Flexible Thermoelectric Reduced Graphene Oxide/Ag2S/Methyl Cellulose Composite Film Prepared by Screen Printing Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
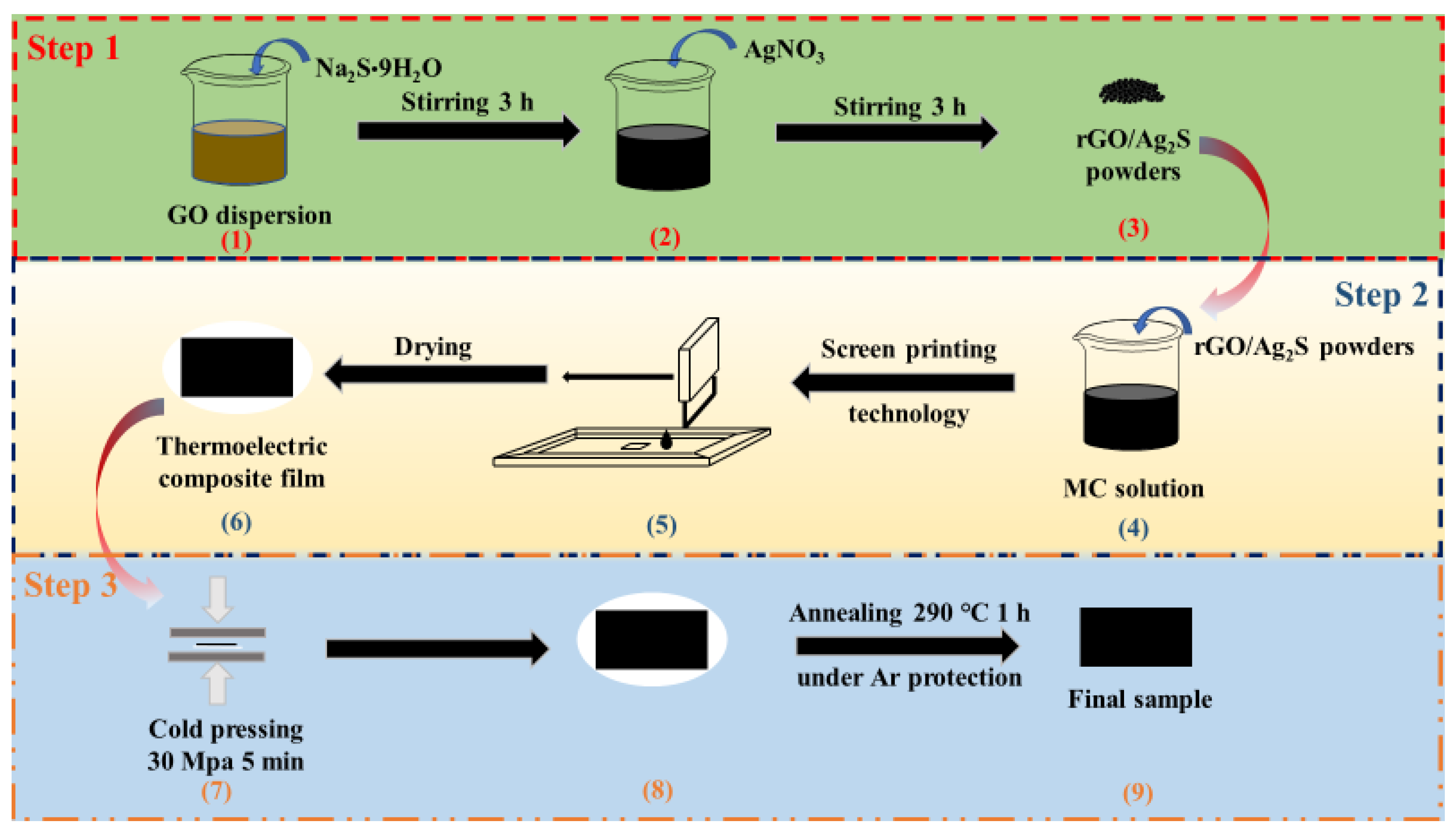
2.2. Preparation of rGO/Ag2S Composite Powders
2.3. Preparation of rGO/Ag2S/MC Composite TE Films
2.4. Post-Treatment of rGO/Ag2S/MC Composite TE Films
2.5. Characterization and Measurement
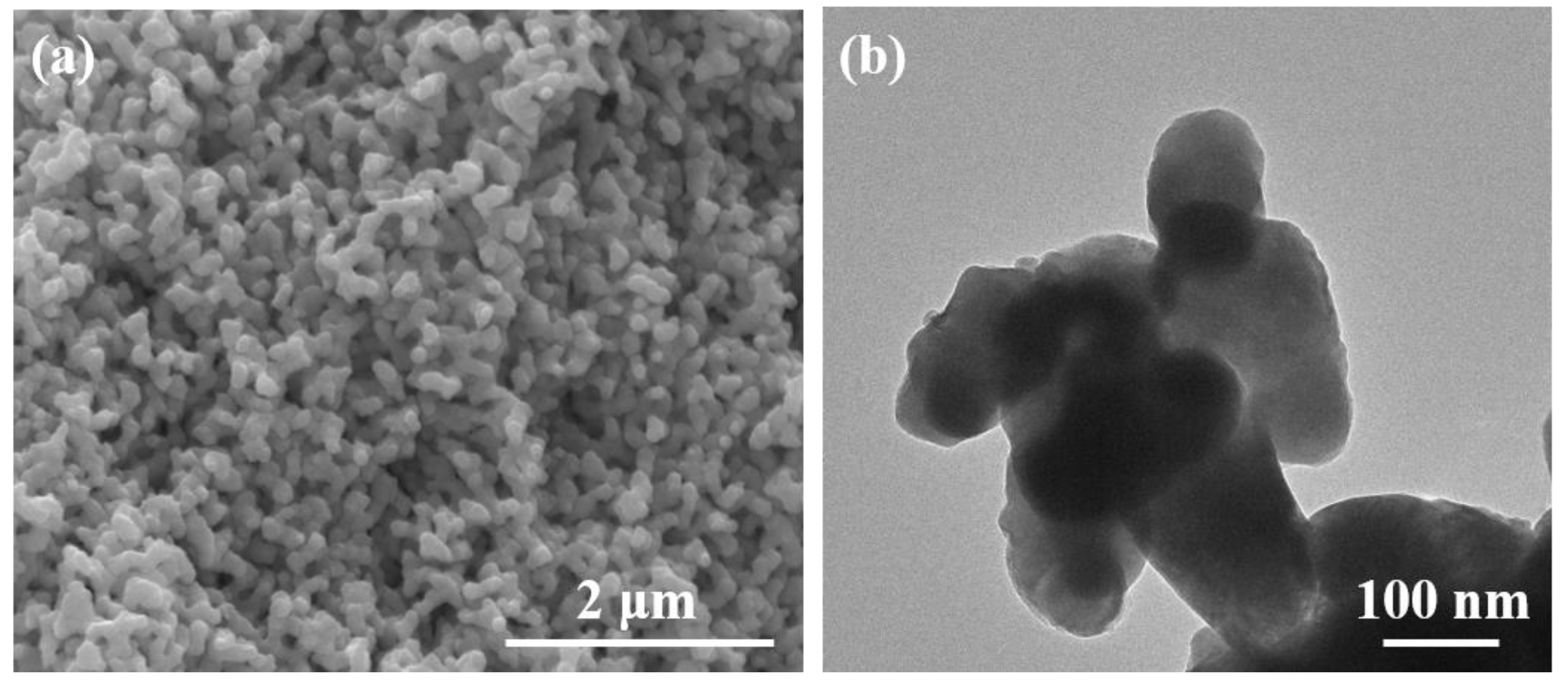
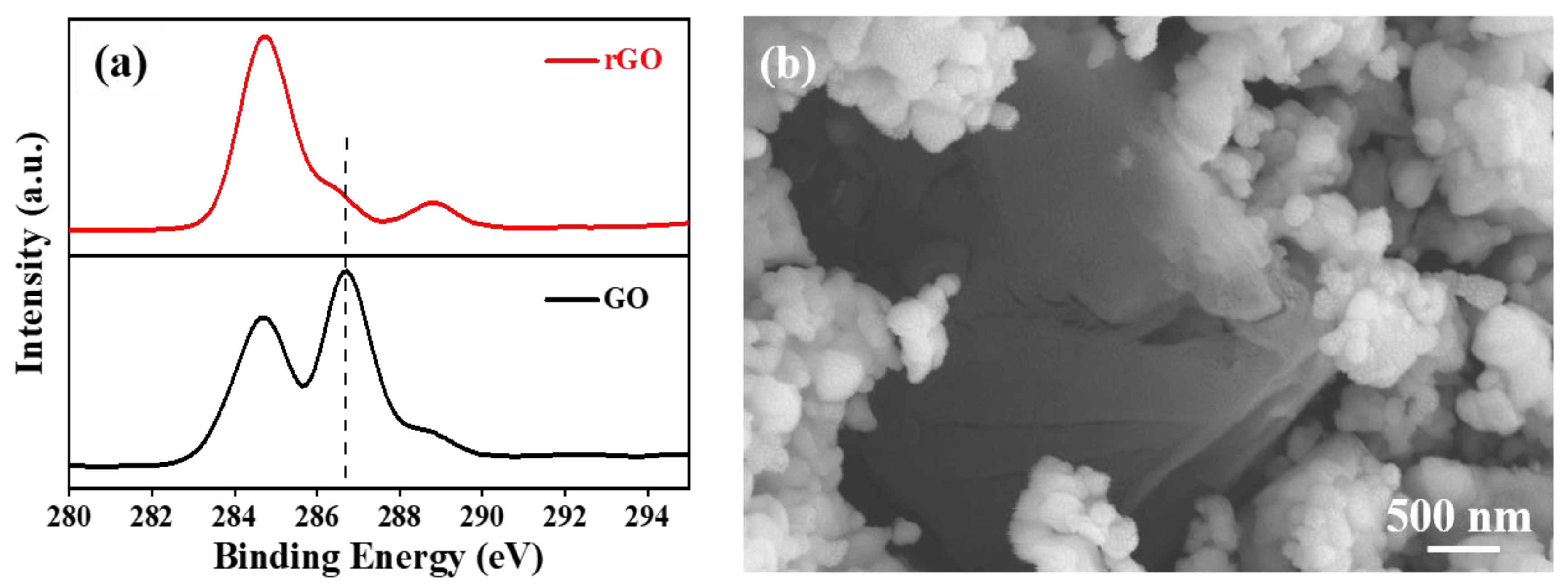
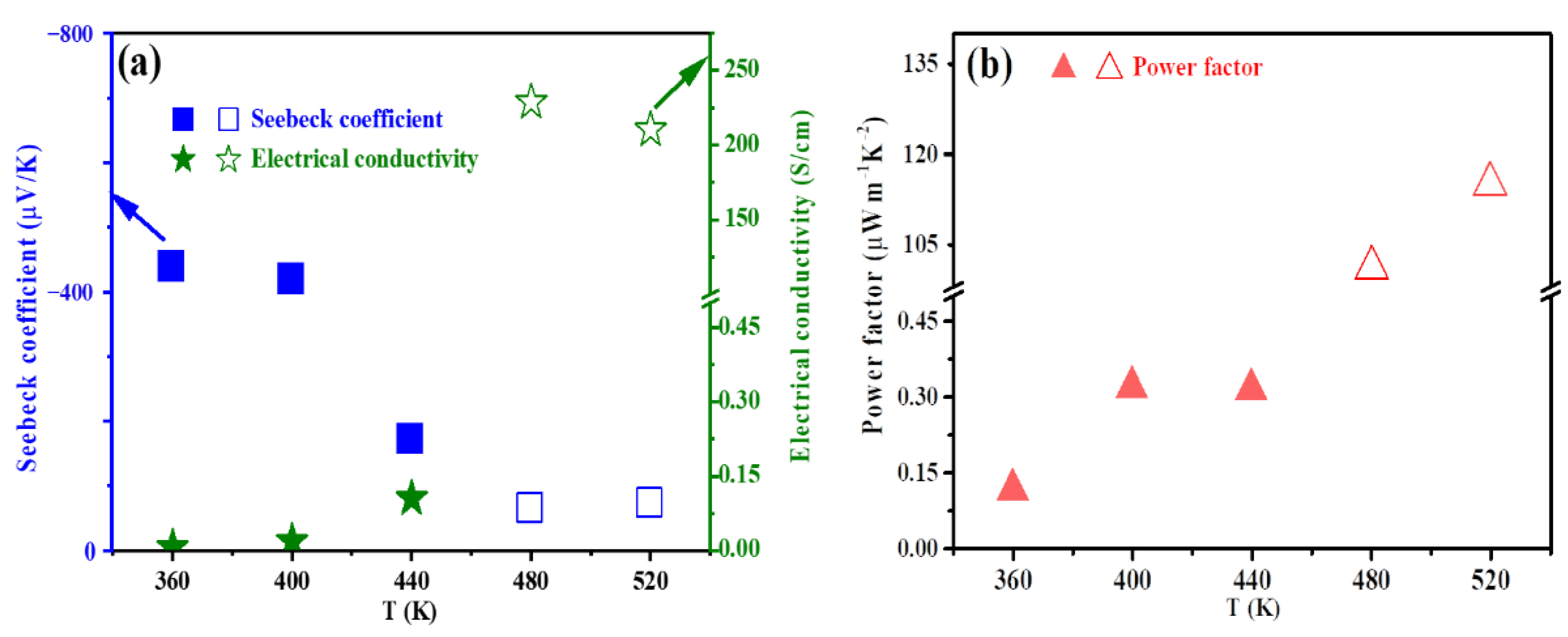
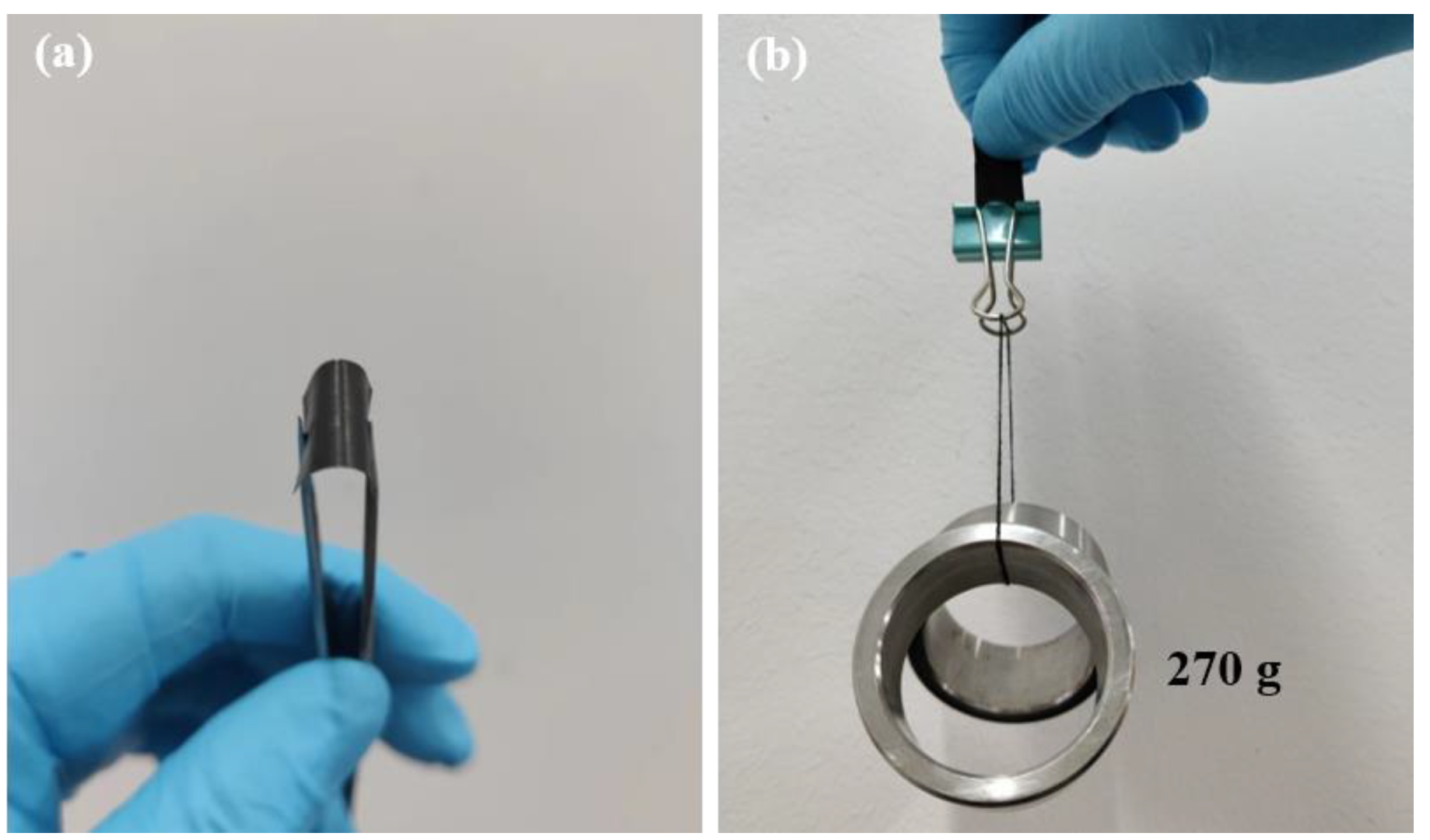
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shakouri, A. Recent developments in semiconductor thermoelectric physics and materials. Annu. Rev. Mater. Res. 2011, 41, 399–431. [Google Scholar] [CrossRef]
- Yang, H.L.; Boulet, P.; Record, M.C. New insight into the structure-property relationships from chemical bonding analysis: Application to thermoelectric materials. J. Solid State Chem. 2020, 286, 121266. [Google Scholar] [CrossRef]
- Bahrami, A.; Schierning, G.; Nielsch, K. Waste recycling in thermoelectric materials. Adv. Energy Mater. 2020, 10, 1904159. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Huang, X.Y.; Bai, S.Q.; Shi, X.; Uher, C.; Chen, L.D. Thermoelectric devices for power generation: Recent progress and future challenges. Adv. Eng. Mater. 2016, 18, 194–213. [Google Scholar] [CrossRef]
- Sarris, A.; Bhatti, B.; Ciampa, F. Thermoelectric energy harvesting using vapour chamber coolers for aerospace applications. J. Intell. Mat. Syst. Struct. 2021, 33, 1602–1612. [Google Scholar] [CrossRef]
- Araiz, M.; Casi, Á.; Catalán, L.; Martínez, Á.; Astrain, D. Prospects of waste-heat recovery from a real industry using thermoelectric generators: Economic and power output analysis. Energ. Convers. Manag. 2020, 205, 112376. [Google Scholar] [CrossRef]
- Kumar, P.M.; Babu, V.J.; Subramanian, A.; Bandla, A.; Thakor, N.; Ramakrishna, S.; Wei, H. The design of a thermoelectric generator and its medical applications. Designs 2019, 3, 22. [Google Scholar] [CrossRef]
- Nozariasbmarz, A.; Collins, H.; Dsouza, K.; Polash, H.M.; Hosseini, M.; Hyland, M.; Liu, J.; Malhotra, A.; Ortiz, F.M.; Mohaddes, F.; et al. Review of wearable thermoelectric energy harvesting: From body temperature to electronic systems. Appl. Energy 2020, 258, 114069. [Google Scholar] [CrossRef]
- Du, Y.; Chen, J.G.; Meng, Q.F.; Dou, Y.C.; Xu, J.Y.; Shen, S.Z. Thermoelectric materials and devices fabricated by additive manufacturing. Vacuum 2020, 178, 109384. [Google Scholar] [CrossRef]
- Du, Y.; Li, J.; Xu, J.Y.; Eklund, P. Thermoelectric properties of reduced graphene oxide/Bi2Te3 nanocomposites. Energies 2019, 12, 2430. [Google Scholar] [CrossRef]
- Pourkiaei, S.M.; Ahmadi, M.H.; Sadeghzadeh, M.; Moosavi, S.; Pourfayaz, F.; Chen, L.G.; Yazdi, M.A.P.; Kumar, R. Thermoelectric cooler and thermoelectric generator devices: A review of present and potential applications, modeling and materials. Energy 2019, 186, 115849. [Google Scholar] [CrossRef]
- Tarachand, A.; Mukherjee, B.; Saxena, M.; Kuo, Y.K.; Okram, G.S.; Dam, S.; Hussain, S.; Lakhani, A.; Deshpande, U.; Shripathi, T. Ag-nanoinclusion-induced enhanced thermoelectric properties of Ag2S. ACS Appl. Energy Mater. 2019, 2, 6383–6394. [Google Scholar] [CrossRef]
- Zhou, W.X.; Wu, D.; Xie, G.F.; Chen, K.Q.; Zhang, G. α-Ag2S: A ductile thermoelectric material with high ZT. ACS Omega 2020, 5, 5796–5804. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.Z.; Yang, L.J.; Wang, J.F.; Fan, C.G.; Hu, J.L. Synthesis and electrochemical properties of Ag2S and Ag2S/Cu2S crystals. J. Surf. Sci. Nanotechnol. 2010, 8, 384–387. [Google Scholar] [CrossRef]
- Li, X.N.; Yang, X.C.; Han, S.S.; Lu, W.; Hou, J.W.; Liu, Y. Synthesis and characterization of high density and high aspect ratio Ag2S nanoparticle nanowires from a paired cell method. Chin. Sci. Bull. 2011, 56, 1828–1831. [Google Scholar] [CrossRef][Green Version]
- Wang, H.Y.; Liu, X.F.; Zhang, B.; Huang, L.S.; Yang, M.L.; Zhang, X.; Zhang, H.; Wang, G.Y.; Zhou, X.Y.; Han, G. General surfactant-free synthesis of binary silver chalcogenides with tuneable thermoelectric properties. Chem. Eng. J. 2020, 393, 124763. [Google Scholar] [CrossRef]
- Wang, H.T.; Ma, H.Q.; Duan, B.; Geng, H.Y.; Zhou, L.; Li, J.L.; Zhang, X.L.; Yang, H.J.; Li, G.D.; Zhai, P.C. High-pressure rapid preparation of high-performance binary silver sulfide thermoelectric materials. ACS Appl. Energy Mater. 2021, 4, 1610–1618. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.Y.; Qiu, P.F.; Shi, X.; Chen, L.D. Thermoelectric properties of Ag2S superionic conductor with intrinsically low lattice thermal conductivity. Acta Phys. Sin. 2019, 68, 20190073. [Google Scholar]
- Zhang, P.; Li, Z.; Zhang, S.J.; Shao, G.S. Recent advances in effective reduction of graphene oxide for highly improved performance toward electrochemical energy storage. Energy Environ. Mater. 2018, 1, 5–12. [Google Scholar] [CrossRef]
- Song, J.; Sui, Y.; Zhao, Q.; Ye, Y.C.; Qin, C.L.; Chen, X.S.; Song, K. A reinforced concrete structure rGO/CNTs/Fe2O3/PEDOT:PSS paper electrode with excellent wettability and flexibility for supercapacitors. New J. Chem. 2021, 45, 14483–14494. [Google Scholar] [CrossRef]
- Ge, G.; Cai, Y.C.; Dong, Q.C.; Zhang, Y.Z.; Shao, J.J.; Huang, W.; Dong, X.C. A flexible pressure sensor based on rGO/polyaniline wrapped sponge with tunable sensitivity for human motion detection. Nanoscale 2018, 10, 10033–10040. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.S.; Lu, J.Z.; Ma, D.W.; Ma, C.M.; Zhang, B.; Wang, H.Y.; Wang, G.Y.; Gregory, D.H.; Zhou, X.Y.; Han, G. Facile in situ solution synthesis of SnSe/rGO nanocomposites with enhanced thermoelectric performance. J. Mater. Chem. A 2020, 8, 1394–1402. [Google Scholar] [CrossRef]
- Gao, J.; Liu, C.Y.; Miao, L.; Wang, X.Y.; Peng, Y.; Chen, Y. Enhanced power factor in flexible reduced graphene oxide/nanowires hybrid films for thermoelectrics. RSC Adv. 2016, 6, 31580–31587. [Google Scholar] [CrossRef]
- Mitra, M.; Kulsi, C.; Chatterjee, K.; Kargupta, K.; Ganguly, S.; Banerjee, D.; Goswami, S. Reduced graphene oxide-polyaniline composites—Synthesis, characterization and optimization for thermoelectric applications. RSC Adv. 2015, 5, 31039–31048. [Google Scholar] [CrossRef]
- Li, F.Y.; Cai, K.F.; Shen, S.; Chen, S. Preparation and thermoelectric properties of reduced graphene oxide/PEDOT:PSS composite films. Synth. Met. 2014, 197, 58–61. [Google Scholar] [CrossRef]
- Gao, J.; Liu, C.Y.; Miao, L.; Wang, X.Y.; Peng, Y.; Chen, Y. Improved thermoelectric performance in flexible tellurium nanowires/reduced graphene oxide sandwich structure hybrid films. J. Electron. Mater. 2017, 46, 3049–3056. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, H.; Kim, Y.; We, J.H.; Shin, J.S.; Lee, H.E.; Oh, M.W.; Lee, K.J.; Cho, B.J. Post ionized defect engineering of the screen-printed Bi2Te2.7Se0.3 thick film for high performance flexible thermoelectric generator. Nano Energy 2017, 31, 258–263. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Du, Y.; Meng, Q.F.; Shen, S.Z. Preparation and thermoelectric properties of screen-printable rGO/Sb2Te3/SV4/PEDOT: PSS composite thermoelectric film. Mater. Res. Express 2021, 8, 065503. [Google Scholar] [CrossRef]
- Cao, Z.; Koukharenko, E.; Tudor, M.J.; Torah, R.N.; Beeby, S.P. Screen printed flexible Bi2Te3-Sb2Te3 based thermoelectric generator. J. Phys. Conf. Ser. 2013, 476, 012031. [Google Scholar] [CrossRef]
- Burton, M.; Howells, G.; Atoyo, J.; Carnie, M. Printed Thermoelectrics. Adv. Mater. 2022, 34, 2108183. [Google Scholar] [CrossRef]
- Wei, Q.S.; Mukaida, M.; Kirihara, K.; Naitoh, Y.; Ishida, T. Polymer thermoelectric modules screen-printed on paper. RSC Adv. 2014, 4, 28802–28806. [Google Scholar] [CrossRef]
- Shin, S.; Kumar, R.; Roh, J.W.; Ko, D.S.; Kim, H.S.; Kim, S.I.; Yin, L.; Schlossberg, S.M.; Cui, S.; You, J.M.; et al. High-performance screen-printed thermoelectric films on fabrics. Sci. Rep. 2017, 7, 7317. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhao, Y.X.; Yan, Z.Q.; Zhang, Z.D.; Zhang, Y.J.; Shi, P.; Xue, C.Y. Screen-printed flexible thermoelectric device based on hybrid silver selenide/PVP composite Films. Nanomaterials 2021, 11, 2042. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Huang, R.; Newbrook, D.; Sethi, V.; Yong, S.; Beeby, S.; Nandhakumar, I. Screen-printed bismuth telluride nanostructured composites for flexible thermoelectric applications. J. Phys. Energy 2022, 4, 024003. [Google Scholar] [CrossRef]
- Li, X.; Du, Y.; Meng, Q.F. Flexible ball-milled Bi0.4Sb1.6Te3/methyl cellulose thermoelectric films fabricated by screen-printing method. Funct. Mater. Lett. 2021, 14, 2151034. [Google Scholar] [CrossRef]
- Niu, H.; Liu, Y.Q.; Song, H.J.; Meng, Q.F.; Du, Y.; Shen, S.Z. Facile preparation of flexible all organic PEDOT: PSS/methyl cellulose thermoelectric composite film by a screen printing process. Synth. Met. 2021, 276, 116752. [Google Scholar] [CrossRef]
- Yang, W.D.; Li, Y.R.; Lee, Y.C. Synthesis of r-GO/TiO2 composites via the UV-assisted photocatalytic reduction of graphene oxide. Appl. Surf. Sci. 2016, 380, 249–256. [Google Scholar] [CrossRef]
- Pham, V.H.; Hur, S.H.; Kim, E.J.; Kim, B.S.; Chung, J.S. Highly efficient reduction of graphene oxide using ammonia borane. Chem. Commun. 2013, 49, 6665–6667. [Google Scholar] [CrossRef]
- Liang, X.; Chen, C.; Dai, F. Effect of plastic deformation on phonon thermal conductivity of α-Ag2S. Appl. Phys. Lett. 2020, 117, 253901. [Google Scholar] [CrossRef]
- Gusev, A.I.; Sadovnikov, S.I. Acanthite–argentite transformation in nanocrystalline silver sulfide and the Ag2S/Ag nanoheterostructure. Semiconductors 2016, 50, 682–687. [Google Scholar] [CrossRef]
- Abdulzadeh, N.N.; Mursakulov, N.N.; Ahmedsadeh, R.G. Temperature Dependence of Thermo-emf of Ag2S. In Proceedings of the TPE-06 3rd International Conference on Technical and Physical Problems in Power Engineering, Ankara, Turkey, 29–31 May 2006. [Google Scholar]
- Cao, Z.; Koukharenko, E.; Tudor, M.J.; Torah, R.N.; Beeby, S.P. Flexible screen printed thermoelectric generator with enhanced processes and materials. Sens. Actuators A Phys. 2016, 238, 196–206. [Google Scholar] [CrossRef]
- Gierczak, M.; Prażmowska-Czajka, J.; Dziedzic, A. Thermoelectric mixed thick-/thin film microgenerators based on constantan/silver. Materials 2018, 11, 115. [Google Scholar] [CrossRef] [PubMed]

| Author | Years | Methods | Post-Treatment | Materials | Type | σa (S/cm) | |S| (µV/K) | PF (µWm−1K−2) | Temperature | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Wei et al. | 2014 | Screen printing | PEDOT: PSS film | P | 550 | 25 | 34 | 473 K | [31] | |
| Shin et al. | 2017 | Screen printing | Sintering and hot-pressing treatment | Bi0.5Sb1.5Te3 | P | 639 | 209 | 2791 | RT | [32] |
| Shin et al. | 2017 | Screen printing | Sintering and hot-pressing treatment | Bi2Te2.7Se0.3 | N | 763 | 165 | 2077 | RT | [32] |
| Liu et al. | 2021 | Screen printing | Sintering treatment | Ag2Se/PVP composites with the content ratio of Ag2Se:PVP = 30:1 | N | ~12.56 | 58.5 | 4.3 | 390 K | [33] |
| Amin et al. | 2022 | Screen printing | Annealing treatment | Bi2Te3 NWs/PVDF composite films with a 10 wt% PVDF | N | 9.8 | 192 | 36 | 225 K | [34] |
| Niu et al. | 2021 | Screen printing | DMSO treatment | PEDOT:PSS/MC composite film with a 25.67 wt% MC | P | 316.8 | 22.6 | 16.2 | 340 K | [36] |
| Li et al. | 2021 | Screen printing | Cold-pressing treatment | Bi0.4Sb1.6Te3/MC composite film with 80 vol.% of Bi0.4Sb1.6Te3 powders | P | 4 | 158.5 | 10.07 | RT | [35] |
| Cao et al. | 2016 | Screen printing | Annealing treatment | Bi3.2Sb1.8/ Epoxy A | N | ~6.85 | 143.5 | 14 | ~RT | [42] |
| Wang et al. | 2022 | Screen printing | Cold-pressing and annealing treatment | rGO/Ag2S/MC composite film with 90 wt% rGO/Ag2S composite powders | N | 210.18 | 73.96 | 115 | 520 K | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Du, Y.; Qin, J.; Wang, L.; Meng, Q.; Li, Z.; Shen, S.Z. Flexible Thermoelectric Reduced Graphene Oxide/Ag2S/Methyl Cellulose Composite Film Prepared by Screen Printing Process. Polymers 2022, 14, 5437. https://doi.org/10.3390/polym14245437
Wang J, Du Y, Qin J, Wang L, Meng Q, Li Z, Shen SZ. Flexible Thermoelectric Reduced Graphene Oxide/Ag2S/Methyl Cellulose Composite Film Prepared by Screen Printing Process. Polymers. 2022; 14(24):5437. https://doi.org/10.3390/polym14245437
Chicago/Turabian StyleWang, Jianjun, Yong Du, Jie Qin, Lei Wang, Qiufeng Meng, Zhenyu Li, and Shirley Z. Shen. 2022. "Flexible Thermoelectric Reduced Graphene Oxide/Ag2S/Methyl Cellulose Composite Film Prepared by Screen Printing Process" Polymers 14, no. 24: 5437. https://doi.org/10.3390/polym14245437
APA StyleWang, J., Du, Y., Qin, J., Wang, L., Meng, Q., Li, Z., & Shen, S. Z. (2022). Flexible Thermoelectric Reduced Graphene Oxide/Ag2S/Methyl Cellulose Composite Film Prepared by Screen Printing Process. Polymers, 14(24), 5437. https://doi.org/10.3390/polym14245437







