Red Disperse Azo Dye Side Chains Influence on Polyethylene Terephthalate Dyeing Performances in Supercritical Carbon Dioxide Media
Abstract
1. Introduction
2. Experimental Process
2.1. Materials
2.2. Preparation of Dye
2.2.1. Diazonium Solution Preparation
2.2.2. Coupling Component Synthesis
2.2.3. Dye Synthesis
2.3. Dye Characterization
2.4. Dyeing Apparatus
2.5. Color Fastness Performance Characterization
2.6. Dyeing Fastness Test
2.7. Water Fastness Test
2.8. Perspiration Fastness Test
2.9. Abrasion and Rubbing Color Fastness Test
3. Results and Discussion
3.1. Dyes Synthesis and Characterization
3.2. Dye Optical Characterization
3.3. Dyeing Performances
3.3.1. Apparent Colorfastness K/S
3.3.2. Color Fastness Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, A.; Chen, B.; Dai, J.; Zhang, K. Using supercritical carbon dioxide as solvent to replace water in polyethylene terephthalate (PET) fabric dyeing procedures. J. Clean. Prod. 2010, 18, 1009–1014. [Google Scholar] [CrossRef]
- Ozturk, E.; Koseoglu, H.; Karaboyacı, M.; Yigit, N.O.; Yetis, U.; Kitis, M. Minimization of water and chemical use in a cotton/polyester fabric dyeing textile mill. J. Clean. Prod. 2016, 130, 92–102. [Google Scholar] [CrossRef]
- Qiu, J.; Xiao, J.; Tang, B.; Ju, B.; Zhang, S. Facile synthesis of novel disperse azo dyes with aromatic hydroxyl group. Dyes Pigm. 2019, 160, 524–529. [Google Scholar] [CrossRef]
- Shukla, C.A.; Kute, M.S.; Kulkarni, A.A. Towards sustainable continuous production of azo dyes: Possibilities and techno-economic analysis. Green Chem. 2021, 23, 6614–6624. [Google Scholar] [CrossRef]
- Liang, F.C.; Jhuang, F.C.; Fang, Y.H.; Benas, J.S.; Chen, W.C.; Yan, Z.L.; Lin, W.C.; Su, C.J.; Sato, Y.; Chiba, T.; et al. Synergistic effect of cation composition engineering of hybrid Cs1−xFAxPbBr3 nanocrystals for self-healing electronics application. Adv. Mater. 2022, 2207617. [Google Scholar] [CrossRef]
- Maeda, S.; Kunitou, K.; Hihara, T.; Mishima, K. One-bath dyeing of polyester/cotton blends with reactive disperse dyes in supercritical carbon dioxide. Text. Res. J. 2016, 74, 989–994. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, J.; Yan, J.; Zheng, L. An industrial scale multiple supercritical carbon dioxide apparatus and its eco-friendly dyeing production. J. CO2 Util. 2016, 16, 272–281. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Abd El-Aziz, E. Supercritical carbon dioxide as a green media in textile dyeing: A review. Text. Res. J. 2017, 88, 1184–1212. [Google Scholar] [CrossRef]
- Huang, T.; Cui, H.; Yang, D.; Kong, X.; Lin, J. Continuous dyeing processes for zipper tape in supercritical carbon dioxide. J. Clean. Prod. 2017, 158, 95–100. [Google Scholar] [CrossRef]
- Liang, F.C.; Chang, Y.W.; Kuo, C.C.; Cho, C.J.; Jiang, D.H.; Jhuang, F.C.; Rwei, S.P.; Borsali, R. A mechanically robust silver nanowire–polydimethylsiloxane electrode based on facile transfer printing techniques for wearable displays. Nanoscale 2019, 11, 1520–1530. [Google Scholar] [CrossRef]
- Park, G.; Kwon, D.E.; Kong, W.; Park, J.; Lee, Y.W. A dissolution kinetic study of disperse dye in supercritical carbon dioxide to design an efficient supercritical dyeing process. Processes 2021, 9, 977. [Google Scholar] [CrossRef]
- Räisänen, R.; Montero, G.A.; Freeman, H.S. A fungal-based anthraquinone emodin for polylactide and polyethylene terephthalate in supercritical carbon dioxide (SC-CO2) dyeing. Color Res. Appl. 2021, 46, 674–680. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Benas, J.S.; Liang, F.C.; Lin, S.M.; Sun, T.W.; Liu, F.C.; Yu, Y.Y.; Kuo, C.C. Synthesis of azo disperse dyes with high absorption for efficient polyethylene terephthalate dyeing performances in supercritical carbon dioxide. Polymers 2022, 14, 3020. [Google Scholar] [CrossRef] [PubMed]
- Regal, M.K.A.; Rafat, E.H.; El-Sattar, N.E.A.A. Synthesis, characterization, and dyeing performance of some azo thienopyridine and thienopyrimidine dyes based on wool and nylon. J. Heterocycl. Chem. 2019, 57, 1173–1182. [Google Scholar] [CrossRef]
- Gong, D.; Jing, X.; Zhao, Y.; Zheng, H.; Zheng, L. One-step supercritical CO2 color matching of polyester with dye mixtures. J. CO2 Util. 2021, 44, 101396. [Google Scholar] [CrossRef]
- Park, S.C.; Tuma, D.; Kim, S.; Lee, Y.R.; Shun, J.J. Sorption of C. I. disperse red 60 in polystyrene and PMMA films and polyester and nylon 66 textiles in the presence of supercritical carbon dioxide. Korean J. Chem. Eng. 2010, 27, 299–309. [Google Scholar] [CrossRef]
- Qian, H.F.; Song, X.Y. The structure of azo disperse dyes and its distribution on polyurethane fiber blend with polyester, or polyamide fiber. Dyes Pigm. 2007, 74, 672–676. [Google Scholar] [CrossRef]
- Qian, H.-F.; Feng, G.; Bai, G.; Liu, Y.-C.; Hu, L.-L. A contrastive study of adsorption behaviors on polyurethane fiber with diester/diurethane tethered and non-tethered azo disperse dyes. Dyes Pigm. 2017, 145, 301–306. [Google Scholar] [CrossRef]
- Bai, T.; Kobayashi, K.; Tamura, K.; Jun, Y.; Zheng, L. Super critical CO2 dyeing for nylon, acrylic, polyester, and casein buttons and their optimum dyeing conditions by design of experiments. J. CO2 Util. 2019, 33, 253–261. [Google Scholar] [CrossRef]
- Penthala, R.; Heo, G.; Kim, H.; Lee, I.Y.; Ko, E.H.; Son, Y.-A. Synthesis of azo and anthraquinone dyes and dyeing of nylon-6,6 in supercritical carbon dioxide. J. CO2 Util. 2020, 38, 49–58. [Google Scholar] [CrossRef]
- Sicardi, S.; Manna, L.; Banchero, M. Diffusion of disperse dyes in PET films during impregnation with a supercritical fluid. J. Supercrit. Fluids 2000, 17, 187–194. [Google Scholar] [CrossRef]
- Liang, F.C.; Kuo, C.C.; Chen, B.Y.; Cho, C.J.; Hung, C.C.; Chen, W.C.; Borsali, R. RGB-switchable porous electrospun nanofiber chemosensor-filter prepared from multifunctional copolymers and their versatile sensing for pH and heavy metal. ACS Appl. Mater. Interfaces 2017, 9, 16381–16396. [Google Scholar] [CrossRef] [PubMed]
- Ketema, A.; Worku, A. Review on intermolecular forces between dyes used for polyester dyeing and polyester fiber. J. Chem. 2020, 2020, 7. [Google Scholar] [CrossRef]
- De Giorgi, M.R.; Cadoni, E.; Maricca, D.; Piras, A. Dyeing polyester fibres with disperse dyes in supercritical CO2. Dye. Pigm. 2000, 45, 75–79. [Google Scholar] [CrossRef]
- He, C.L.; Liang, F.C.; Veeramuthu, L.; Cho, C.J.; Benas, J.S.; Tzeng, Y.R.; Tseng, Y.L.; Chen, W.C.; Rwei, A.; Kuo, C.C. Super tough and spontaneous water-assisted autonomous self-healing elastomer for underwater wearable electronics. Adv. Sci. 2021, 8, e2102275. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, D. Determination of diffusion and mass transfer coefficients during drying of solvent-absorbed polymer films. Polym. J. 2000, 32, 415–421. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’Rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef]
- Qiu, J.; Tang, B.; Ju, B.; Zhang, S.; Jin, X. Clean synthesis of disperse azo dyes based on peculiar stable 2,6-dibromo-4-nitrophenyl diazonium sulfate. Dyes Pigm. 2020, 173, 107920. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, G.; Wang, J.; Sun, S.; Zhang, Z. The mechanisms of large stokes shift and fluorescence quantum yields in anilino substituted rhodamine analogue: TICT and PICT. Comput. Theor. Chem. 2016, 1095, 44–53. [Google Scholar] [CrossRef]
- Jie, S.; Chunlin, Z.; Hökelek, T. X-ray crystal structure analysis for CI disperse orange 61. Color. Technol. 2012, 128, 91–94. [Google Scholar] [CrossRef]
- Abate, M.T.; Ferri, A.; Guan, J.; Chen, G.; Nierstrasz, V. Colouration and bio-activation of polyester fabric with curcumin in supercritical CO2: Part I—Investigating colouration properties. J. Supercrit. Fluids 2019, 152, 104548. [Google Scholar] [CrossRef]
- Shaki, H. Color, fastness, antibacterial, and skin sensitivity properties of high lightfastness azo disperse dyes incorporating sulfamide groups. Fibers Polym. 2020, 21, 2530–2538. [Google Scholar] [CrossRef]
- Feng, G.; Qian, H.-F.; Bai, G.; Liu, Y.-C.; Hu, L.-L. Synthesis, characterization, and application of diester/diurethane tethered azo disperse dyes: A new strategy to improve dye’s fastness properties. Dye. Pigment. 2016, 129, 54–59. [Google Scholar] [CrossRef]
- De Rossi, U.; Moll, J.; Kriwanek, J.; Daehne, S. Influence of the N-alkyl chain length on the J-aggregation behavior of a cyanine dye. J. Fluoresc. 1994, 4, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Jiang, H.; Chai, L.; Cui, Z.; Chen, W. Design and synthesis of diazirine-containing dyes for polypropylene fibre: A study on the effect of alkyl chain. Color. Technol. 2022, 138, 551–564. [Google Scholar] [CrossRef]
- Pan, J.; Gao, L.; Sun, W.; Wang, S.; Shi, X. Length effects of short alkyl side chains on phase-separated structure and dynamics of hydrophobic association hydrogels. Macromolecules 2021, 54, 5962–5973. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. An article on optics of paint layers. Fuer. Tekn. Physik. 1931, 12, 593–609. [Google Scholar]
- Qiu, J.; Tang, B.; Ju, B.; Xu, Y.; Zhang, S. Stable diazonium salts of weakly basic amines—Convenient reagents for synthesis of disperse azo dyes. Dyes. Pigm. 2017, 136, 63–69. [Google Scholar] [CrossRef]
- Grishanov, S. Structure and properties of textile materials. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2011; Volume 1, pp. 28–36. [Google Scholar]
- Richards, P.R. Fabric Finishing: Dyeing and Colouring. In Textiles and Fashion: Materials. Design and Technology; Sinclair, R., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2015; pp. 475–505. [Google Scholar]
- Elsherbiny, A.S.; Salem, M.A.; Ismail, A.A. Influence of the alkyl chain length of cyanine dyes on their adsorption by Na+-montmorillonite from aqueous solutions. Chem. Eng. J. 2012, 200–202, 283–290. [Google Scholar] [CrossRef]
- Lu, L.; He, L.; Zhang, S.; Freeman, H.S. Novel yellow azo-anthraquinone dyes for polylactide fibres: Effects of alkyl chains length. Color. Technol. 2012, 128, 121–126. [Google Scholar] [CrossRef]

| Dye | R1 | R2 | R3 | General Structure | Mass | Molecular Formula | Yield |
|---|---|---|---|---|---|---|---|
| 161-A | CH3 | NO2 | OCH3 | 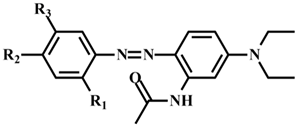 | 399.19 | C20H25N5O4 | 78.7% |
| 161-B | - | NO2 | - | 355.16 | C18H21N5O3 | 81.6% | |
| Dye | R | General Structure | Mass | Molecular Formula | Yield | ||
| X-377-2-D | C2H5 |  | 415.19 | C20H25N5O5 | 82.6% | ||
| X-377-4-D | C4H9 | 471.25 | C24H33N5O5 | 77.2% | |||
| X-377-6-D | C6H13 | 527.31 | C28H41N5O5 | 76.3% | |||
| X-377-8-D | C8H17 | 583.37 | C32H9N5O5 | 62.5% | |||
| Dye | Maximum Absorption Wavelength (nm) | Absorbance | Absorption Coefficient (L·mol−1·cm−1) | Log (e) |
|---|---|---|---|---|
| 161-A | 503 | 0.870034 | 87,003.4 | 4.939 |
| 161-B | 511 | 0.828747 | 82,874.7 | 4.918 |
| X-377-2-D | 501 | 0.929598 | 92,959.8 | 4.968 |
| X-377-4-D | 503 | 0.649098 | 64,909.8 | 4.812 |
| X-377-6-D | 502 | 0.321163 | 32,116.3 | 4.506 |
| X-377-8-D | 502 | 0.143213 | 14,321.3 | 4.155 |
| Dye | Apparent Color Density | Relative Color First Time |
|---|---|---|
| 161-A | 21.0380 | 87.07% |
| 161-B | 13.51 | 72.33% |
| X-377-2-D | 17.8810 | 83.23% |
| X-377-4-D | 15.6820 | 75.32% |
| X-377-6-D | 9.3760 | 67.03% |
| X-377-8-D | 3.2750 | 65.21% |
| Dye | Cellulose Acetate | Cotton | Nylon | Polyester | Acrylic | Wool |
|---|---|---|---|---|---|---|
| 161-A | 4 | 4–5 | 3–4 | 4–5 | 5 | 5 |
| 161-B | 4–5 | 4–5 | 3–4 | 4–5 | 5 | 5 |
| X-377-2-D | 4 | 4–5 | 3–4 | 4–5 | 5 | 5 |
| X-377-4-D | 4–5 | 4–5 | 4 | 5 | 5 | 4–5 |
| X-377-6-D | 4–5 | 4–5 | 4–5 | 4–5 | 5 | 5 |
| X-377-8-D | 5 | 5 | 5 | 5 | 5 | 4–5 |
| Dye | Cellulose Acetate | Cotton | Nylon | Polyester | Acrylic | Wool |
|---|---|---|---|---|---|---|
| 019-A | 4–5 | 5 | 4–5 | 5 | 5 | 5 |
| 019-B | 4–5 | 4–5 | 4 | 4–5 | 5 | 5 |
| X-377-2-D | 4–5 | 4–5 | 4 | 4–5 | 5 | 5 |
| X-377-4-D | 4–5 | 5 | 4–5 | 5 | 5 | 5 |
| X-377-6-D | 4–5 | 4–5 | 4–5 | 4–5 | 5 | 5 |
| X-377-8-D | 5 | 5 | 5 | 5 | 5 | 4–5 |
| Dye | Dry Friction | Wet Friction |
|---|---|---|
| 019-A | 4 | 4–5 |
| 019-B | 4 | 4–5 |
| X-377-2-D | 4 | 4–5 |
| X-377-4-D | 4 | 4–5 |
| X-377-6-D | 4–5 | 4–5 |
| X-377-8-D | 3–4 | 4–5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-W.; Benas, J.-S.; Liang, F.-C.; Lin, S.-M.; Huang, Y.-H.; Chen, W.-W.; Chen, Y.-T.; Lee, C.-H.; Yu, Y.-Y.; Kuo, C.-C. Red Disperse Azo Dye Side Chains Influence on Polyethylene Terephthalate Dyeing Performances in Supercritical Carbon Dioxide Media. Polymers 2022, 14, 5487. https://doi.org/10.3390/polym14245487
Cheng Y-W, Benas J-S, Liang F-C, Lin S-M, Huang Y-H, Chen W-W, Chen Y-T, Lee C-H, Yu Y-Y, Kuo C-C. Red Disperse Azo Dye Side Chains Influence on Polyethylene Terephthalate Dyeing Performances in Supercritical Carbon Dioxide Media. Polymers. 2022; 14(24):5487. https://doi.org/10.3390/polym14245487
Chicago/Turabian StyleCheng, Yu-Wen, Jean-Sebastien Benas, Fang-Cheng Liang, Shang-Ming Lin, Yu-Hang Huang, Wei-Wen Chen, Yu-Ting Chen, Chen-Hung Lee, Yang-Yen Yu, and Chi-Ching Kuo. 2022. "Red Disperse Azo Dye Side Chains Influence on Polyethylene Terephthalate Dyeing Performances in Supercritical Carbon Dioxide Media" Polymers 14, no. 24: 5487. https://doi.org/10.3390/polym14245487
APA StyleCheng, Y.-W., Benas, J.-S., Liang, F.-C., Lin, S.-M., Huang, Y.-H., Chen, W.-W., Chen, Y.-T., Lee, C.-H., Yu, Y.-Y., & Kuo, C.-C. (2022). Red Disperse Azo Dye Side Chains Influence on Polyethylene Terephthalate Dyeing Performances in Supercritical Carbon Dioxide Media. Polymers, 14(24), 5487. https://doi.org/10.3390/polym14245487







