratio Measurement
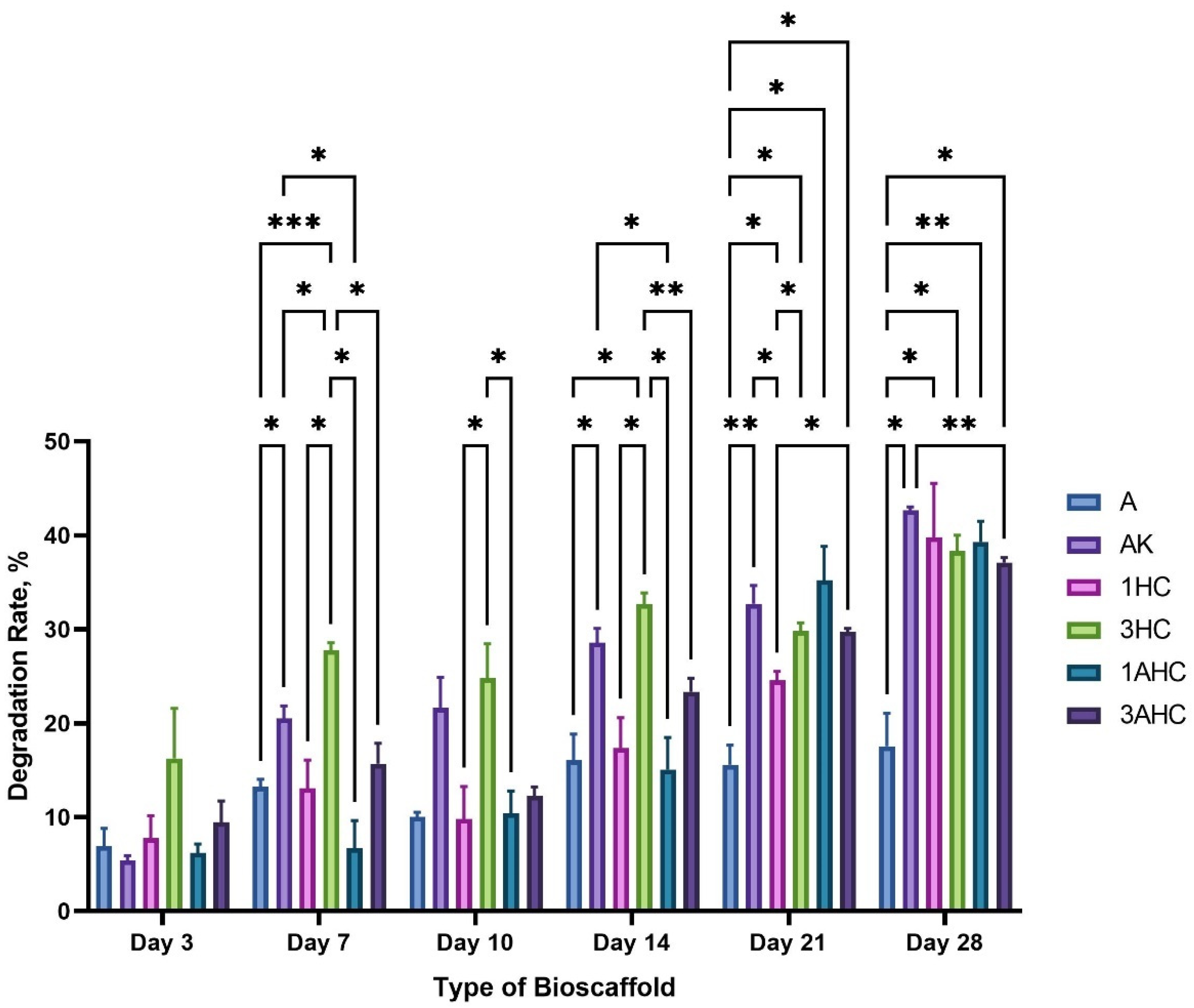
3.2.5. Degradation Analysis
3.2.6. Tensile Strength Analysis
3.2.7. Electrical Conductivity Analysis
3.3. Cell Culture
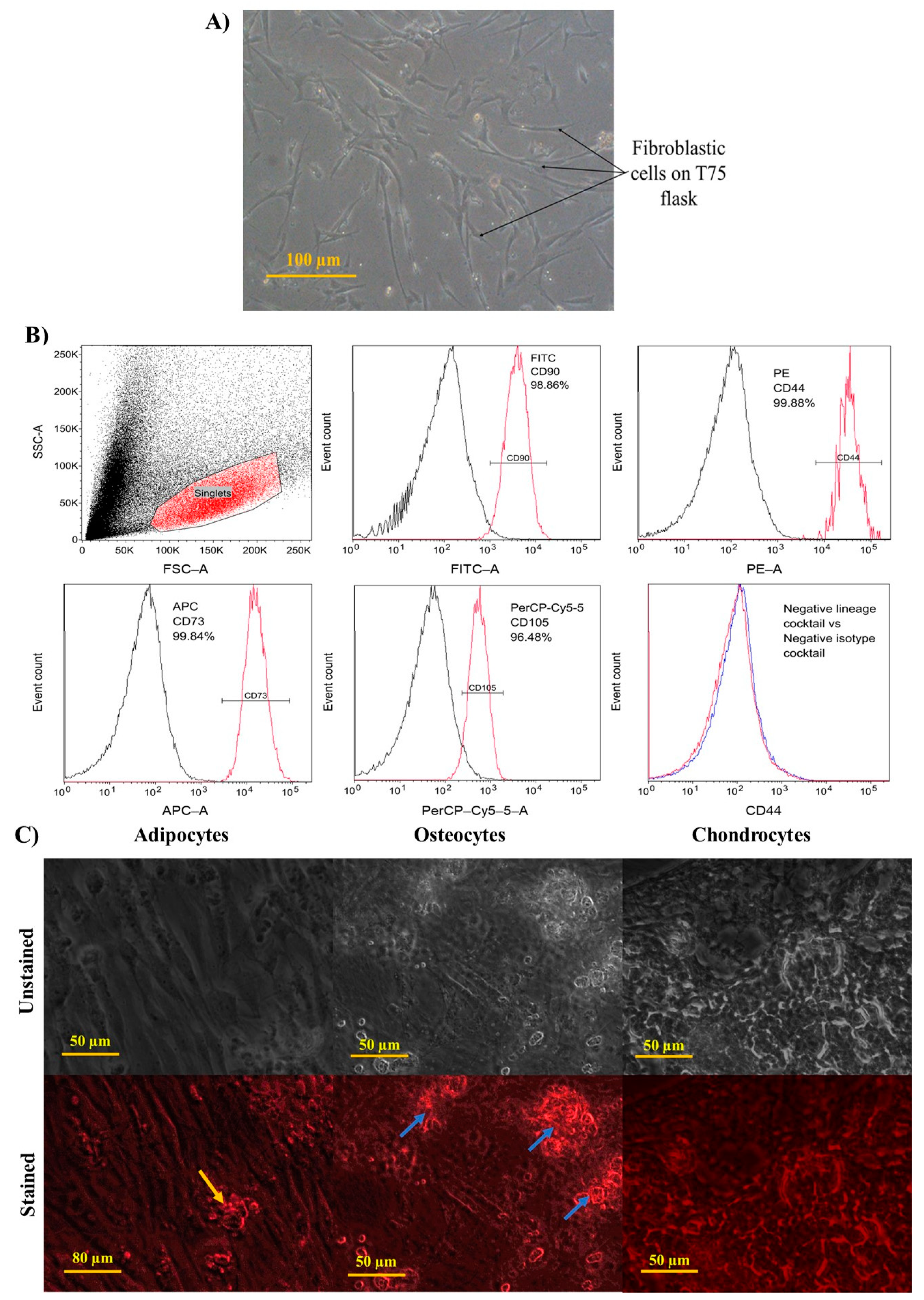
3.3.1. Characterisation of WJMSCs
Morphology of WJMSCs
Immunophenotyping of WJMSCs
Differentiation Ability of WJMSCs
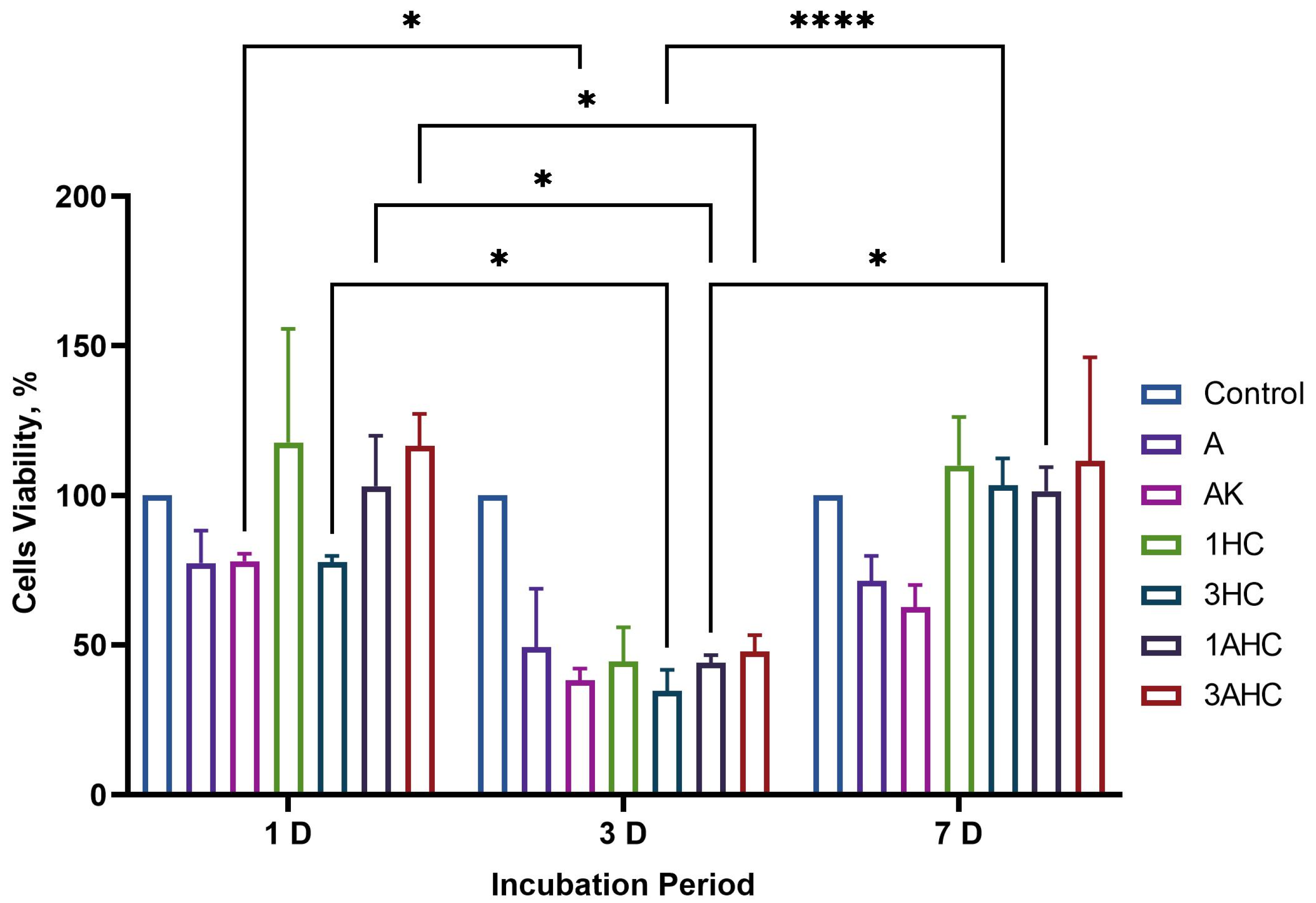
3.3.2. Biocompatibility Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Pramanick, B.; Maiti, T.K.; Bhattacharyya, T.K. Glassy carbon microneedles—New transdermal drug delivery device derived from a scalable C-MEMS process. Microsyst. Nanoeng. 2018, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Hasirci, V.; Hasirci, N. Carbon as a Biomaterial. In Fundamentals of Biomaterials; Springer: Berlin/Heidelberg, Germany, 2018; pp. 83–94. [Google Scholar]
- Yan, C.; Ren, Y.; Sun, X.; Jin, L.; Liu, X.; Chen, H.; Wang, K.; Yu, M.; Zhao, Y. Photoluminescent functionalized carbon quantum dots loaded electroactive Silk fibroin/PLA nanofibrous bioactive scaffolds for cardiac tissue engineering. J. Photochem. Photobiol. B Biol. 2020, 202, 111680. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Pillai, M.M.; Shanthakumari, S.; Gnanapoongothai, S.; Dinakar Rai, B.K.; Santosh Sahanand, K.; Selvakumar, R.; Bhattacharyya, A. Carbon nanofiber amalgamated 3D poly-ε-caprolactone scaffold functionalized porous-nanoarchitectures for human meniscal tissue engineering: In vitro and in vivo biocompatibility studies. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Tashakori-Miyanroudi, M.; Rakhshan, K.; Ramez, M.; Asgarian, S.; Janzadeh, A.; Azizi, Y.; Seifalian, A.; Ramezani, F. Conductive carbon nanofibers incorporated into collagen bio-scaffold assists myocardial injury repair. Int. J. Biol. Macromol. 2020, 163, 1136–1146. [Google Scholar] [CrossRef]
- Zheng, N.; Fitzpatrick, V.; Cheng, R.; Shi, L.; Kaplan, D.L.; Yang, C. Photoacoustic carbon nanotubes embedded silk scaffolds for neural stimulation and regeneration. ACS Nano 2022, 16, 2292–2305. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, P.; Nazeri, N.; Derakhshan, M.A.; Ghanbari, H. Preparation and characterization of polyurethane/chitosan/CNT nanofibrous scaffold for cardiac tissue engineering. Int. J. Biol. Macromol. 2021, 180, 590–598. [Google Scholar] [CrossRef]
- Yan, Y.; Nashath, F.Z.; Chen, S.; Manickam, S.; Lim, S.S.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of graphene: Potential carbon precursors and approaches. Nanotechnol. Rev. 2020, 9, 1284–1314. [Google Scholar] [CrossRef]
- Tripathi, N.; Pavelyev, V.; Islam, S.S. Synthesis of carbon nanotubes using green plant extract as catalyst: Unconventional concept and its realization. Appl. Nanosci. 2017, 7, 557–566. [Google Scholar] [CrossRef]
- Smets, K.; De Jong, M.; Lupul, I.; Gryglewicz, G.; Schreurs, S.; Carleer, R.; Yperman, J. Rapeseed and raspberry seed cakes as inexpensive raw materials in the production of activated carbon by physical activation: Effect of activation conditions on textural and phenol adsorption characteristics. Materials 2016, 9, 565. [Google Scholar] [CrossRef]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis–A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Wang, C.; Huang, J.; Qi, H.; Cao, L.; Xu, Z.; Cheng, Y.; Zhao, X.; Li, J. Controlling pseudographtic domain dimension of dandelion derived biomass carbon for excellent sodium-ion storage. J. Power Sources 2017, 358, 85–92. [Google Scholar] [CrossRef]
- Adamson, A.; Väli, R.; Paalo, M.; Aruväli, J.; Koppel, M.; Palm, R.; Härk, E.; Nerut, J.; Romann, T.; Lust, E. Peat-derived hard carbon electrodes with superior capacity for sodium-ion batteries. RSC Adv. 2020, 10, 20145–20154. [Google Scholar] [CrossRef]
- Li, S.C.; Hu, B.C.; Ding, Y.W.; Liang, H.W.; Li, C.; Yu, Z.Y.; Wu, Z.Y.; Chen, W.S.; Yu, S.H. Wood-Derived Ultrathin Carbon Nanofiber Aerogels. Angew. Chem. 2018, 130, 7203–7208. [Google Scholar] [CrossRef]
- Redko, T.; Volford, A.; Marek, E.; Scott, S.; Hayhurst, A. Measurement of the times for pyrolysis and the thermal diffusivity of a pyrolysing particle of wood and also of the resulting char. Combust. Flame 2020, 212, 510–518. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Q.; Gao, S.; Tang, G.; Liu, K.; He, S.; Huang, C. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 2019, 155, 706–726. [Google Scholar] [CrossRef]
- Pramanick, B.; Martinez-Chapa, S.O.; Madou, M.; Hwang, H. Fabrication of 3D carbon microelectromechanical systems (C-MEMS). JoVE (J. Vis. Exp.) 2017, 124, e55649. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Niu, A.; Li, J.; Fu, J.-W.; Xu, Q.; Pei, D.-S. In vivo characterization of hair and skin derived carbon quantum dots with high quantum yield as long-term bioprobes in zebrafish. Sci. Rep. 2016, 6, 37860. [Google Scholar] [CrossRef]
- Bal Altuntaş, D.; Aslan, S.; Akyol, Y.; Nevruzoğlu, V. Synthesis of new carbon material produced from human hair and its evaluation as electrochemical supercapacitor. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 2346–2356. [Google Scholar] [CrossRef]
- Duan, N.; Li, Q.; Meng, X.; Wang, Z.; Wu, S. Preparation and characterization of k-carrageenan/konjac glucomannan/TiO2 nanocomposite film with efficient anti-fungal activity and its application in strawberry preservation. Food Chem. 2021, 364, 130441. [Google Scholar] [CrossRef]
- Guo, L.; Yokoyama, W.; Chen, L.; Liu, F.; Chen, M.; Zhong, F. Characterization and physicochemical properties analysis of konjac glucomannan: Implications for structure-properties relationships. Food Hydrocoll. 2021, 120, 106818. [Google Scholar] [CrossRef]
- Ni, Y.; Sun, J.; Wang, J. Enhanced antimicrobial activity of konjac glucomannan nanocomposite films for food packaging. Carbohydr. Polym. 2021, 267, 118215. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yokoyama, W.; Chen, M.; Zhong, F. Konjac glucomannan molecular and rheological properties that delay gastric emptying and improve the regulation of appetite. Food Hydrocoll. 2021, 120, 106894. [Google Scholar] [CrossRef]
- Du, Q.; Liu, J.; Ding, Y. Recent progress in biological activities and health benefits of konjac glucomannan and its derivatives. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100270. [Google Scholar] [CrossRef]
- Xiang, F.; Xia, Y.; Wang, Y.; Wang, Y.; Wu, K.; Ni, X. Preparation of konjac glucomannan based films reinforced with nanoparticles and its effect on cherry tomatoes preservation. Food Packag. Shelf Life 2021, 29, 100701. [Google Scholar] [CrossRef]
- Ueno, H.; Haraguchi, N.; Azuma, M.; Shiiya, T.; Noda, T.; Ebihara, E.; Uehira, Y.; Uchida, T.; Sasaba, K.; Nakamura, M. Active Consumption of Konjac and Konjac Products Improves Blood Glucose Control in Patients with Type 2 Diabetes Mellitus. J. Am. Nutr. Assoc. 2021, 29, 1–7. [Google Scholar] [CrossRef]
- Dai, J.; Chen, J.; Qi, J.; Ding, M.; Liu, W.; Shao, T.; Han, J.; Wang, G. Konjac Glucomannan from Amorphophallus konjac enhances immunocompetence of the cyclophosphamide-induced immunosuppressed mice. Food Sci. Nutr. 2021, 9, 728–735. [Google Scholar] [CrossRef]
- Xu, M.; Huang, J.; Jiang, S.; He, J.; Wang, Z.; Qin, H.; Guan, Y.-Q. Glucose sensitive konjac glucomannan/concanavalin A nanoparticles as oral insulin delivery system. Int. J. Biol. Macromol. 2022, 202, 296–308. [Google Scholar] [CrossRef]
- Wu, H.; Bu, N.; Chen, J.; Chen, Y.; Sun, R.; Wu, C.; Pang, J. Construction of konjac glucomannan/oxidized hyaluronic acid hydrogels for controlled drug release. Polymers 2022, 14, 927. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, G.; Liu, J.; Li, M.; Li, Q.; Tang, K. Gelatin/oxidized konjac glucomannan composite hydrogels with high resistance to large deformation for tissue engineering applications. ACS Appl. Bio Mater. 2021, 4, 1536–1543. [Google Scholar] [CrossRef]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Mahmoodzadeh, F. Incorporation of Oxidized Pectin to Reinforce Collagen/Konjac Glucomannan Hydrogels Designed for Tissue Engineering Applications. Macromol. Res. 2021, 29, 289–296. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Mao, C.; Song, Z.; Li, X.; Liu, C. Preparation and characterization of konjac glucomannan and gum arabic composite gel. Int. J. Biol. Macromol. 2021, 183, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Liu, Y.; Liu, J. Properties of the konjac glucomannan and zein composite gel with or without freeze-thaw treatment. Food Hydrocoll. 2021, 117, 106700. [Google Scholar] [CrossRef]
- Xiao, M.; Luo, L.; Tang, B.; Qin, J.; Wu, K.; Jiang, F. Physical, structural, and water barrier properties of emulsified blend film based on konjac glucomannan/agar/gum Arabic incorporating virgin coconut oil. LWT 2022, 154, 112683. [Google Scholar] [CrossRef]
- Zhu, J.; Eid, M.; Li, J.; Geng, F.; Li, B. Synergistic interactions between konjac glucomannan and welan gum mixtures. LWT 2022, 162, 113425. [Google Scholar] [CrossRef]
- Qiao, D.; Tu, W.; Zhong, L.; Wang, Z.; Zhang, B.; Jiang, F. Microstructure and mechanical/hydrophilic features of agar-based films incorporated with konjac glucomannan. Polymers 2019, 11, 1952. [Google Scholar] [CrossRef]
- Chen, X.; Fu, X.; Huang, L.; Xu, J.; Gao, X. Agar oligosaccharides: A review of preparation, structures, bioactivities and application. Carbohydr. Polym. 2021, 265, 118076. [Google Scholar] [CrossRef]
- Qiao, D.; Li, H.; Jiang, F.; Zhao, S.; Chen, S.; Zhang, B. Incorporation of κ-carrageenan improves the practical features of agar/konjac glucomannan/κ-carrageenan ternary system. Food Sci. Hum. Wellness 2023, 12, 512–519. [Google Scholar] [CrossRef]
- Mehta, P.; Kaith, B.S. Green Synthesis of Agar/Gelatin Based Superabsorbent (BGCP) Through Gamma Radiation Cross-Linking Polymerization for Castoff as Sustained Drug Delivery Device and in Soil Treatment for Improved Water Retention. J. Polym. Env. 2021, 29, 647–661. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, J.; Zhang, T.; Hati, S.; Mo, H.; Xu, D.; Li, H.; Hu, L.; Liu, Z. Characterization of carvacrol incorporated antimicrobial film based on agar/konjac glucomannan and its application in chicken preservation. J. Food Eng. 2022, 330, 111091. [Google Scholar] [CrossRef]
- GrandViewResearch Scaffold Technology Market Size, Share & Trends Analysis Report by Type, by Application (Stem Cell, Regenerative Medicine, Drug Discovery), by Disease Type (Cancer, Dental, Neurology), by End-Use, and Segment Forecasts, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/scaffold-technology-market (accessed on 6 April 2022).
- Sahmani, S.; Shahali, M.; Ghadiri Nejad, M.; Khandan, A.; Aghdam, M.; Saber-Samandari, S. Effect of copper oxide nanoparticles on electrical conductivity and cell viability of calcium phosphate scaffolds with improved mechanical strength for bone tissue engineering. Eur. Phys. J. Plus 2019, 134, 7. [Google Scholar] [CrossRef]
- Tohmyoh, H.; Fujita, K.; Suzuki, H.; Futada, K. Structural elasticity for tensile deformation of a single human hair and the comparison with it for the bending deformation. J. Mech. Behav. Biomed. Mater. 2021, 113, 104166. [Google Scholar] [CrossRef] [PubMed]
- Pramanick, B.; Cadenas, L.B.; Kim, D.-M.; Lee, W.; Shim, Y.-B.; Martinez-Chapa, S.O.; Madou, M.J.; Hwang, H. Human hair-derived hollow carbon microfibers for electrochemical sensing. Carbon 2016, 107, 872–877. [Google Scholar] [CrossRef]
- Ahmed, M.; Islam, M.A.; Asif, M.; Hameed, B. Human hair-derived high surface area porous carbon material for the adsorption isotherm and kinetics of tetracycline antibiotics. Bioresour. Technol. 2017, 243, 778–784. [Google Scholar] [CrossRef]
- Chayid, M.A.; Ahmed, M.J. Amoxicillin adsorption on microwave prepared activated carbon from Arundo donax Linn: Isotherms, kinetics, and thermodynamics studies. J. Environ. Chem. Eng. 2015, 3, 1592–1601. [Google Scholar] [CrossRef]
- Choi, S.W.; Tang, J.; Pol, V.G.; Lee, K.B. Pollen-derived porous carbon by KOH activation: Effect of physicochemical structure on CO2 adsorption. J. CO2 Util. 2019, 29, 146–155. [Google Scholar] [CrossRef]
- Rodríguez, F.; Montoya-Ruiz, C.; Estiati, I.; Saldarriaga, J.F. Removal of drugs in polluted waters with char obtained by pyrolysis of hair waste from the tannery process. ACS Omega 2020, 5, 24389–24402. [Google Scholar] [CrossRef]
- Yagmur, E.; Gokce, Y.; Tekin, S.; Semerci, N.I.; Aktas, Z. Characteristics and comparison of activated carbons prepared from oleaster (Elaeagnus angustifolia L.) fruit using KOH and ZnCl2. Fuel 2020, 267, 117232. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Kaur, A.; Maheshwari, P.H.; Sundriyal, S. Surface and Diffusion Charge Contribution Studies of Human Hair-Derived Heteroatom-Doped Porous Carbon Electrodes for Supercapacitors. Energy Fuels 2021, 36, 626–637. [Google Scholar] [CrossRef]
- Zopf, D.A.; Flanagan, C.L.; Mitsak, A.G.; Brennan, J.R.; Hollister, S.J. Pore architecture effects on chondrogenic potential of patient-specific 3-dimensionally printed porous tissue bioscaffolds for auricular tissue engineering. Int. J. Pediatr. Otorhinolaryngol. 2018, 114, 170–174. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Xu, T.; Li, J.; Guo, T.; Lu, X.; Ren, J.; Ren, X.; Mu, Y.; Weng, J. Effect of the interconnecting window diameter of hydroxyapatite scaffolds on vascularization and osteoinduction. Ceram. Int. 2022, 48, 25070–25078. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, B.; Zhao, S.; Qiao, D.; Xie, F. Plasticized Starch/Agar Composite Films: Processing, Morphology, Structure, Mechanical Properties and Surface Hydrophilicity. Coatings 2021, 11, 311. [Google Scholar] [CrossRef]
- Salleh, A.; Mustafa, N.; Yeit Haan, T.; Mohd Nor, F.; Fauzul Azim, K.; Ishak, A.; Mh Busra, F. Dual-Layered Approach of Ovine Collagen-Gelatin/Cellulose Hybrid Biomatrix Containing Graphene Oxide-Silver Nanoparticles for Cutaneous Wound Healing: Fabrication, Physicochemical, Cytotoxicity and Antibacterial Characterisation. Biomedicines 2022, 10, 816. [Google Scholar] [CrossRef]
- Nokoorani, Y.D.; Shamloo, A.; Bahadoran, M.; Moravvej, H. Fabrication and characterization of scaffolds containing different amounts of allantoin for skin tissue engineering. Sci. Rep. 2021, 11, 16164. [Google Scholar] [CrossRef]
- Guo, S.; Li, H.; Li, Y.; Han, Y.; Chen, K.; Xu, G.; Zhu, Y.; Hu, X. SiO2-enhanced structural stability and strong adhesion with a new binder of konjac glucomannan enables stable cycling of silicon anodes for lithium-ion batteries. Adv. Energy Mater. 2018, 8, 1800434. [Google Scholar] [CrossRef]
- Lewandowski, J.; Rozwadowska, N.; Kolanowski, T.J.; Malcher, A.; Zimna, A.; Rugowska, A.; Fiedorowicz, K.; Łabędź, W.; Kubaszewski, Ł.; Chojnacka, K. The impact of in vitro cell culture duration on the maturation of human cardiomyocytes derived from induced pluripotent stem cells of myogenic origin. Cell Transplant. 2018, 27, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Mohmad, M.; Abdollah, M.F.B.; Khudhair, A.Q.; Tamaldin, N.; Amiruddin, H.; Zin, M.R.B.M.; Tunggal, D. Physical-mechanical properties of palm kernel activated carbon reinforced polymeric composite: Potential as a self-lubricating material. J. Tribol. 2018, 17, 77–92. [Google Scholar]
- Uddin, M.; Dhanasekaran, P.S.; Asmatulu, R. Mechanical properties of highly porous PEEK bionanocomposites incorporated with carbon and hydroxyapatite nanoparticles for scaffold applications. Prog. Biomater. 2019, 8, 211–221. [Google Scholar] [CrossRef]
- Ashtari, K.; Nazari, H.; Ko, H.; Tebon, P.; Akhshik, M.; Akbari, M.; Alhosseini, S.N.; Mozafari, M.; Mehravi, B.; Soleimani, M.; et al. Electrically conductive nanomaterials for cardiac tissue engineering. Adv. Drug Deliv. Rev. 2019, 144, 162–179. [Google Scholar] [CrossRef]
- Palmieri, V.; Sciandra, F.; Bozzi, M.; De Spirito, M.; Papi, M. 3D graphene scaffolds for skeletal muscle regeneration: Future perspectives. Front. Bioeng. Biotechnol. 2020, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Azevêdo, H.V.S.B.; Raimundo, R.A.; Ferreira, L.S.; Silva, M.M.S.; Morales, M.A.; Macedo, D.A.; Gomes, U.U.; Cavalcante, D.G.L. Green synthesis of CoWO4 powders using agar-agar from red seaweed (Rhodophyta): Structure, magnetic properties and battery-like behavior. Mater. Chem. Phys. 2020, 242, 122544. [Google Scholar] [CrossRef]
- Selvalakshmi, S.; Mathavan, T.; Selvasekarapandian, S.; Premalatha, M. Effect of ethylene carbonate plasticizer on agar-agar: NH4Br-based solid polymer electrolytes. Ionics 2018, 24, 2209–2217. [Google Scholar] [CrossRef]
- Vinayagam, M.; Babu, R.S.; Sivasamy, A.; de Barros, A.L.F. Biomass-derived porous activated carbon from Syzygium cumini fruit shells and Chrysopogon zizanioides roots for high-energy density symmetric supercapacitors. Biomass Bioenergy 2020, 143, 105838. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [PubMed]
- Ranjbaran, H.; Abediankenari, S.; Mohammadi, M.; Jafari, N.; Khalilian, A.; Rahmani, Z.; Amiri, M.M.; Ebrahimi, P. Wharton’s jelly derived-mesenchymal stem cells: Isolation and characterization. Acta Med. Iran 2018, 56, 28–33. [Google Scholar] [PubMed]
- Alizadeh, R.; Bagher, Z.; Kamrava, S.K.; Falah, M.; Ghasemi Hamidabadi, H.; Eskandarian Boroujeni, M.; Mohammadi, F.; Khodaverdi, S.; Zare-Sadeghi, A.; Olya, A.; et al. Differentiation of human mesenchymal stem cells (MSC) to dopaminergic neurons: A comparison between Wharton’s Jelly and olfactory mucosa as sources of MSCs. J. Chem. Neuroanat. 2019, 96, 126–133. [Google Scholar] [CrossRef]
- Widowati, W.; Gunanegara, R.; Rizal, R.; Widodo, W.; Amalia, A.; Wibowo, S.; Handono, K.; Marlina, M.; Lister, I.; Chiuman, L. Comparative Analysis of Wharton’s Jelly Mesenchymal Stem Cell (WJ-MSCs) Isolated Using Explant and Enzymatic Methods; Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1374, p. 012024. [Google Scholar]
- Rizal; Syaidah, R.; Aqsha, Z.M.; Josephin, A.; Pakpahan, V.M. Characterization, Differentiation, and Population Doubling Time of Wharton’s Jelly Mesenchymal Stem Cells (WJ-MSCs) in Passage 5 and 8; AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2021; Volume 2344, p. 040002. [Google Scholar]
- Sabzevari, R.; Roushandeh, A.M.; Mehdipour, A.; Alini, M.; Roudkenar, M.H. SA/G hydrogel containing hCAP-18/LL-37-engineered WJ-MSCs-derived conditioned medium promoted wound healing in rat model of excision injury. Life Sci. 2020, 261, 118381. [Google Scholar] [CrossRef]
- Serafin, A.; Murphy, C.; Rubio, M.C.; Collins, M.N. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Mater. Sci. Eng. C 2021, 122, 111927. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitus, V.; Ibrahim, F.; Shamsuddin, S.A.A.; Razali, N.; Noor Azlan, N.A.B.; Zaman, W.S.W.K. Carbonised Human Hair Incorporated in Agar/KGM Bioscaffold for Tissue Engineering Application: Fabrication and Characterisation. Polymers 2022, 14, 5489. https://doi.org/10.3390/polym14245489
Vitus V, Ibrahim F, Shamsuddin SAA, Razali N, Noor Azlan NAB, Zaman WSWK. Carbonised Human Hair Incorporated in Agar/KGM Bioscaffold for Tissue Engineering Application: Fabrication and Characterisation. Polymers. 2022; 14(24):5489. https://doi.org/10.3390/polym14245489
Chicago/Turabian StyleVitus, Vieralynda, Fatimah Ibrahim, Shamsul Azlin Ahmad Shamsuddin, Nuguelis Razali, Noor Anastasha Balqis Noor Azlan, and Wan Safwani Wan Kamarul Zaman. 2022. "Carbonised Human Hair Incorporated in Agar/KGM Bioscaffold for Tissue Engineering Application: Fabrication and Characterisation" Polymers 14, no. 24: 5489. https://doi.org/10.3390/polym14245489
APA StyleVitus, V., Ibrahim, F., Shamsuddin, S. A. A., Razali, N., Noor Azlan, N. A. B., & Zaman, W. S. W. K. (2022). Carbonised Human Hair Incorporated in Agar/KGM Bioscaffold for Tissue Engineering Application: Fabrication and Characterisation. Polymers, 14(24), 5489. https://doi.org/10.3390/polym14245489