Molecular Dynamics Simulations of Polydopamine Nanosphere’s Structure Based on Experimental Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Synthesis of PDA Spheres
2.4. Synthesis of Au-PDA Spheres
2.5. Simulation Procedures
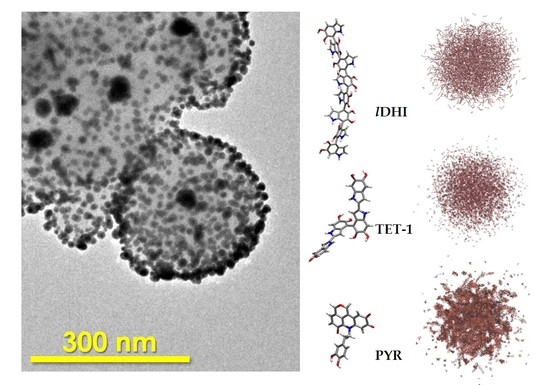
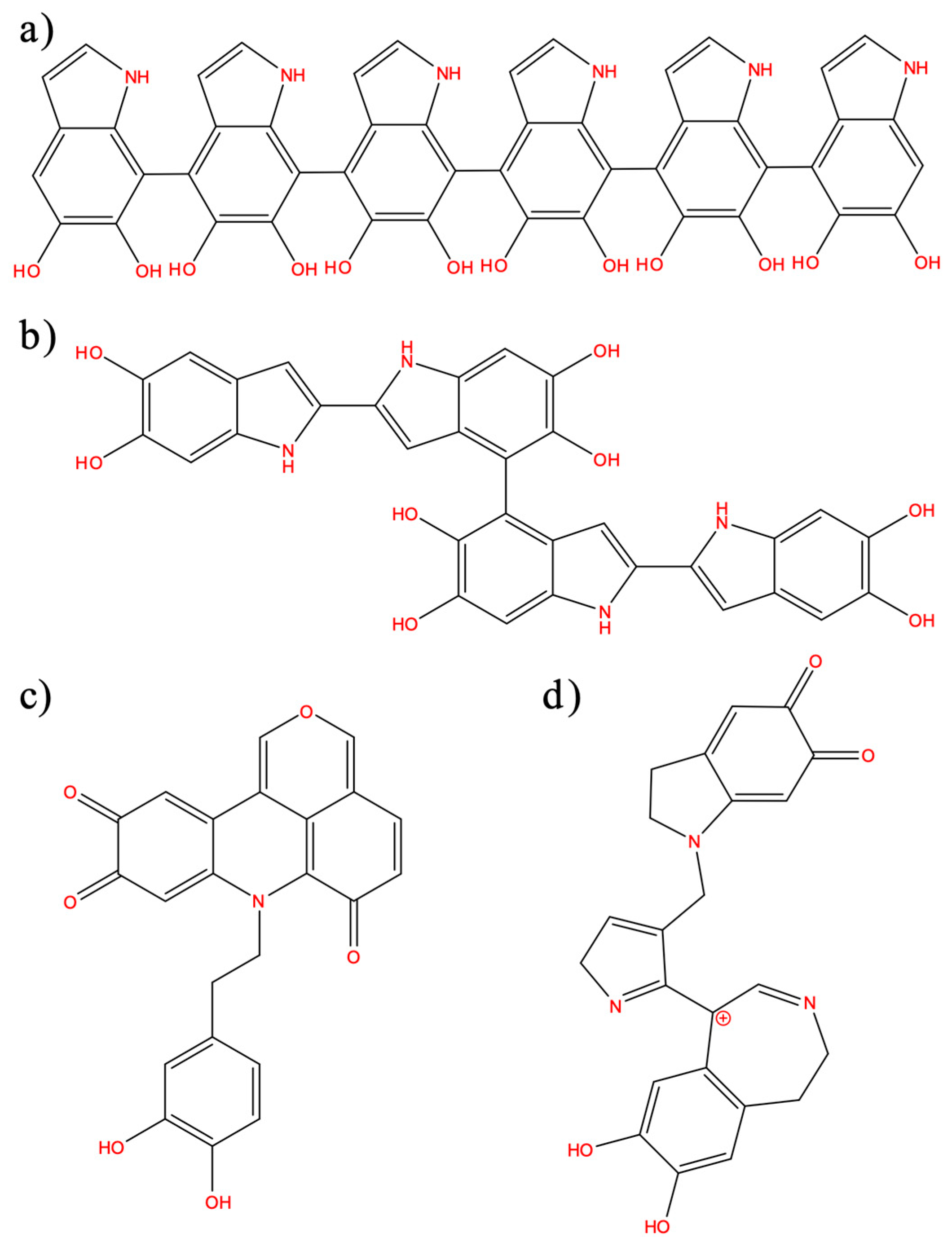
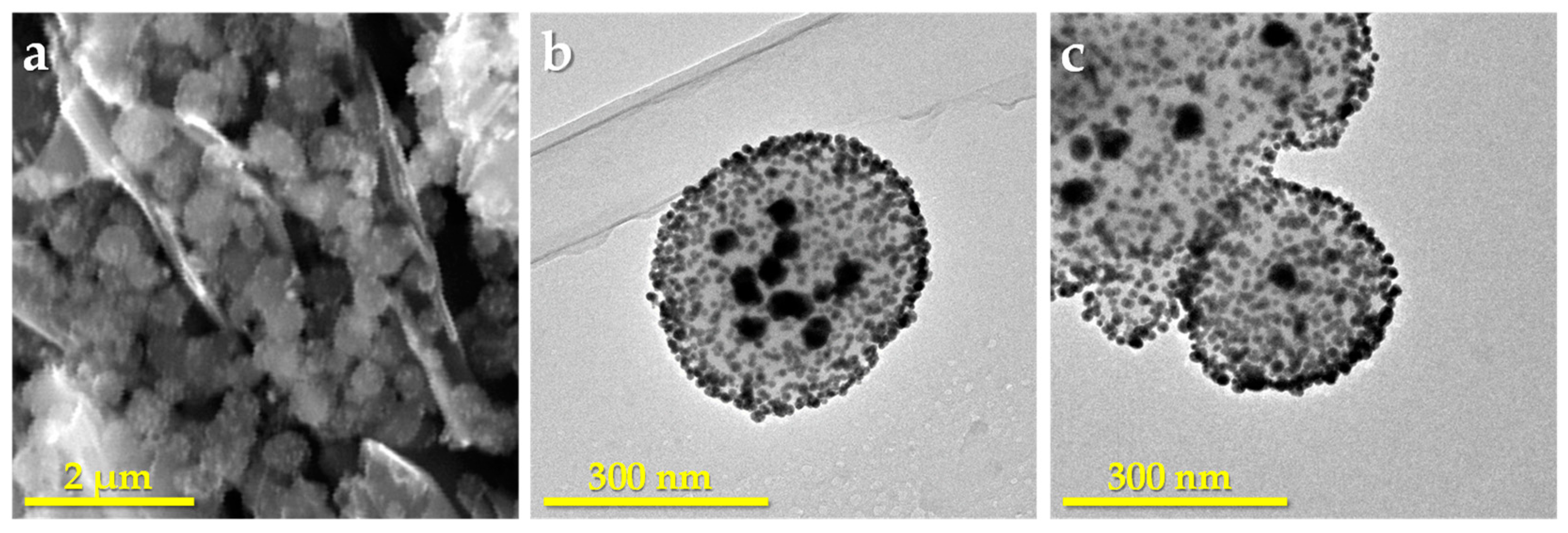
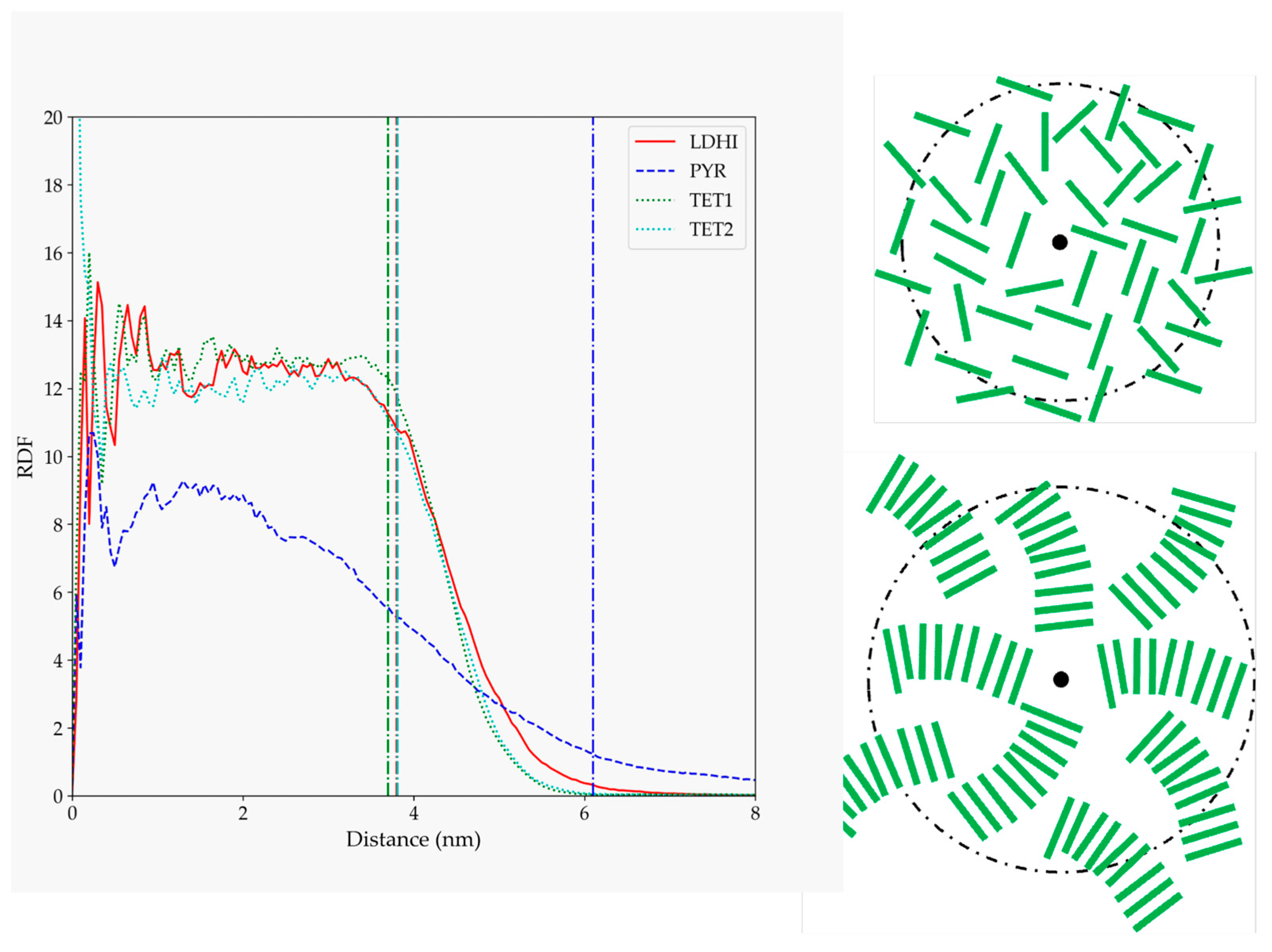
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Zhu, J.; Zhang, B.; Jiao, Y.; Huang, J.; Wang, F. Manganese dioxide nanosheets decorated on MXene (Ti3C2Tx) with enhanced performance for asymmetric supercapacitors. Ceram. Int. 2021, 47, 12211–12220. [Google Scholar] [CrossRef]
- Mei, H.; Gao, Z.; Wang, Q.; Sun, H.; Zhao, K.; Zhang, P.; Hao, J.; Ashokkumar, M.; Cui, J. Ultrasound expands the versatility of polydopamine coatings. Ultrason. Sonochem. 2021, 74, 105571. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.L.; Weil, T.; Wah, D.Y.; Ball, V. Polydopamine at biological interfaces. Adv. Colloid Interface Sci. 2022, 305, 102689. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, H.; Hanks, T.W. Polydopamine: A bioinspired adhesive and surface modification platform. Polym. Int. 2022, 71, 578–582. [Google Scholar] [CrossRef]
- Eom, T.; Lee, J.; Lee, S.; Ozlu, B.; Kim, S.; Martin, D.C.; Shim, B.S. Highly Conductive Polydopamine Coatings by Direct Electrochemical Synthesis on Au. ACS Appl. Polym. Mater. 2022, 4, 5319–5329. [Google Scholar] [CrossRef]
- Qi, X.; Pan, W.; Tong, X.; Gao, T.; Xiang, Y.; You, S.; Mao, R.; Chi, J.; Hu, R.; Zhang, W.; et al. ε-Polylysine-stabilized agarose/polydopamine hydrogel dressings with robust photothermal property for wound healing. Carbohydr. Polym. 2021, 264, 118046. [Google Scholar] [CrossRef]
- Du, Y.; Huang, H.; Hu, X.; Liu, S.; Sheng, X.; Li, X.; Lu, X.; Qu, J. Melamine foam/polyethylene glycol composite phase change material synergistically modified by polydopamine/MXene with enhanced solar-to-thermal conversion. Renew. Energy 2021, 171, 1–10. [Google Scholar] [CrossRef]
- Zhu, X.; Yan, B.; Yan, X.; Wei, T.; Yao, H.; Mia, M.S.; Xing, T.; Chen, G. Fabrication of non-iridescent structural color on silk surface by rapid polymerization of dopamine. Prog. Org. Coat. 2020, 149, 105904. [Google Scholar] [CrossRef]
- Khan, Z.; Shanker, R.; Um, D.; Jaiswal, A.; Ko, H. Bioinspired Polydopamine and Composites for Biomedical Applications. In Electrically Conductive Polymers and Polymer Composites: From Synthesis to Biomedical Applications; Khan, A., Jawaid, M., Parwaz Khan, A.A., Asiri, A.M., Eds.; Wiley-VCH: Weinheim, Germany, 2018; pp. 1–24. [Google Scholar]
- Wu, M.; Wanga, T.; Müllerb, L.; Müller, F.A. Adjustable synthesis of polydopamine nanospheres and their nucleation and growth. Colloids Surf. A 2020, 603, 125196. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.Y.; Wang, L.L.; Wang, Y.Y. A Sensitive Electrochemical Sensor for Paracetamol Based on a glassy carbon electrode modified with Multiwalled Carbon Nanotubes and Dopamine Nanospheres Functionalized with Gold Nanoparticles. Microchim. Acta 2014, 181, 1439–1446. [Google Scholar] [CrossRef]
- Han, X.; Chen, X.; Yan, M.; Liu, H. Synergetic effect of polydopamine particles and in-situ fabricated gold nanoparticles on charge-dependent catalytic behaviours. Particuology 2019, 44, 63–70. [Google Scholar] [CrossRef]
- Xu, M.; Yan, L.; Zhu, Y.; Li, Y.; Song, X.; Yin, L. Polydopamine-coated gold nanoparticles used as modifier of the electron transport layer for TB7:PC71BM polymer solar cells. J. Mater. Sci. Mater. Electron. 2020, 31, 6698–6705. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, S.; Xing, J.; Pi, Z.; Song, F.; Liu, Z. Trace determination and characterization of ginsenosides in rat plasma through magnetic dispersive solid-phase extraction based on core-shell polydopamine-coated magnetic nanoparticles. J. Pharm. Anal. 2020, 10, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.; Lee, Y.; Lee, S.; Park, S.B.; Lee, J. Bioinspired Polymerization of Dopamine to Generate Melanin-Like Nanoparticles Having an Excellent Free-Radical-Scavenging Property. Biomacromolecules 2011, 12, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, Y.; Odusote, G.; Qu, F.; Priestley, R.D. Core–Shell Fe3O4 Polydopamine Nanoparticles Serve Multipurpose as Drug Carrier, Catalyst Support and Carbon Adsorbent. ACS Appl. Mater. Interfaces 2013, 5, 9167–9171. [Google Scholar] [CrossRef]
- Oroujeni, M.; Kaboudin, B.; Xia, W.; Jöhsson, P.; Ossipov, D.A. Conjugation of Cyclodextrin to Magnetic Fe3O4 Nanoparticles via Polydopamine Coating for Drug Delivery. Prog. Org. Coat. 2018, 114, 154–161. [Google Scholar] [CrossRef]
- Li, H.; Yin, D.; Li, W.; Tang, Q.; Zou, L.; Peng, Q. Polydopamine-based nanomaterials and their potentials in advanced drug delivery and therapy. Colloids Surf. B Biointerfaces 2021, 199, 111502. [Google Scholar] [CrossRef]
- Dong, Z.; Gong, H.; Gao, M.; Zhu, W.; Sun, X.; Feng, L.; Fu, T.; Li, Y.; Liu, Z. Polydopamine Nanoparticles as a Versatile Molecular Loading Platform to Enable Imaging-guided Cancer Combination Therapy. Theranostics 2016, 6, 1031–1042. [Google Scholar] [CrossRef]
- Antidormi, A.; Melis, C.; Canadell, E.; Colombo, L. Assessing the Performance of Eumelanin/Si Interface for Photovoltaic Applications. J. Phys. Chem. C 2017, 121, 11576–11584. [Google Scholar] [CrossRef]
- Pinna, E.; Melis, C.; Antidormi, A.; Cardia, R.; Sechi, E.; Cappellini, G.; d’Ischia, M.; Colombo, L.; Mula, G. Deciphering Molecular Mechanisms of Interface Buildup and Stability in Porous Si/Eumelanin Hybrids. Int. J. Mol. Sci. 2017, 18, 1567. [Google Scholar] [CrossRef]
- Zeng, Q.; Qian, Y.; Huang, Y.; Ding, F.; Qi, X.; Shen, J. Polydopamine nanoparticle-dotted food gum hydrogel with excellent antibacterial activity and rapid shape adaptability for accelerated bacteria-infected wound healing. Bioact. Mater. 2021, 6, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Afrash, H.; Nazeri, N.; Davoudi, P.; Majidi, R.F.; Ghanbari, H. Development of a Bioactive Scaffold based on NGF Containing PCL/Chitosan Nanofibers for Nerve Regeneration. Biointerface Res. Appl. Chem. 2021, 11, 12606–12617. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Zare, M.; Khodadadi, A.; Seidi, F.; Sajadi, S.M.; Zarrintaj, P.; Arefi, A.; Saeb, M.R.; Mozafari, M. Polydopamine Biomaterials for Skin Regeneration. ACS Biomater. Sci. Eng. 2022, 8, 2196–2219. [Google Scholar] [CrossRef] [PubMed]
- Chinchulkar, S.A.; Patra, P.; Dehariya, D.; Yu, A.; Rengan, A.K. Polydopamine nanocomposites and their biomedical applications: A review. Polym. Adv. Technol. 2022, 33, 3935–3956. [Google Scholar] [CrossRef]
- Guo, J.; Liu, D.; Yang, Z.; Wenge, W.; Chan, E.W.C.; Zeng, Z.; Wong, K.Y.; Lin, P.; Chen, S. A photoelectrochemical biosensor for rapid and ultrasensitive norovirus detection. Bioelectrochemistry 2020, 136, 107591. [Google Scholar] [CrossRef]
- Szewczyk, J.; Aguilar-Ferrer, D.; Coy, E. Polydopamine films: Electrochemical growth and sensing applications. Eur. Polym. J. 2022, 174, 111346. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, T.; Liu, J. Recent Development of Polydopamine Anti-Bacterial Nanomaterials. Int. J. Mol. Sci. 2022, 23, 7278. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; d’Ischia, M.; Napolitano, A.; Pezella, A. Structure of Melanins. In Melanins and Melanosomes: Biosynthesis, Biogenesis, Physiological, and Pathological Functions; Borovanský, J., Riley, P.A., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 167–185. [Google Scholar]
- d’Ischia, M.; Napolitano, A.; Pezella, A.; Meredith, P.; Sarna, T. Chemical and structural diversity in eumelanins: Unexplored bio-optoelectronic materials. Angew. Chem. Int. Ed. 2009, 48, 3914–3921. [Google Scholar] [CrossRef]
- Panzella, L.; Gentile, G.; D’Errico, G.; Della Vecchia, N.F.; Errico, M.E.; Napolitano, A.; Carfagna, C.; d’Ischia, M. Atypical structural and π-electron features of a melanin polymer that lead to superior free-radical-scavenging properties. Angew. Chem. Int. Ed. 2013, 52, 12684–12687. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 33, 10539–10548. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.T.; Buehler, M.J. Polydopamine and eumelanin: From structure-property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, C.T.; Bdikin, I.; Ball, V.; Grácio, J.; Buehler, M.J. Tuning heterogenous poly(dopamine) structure and mechanics: In silico covalent cross-linking and thin film nanoindentation. Soft Matter 2014, 10, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, J. Chemistry of Polydopamine—Scope, Variation and Limitation. Eur. J. Org. Chem. 2019, 2019, 4976–4994. [Google Scholar] [CrossRef]
- Lyu, Q.; Hsueh, N.; Chai, C.L.L. Unravelling the polydopamine mystery: Is the end in sight? Polym. Chem. 2019, 10, 5771. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.H. Hydroxide ion-mediated synthesis of monodisperse dopamine-melanin nanospheres. J. Colloid Interface Sci. 2015, 458, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Zhang, X.L.; Niu, H.Y.; Ma, Y.R.; Li, W.H.; Cai, Y.Q. In situ growth of gold nanoparticles onto polydopamine-encapsulated magnetic microspheres for catalytic reduction of nitrobenzene. Appl. Catal. B Environ. 2013, 134, 26–33. [Google Scholar] [CrossRef]
- Lee, Y.S.; Bae, J.Y.; Koo, H.Y.; Lee, Y.B.; Cho, W.S. A remote-controlled generation of gold@polydopamine (core@shell) nanoparticles via physical-chemical stimuli of polydopamine/gold composites. Sci. Rep. 2016, 6, 22650. [Google Scholar] [CrossRef]
- Lin, K.; Gan, Y.; Zhu, P.; Li, S.; Lin, C.; Yu, S.; Zhao, S.; Shi, J.; Li, R.; Yuan, J. Hollow mesoporous polydopamine nanospheres: Synthesis, biocompatibility and drug delivery. Nanotechnology 2021, 32, 285602. [Google Scholar] [CrossRef]
- Li, S.; Gan, Y.; Lin, C.; Lin, K.; Hu, P.; Liu, L.; Yu, S.; Zhao, S.; Shi, J. NIR-/pH-Responsive Nanocarriers Based on Mesoporous Hollow Polydopamine for Codelivery of Hydrophilic/Hydrophobic Drugs and Photothermal Synergetic Therapy. ACS Appl. Bio Mater. 2021, 4, 1605–1615. [Google Scholar] [CrossRef]
- Arroquia, A.; Acosta, I.; García Armada, M.P. Self-assembled gold decorated polydopamine nanospheres as electrochemical sensor for simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan. Mater. Sci. Eng. C 2020, 109, 110602. [Google Scholar] [CrossRef]
- Cîrcu, M.; Filip, C. Closer to the polydopamine structure: New insights from a combined 13C/1H/2H solid-state NMR study on deuterated samples. Polym. Chem. 2018, 9, 3379–3387. [Google Scholar] [CrossRef]
- Chen, C.-T.; Martin-Martinez, F.J.; Seob Jung, G.; Buehler, M.J. Polydopamine and eumelanin molecular structures investigated with ab initio calculations. Chem. Sci. 2017, 8, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the structure of poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Moss, S.C.; Eisner, M.; Zschack, P. X-Ray characterization of melanins I. Pigm. Cell. Res. 1994, 7, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-T.; Chuang, C.; Cao, J.; Ball, V.; Ruch, D.; Buehler, M.J. Excitonic effects from geometric order and disorder explain broadband optical absorption in eumelanin. Nat. Commun. 2014, 5, 3859. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.L.; Micillo, R.; Panzella, L.; Crescenzi, O.; Oscurato, S.L.; Maddalena, P.; Napolitano, A.; Ball, V.; d’Ischia, M. Structural Basis of Polydopamine Film Formation: Probing 5,6-Dihydroxyindole-Based Eumelanin Type Units and the Porphyrin Issue. ACS Appl. Mater. Interfaces 2018, 10, 7670–7680. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulte, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. Potential energy functions for atomic-level simulations of water and organic and biomolecular systems. Proc. Natl. Acad. Sci. USA 2005, 102, 6665–6670. [Google Scholar] [CrossRef]
- Dodda, L.S.; Vilseck, J.Z.; Tirado-Rives, J.; Jorgensen, W.L. 1.14*CM1A-LBCC: Localized Bond-Charge Corrected CM1A Charges for Condensed-Phase Simulations. J. Phys. Chem. B 2017, 121, 3864–3870. [Google Scholar] [CrossRef]
- Dodda, L.S.; Cabeza de Vaca, I.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen web server: An automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. Packmol: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Qiu, D.; Shenkin, P.S.; Hollinger, F.P.; Still, W.C. The GB/SA continuum model for solvation. A fast analytical method for the calculation of approximate Born radii. J. Phys. Chem. 1997, 101, 3005–3014. [Google Scholar] [CrossRef]
- Baruwati, B.; Varma, R.S. High Value Products from Waste: Grape Pomace Extract—A Three-in-One Package for the Synthesis of Metal Nanoparticles. ChemSusChem 2009, 2, 1041–1044. [Google Scholar] [CrossRef]
- Zhao, Y.; Yeh, Y.; Liu, R.; You, J.; Qu, F. Facile deposition of gold nanoparticles on core-shell Fe3O4@polydopamine as recyclable nanocatalyst. Solid State Sci. 2015, 45, 9–14. [Google Scholar] [CrossRef]
- Panigrahi, S.; Kundu, S.; Ghosh, S.; Nath, S.; Pal, T. General method of synthesis for metal nanoparticles. J. Nanopart. Res. 2004, 6, 411–414. [Google Scholar] [CrossRef]
- Lu, T. Simple, reliable, and universal metrics of molecular planarity. J. Mol. Model. 2021, 27, 263. [Google Scholar] [CrossRef]
- Sarkisov, L.; Bueno-Perez, R.; Sutharson, M.; Fairen-Jimenez, D. Material Informatics with PoreBlazer v4.0 and the CSD MOF Database. Chem. Mater. 2020, 32, 9849–9867. [Google Scholar] [CrossRef]
- Lee, M.-T. Designing Highly Conductive Block Copolymer-Based Anion Exchange Membranes by Mesoscale Simulations. J. Phys. Chem. B 2021, 125, 2729–2740. [Google Scholar] [CrossRef]
- Lee, M.-T. Designing Anion Exchange Membranes with Enhanced Hydroxide Ion Conductivity by Mesoscale Simulations. J. Phys. Chem. C 2020, 124, 4470–4482. [Google Scholar] [CrossRef]

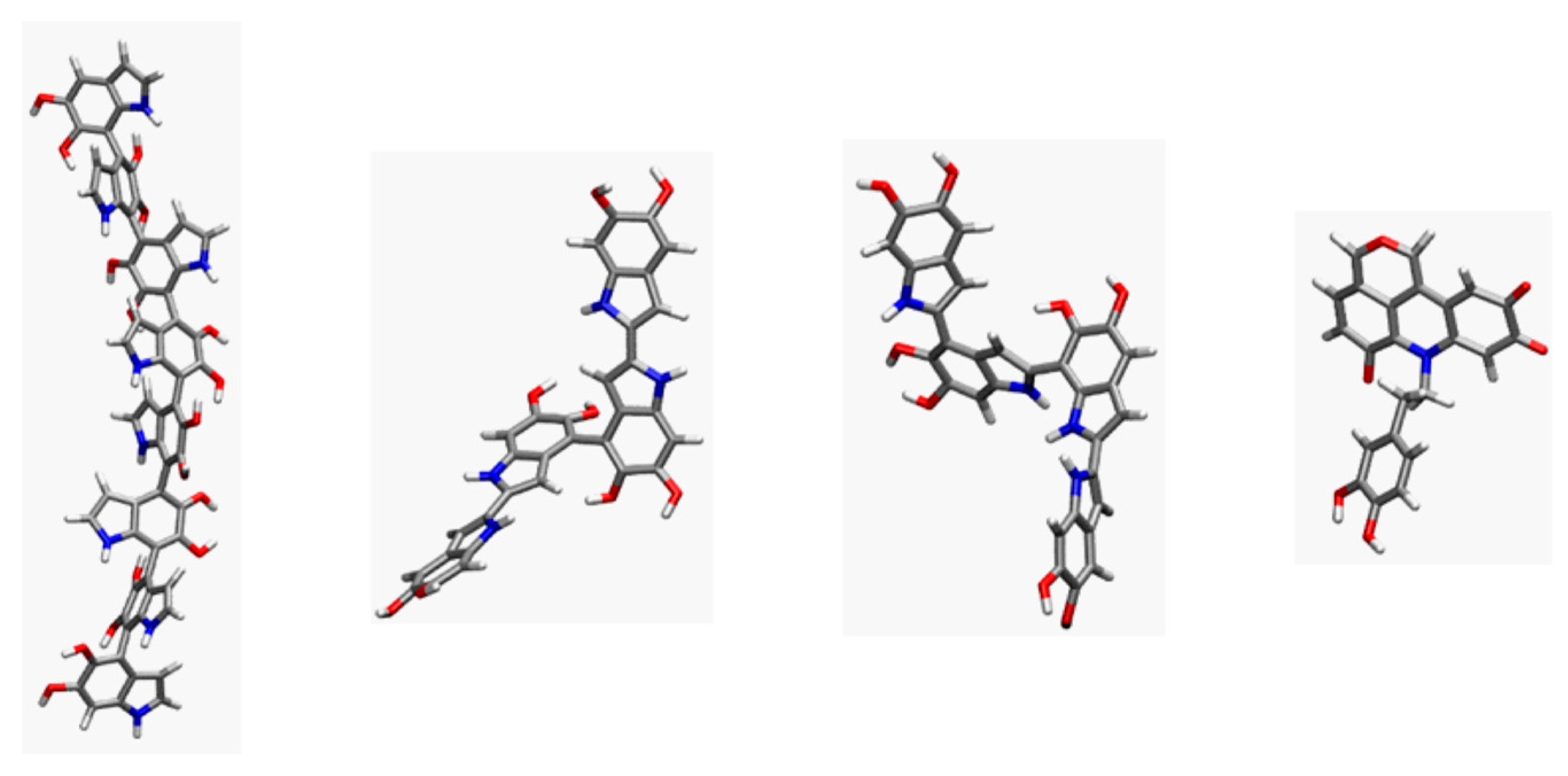
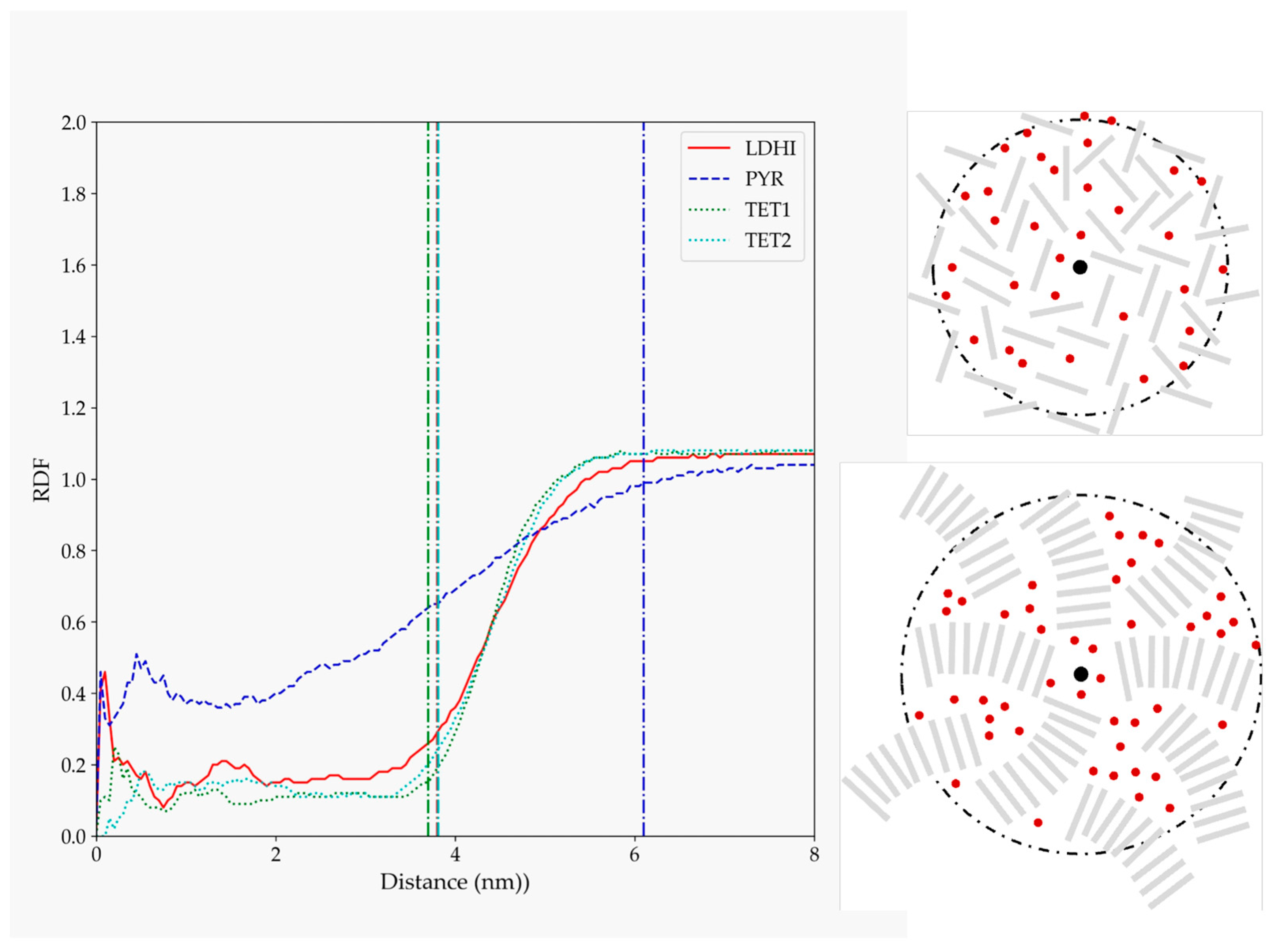

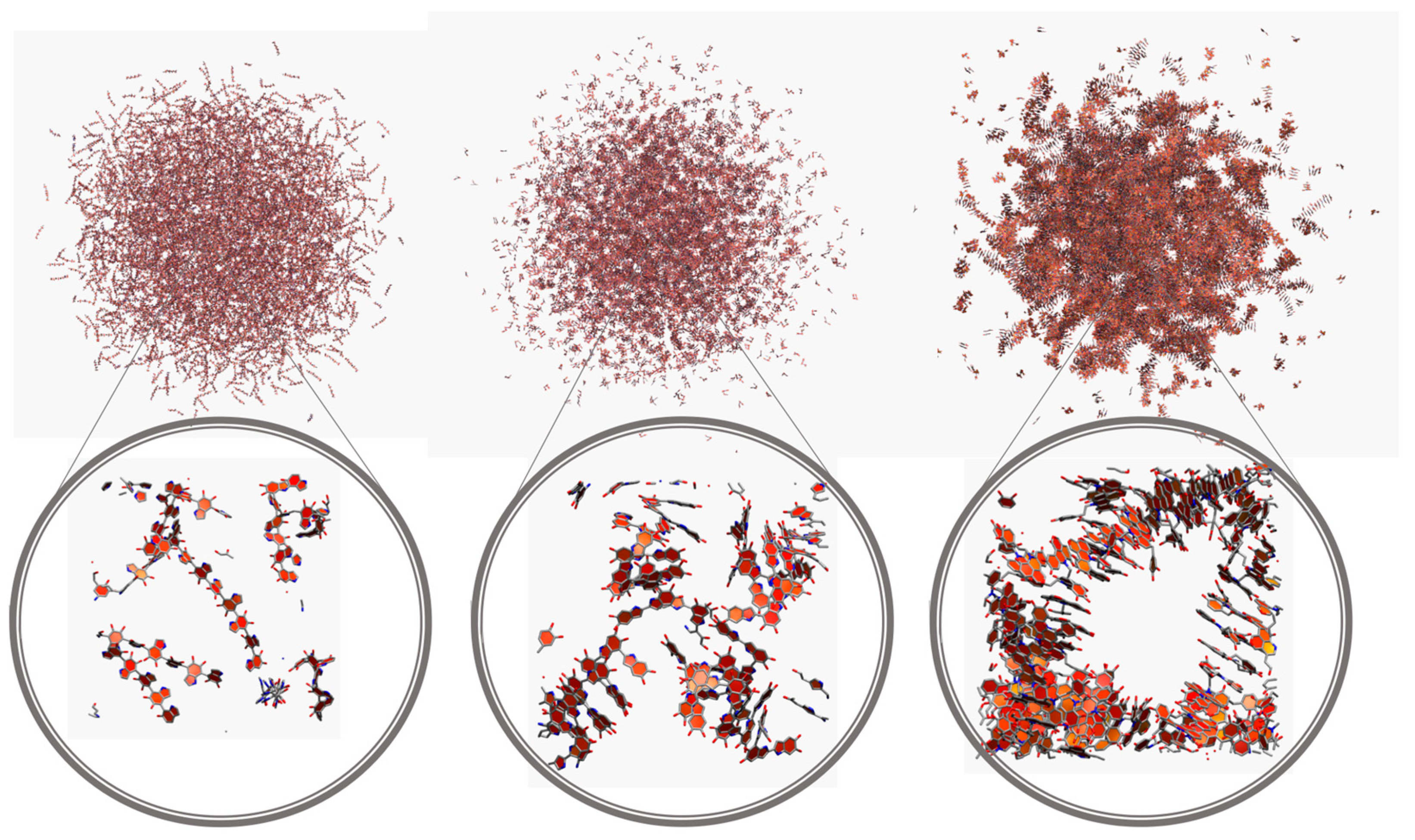
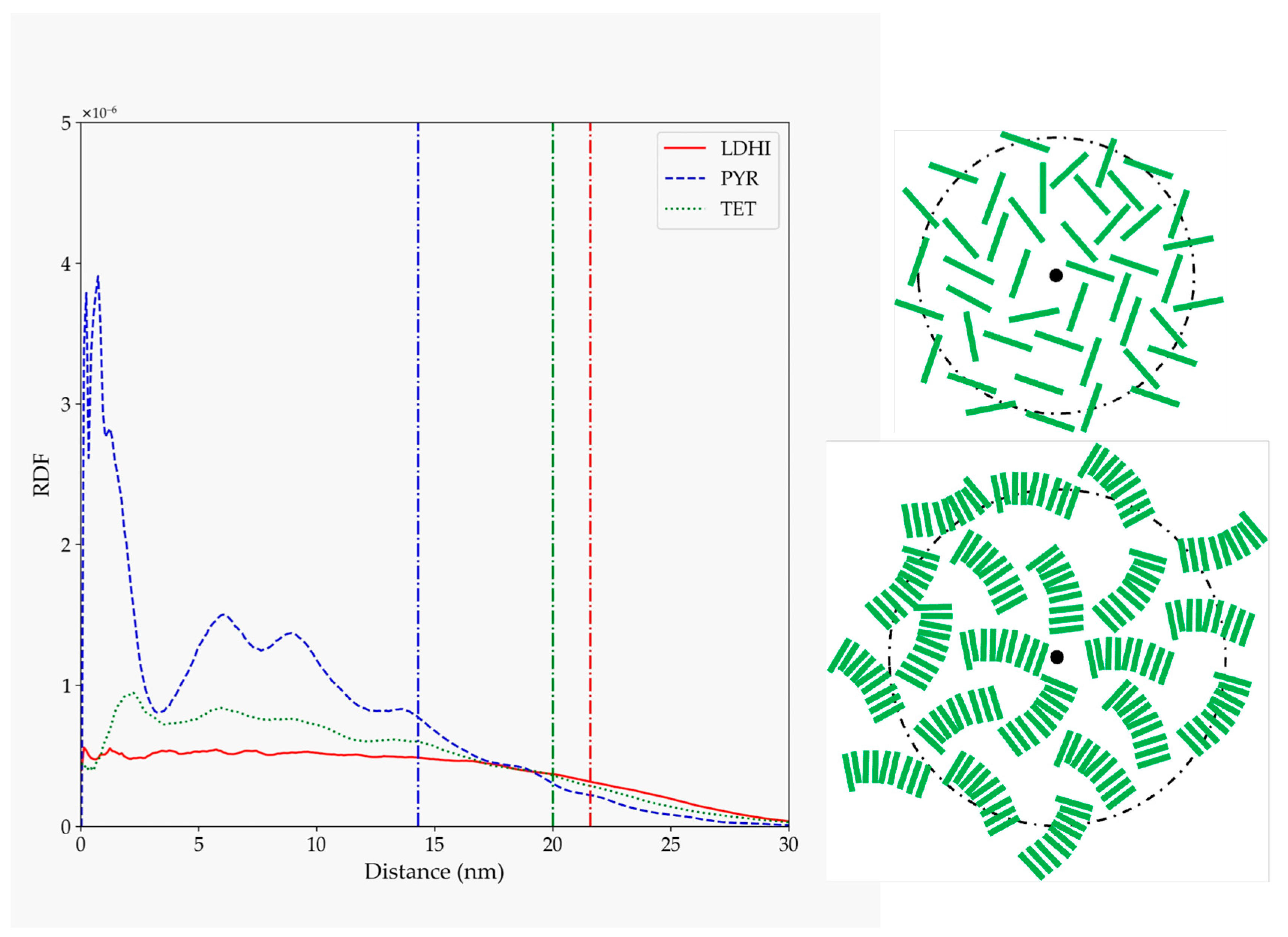
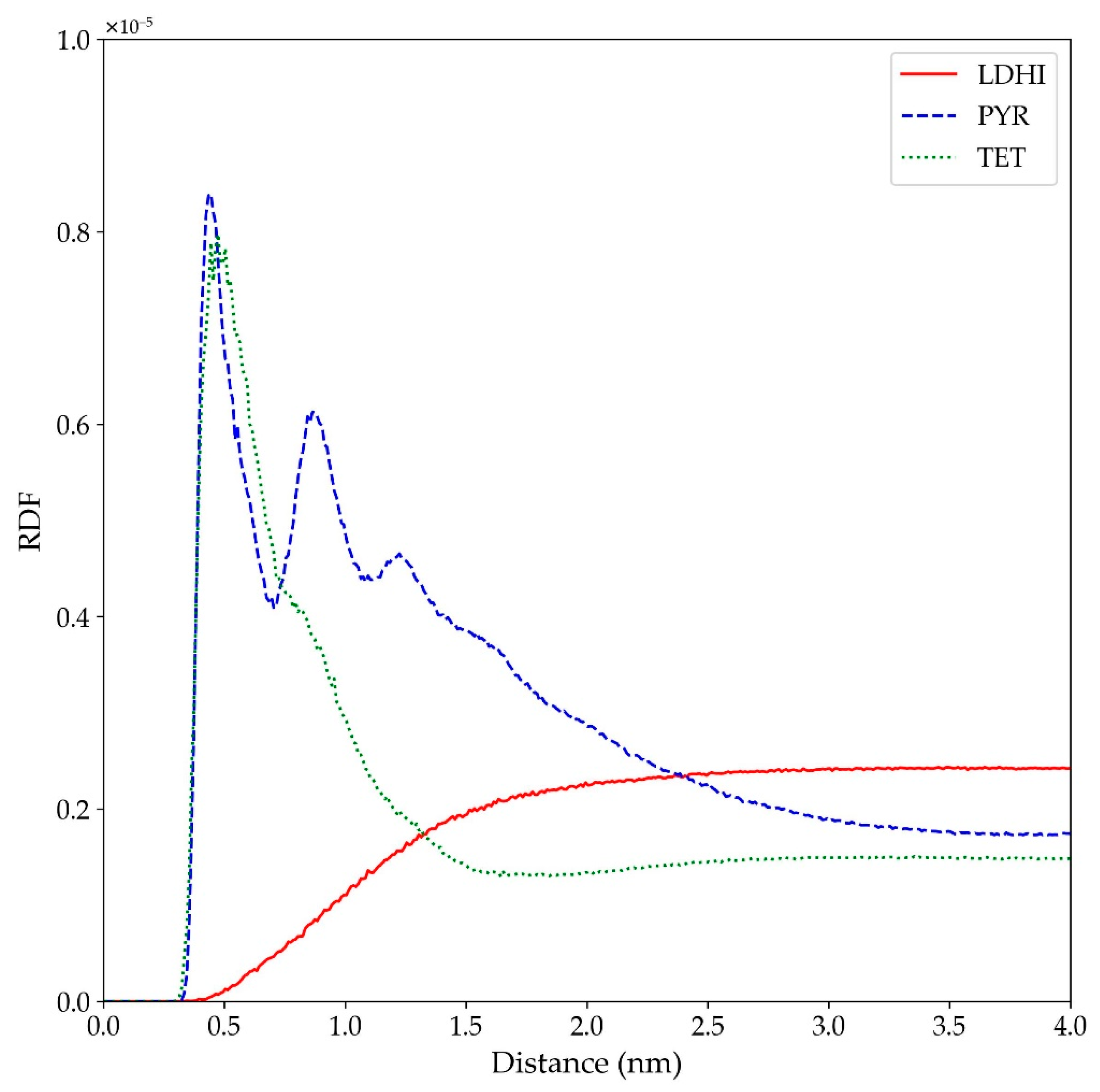
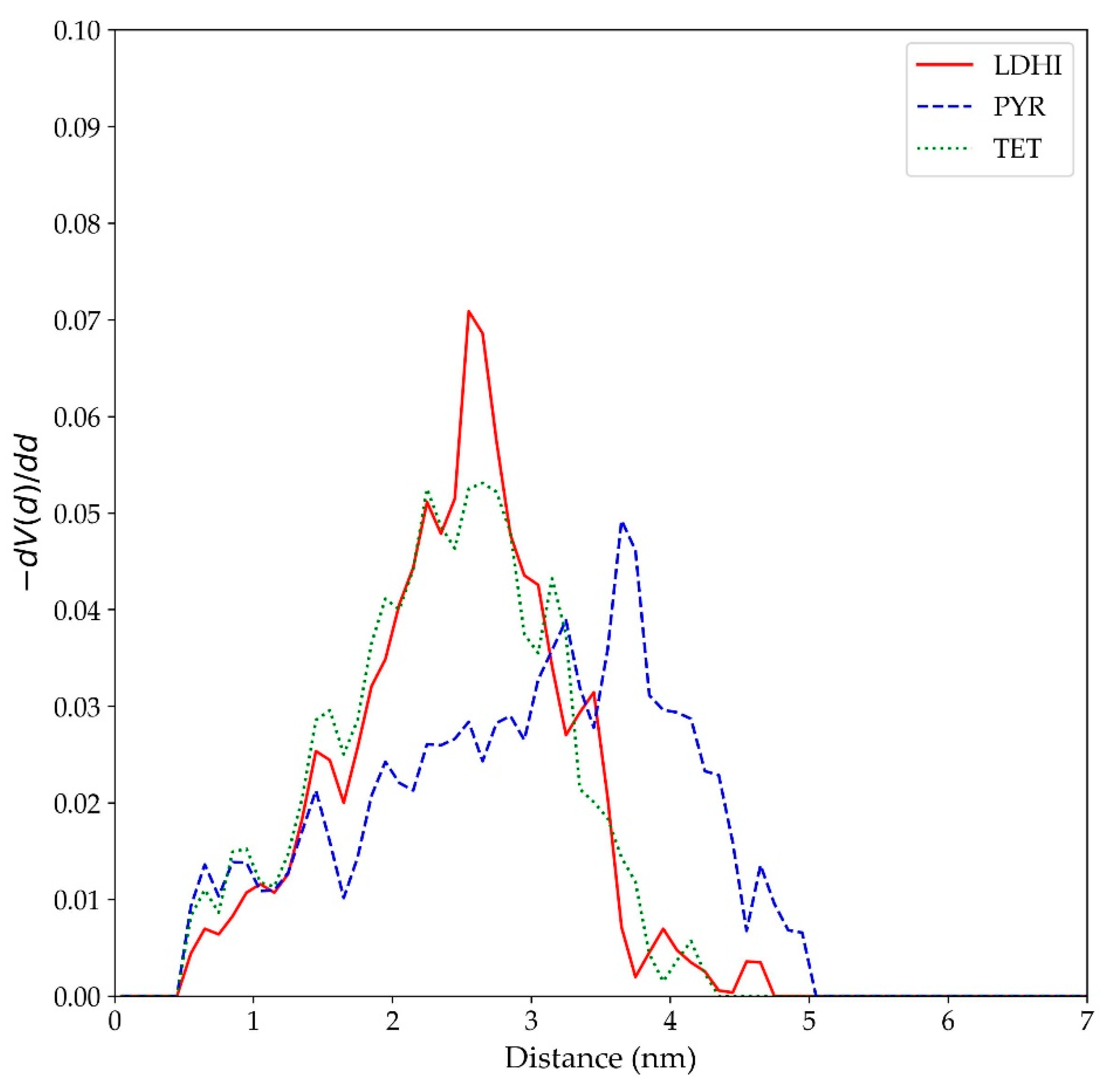
| Model | MPP | SDP |
|---|---|---|
| lDHI | 6.12 | 21.78 |
| TET-1 | 3.20 | 12.05 |
| TET-2 | 3.04 | 11.55 |
| PYR | 2.34 | 8.39 |
| Model | κ2 |
|---|---|
| lDHI | 2.5·10−4 |
| TET-1 | 4.5·10−4 |
| PYR | 3.2·10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzanares-Gómez, J.; León, S.; Climent-Pascual, E.; García-Armada, M.P. Molecular Dynamics Simulations of Polydopamine Nanosphere’s Structure Based on Experimental Evidence. Polymers 2022, 14, 5486. https://doi.org/10.3390/polym14245486
Manzanares-Gómez J, León S, Climent-Pascual E, García-Armada MP. Molecular Dynamics Simulations of Polydopamine Nanosphere’s Structure Based on Experimental Evidence. Polymers. 2022; 14(24):5486. https://doi.org/10.3390/polym14245486
Chicago/Turabian StyleManzanares-Gómez, Jesús, Salvador León, Esteban Climent-Pascual, and María Pilar García-Armada. 2022. "Molecular Dynamics Simulations of Polydopamine Nanosphere’s Structure Based on Experimental Evidence" Polymers 14, no. 24: 5486. https://doi.org/10.3390/polym14245486
APA StyleManzanares-Gómez, J., León, S., Climent-Pascual, E., & García-Armada, M. P. (2022). Molecular Dynamics Simulations of Polydopamine Nanosphere’s Structure Based on Experimental Evidence. Polymers, 14(24), 5486. https://doi.org/10.3390/polym14245486