Removal Efficiency of Insoluble β-Cyclodextrin Polymer from Water–Soluble Carcinogenic Direct Azo Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent
2.2. Apparatus
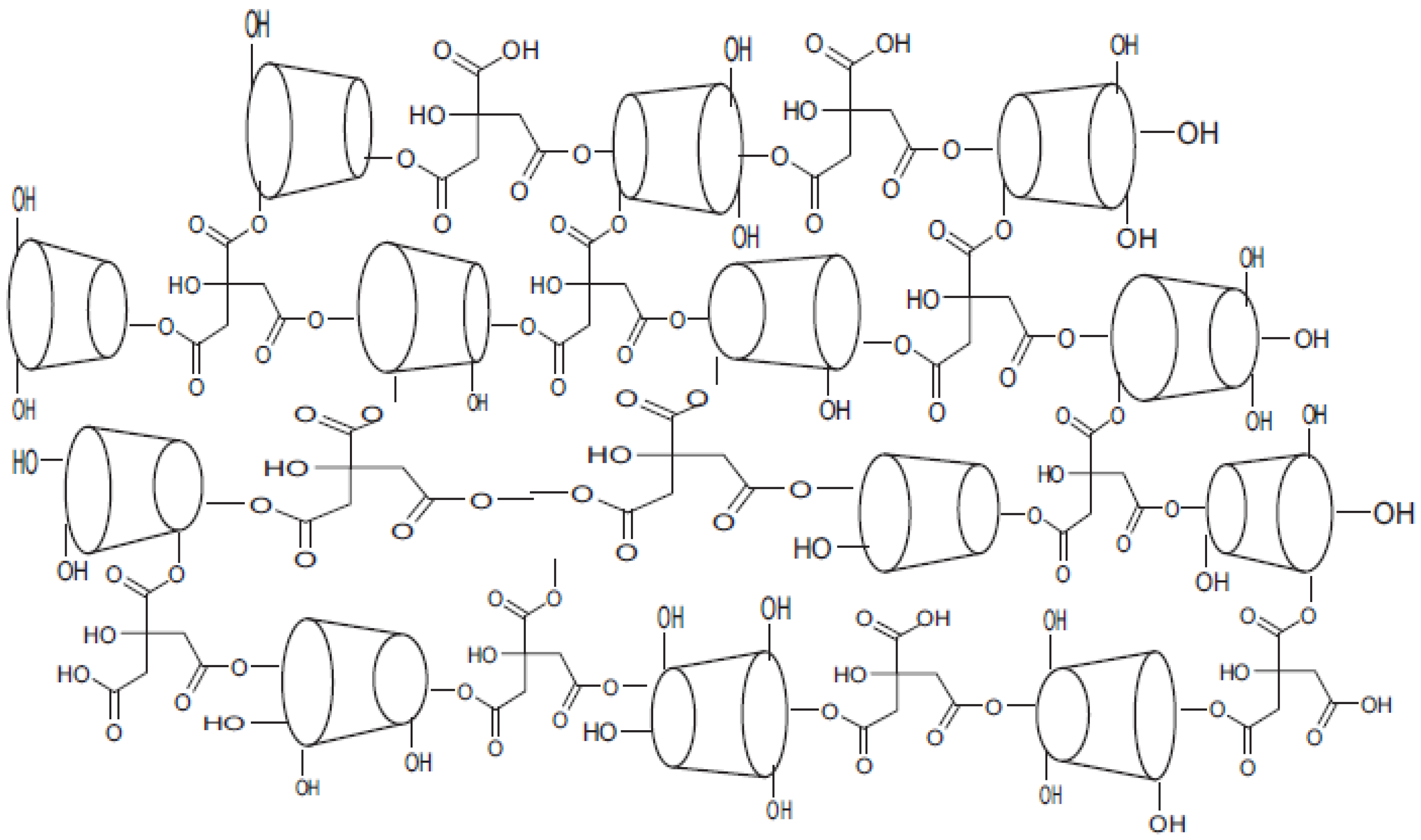
2.3. Preparation of Polymer
2.4. Adsorption Studies
2.5. Adsorption Isotherms
3. Results and Discussion
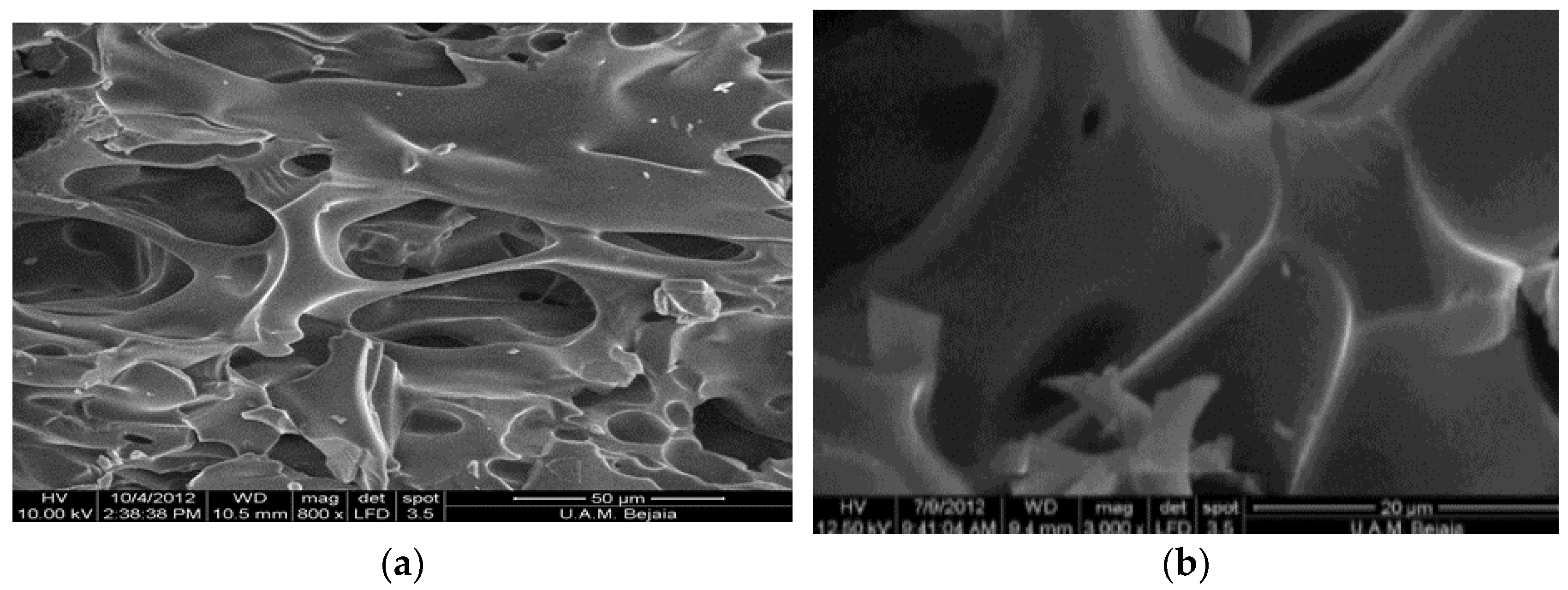
3.1. Cyclodextrin Polymer Characterization
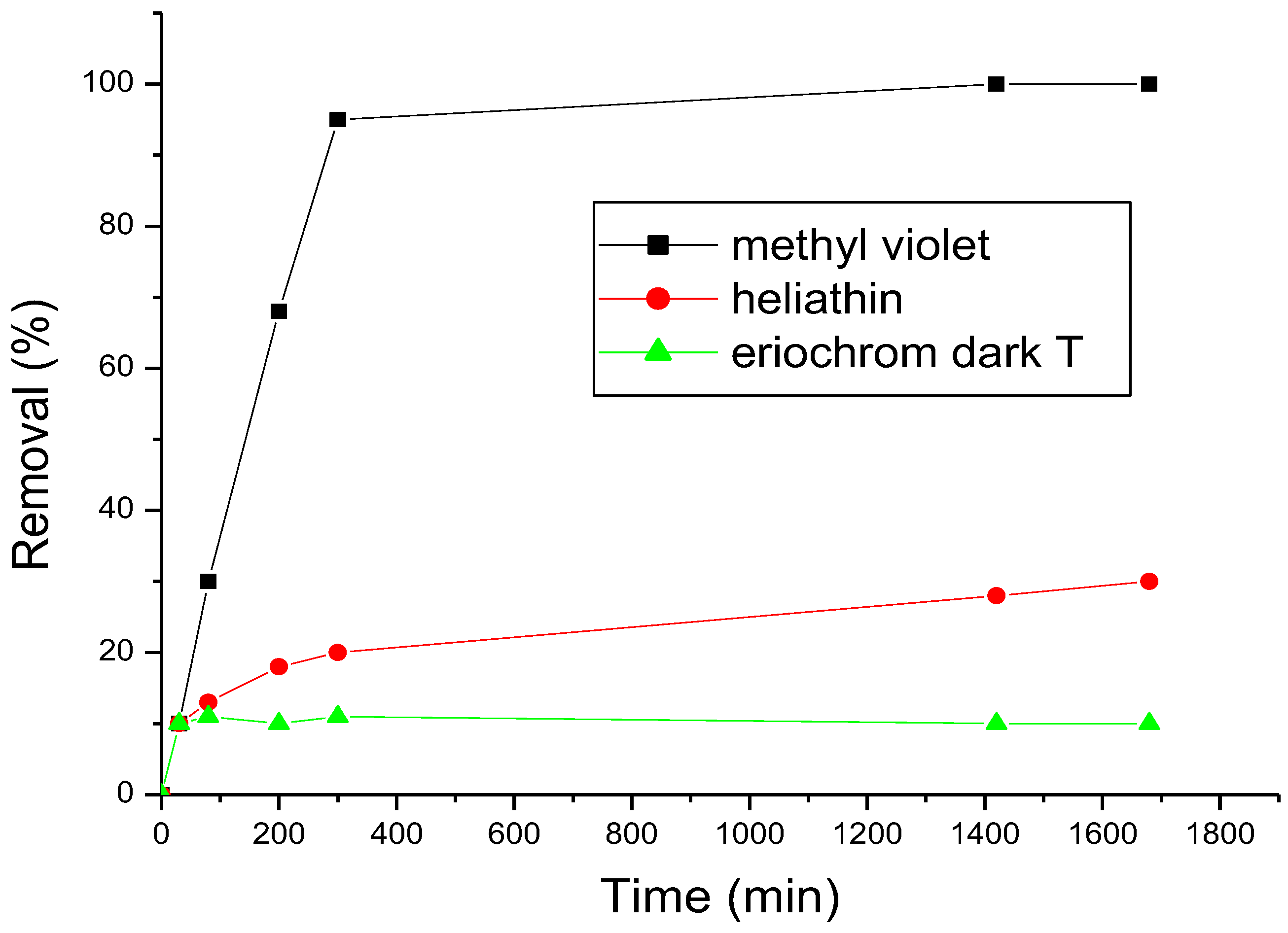
3.2. Adsorption of Dyes onto β-Cyclodextrin Polymer
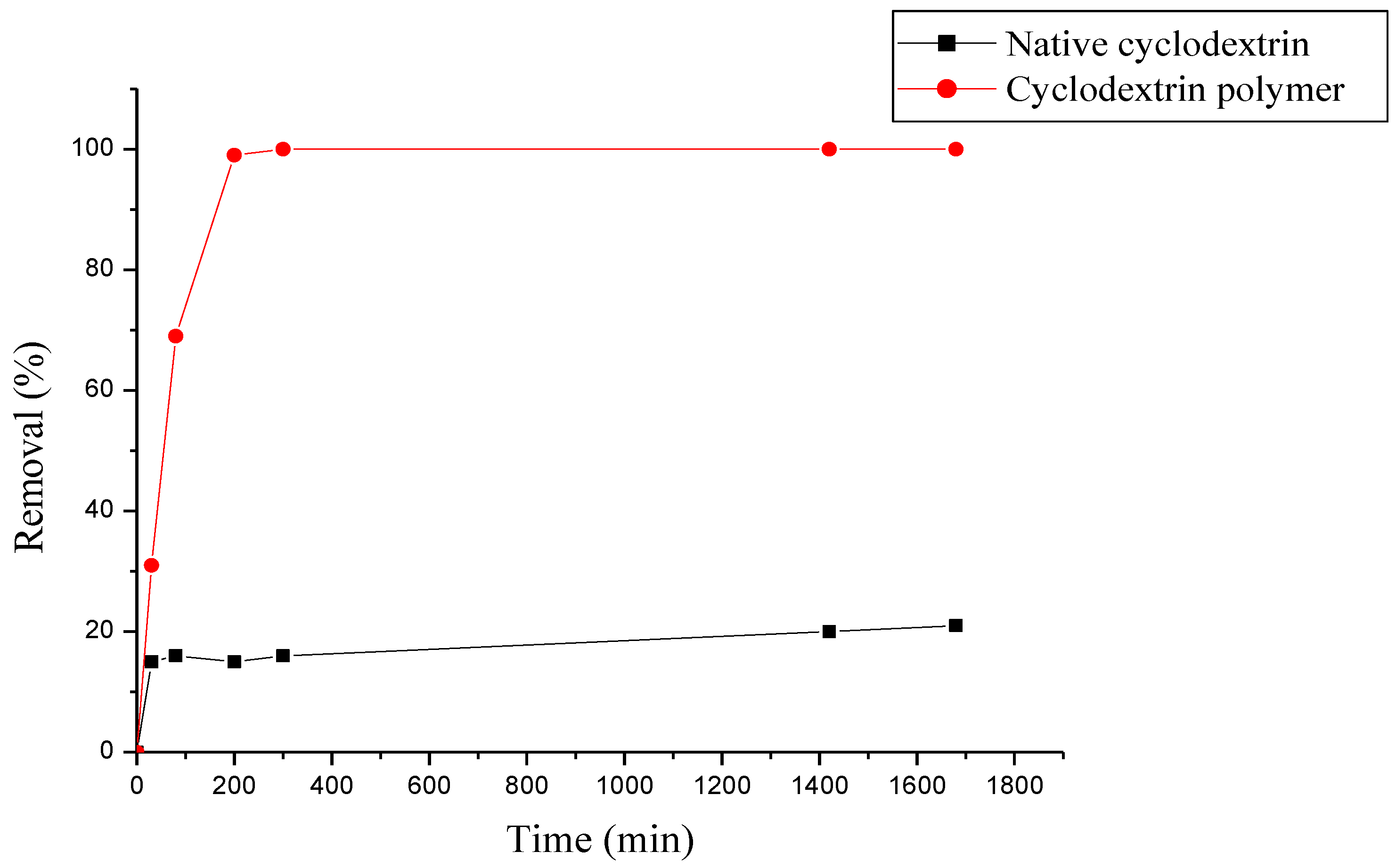
3.3. Comparison between Native β-Cyclodextrine and β-Cyclodextrin Polymer
3.4. Effect of Operators Variables on Methyl Violet Adsorption
3.4.1. Effect of pH and Ionic Strength
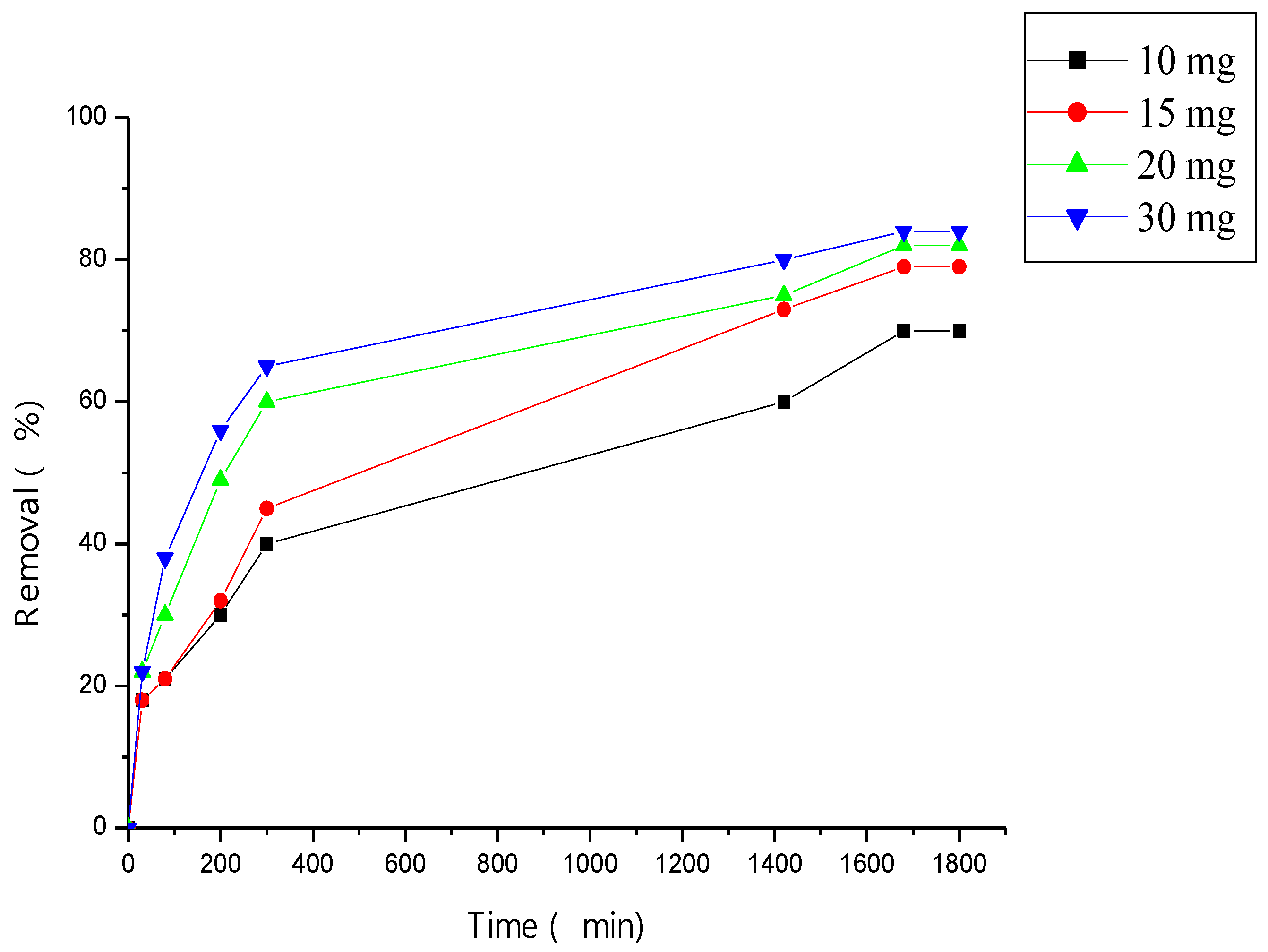
3.4.2. Effect of Adsorbent Amount
3.4.3. Effect of Temperature
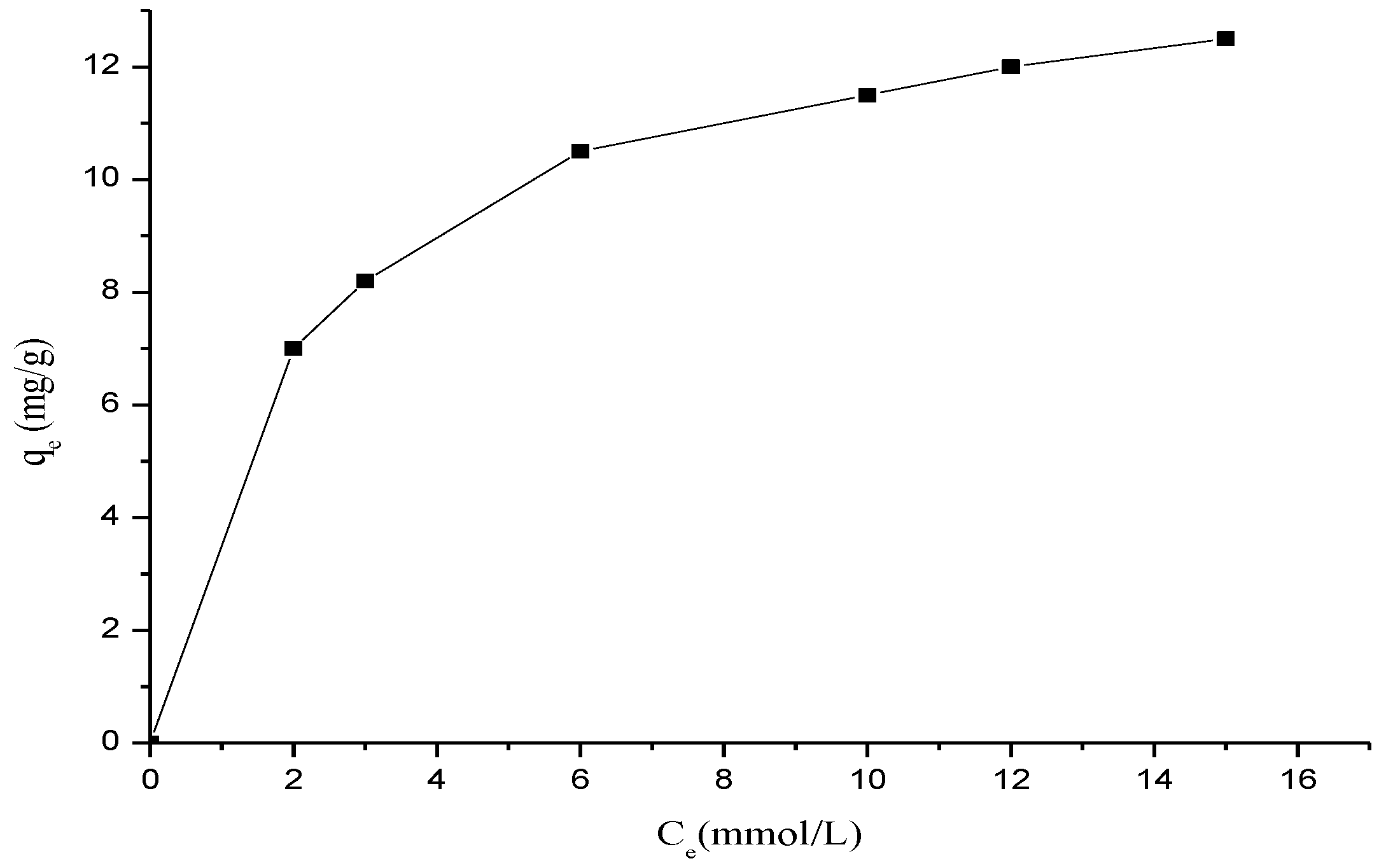
3.5. Adsorption Isotherms
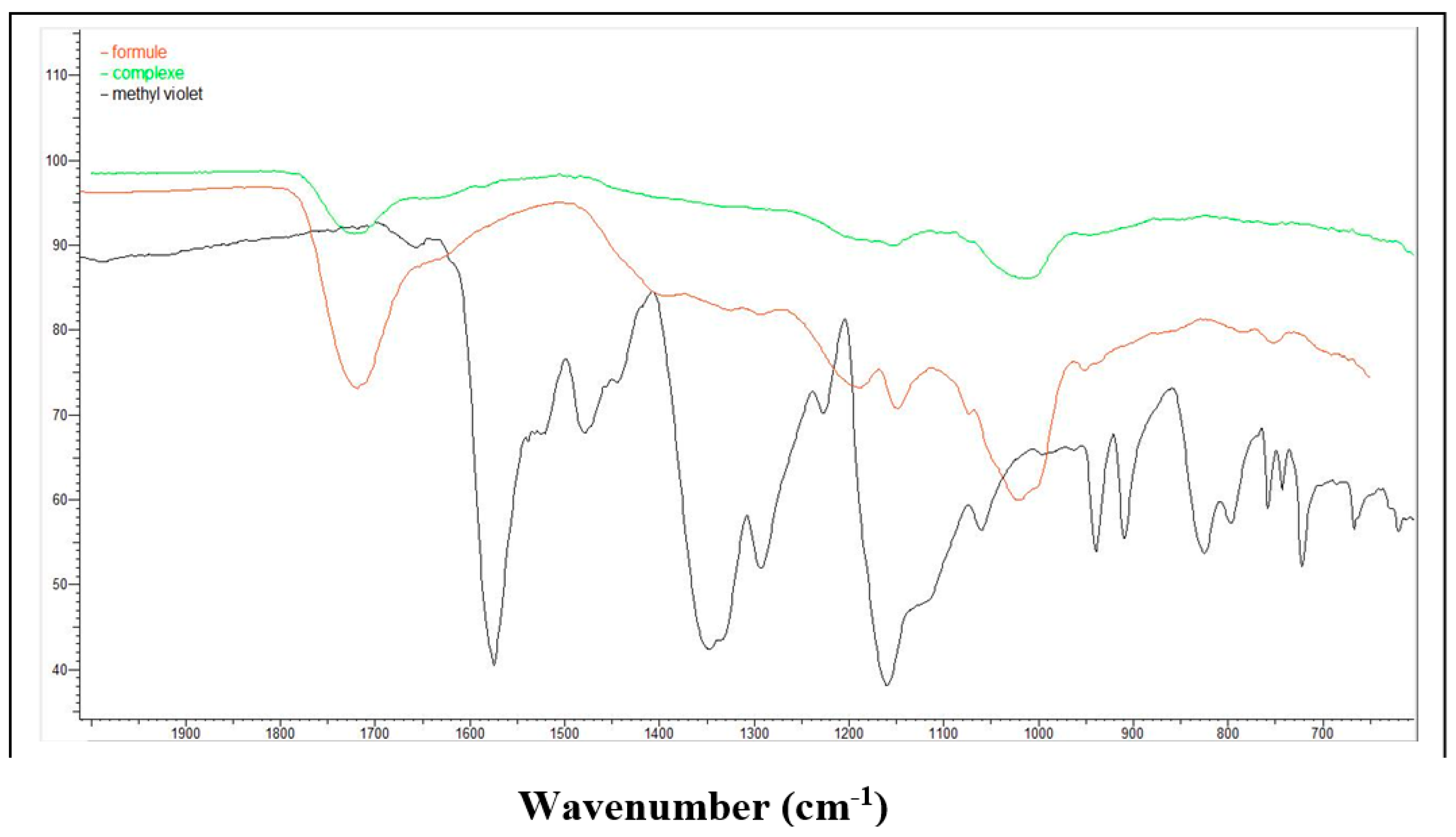
3.6. Physicochemical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raval, A.; Kohli, H.P.; Mahadwad, O.M. Application of emulsion liquid membrane for removal of malachite green dye from aqueous solution: Extraction and stability studies. Chem. Eng. J. Adv. 2022, 12, 100398. [Google Scholar] [CrossRef]
- Saeed, M.; Alwadi, N.; Ferhat, L.B.; Baig, A.; Nabgan, W.; Iqbal, M. Co3O4-Bi2O3 heterojunction: An effective photocatalyst for rhodamine B dye. Arab. J. Chem. 2022, 15, 103732. [Google Scholar] [CrossRef]
- Salih, S.J.; Kareem, A.S.A.; Anwer, S.S. Adsorption of anionic dye from textile wastewater raw corncob. Hellyone 2022, 8, e10092. [Google Scholar] [CrossRef] [PubMed]
- Onu, C.E.; Nwabanne, J.T.; Ohale, P.E.; Asadu, C.O. Comparative analysisRSM, ANN and the mechanistic modeling in eriochrome black-T dye adsorption using modified clay. S. Afr. J. Chem. 2021, 36, 24–42. [Google Scholar]
- Dubey, S.P.; Dwivedi, A.D.; Sillanpää, M.; Gopal, K. Artemisi vulgaris-derived mesoporous honeycomb-shaped activated carbon for ibuprofen adsorption. Chem. Eng. J. 2010, 165, 537–544. [Google Scholar] [CrossRef]
- Ofomaja, A.-E.; Ukpebor, E.-E.; Uzoekwe, S.-A. Biosorption of Methyl violet onto palm kernel fiber: Diffusion studies and multistage process design to minimize biosorbent mass and contact time. Biomass. Bioenergy 2011, 35, 4112–4123. [Google Scholar] [CrossRef]
- Musyoka, S.-M.; Mittal, H.; Mishra, S.-B.; Ngila, J.-C. Effect of functionalization on the adsorption capacity of cellulose for the removal of methyl violet. Int. J. Biol. Macromol. 2014, 65, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Y.; Bin, J.-P.; Bu, H.-T.; Jiang, G.-B. Removal of anionic dye from aqueous solution using ethylenediamine modified chitosane. Carbohydr. Polym. 2011, 84, 1350–1356. [Google Scholar] [CrossRef]
- Moulahcene, L.; Skiba, M.; Senhadji, O.; Milon, N.; Lahiani-Skiba, M. Inclusion and removal of pharmaceutical residues from aqueous solution using water-insoluble cyclodextrin polymers. Chem Eng Res Des. 2015, 97, 145–158. [Google Scholar] [CrossRef]
- Crini, G. Studies on adsorption of dyes on beta-cyclodextrin polymer. Bioresour. Technol. 2003, 90, 193–198. [Google Scholar] [CrossRef]
- Skiba, M.; Lahiani-Skiba, M. Novel method for preparation of cyclodextrin polymers: Physico-chemical characterization and cytotoxicity. J. Incl. Phenom. 2013, 75, 341–349. [Google Scholar] [CrossRef]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Crini, G.; Peindy, H.N. Adsorption of C.I. Basic Blue 9 on cyclodextrin-based material containing carboxylic groups. Dyes. Pigm. 2006, 70, 204–211. [Google Scholar] [CrossRef]
- Crini, G. Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dye. Pigment. 2008, 77, 415–426. [Google Scholar] [CrossRef]
- Brevet Mohamed SKIBA. Method for synthetizing Calixaren and/or Cyclodextrin copolymers, terpolymers and tetrapolymers. PCT-FR2010 000875.
- Li, N.; Wei, X.; Mei, Z.; Xiong, X.; Chen, S.; Ye, M.; Ding, S. Synthesis and characterization of a novel polyamidoamine-cyclodextrin crosslinked copolymer. Carbohydr. Res. 2011, 346, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Chabani, M.; Amrane, A.; Bensmaili, A. Kinetics of nitrates, adsorption on Amberlite IRA 400 resin. Desalination 2007, 206, 560–567. [Google Scholar] [CrossRef]
- Giles, C.; Ewan, T.M.; Nakhwa, S.; Smith, D. Studies of adsorption isotherms and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. Lond. 1960, 56, 1973–2993. [Google Scholar]
- Persoff, P.; Thomas, J. Estimating Michaelis–Menten or Langmuir isotherm constants by weighted nonlinear least squares. Soil Sci. Soc. Amer. J. 1998, 52, 886–889. [Google Scholar] [CrossRef]
- Bonettoa, L.; Ferrarinia, F.; de Marcoa, C.; Crespoa, J.; Guéganb, R.; Giovanela, M. Removal of methyl violet 2B dye from aqueous solution using amagnetic composite as an adsorbent. J. Water Process. Eng. 2015, 6, 11–20. [Google Scholar] [CrossRef]




| Proprity | Swelling Capacity (%) | Total Acidic Groups (mmol/g) | Surface Area (m2/g) |
|---|---|---|---|
| β-cyclodextrin polymer | 70.38 | 9.75 | 1096 |
| Model | Langumir | Frendlich | Temkin |
|---|---|---|---|
| r2 | 0.9997 | 0.9898 | 0.9945 |
| KL (L/mg) | 0.65091 | - | - |
| qmax (mg/g) | 13.21 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulahcene, L.; Skiba, M.; Milon, N.; Fadila, H.; Bounoure, F.; Lahiani-Skiba, M. Removal Efficiency of Insoluble β-Cyclodextrin Polymer from Water–Soluble Carcinogenic Direct Azo Dyes. Polymers 2023, 15, 732. https://doi.org/10.3390/polym15030732
Moulahcene L, Skiba M, Milon N, Fadila H, Bounoure F, Lahiani-Skiba M. Removal Efficiency of Insoluble β-Cyclodextrin Polymer from Water–Soluble Carcinogenic Direct Azo Dyes. Polymers. 2023; 15(3):732. https://doi.org/10.3390/polym15030732
Chicago/Turabian StyleMoulahcene, Lamia, Mohamed Skiba, Nicolas Milon, Hammache Fadila, Frédéric Bounoure, and Malika Lahiani-Skiba. 2023. "Removal Efficiency of Insoluble β-Cyclodextrin Polymer from Water–Soluble Carcinogenic Direct Azo Dyes" Polymers 15, no. 3: 732. https://doi.org/10.3390/polym15030732
APA StyleMoulahcene, L., Skiba, M., Milon, N., Fadila, H., Bounoure, F., & Lahiani-Skiba, M. (2023). Removal Efficiency of Insoluble β-Cyclodextrin Polymer from Water–Soluble Carcinogenic Direct Azo Dyes. Polymers, 15(3), 732. https://doi.org/10.3390/polym15030732






