Assessment of Physical/Mechanical Performance of Dental Resin Sealants Containing Sr-Bioactive Glass Nanoparticles and Calcium Phosphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Experimental Dental Sealants
2.2. Degree of Monomer Conversion (DC)
2.3. Biaxial Flexural Strength (BFS) and Modulus (BFM)
2.4. Vickers Surface Microhardness
2.5. Surface Calcium Phosphate Precipitation
2.6. Ion Release
2.7. Statistical Analysis
3. Results
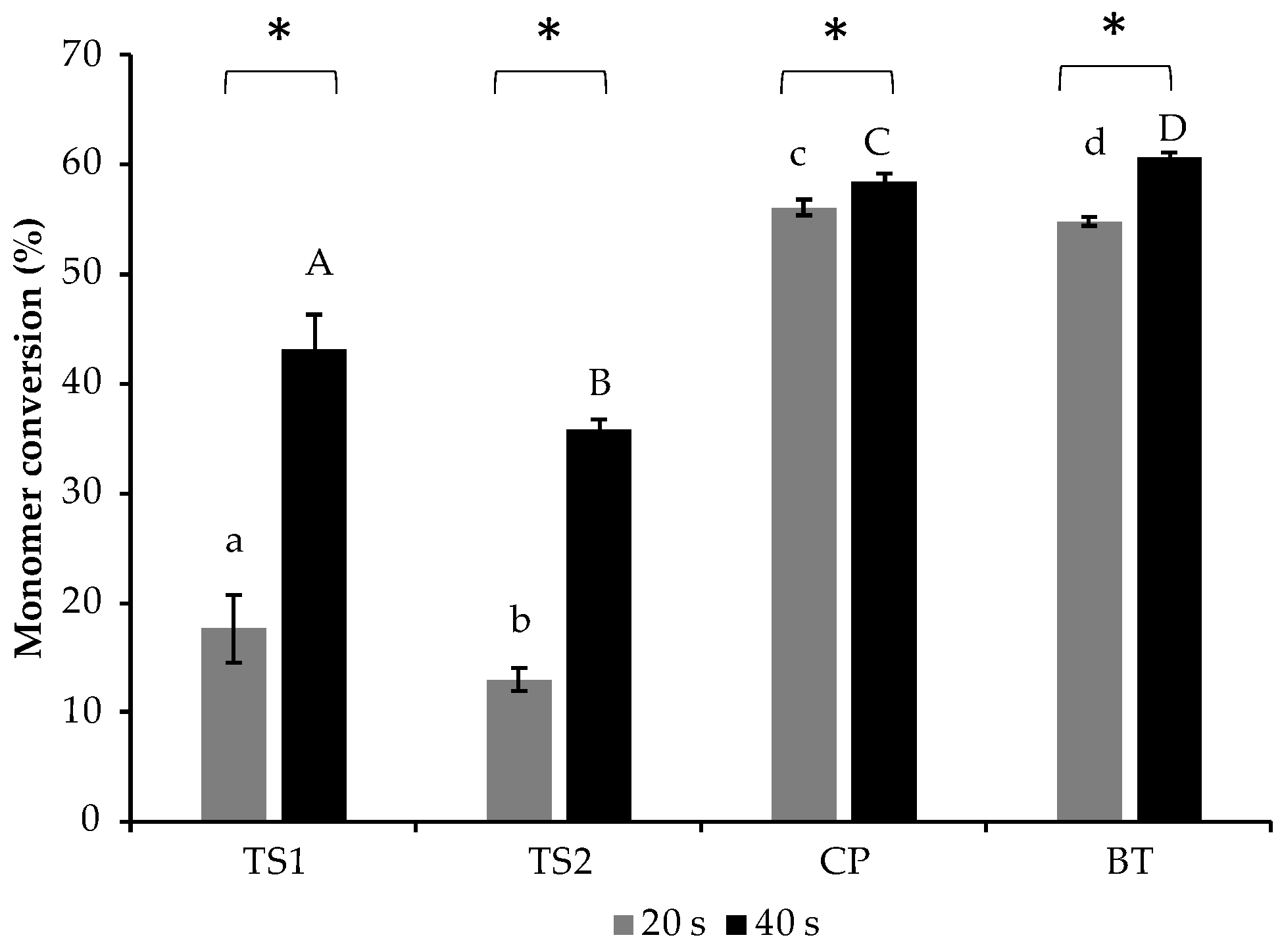
3.1. Degree of Monomer Conversion
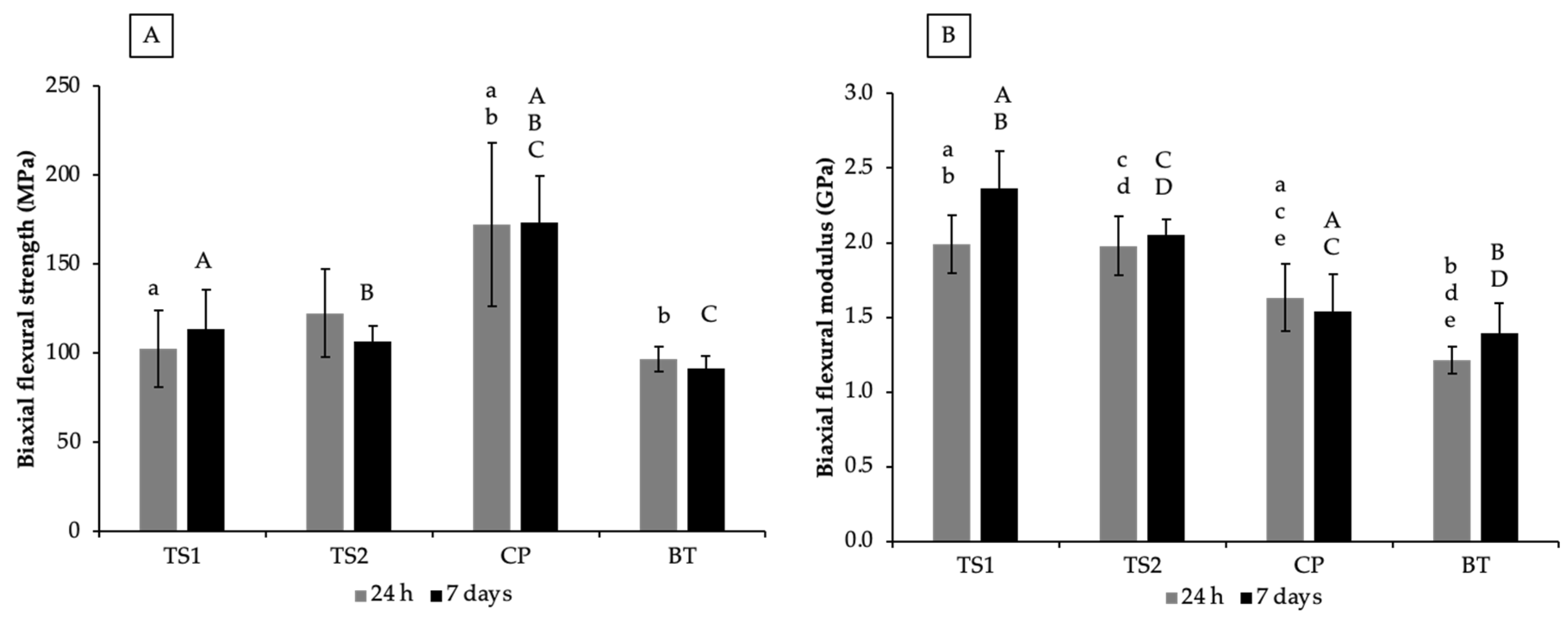
3.2. Biaxial Flexural Strength (BFS) and Biaxial Flexural Modulus (BFM)
3.3. Vickers Surface Microhardness (SH)
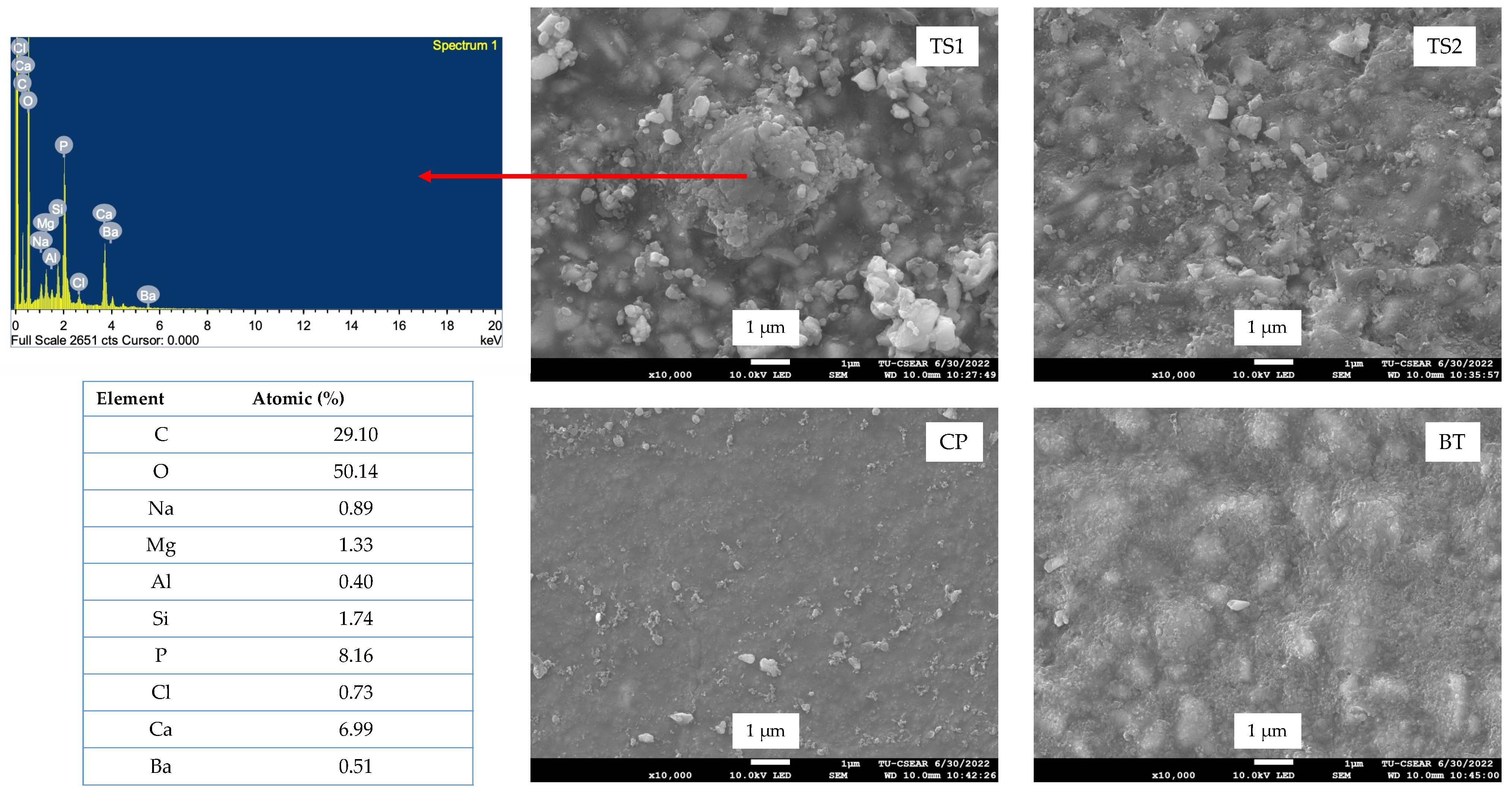
3.4. Surface Calcium Phosphate Precipitation
3.5. Ion Release
4. Discussion
5. Conclusions
- Required a curing time of 40 s to achieve a high DC;
- Had a BFS that was between the BFSs of commercial resin sealants;
- Had a BFM and SH higher than those of the commercial products;
- Exhibited ion release that could potentially encourage calcium phosphate precipitation and help to reduce secondary caries around the resin sealant.
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desai, H.; Stewart, C.A.; Finer, Y. Minimally Invasive Therapies for the Management of Dental Caries-A Literature Review. Dent. J. 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.P.; Sardana, D.; Lo, E.C.; Yiu, C.K. Fissure Sealant in a Nutshell. Evidence-Based Meta-Evaluation of Sealants’ Effectiveness in Caries Prevention and Arrest. J. Evid. Based Dent. Pract. 2021, 21, 101587. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M. Nonrestorative Management of Cavitated and Noncavitated Caries Lesions. Dent. Clin. N. Am. 2019, 63, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Behroozian, A.; Aghazadeh, Z.; Sadrabad, Z.K.; Aghazadeh, M.; Alizadeh, V.; Esmaili, Z.; Pirzadeh Ashraf, M. Evaluation of the success rate of pit and fissure sealants on first molars: 12 months follow-up study. Int. J. Dent. Hyg. 2022, 20, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, X. Clinical comparison of Fuji VII and a resin sealant in children at high and low risk of caries. Dent. Mater J. 2013, 32, 512–518. [Google Scholar] [CrossRef]
- Hevinga, M.A.; Opdam, N.J.; Bronkhorst, E.M.; Truin, G.J.; Huysmans, M.C. Long-term performance of resin based fissure sealants placed in a general dental practice. J. Dent. 2010, 38, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Choi, J.W.; Kim, K.M.; Kwon, J.S. Prevention of Secondary Caries Using Resin-Based Pit and Fissure Sealants Containing Hydrated Calcium Silicate. Polymers 2020, 12, 1200. [Google Scholar] [CrossRef]
- Aljabo, A.; Abou Neel, E.A.; Knowles, J.C.; Young, A.M. Development of dental composites with reactive fillers that promote precipitation of antibacterial-hydroxyapatite layers. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 285–292. [Google Scholar] [CrossRef]
- Panpisut, P.; Khan, M.A.; Main, K.; Arshad, M.; Xia, W.; Petridis, H.; Young, A.M. Polymerization kinetics stability, volumetric changes, apatite precipitation, strontium release and fatigue of novel bone composites for vertebroplasty. PLoS ONE 2019, 14, e0207965. [Google Scholar] [CrossRef]
- Panpisut, P.; Liaqat, S.; Zacharaki, E.; Xia, W.; Petridis, H.; Young, A.M. Dental composites with calcium/strontium phosphates and polylysine. PLoS ONE 2016, 11, e0164653. [Google Scholar] [CrossRef]
- Panpisut, P.; Suppapatpong, T.; Rattanapan, A.; Wongwarawut, P. Monomer conversion, biaxial flexural strength, apatite forming ability of experimental dual-cured and self-adhesive dental composites containing calcium phosphate and nisin. Dent. Mater. J. 2021, 40, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Xia, W.; Ashley, P.F.; Young, A.M. Renewal MI Dental Composite Etch and Seal Properties. Materials 2022, 15, 5438. [Google Scholar] [CrossRef] [PubMed]
- Raszewski, Z.; Brzakalski, D.; Derpenski, L.; Jalbrzykowski, M.; Przekop, R.E. Aspects and Principles of Material Connections in Restorative Dentistry-A Comprehensive Review. Materials 2022, 15, 7131. [Google Scholar] [CrossRef]
- Bao, Z.; Sun, H.; Fan, D.; Wang, X.; Wang, Q. Shear Bond Strength and Microleakage of Pit and Fissure Sealants Placed after Saliva-Contaminated Etched Enamel. Coatings 2022, 12, 441. [Google Scholar] [CrossRef]
- Brauer, D.S.; Karpukhina, N.; Kedia, G.; Bhat, A.; Law, R.V.; Radecka, I.; Hill, R.G. Bactericidal strontium-releasing injectable bone cements based on bioactive glasses. J. R. Soc. Int. 2013, 10, 20120647. [Google Scholar] [CrossRef]
- Baheiraei, N.; Eyni, H.; Bakhshi, B.; Najafloo, R.; Rabiee, N. Effects of strontium ions with potential antibacterial activity on in vivo bone regeneration. Sci. Rep. 2021, 11, 8745. [Google Scholar] [CrossRef]
- Manoochehri, H.; Ghorbani, M.; Moosazadeh Moghaddam, M.; Nourani, M.R.; Makvandi, P.; Sharifi, E. Strontium doped bioglass incorporated hydrogel-based scaffold for amplified bone tissue regeneration. Sci. Rep. 2022, 12, 10160. [Google Scholar] [CrossRef]
- Chaichana, W.; Insee, K.; Chanachai, S.; Benjakul, S.; Aupaphong, V.; Naruphontjirakul, P.; Panpisut, P. Physical/mechanical and antibacterial properties of orthodontic adhesives containing Sr-bioactive glass nanoparticles, calcium phosphate, and andrographolide. Sci. Rep. 2022, 12, 6635. [Google Scholar] [CrossRef]
- Tiskaya, M.; Shahid, S.; Gillam, D.; Hill, R. The use of bioactive glass (BAG) in dental composites: A critical review. Dent. Mater. 2021, 37, 296–310. [Google Scholar] [CrossRef]
- Saikaew, P.; Phimolthares, P.; Phitakthanaakul, P.; Sirikul, P.; Mekrakseree, S.; Panpisut, P. Effects of Color Modifier on Degree of Monomer Conversion, Biaxial Flexural Strength, Surface Microhardness, and Water Sorption/Solubility of Resin Composites. Polymers 2021, 13, 3902. [Google Scholar] [CrossRef]
- Delgado, A.H.S.; Young, A.M. Methacrylate peak determination and selection recommendations using ATR-FTIR to investigate polymerisation of dental methacrylate mixtures. PLoS ONE 2021, 16, e0252999. [Google Scholar] [CrossRef] [PubMed]
- Higgs, W.A.; Lucksanasombool, P.; Higgs, R.J.; Swain, M.V. A simple method of determining the modulus of orthopedic bone cement. J. Biomed. Mater. Res. 2001, 58, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Thepveera, W.; Potiprapanpong, W.; Toneluck, A.; Channasanon, S.; Khamsuk, C.; Monmaturapoj, N.; Tanodekaew, S.; Panpisut, P. Rheological Properties, Surface Microhardness, and Dentin Shear Bond Strength of Resin-Modified Glass Ionomer Cements Containing Methacrylate-Functionalized Polyacids and Spherical Pre-Reacted Glass Fillers. J. Funct. Biomater. 2021, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- BS ISO 23317:2014; Implants for Surgery. In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. British Standard: London, UK, 2014.
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Sarosi, C.; Moldovan, M.; Soanca, A.; Roman, A.; Gherman, T.; Trifoi, A.; Chisnoiu, A.M.; Cuc, S.; Filip, M.; Gheorghe, G.F.; et al. Effects of Monomer Composition of Urethane Methacrylate Based Resins on the C=C Degree of Conversion, Residual Monomer Content and Mechanical Properties. Polymers 2021, 13, 4415. [Google Scholar] [CrossRef]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the Degree of Conversion, Residual Monomers and Mechanical Properties of Some Light-Cured Dental Resin Composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef]
- Lara, L.; Rocha, M.G.; Menezes, L.R.; Correr, A.B.; Sinhoreti, M.A.C.; Oliveira, D. Effect of combining photoinitiators on cure efficiency of dental resin-based composites. J. Appl. Oral. Sci. 2021, 29, e20200467. [Google Scholar] [CrossRef]
- Faria, E.S.A.L.; Pfeifer, C.S. Impact of thio-urethane additive and filler type on light-transmission and depth of polymerization of dental composites. Dent. Mater. 2017, 33, 1274–1285. [Google Scholar] [CrossRef]
- Shortall, A.C.; Palin, W.M.; Burtscher, P. Refractive index mismatch and monomer reactivity influence composite curing depth. J. Dent. Res. 2008, 87, 84–88. [Google Scholar] [CrossRef]
- Goncalves, F.; Kawano, Y.; Pfeifer, C.; Stansbury, J.W.; Braga, R.R. Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites. Eur. J. Oral. Sci. 2009, 117, 442–446. [Google Scholar] [CrossRef]
- Walters, N.J.; Xia, W.; Salih, V.; Ashley, P.F.; Young, A.M. Poly(propylene glycol) and urethane dimethacrylates improve conversion of dental composites and reveal complexity of cytocompatibility testing. Dent. Mater. 2016, 32, 264–277. [Google Scholar] [CrossRef]
- Achilias, D.S.; Sideridou, I.D. Kinetics of the Benzoyl Peroxide/Amine Initiated Free-Radical Polymerization of Dental Dimethacrylate Monomers: Experimental Studies and Mathematical Modeling for TEGDMA and Bis-EMA. Macromolecules 2004, 37, 4254–4265. [Google Scholar] [CrossRef]
- Kangwankai, K.; Sani, S.; Panpisut, P.; Xia, W.; Ashley, P.; Petridis, H.; Young, A.M. Monomer conversion, dimensional stability, strength, modulus, surface apatite precipitation and wear of novel, reactive calcium phosphate and polylysine-containing dental composites. PLoS ONE 2017, 12, e0187757. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Sokolowski, J.; Bociong, K. The Photoinitiators Used in Resin Based Dental Composite-A Review and Future Perspectives. Polymers 2021, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Meena, A.; Patnaik, A. Biomaterials for dental composite applications: A comprehensive review of physical, chemical, mechanical, thermal, tribological, and biological properties. Polym. Adv. Technol. 2022, 33, 1762–1781. [Google Scholar] [CrossRef]
- BS EN ISO 6874:2015; Dentistry. Polymer-Based Pit and Fissure Sealants. British Standard: London, UK, 2015.
- Ilie, N.; Hilton, T.J.; Heintze, S.D.; Hickel, R.; Watts, D.C.; Silikas, N.; Stansbury, J.W.; Cadenaro, M.; Ferracane, J.L. Academy of Dental Materials guidance-Resin composites: Part I-Mechanical properties. Dent. Mater. 2017, 33, 880–894. [Google Scholar] [CrossRef]
- Alania, Y.; Chiari, M.D.; Rodrigues, M.C.; Arana-Chavez, V.E.; Bressiani, A.H.; Vichi, F.M.; Braga, R.R. Bioactive composites containing TEGDMA-functionalized calcium phosphate particles: Degree of conversion, fracture strength and ion release evaluation. Dent. Mater. 2016, 32, e374–e381. [Google Scholar] [CrossRef]
- Loy, C.W.; Whitten, A.E.; de Campo, L.; Appadoo, D.; Zainuddin, N.; Matori, K.A.; Rehm, C.; Sokolova, A.; Wang, C.; Xia, Q.; et al. Investigation of the siliceous hydrogel phase formation in glass-ionomer cement paste. Phys. B Condens. Matter 2018, 551, 287–290. [Google Scholar] [CrossRef]
- Faria, M.; Guedes, A.; Rompante, P.; Carvalho, O.; Silva, F.; Henriques, B.; Özcan, M.; Souza, J.C.M. Wear Pathways of Tooth Occlusal Fissure Sealants: An Integrative Review. Biotribology 2021, 27, 100190. [Google Scholar] [CrossRef]
- Sokolowski, G.; Szczesio, A.; Bociong, K.; Kaluzinska, K.; Lapinska, B.; Sokolowski, J.; Domarecka, M.; Lukomska-Szymanska, M. Dental Resin Cements-The Influence of Water Sorption on Contraction Stress Changes and Hydroscopic Expansion. Materials 2018, 11, 973. [Google Scholar] [CrossRef]
- Nomura, R.; Kitamura, T.; Matayoshi, S.; Ohata, J.; Suehiro, Y.; Iwashita, N.; Okawa, R.; Nakano, K. Inhibitory effect of a gel paste containing surface pre-reacted glass-ionomer (S-PRG) filler on the cariogenicity of Streptococcus mutans. Sci. Rep. 2021, 11, 23495. [Google Scholar] [CrossRef] [PubMed]
- Al-Eesa, N.A.; Johal, A.; Hill, R.G.; Wong, F.S.L. Fluoride containing bioactive glass composite for orthodontic adhesives—Apatite formation properties. Dent. Mater. 2018, 34, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
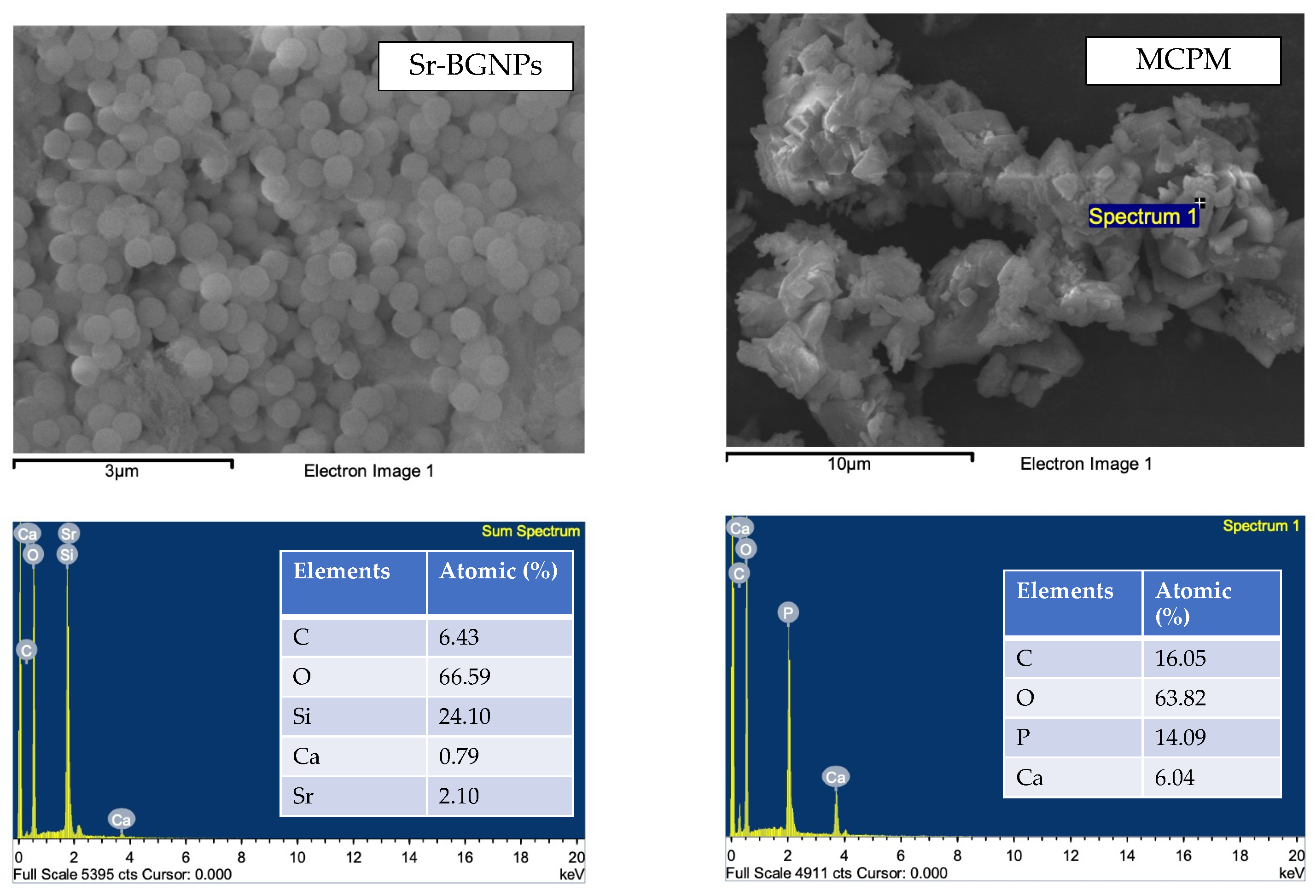


| Formulations | Sr/F-BGNPs (wt%) | MCPM (wt%) | Glass (wt%) |
|---|---|---|---|
| TS1 | 10 | 2 | 88 |
| TS2 | 5 | 4 | 91 |
| Name | Composition | Manufacturer | Lot No. |
|---|---|---|---|
| ClinproTM Sealant (CP) | Triethylene glycol dimethacrylate (40–50 wt%), bisphenol A-glycidyl methacrylate (40–50 wt%), silane treated silica (5–10 wt%), tetrabutylammonium tetrafluoroborate (<5 wt%), diphenyliodonium hexafluorophosphate (<1 wt%), triphenylantimony (<0.5 wt%), DL-camphoquinone (<2 wt%, ethyl 4-(dimethylamino)benzoate (EDMAB, <2 wt%), titanium dioxide (<0.5 wt%), hydroquinone (<0.05 wt%) | 3M, Saint Paul, MN, USA | 09997 |
| BeautiSealant (BT) | Surface pre-reacted glass fillers (S-PRG, 30 wt%), micro fumed silica, urethane dimethacrylate (25–30 wt%), triethyleneglycol dimethacrylate, dimethyl aminoethyl methacrylate (0.1–2 wt%) | SHOFU INC., Kyoto, Japan | 092101 |
| Formulations | Ca | P | Sr |
|---|---|---|---|
| TS1 | 1421 ± 1043 (a) | 214 ± 54 (a) | 1620 ± 1329 |
| TS2 | 611 ± 310 | 236 ± 74 (b) | 666 ± 358 |
| CP | 15 ± 10 (a,b) | 24 ± 7 (a,b) | ND |
| BT | 1650 ± 214 (b) | ND | 1614 ± 210 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panpisut, P.; Praesuwatsilp, N.; Bawornworatham, P.; Naruphontjirakul, P.; Patntirapong, S.; Young, A.M. Assessment of Physical/Mechanical Performance of Dental Resin Sealants Containing Sr-Bioactive Glass Nanoparticles and Calcium Phosphate. Polymers 2022, 14, 5436. https://doi.org/10.3390/polym14245436
Panpisut P, Praesuwatsilp N, Bawornworatham P, Naruphontjirakul P, Patntirapong S, Young AM. Assessment of Physical/Mechanical Performance of Dental Resin Sealants Containing Sr-Bioactive Glass Nanoparticles and Calcium Phosphate. Polymers. 2022; 14(24):5436. https://doi.org/10.3390/polym14245436
Chicago/Turabian StylePanpisut, Piyaphong, Nannapat Praesuwatsilp, Phubet Bawornworatham, Parichart Naruphontjirakul, Somying Patntirapong, and Anne M. Young. 2022. "Assessment of Physical/Mechanical Performance of Dental Resin Sealants Containing Sr-Bioactive Glass Nanoparticles and Calcium Phosphate" Polymers 14, no. 24: 5436. https://doi.org/10.3390/polym14245436
APA StylePanpisut, P., Praesuwatsilp, N., Bawornworatham, P., Naruphontjirakul, P., Patntirapong, S., & Young, A. M. (2022). Assessment of Physical/Mechanical Performance of Dental Resin Sealants Containing Sr-Bioactive Glass Nanoparticles and Calcium Phosphate. Polymers, 14(24), 5436. https://doi.org/10.3390/polym14245436










