Abstract
To improve the mechanical properties of polyurethane cross-linked poly (ethylene oxide-co-tetrahydrofuran) (P(E-co-T)) elastomers at room temperature, using poly (ethylene oxide-co-tetrahydrofuran) and high-molecular-weight polyethylene glycol (PEG) as raw materials and polyisocyanate N100 as curing agent, a series of polyurethane cross-linked blended polyether elastomers were prepared by changing the elastomer-curing parameter R value (n(-NCO)/n(-OH)) and P(E-co-T)/PEG ratio. Equilibrium swelling measurements showed that the chemical cross-linkage of the elastomers tended to decrease with the decreasing R value, the average molecular weight (Mc) of the network chain increased, and the density of the network chain (N0) decreased. Wide-angle X-ray diffraction (WAXD) and differential scanning calorimetry (DSC) tests showed that PEG chain segments within the elastomers crystallized at room temperature, while the crystallinity increased with decreasing R value and increasing PEG content. The mechanical property tests showed that the elongation at break tended to decrease with increasing R value; the tensile strength first increased and then decreased. At R value 0.9, the elastomer presented good comprehensive mechanical properties. In addition, the mechanical properties of polyurethane cross-linked P(E-co-T)/PEG blended polyether elastomer showed an increasing trend with the increase in PEG content when the curing parameter of 0.9 remained unchanged.
1. Introduction
Polyurethane elastomer is a kind of polymer material made from polyisocyanate and macromolecular polyol, which has good chemical and mechanical properties and is widely used in many fields, such as the automobile industry, the printing industry, the leather industry, and so on [1,2,3]. Among them, thermoset polyurethane elastomer is a very important category of polyurethane elastomers, which has the advantages of good topological structure stability, chemical resistance, wear resistance, and thermal stability [3,4,5], and can be applied in the aerospace industry and other industries [6,7,8].
Until now, the mechanical properties of thermoset polyurethane elastomers have been focused on the chemical crosslinking density of the elastomer [5,9,10,11], the chemical structure of the cross-linking point [5,12,13], and the type of isocyanate curing agent used [14,15,16]. Meiorin et al. [5] have used MDI (4,4′-diphenylmethane diisocyanate) as the curing agent, and glycerol and trimethylolpropane as the cross-linking agents, and prepared a series of polyurethane elastomers with different crosslinking density, mechanical properties, and thermal stability. Zhai et al. [12] have prepared a series of polyurethane crosslinked poly(3,3-bis(azidomethy)oxetane-tetrahydrofuran) elastomers with identical chemical crosslinking networks and different chemical structures at the crosslinking points, and the results show that the hydrogen bonding between the crosslinking points has an important effect on the mechanical properties of the elastomers. Liu et al. [14] have used isophorone diisocyanate and dicyclohexylmethane 4,4′-diisocyanate as curing agents to prepare thermoset polyurethane elastomers, and the results show that the structure of the isocyanate curing agents has an important influence on the mechanical properties of the elastomers. Kojio et al. [15] have used TEGDI (1,2-diisocyanatoethoxyethane) and HDI (1,6-diisocyanatohexane) as curing agents, respectively, and prepared polyurethane elastomers, and the results show that the elastomers prepared by TEGDI have lower tensile modulus and higher elongation than HDI due to the flexible ether bond structure in the TEGDI molecule. Additionally, recent studies have shown that polyurethane crosslinking reaction kinetics is also an important factor affecting the chemical crosslinking network and the macroscopic properties of elastomers [17,18].
In addition to using the chemical crosslinking structures to modulate the mechanical properties of polyurethane elastomers, using the crystallization behavior of macromolecular soft segments is also an important method for preparing elastomers with excellent mechanical properties [19,20,21]. Eceiza et al. [22] have reported that the strain-induced crystallization of polycarbonate chain segments can strengthen the physical crosslinking interaction between the chains and improve the mechanical properties of the elastomer. Anokhin et al. [23] blended PCL (poly-ε-caprolactone) and PBA (poly(1,4-butylene adipate)), whose crystallization behavior is different from PCL, and obtained a series of polyurethane chain-extended PCL/PBA elastomers with different mechanical properties. Given that the crystallinity is related to elastomer thermal history, Gorbunova et al. [24] subjected polyurethane chain-extended PBA elastomers to different heat treatments to modulate the crystallinity of PBA segments within the elastomers and achieved elastomers with different mechanical properties. However, due to the different thermodynamic properties of macromolecule chains, the blended parts within thermoplastic elastomers would show some de-mixing phenomena during long-term storage because of molecular chain slow creep, causing the mechanical properties to change [25,26,27]. In contrast, within a thermoset elastomer, the molecular chains are fixed into a crosslinked network structure by a crosslinking agent, and even if the resultant material tends toward de-mixing thermodynamically, the de-mixing phenomena would not occur. In combination with the soft segment crystallization mechanism of thermoplastic elastomers and the thermal stability advantage of thermoset elastomers, the mechanical properties of thermoset elastomers could be further improved.
Poly(ethylene oxide-co-tetrahydrofuran) is amorphous at room temperature, and it is difficult to form effective crystallization physical crosslinking [28]; large molecule polyethylene glycol has a regular molecular chain structure, is prone to crystallizing, and forms physical crosslinks [29]. Given that P(E-co-T) and PEG have similar chemical composition of chain segments and similar solubility parameters, they have good mutual solubility [30]. In this paper, polyurethane cross-linked elastomers were prepared by blending P(E-co-T) and PEG and the influence mechanism of the elastomer crystallization behaviors on the mechanical properties was investigated in detail (Figure 1).
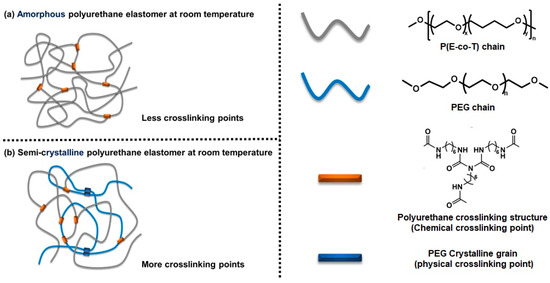
Figure 1.
Aggregations of polyurethane crosslinked thermoset elastomers.
2. Materials and Methods
2.1. Materials
Hydroxyl terminated poly (ethylene oxide-co-tetrahydrofuran) (P(E-co-T), Mn 4038 g mol−1, hydroxyl value 0.467 mmol g−1) and polyisocyanate cross-linker N100 (Mn 741 g mol−1, isocyanate concentration 5.36 mmol g−1) were provided by Luoyang Liming Chemical Research Institute. Polyethylene glycol (PEG, Mn 4000 g mol−1, hydroxyl value 0.5 mmol g−1,) was purchased from Aladdin Chemical Company. Curing catalyst ditin butyl dilaurate (T12) was purchased from Maclin Chemical Company.
2.2. Preparation of Elastomers
Fixing PEG content at 12%, the formulations of blended polyether elastomers with different curing parameter R values (the molar ratios of the isocyanate group to the hydroxyl group, n(-NCO)/n(-OH)) are listed in Table 1. According to Table 1, all components were uniformly mixed at 65 °C, poured into Teflon molds, and degassed in a vacuum. The resultant mixtures were cured at 65 °C until the isocyanate absorption peak at 2260 cm−1 disappeared by FTIR analysis. Thus, a series of polyurethane cross-linked blended polyether elastomers S1-1~S1-5 were obtained.

Table 1.
Formulation composition of elastomers with different R values.
Fixing R value at 0.9, the elastomer formulations with different PEG contents are listed in Table 2. Similarly, as shown in Table 2, all components were uniformly mixed at 65 °C, poured into Teflon molds, and degassed in a vacuum. The mixtures were cured at 65 °C until the isocyanate absorption peak at 2260 cm−1 disappeared by FTIR analysis. Thus, a series of polyurethane cross-linked blended polyether elastomers S2-1~S2-4 were obtained.

Table 2.
Formulation composition of elastomers with different PEG contents.
2.3. Characterization
2.3.1. FTIR
Elastomer samples were tested at 20 °C by using a Nicolet 6700 infrared spectrometer (Thermo) with an attenuated total reflection (ATR) assembly for FTIR investigation. The tests were carried out with a resolution of 2 cm−1, 32 scans, and a scan range of 4000–500 cm−1.
2.3.2. Density Test
The densities of elastomer samples were tested by AccuPyc II 1345 true density analyzer (Micromeritics). Nitrogen was used as a test gas, and the test temperature was 20 °C. Before testing, nitrogen was purged 10 times. For each sample, the number of tests was 10 times, and the average of the test results was taken as the density of the sample.
2.3.3. Equilibrium Swelling Measurement
Equilibrium swelling measurements were performed in the solvent toluene at 20 °C. The elastomer sample (about 6 × 5 × 3 mm3, ~0.1 g) was immersed in toluene, and the sample was taken out at intervals, the residual solvent on the surface was swept and weighed, and the sample was put back into the toluene solvent. The above-mentioned steps were repeated until the mass difference between two consecutive steps was less than 0.001 g. The equilibrium volume-swelling ratio () of the elastomer was calculated using Equation (1) [31], where is the initial mass of the sample, is the mass after swelling, is the solvent density, and is the elastomer density.
2.3.4. DSC Test
Elastomer samples were tested by using F204 differential scanning calorimeter (DSC, Netzsch). The instrument was temperature-corrected using indium standard (Tm = 156.6 °C). All tests were conducted under dry nitrogen atmosphere. Samples of 5–10 mg were first heated to 100 °C at a heating rate of 30 °C min−1, then held for 5 min to eliminate the thermal history. Then the samples were cooled to −50 °C at a cooling rate of 5 °C min−1 using liquid nitrogen. After that, the samples were heated again to 100 °C at a heating rate of 10 °C min−1, and the data of the secondary heating were recorded.
2.3.5. Wide-Angle X-ray Diffraction Test
Wide-angle X-ray diffraction (WAXD) was performed on elastomer samples by using a MiniFlex 600 X-ray diffractometer (Gigaku) with Ni-filtered Cu Kα radiation (40 kV, 40 mA). The test was conducted at 20 °C with a scanning speed of 2° min−1, and the 2θ angle ranged from 5° to 40°.
2.3.6. Mechanical Properties Test
Elastomer samples were tested by using a CMT4104 tensile tester (MTS). Elastomer samples were cut into dumbbell-shaped specimens (central portion 4 mm × 3 mm, gauge length 15 mm). The test temperature was 20 °C and the tensile rate was 20 mm min−1. The stress–strain curves were recorded with a minimum of three valid values for each group sample.
3. Result and Discussion
3.1. FT-IR of Elastomers with Different R Values
The infrared spectra of elastomers S1-1~S1-5, which were prepared according to Table 1, are shown in Figure 2. It can be seen that there existed some typical group absorption peaks, among which: ~2900 cm−1 absorption peak corresponds to the C-H stretching vibration of -CH2- on the polyether chain of P(E-co-T) and PEG, and the absorption peak at ~1100 cm−1 is the stretching vibration of the ether bonds. All infrared spectra of elastomers S1-1~1–5 had no obvious absorption peaks at ~2260 cm−1, indicating that the isocyanate curing agent had completely reacted with the terminal hydroxyl of the blended polyether P(E-co-T)/PEG, having formed the carbamate structure. As a result, all infrared absorption spectra of elastomer S1-1~1–5 gave a characteristic peak at ~1700 cm−1 that was attributed to the formed carbamate group.
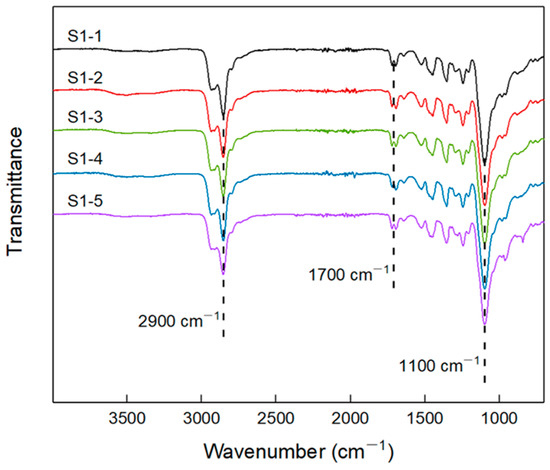
Figure 2.
FTIR spectra of elastomers S1-1~S1-5.
3.2. Chemical Crosslinking Networks of Elastomers with Different R Values
The chemical crosslinking network is one of the most important factors affecting elastomer mechanical properties. Elastomers S1-1~S1-5 were tested for swelling behaviors, and the relationship of equilibrium volume-swelling ratio (qv) dependent on R value is shown in Figure 3. Obviously, the equilibrium volume–swelling ratio of elastomers S1-1~S1-5 decreased monotonically with the increasing R value. Based on the Flory–Huggins theory [32], the apparent average molecular weight (Mc) of the cross-linked elastomer network chain and the network chain density (N0) can be estimated using Equations (2)–(6) [28,31], where ρ is the density of the elastomer, g cm−3; is the molar volume of the solvent toluene, 106.4 mL mol−1; is the volume fraction of the elastomer; is the Flory–Huggins interaction parameter between elastomer and solvent, which was obtained using the Bristow–Watson equation (Equation (4)) [33]; the solvent toluene solubility parameter (δs) is 18.241 (J cm−3)1/2 [30]; the PEG solubility parameter (δPEG) is 18.473 (J cm−3)1/2 [34]; the P(E-co-T) solubility parameter (δP(E-co-T)) is 18.357 (J cm−3)1/2; the solubility parameter of P(E-co-T)/PEG blended polyether elastomer (δP) is obtained by weighting PEG and P(E-co-T) solubility parameters according to Equation (5), where p is the mass fraction of PEG in P(E-co-T)/PEG blended polyether.
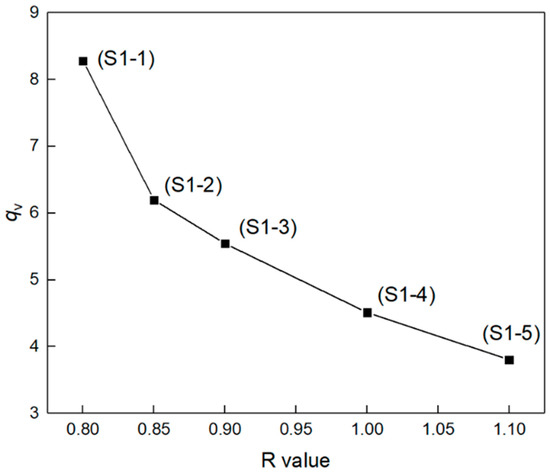
Figure 3.
Equilibrium volume-swelling ratios of elastomers with different R values.
The network structure parameters of elastomer S1-1~S1-5 are listed in Table 3. With the increase in R value, the apparent average molecular weight of the elastomer S1-1~S1-5 decreased monotonically from 16416 g mol−1 to 3086 g mol−1, and the network chain density N0 increased monotonically from 0.0645 mmol cm−3 to 0.3437 mmol cm−3. This was because, under the condition of higher R value, the terminal hydroxyl groups of the blended polyether and the curing agent isocyanate had completely reacted; meanwhile, the excessive isocyanate group could further react with the resultant carbamate group to form a urea group. The elastomer network formed more chemical cross-linking points and the network chains presented lower average molecular weight Mc and higher network chain density N0. At lower R value, the terminal hydroxyl group of blended polyether could not react completely with the isocyanate curing agent, so that there existed a lot of suspended chains within the elastomer network. Consequently, the elastomer exhibited a higher apparent average molecular weight and a lower network chain density N0 [12].

Table 3.
Network structure parameters of elastomers with different R values.
3.3. Aggregation of Elastomers with Different R Values
In addition to the chemical crosslinking, a physical crosslinking of the elastomers is also one of the important factors affecting the macro-mechanical properties. Figure 4 shows the wide-angle X-ray diffraction spectra of elastomers S1-1~S1-5 at room temperature. It can be seen that elastomers S1-1~S1-5 showed different X-ray diffraction characteristics. Elastomers S1-5 just showed a halo peak without sharp diffraction spikes. In contrast, the diffraction spectra of elastomers S1-1~S1-4 showed obvious diffraction spikes at 2θ angles 19.4° and 23.4°; moreover, the intensity of diffraction spikes gradually decreased with the increasing curing parameter R value. Given that the characteristic diffraction spikes of polytetrahydrofuran crystals appeared at 2θ angle 19.8°~20.0° and 24.1°~24.6° [35], while PEG crystals appeared at 2θ angle 19.2°~19.5° and 23.4°~23.6° [36], at room temperature, the diffraction spikes presented in elastomers S1-1~S1-4 should originate from the PEG crystalline structure.
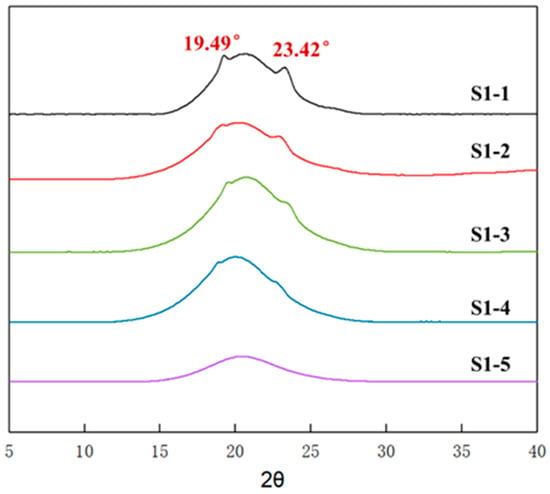
Figure 4.
WAXD spectra of elastomers with different R values.
In addition, the thermal scans of elastomers S1-1~S1-5 were performed using DSC, and the secondary heating DSC curves are shown in Figure 5. It can be seen that two obvious endothermic peaks appeared during the secondary heating process for all samples. Given that the crystalline melting point of tetrahydrofuran micro-blocks occurred in the low-temperature region [9], while the crystalline melting point of polyethylene glycol occurred in the high temperature region [37], combined with the X-ray diffraction spectra of elastomer S1-1~S1-5 at room temperature (Figure 4), it can be inferred that, in Figure 5 the endothermic peak in the low-temperature region originated from the crystalline melting of the tetrahydrofuran micro-block on the P(E-co-T) chains [28], while the endothermic peak in the high-temperature region originated from the crystalline melting of polyethylene glycol.
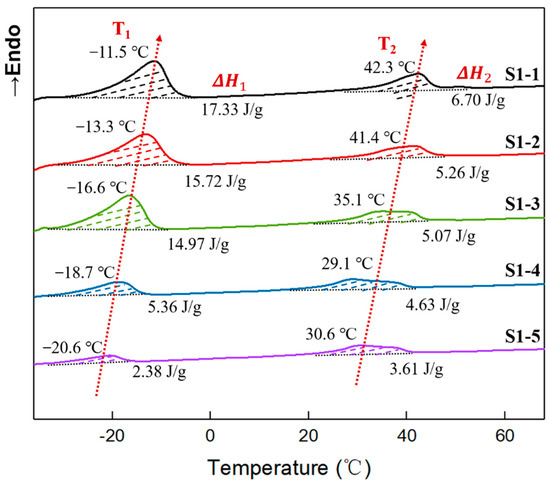
Figure 5.
DSC curves of elastomers with different R values.
Comparing the crystalline melt peaks in the high-temperature region, it was also found that the endothermic melting peaks of elastomers S1-3, S1-4, and S1-5, which were prepared by using higher curing parameter R values, was characteristic of a significantly wide distribution. Considering the high R value causing high network cross-linked density, the movement of PEG segments within the elastomers was limited, making them difficult to completely form thermodynamically stable aggregated structures. In the elastomer matrices, both PEG microcrystals and PEG structural intact crystal grains coexisted [37,38,39,40]. During heating, the PEG microcrystals first melted, followed by the structural intact crystal grains at higher temperatures, so the elastomers gave a wider distribution of endothermic peaks in the high-temperature region. As the R value decreased, the restriction of chain segment movement weakened, and PEG segment mobility increased, forming more thermodynamically stable and structurally intact crystal grains. Correspondingly, the PEG crystalline melting peak became narrower and shifted toward higher temperatures. In contrast, the microcrystalline structure formed by tetrahydrofuran micro-blocks exhibited only a single melting peak at low temperature. This indicates that, at room temperature, elastomers S1-1~S1-5 were all semi-crystalline aggregations caused by PEG crystallization.
3.4. Crystallinity of Elastomers with Different R Values
To further reveal the effect of crystallization physical crosslinking on the mechanical properties of elastomers S1-1~S1-5, the crystallinities have been quantitatively analyzed. As can be seen from Figure 5, the crystallization enthalpies of tetrahydrofuran micro-blocks and PEG gradually decreased with the increasing R value. The crystallization enthalpy of tetrahydrofuran micro-blocks decreased from 17.33 J g−1 for elastomer S 1-1 at R value of 0.80 to 2.38 J g−1 for elastomer S1-5 at R value of 1.10, while the crystallization enthalpy of PEG decreased from 6.70 J g−1 to 3.61 J g−1. Based on that polytetrahydrofuran crystallization enthalpy is 172.0 J g−1 [41], PEG crystallization enthalpy 156.98 J g−1 [38], for P(E-co-T)/PEG blended polyether elastomers, the crystallinities of tetrahydrofuran micro-blocks and PEG can be estimated using Equation (7), where is the melting enthalpy per unit mass of the elastomer.
The crystallinities of elastomers S1-1~S1-5 are listed in Table 4. It can be seen that, within the P(E-co-T)/PEG elastomer, tetrahydrofuran micro-block crystallinity decreased from 10.08% for elastomer S1-1 at R value 0.80 to 1.38% for elastomer S1-5 at R value of 1.10, and PEG crystallinity decreased from 4.27% to 2.30%. The crystallinities of elastomers S1-1~S1-5 decreased with the increase of the curing parameter R value. As is consistent with XRD analysis. Considering that the crystalline melting peak of tetrahydrofuran micro-block is far lower than room temperature, while the melting peak of PEG is higher than room temperature, it can be inferred that PEG crystallinity, which dominates elastomer physical crosslinking degree at room temperature, would have a significant influence on the mechanical properties of P(E-co-T)/PEG blended polyether elastomer at room temperature.

Table 4.
Melting enthalpy and crystallinity of elastomers with different R value.
3.5. Mechanical Properties of Elastomers with Different R Values
At room temperature, the typical stress–strain curves of P(E-co-T)/PEG blended polyether elastomer S1-1~S1-5 are shown in Figure 6. The tensile moduli (E) of elastomer S1-1~S1-5 gradually increase with the increase in R value, indicating that, under the condition that P(E-co-T) and PEG content remain unchanged, the elastomer tensile modulus mainly depended on the elastomer curing parameters. Higher curing parameters and higher chemical crosslinking density are favorable to the elastomer tensile modulus. As to the elongation at break (εb) and the tensile strength at break (σb) of the elastomers, the εb gradually decreased as the curing parameter increased, while the σb first increased and then decreased.
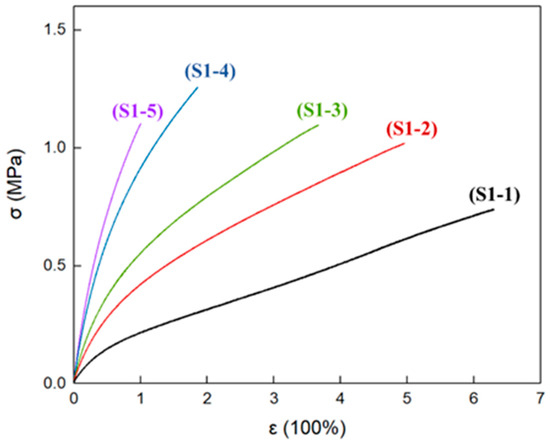
Figure 6.
Stress–strain curves of elastomers with different R values at room temperature.
Table 5 lists the mechanical property results of elastomers S1-1~S1-5. Clearly, elastomers S1-2 and S1-3 had similar tensile strength at break as elastomer S1-5, but had higher elongation. Combined with the crystallinity of the elastomers at room temperature (Figure 4 and Table 4), it can be inferred that, within elastomer S1-2 and S1-3 matrices, the physical crosslinking structure formed by PEG crystallization compensated for the adverse effect of the lower curing parameter R value on the mechanical properties of the elastomers, making elastomers S1-2 and S1-3 simultaneously give excellent elongation at break and tensile strength at break.

Table 5.
Mechanical properties of elastomers with different R values.
Mechanical properties are a macroscopic manifestation of elastomer microstructure characteristics. To confirm the micro-aggregation thermal stability of elastomers S1-1~S1-5, the elastomers were heated and aged at 60 °C for 7 days, and then the mechanical properties were tested at room temperature. Table 6 lists the mechanical property results of the elastomers after aging. Comparing with Table 5, there was no obvious difference in mechanical properties of the elastomers before and after aging. This suggests that the micro-aggregation of elastomers S1-1~S1-5 had not noticeably changed after long-term high-temperature aging, and the micro-aggregation of polyurethane crosslinked P(E-co-T)/PEG elastomer had good thermal stability.

Table 6.
Mechanical properties of aged elastomers with different R values.
3.6. Aggregation of Elastomers with Different PEG Contents
To further explore the effect of PEG content on the mechanical properties of P(E-co-T)/PEG blended polyether elastomers, fixing the curing parameter at 0.9 and varying the PEG content according to Table 2, elastomers S2-1~S2-4 with different PEG contents were prepared and analyzed by using X-ray diffraction. Figure 7 shows the X-ray diffraction spectra of elastomer S2-1~S2-4 at room temperature. Obviously, there emerged noticeable diffraction spikes around 2θ angle 19.2° and 23.2°, and the intensity gradually increased as PEG content increased from 8% of S2-1 to 20% of S2-4. Referring to Figure 4, it can be inferred that the diffraction spikes originated from the crystalline structure of PEG segments within P(E-co-T)/PEG blended polyether elastomer.
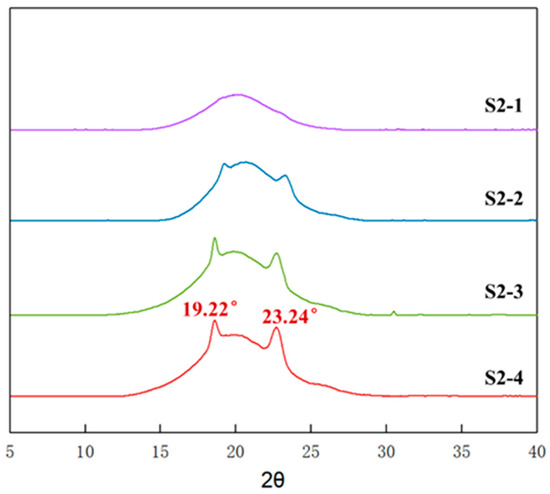
Figure 7.
WAXD spectra of elastomers with different PEG contents.
3.7. Crystallinity of Elastomers with Different PEG Contents
The secondary heating DSC curves of elastomers S2-1~S2-4 are shown in Figure 8. All elastomer samples presented two obvious endothermic peaks. Similarly, the endothermic peak below room temperature is attributed to the crystalline melting of the tetrahydrofuran micro-block on the P(E-co-T) chain, while the one above room temperature was the crystalline melting of PEG. As in Figure 5, both crystalline melting peak temperatures showed an overall trend toward higher temperatures with increasing PEG content. This indicates that more structural intact crystal grains were formed within P(E-co-T)/PEG blended polyether elastomer as the PEG content increased, leading to a shift of the crystalline melting peak toward higher temperatures. Additionally, within P(E-co-T)/PEG blended polyether elastomer matrices, increasing PEG content contributed to the integrity of the crystalline structure.
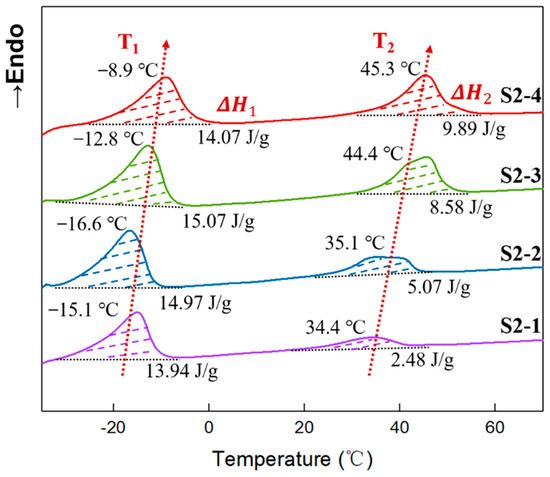
Figure 8.
DSC curves of elastomers with different PEG contents.
Based on the melting enthalpy of Figure 8, the crystallinity of P(E-co-T)/PEG elastomers S2-1 to S2-4, which were calculated using Equation (7), are shown in Table 7. It can be seen that the crystallinity of tetrahydrofuran micro-block was in the range of 8.10–8.76% with the increase in PEG content; in contrast, the crystallinity of PEG rose from 1.58% of elastomer S2-1 to 6.30% of elastomer S2-4, having increased by ~300%. This indicates that, within P(E-co-T)/PEG blended polyether elastomers, fixing curing parameter R value, increasing PEG content could significantly enhance the physical crosslinking degree of the elastomer at room temperature.

Table 7.
Melting enthalpy and crystallinity of elastomers with different PEG contents.
3.8. Mechanical Properties of Elastomers with Different PEG Contents
The mechanical properties of elastomers S2-1~S2-4 were tested at room temperature, and the typical stress–strain curves are shown in Figure 9. At the beginning of the straining, the stress–strain curves of elastomers S2-1~S2-4 all exhibited a typical tensile behavior of amorphous polymer elastomers, and the tensile modulus gradually decreased with increasing strain. In addition, combined with Table 7, it can be inferred that high crystallinity increased the physical crosslinking density of the elastomers. This made the tensile modulus of elastomers S2-1~S2-4 gradually increase with the increase in PEG content.
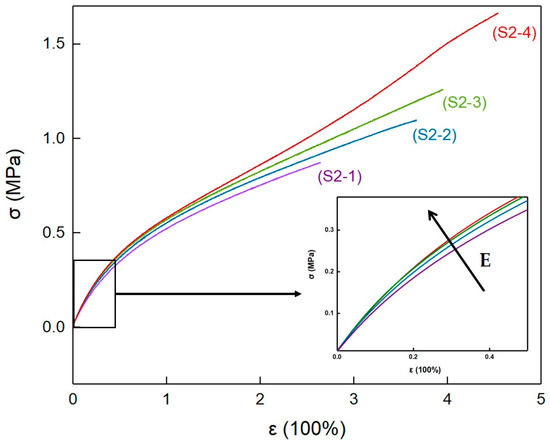
Figure 9.
Stress–strain curves of elastomers with different PEG contents.
At the late strain stage, the tensile modulus of elastomer S2-4 showed a significant upturn trend with increasing strain. This indicates that, within P(E-co-T)/PEG blended polyether elastomers, when PEG content increased to 20%, the elastomer network chains orientated and further crystallized during the strain process, increasing the physical crosslinking density and causing the modulus increase. At the same time, the oriented crystallization allowed the network chains to further relax, making elastomers S2-4 have a higher strain and tensile strength at break.
Table 8 lists the mechanical property results of elastomers S2-1~S2-4. The tensile modulus increased from 0.78 MPa for elastomer S2-1 to 0.93 MPa for elastomer S2-4, and the elongation at break increased from 237% for elastomer S2-1 to 443% for elastomer S2-4, and the tensile strength at break increased from 0.80 MPa for elastomer S2-1 to 1.49 MPa for elastomer S2-4. The initial tensile modulus, elongation at break, and tensile strength all gradually increased with the increase in PEG content. The introduction of crystallizable PEG to polyurethane cross-linked P(E-co-T) elastomers could change the micro-aggregation structure of the elastomers at room-temperature, and significantly improve the mechanical properties.

Table 8.
Mechanical properties of elastomers with different PEG contents.
4. Conclusions
The polyurethane cross-linked P(E-co-T)/PEG blended polyether elastomers with different curing parameter R values and different PEG contents were prepared by using N100 as the curing agent. When PEG content was 12%, polyurethane cross-linked P(E-co-T)/PEG blended polyether elastomer presented a semi-crystalline structure at room temperature, and the crystallinity gradually increased with the decrease in curing parameter R value. At R value 0.9, the elastomer exhibited both good tensile strength and elongation at break. Fixing the curing parameter R value at 0.9, all the elastomer tensile strength, tensile modulus, and elongation at break gradually increased with increasing PEG content. Introducing crystallizable PEG into polyurethane cross-linked P(E-co-T) elastomers could effectively adjust elastomer micro-aggregation at room temperature and improve the elastomer mechanical properties.
Apart from utilizing prepolymer molecular weight, curing agent type, and curing parameters to regulate the mechanical properties of thermoset elastomers, introducing crystallizable prepolymers and using the crystallization physical crosslinking are important ways to regulate the mechanical properties of amorphous thermoset elastomers.
Author Contributions
Conceptualization, P.J. and J.Z.; methodology, P.J. and J.Z.; validation, P.J. and J.Z.; formal analysis, P.J.; investigation, P.J.; resources, J.Z.; data curation, P.J.; writing—original draft preparation, P.J.; writing—review and editing, J.Z., A.P., R.Y., X.G. and J.H.; visualization, P.J.; supervision, J.Z.; project administration, J.Z., A.P., R.Y., X.G. and J.H.; funding acquisition, J.Z. and R.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 51473022, and the Pre-Research Field Fund, grant number 61407200114.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the National Natural Science Foundation of China (51473022) and the Pre-Research Field Fund (61407200114).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Engels, H.W.; Pirkl, H.G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile materials and sustainable problem solvers for today’s challenges. J. Ger. Chem. Soc. 2013, 52, 9422–9441. [Google Scholar] [CrossRef] [PubMed]
- Berezkin, Y.; Urick, M. Modern Polyurethanes: Overview of Structure Property Relationship. In Polymers for Personal Care and Cosmetics; ACS Publication: Washington, DC, USA, 2013; pp. 65–81. [Google Scholar] [CrossRef]
- Tian, S. Recent Advances in Functional Polyurethane and Its Application in Leather Manufacture: A Review. Polymers 2020, 12, 1996. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Li, X.P.; Chen, J.; He, X.D.; Huang, W.M.; Zhu, K.; Yu, W.H.; Ni, H.L.; Zhao, K.Q.; Hu, P. Unified method to prepare thermoplastic/thermoset soft polyurethanes reshape-able around room temperature on-demand. J. Polym. Res. 2021, 28, 201. [Google Scholar] [CrossRef]
- Meiorin, C.; Calvo-Correas, T.; Mosiewicki, M.A.; Aranguren, M.I.; Corcuera, M.A.; Eceiza, A. Comparative effects of two different crosslinkers on the properties of vegetable oil-based polyurethanes. J. Appl. Polym. Sci. 2019, 137, 48741. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Y.; Liu, G.; Li, X.; Luo, Y. Mechanical properties and thermal decomposition of PBAMO/GAP random block ETPE. J. Therm. Anal. Calorim. 2016, 126, 717–724. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Luo, Y. Synthesis and Characterization of 3,3′-Bisazidomethyl Oxetane-3-Azidomethyl-3′-Methyl Oxetane Alternative Block Energetic Thermoplastic Elastomer. Propellants Explos. Pyrotech. 2012, 37, 235–240. [Google Scholar] [CrossRef]
- Eroglu, M.S.; Guven, O. Characterization of network structure of poly(glycidyl azide) elastomers by swelling, solubility and mechanical measurements. Polymer 1998, 39, 1173–1176. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Ma, S.; Luo, Y. Compatibility, mechanical and thermal properties of GAP/P(EO-co-THF) blends obtained upon a urethane-curing reaction. Polym. Bull. 2017, 74, 4607–4618. [Google Scholar] [CrossRef]
- Chen, K.; Yuan, S.; Wen, X.; Sang, C.; Luo, Y. Effect of Mixed Isocyanate Curing Agents on the Performance of In Situ-Prepared HTPE Binder Applied in Propellant. Propellants Explos. Pyrotech. 2021, 46, 428–439. [Google Scholar] [CrossRef]
- Jutrzenka Trzebiatowska, P.; Santamaria Echart, A.; Calvo Correas, T.; Eceiza, A.; Datta, J. The changes of crosslink density of polyurethanes synthesised with using recycled component. Chemical structure and mechanical properties investigations. Prog. Org. Coat. 2018, 115, 41–48. [Google Scholar] [CrossRef]
- Zhai, J.X.; Pang, A.M.; Ding, T.F.; Liu, R.T.; Guo, X.Y.; Song, T.L. Effect of crosslinking point structures on properties of polyurethane end-crosslinked PBT elastomers. Iran. Polym. J. 2022, 31, 333–341. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, G.; Du, J.; Li, J.; Li, J.; Tang, Q.; Fan, X. Investigation of Hydroxyl-Terminated Polyether Cured with Different Isocyanates: Curing Process and Mechanical Property. Propellants Explos. Pyrotech. 2020, 45, 1972–1979. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Zheng, H.; Li, C.; Zhang, Y.; Zhang, Q. Design and development of self-repairable and recyclable crosslinked poly(thiourethane-urethane) via enhanced aliphatic disulfide chemistry. J. Polym. Sci. 2020, 58, 1092–1104. [Google Scholar] [CrossRef]
- Kojio, K.; Fukumaru, T.; Furukawa, M. Highly Softened Polyurethane Elastomer Synthesized with Novel 1,2-Bis(isocyanate)ethoxyethane. Macromolecules 2004, 37, 3287–3291. [Google Scholar] [CrossRef]
- Geng, Z.; Pang, A.; Ding, T.; Guo, X.; Yang, R.; Luo, Y.; Zhai, J. Overlooked Impact of Interchain H-Bonding between Cross-Links on the Mechanical Properties of Thermoset Polyurethane Elastomers. Macromolecules 2022, 55, 8749–8756. [Google Scholar] [CrossRef]
- de Keer, L.; Kilic, K.I.; Van Steenberge, P.H.M.; Daelemans, L.; Kodura, D.; Frisch, H.; De Clerck, K.; Reyniers, M.F.; Barner-Kowollik, C.; Dauskardt, R.H.; et al. Computational prediction of the molecular configuration of three-dimensional network polymers. Nat. Mater. 2021, 20, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- de Keer, L.; van Steenberge, P.H.M.; Reyniers, M.F.; D’Hooge, D.R. Going Beyond the Carothers, Flory and Stockmayer Equation by Including Cyclization Reactions and Mobility Constraints. Polymers 2021, 13, 2410. [Google Scholar] [CrossRef] [PubMed]
- Nofar, M.; Mohammadi, M.; Carreau, P.J. Effect of TPU hard segment content on the rheological and mechanical properties of PLA/TPU blends. J. Appl. Polym. Sci. 2020, 137, 49387. [Google Scholar] [CrossRef]
- Shen, Z.; Zheng, L.; Li, C.; Liu, G.; Xiao, Y.; Wu, S.; Liu, J.; Zhang, B. A comparison of non-isocyanate and HDI-based poly(ether urethane): Structure and properties. Polymer 2019, 175, 186–194. [Google Scholar] [CrossRef]
- Schimpf, V.; Max, J.B.; Stolz, B.; Heck, B.; Mülhaupt, R. Semicrystalline Non-Isocyanate Polyhydroxyurethanes as Thermoplastics and Thermoplastic Elastomers and Their Use in 3D Printing by Fused Filament Fabrication. Macromolecules 2018, 52, 320–331. [Google Scholar] [CrossRef]
- Eceiza, A.; Martin, M.D.; de la Caba, K.; Kortaberria, G.; Gabilondo, N.; Corcuera, M.A.; Mondragon, I. Thermoplastic polyurethane elastomers based on polycarbonate diols with different soft segment molecular weight and chemical structure: Mechanical and thermal properties. Polym. Eng. Sci. 2008, 48, 297–306. [Google Scholar] [CrossRef]
- Anokhin, D.V.; Gorbunova, M.A.; Abukaev, A.F.; Ivanov, D.A. Multiblock Thermoplastic Polyurethanes: In Situ Studies of Structural and Morphological Evolution under Strain. Materials 2021, 14, 3009. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, M.A.; Komov, E.V.; Grunin, L.Y.; Ivanova, M.S.; Abukaev, A.F.; Imamutdinova, A.M.; Ivanov, D.A.; Anokhin, D.V. The effect of separation of blocks on the crystallization kinetics and phase composition of poly(butylene adipate) in multi-block thermoplastic polyurethanes. Phys. Chem. Chem. Phys. 2022, 24, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, J.; Wang, Y.; Zhou, J.; Cao, L.; He, Z.; Zhang, Y.; Tang, C.; Zhong, L. Enhanced Temperature Stability of High Energy Density Ferroelectric Polymer Blends: The Spatial Confinement Effect. Macromol. Rapid Commun. 2019, 40, e1900406. [Google Scholar] [CrossRef]
- Alvarado, N.; Alegría, L.; Sandoval, C.; Kortaberría, G.; Leiva, A.; Gargallo, L.; Radic, D. Synthesis and Characterization of a Branched Poly(Methacrylamide): Thermal Stability and Molecular Simulation Studies of Their Blends With Vinylic Polymers. J. Macromol. Sci. Part A 2014, 51, 864–872. [Google Scholar] [CrossRef]
- Karim, A.; Liu, D.W.; Douglas, J.F.; Nakatani, A.I.; Amis, E.J. Modification of the phase stability of polymer blends by fillers. Polymer 2000, 41, 8455–8458. [Google Scholar] [CrossRef]
- Zou, Y.C.; Yang, R.J.; Zhai, J.X. Polytriazole polyether elastomers with widely tunable mechanical properties: The role of network structure and crystallization behavior. J. Appl. Polym. Sci. 2017, 134, 45298. [Google Scholar] [CrossRef]
- Kambe, Y. Thermal-Behavior of Poly(Ethylene Oxide) as Revealed by Differential Scanning Calorimetry. Polymer 1980, 21, 352–355. [Google Scholar] [CrossRef]
- van Krevelen, D.W. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–1004. [Google Scholar]
- Qu, Z.; Zhai, J.; Yang, R. Comparison between properties of polyether polytriazole elastomers and polyether polyurethane elastomers. Polym. Adv. Technol. 2014, 25, 314–321. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Bristow, G.M.; Watson, W.F. Cohesive Energy Densities of Polymers Part 1—Cohesive Energy Densities of Rubbers by Swelling Measurements. Trans. Faraday Soc. 1958, 54, 1731–1741. [Google Scholar] [CrossRef]
- Ozdemir, C.; Guner, A. Solubility profiles of poly(ethylene glycol)/solvent systems, I: Qualitative comparison of solubility parameter approaches. Eur. Polym. J. 2007, 43, 3068–3093. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, H.; Li, W.; Han, X.; Zhang, X. Thermal performance and crystallization behavior of poly(ethylene glycol) hexadecyl ether in confined environment. Polym. Int. 2014, 63, 982–988. [Google Scholar] [CrossRef]
- Rachmawati, R.; Woortman, A.J.; Loos, K. Facile preparation method for inclusion complexes between amylose and polytetrahydrofurans. Biomacromolecules 2013, 14, 575–583. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, T.; Cui, J.; Liu, L.; Zhou, Y.; Li, G.; Zhou, E.; Chen, X. Nonisothermal crystallization behavior of the poly(ethylene glycol) block in poly(L-lactide)–poly(ethylene glycol) diblock copolymers: Effect of the poly(L-lactide) block length. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 3215–3226. [Google Scholar] [CrossRef]
- Buckley, C.P.; Kovacs, A.J. Melting behavior of low-molecular weight poly (ethylene-oxide) fractions. 2. folded chain crystals. Colloid Polym. Sci. 1976, 254, 695–715. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Q.; Wang, Y.; Zhang, C.; Mo, Z.; Cao, S. Melting behaviors, isothermal and non-isothermal crystallization kinetics of nylon 1212. Polymer 2003, 44, 2537–2545. [Google Scholar] [CrossRef]
- Run, M.; Wu, S.; Zhang, D.; Wu, G. Melting behaviors and isothermal crystallization kinetics of poly(ethylene terephthalate)/mesoporous molecular sieve composite. Polymer 2005, 46, 5308–5316. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Cao, Y.; Kukureka, S.N. The effect of molecular weight on the crystallization kinetics and equilibrium melting temperature of poly(tetramethylene ether glycol). Polym. Adv. Technol. 2006, 17, 1–5. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).