Enhancing the Mechanical Properties of Waterborne Polyurethane Paint by Graphene Oxide for Wood Products
Abstract
1. Introduction
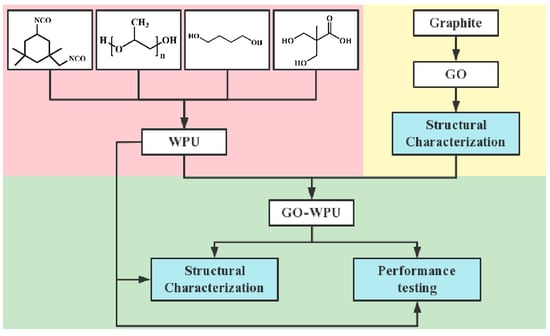
2. Materials and Experiments
2.1. Materials
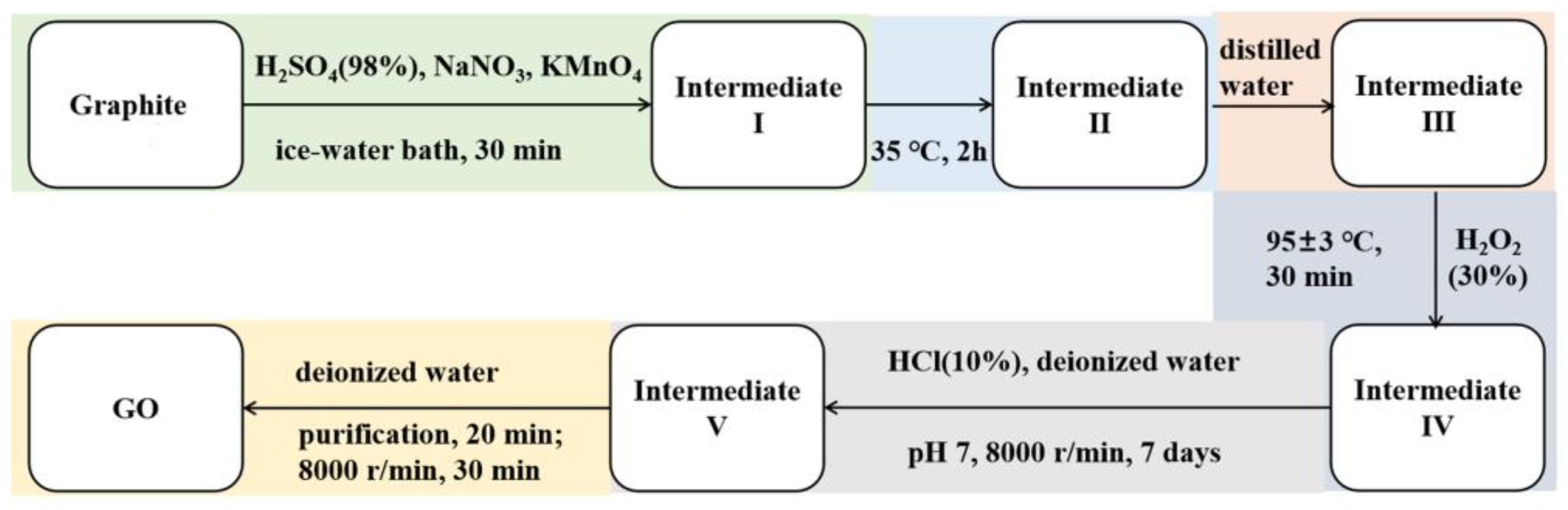
2.2. Preparation of GO, Composite Emulsions, and Paint Film
2.3. Structural Characterization
2.4. Performance Testing
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.G.; Zou, X.B.; Liu, X.L.; Liang, Z.; Ge, Z.; Luo, Y.J. Preparation and properties of waterborne polyurethane modified by aminoethylaminopropyl polydimethylsiloxane for fluorine-free water repellents. Prog. Org. Coat. 2020, 139, 105407. [Google Scholar] [CrossRef]
- Hu, X.H.; Ding, Y.S.; Liu, J.; Deng, Y.; Cheng, C.L. Synthesis and fluorescence properties of a waterborne polyurethane-acrylic hybrid polymeric dye. Polym. Bull. 2017, 74, 555–569. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Li, X.Q.; Li, B.X.; Feng, X.; Zhang, B.B.; Liu, Y. Preparation and antibacterial properties of BP-GO-AgNPs composite coating. Rare. Metal. Mat. Eng. 2022, 51, 1017–1023. [Google Scholar]
- Li, B.S.; Yuan, Z.W.; Chang, F.Q.; Zhang, W.W.; Zhang, Z.; Chen, S.Q. Preparation and characterization of Cu-GO and Cu-GO-YSZ nanocomposite coating by electrochemical deposition for improved mechanical and anti-corrosion properties. Surf. Coat. Technol. 2022, 439, 128441. [Google Scholar] [CrossRef]
- Li, J.; Feng, Q.K.; Cui, J.C.; Yuan, Q.Q.; Qiu, H.X.; Gao, S.L.; Yang, J.H. Self-assembled graphene oxide microcapsules in Pickering emulsions for self-healing waterborne polyurethane coatings. Compos. Sci. Technol. 2017, 151, 282–290. [Google Scholar] [CrossRef]
- Xi, P.Y.; Wu, L.; Quan, F.Y.; Xia, Y.Z.; Fang, K.J.; Jiang, Y.J. Scalable nano building blocks of waterborne polyurethane and nanocellulose for tough and strong bioinspired nanocomposites by a self-healing and shape-retaining strategy. ACS Appl. Mater. Interfaces 2022, 21, 14. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, J.H.; Son, Y. Effect of a monomer composition on the mechanical properties and glass transition temperature of a waterborne polyurethane/graphene oxide and waterborne polyurethane/MWCNT nanocomposite. Polymers 2020, 12, 2013. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, X.L.; Luo, L.; Huang, Y.C.; Li, K.; Li, J.B. Enhancement effect of acylated cellulose nanocrystals on waterborne polyurethane. J. Polym. Res. 2022, 8, 29. [Google Scholar] [CrossRef]
- Chen, Y.J.; He, J.X.; Xu, L.F.; Xu, B.; Qian, L.J. Mechanical properties and flame retardancy of PLA composites containing zinc oxide and chain extender. J. Appl. Polym. Sci. 2021, 38, 138. [Google Scholar] [CrossRef]
- Zhu, M.J.; Li, S.Y.; Sun, Q.Y.; Shi, B. Enhanced mechanical property, chemical resistance and abrasion durability of waterborne polyurethane based coating by incorporating highly dispersed polyacrylic acid modified graphene oxide. Prog. Org. Coat. 2022, 170, 106949. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.J.; Chen, P.G.; Wang, W.; Cao, J.Z. Enhancing weathering resistance of wood by using bark extractives as natural photostabilizers in polyurethane-acrylate coating. Prog. Org. Coat. 2020, 145, 105665. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Santos, L.S.; Vitry, V.; Paint, Y.; Olivier, M.G. Improvement of the corrosion performance of AA2024 alloy by a duplex PEO/clay modified sol-gel nanocomposite coating. Surf. Coat. Technol. 2022, 434, 128168. [Google Scholar] [CrossRef]
- Asif, A.H.; Mahajan, M.S.; Sreeharsha, N.; Gite, V.V.; Al-Dhubiab, B.E.; Kaliyadan, F.; Nanjappa, S.H.; Meravanige, G.; Aleyadhy, D.M. Enhancement of anticorrosive performance of cardanol based polyurethane coatings by incorporating magnetic hydroxyapatite nanoparticles. Materials 2022, 15, 2308. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Q.; Liang, Z.; Li, Y.X.; Wu, Z.M.; Yi, Z.M. Synthesis of waterborne polyurethane by inserting polydimethylsiloxane and constructing dual crosslinking for obtaining the superior performance of waterborne coatings. Compos. Part. B-Eng. 2022, 238, 109889. [Google Scholar] [CrossRef]
- Sun, Y.F.; Liu, C.Y.; Hong, Y.; Liu, R.P.; Zhou, X.D. Synthesis and application of self-crosslinking and flame retardant waterborne polyurethane as fabric coating agent. Prog. Org. Coat. 2019, 137, 105323. [Google Scholar] [CrossRef]
- Fan, W.W.; Wang, J.C.; Li, Z.J. Antiglare waterborne polyurethane/modified silica nanocomposite with balanced comprehensive properties. Polym. Test. 2021, 99, 107072. [Google Scholar] [CrossRef]
- Deng, F.F.; Zhang, Y.L.; Li, X.; Liu, Y.W.; Shi, Z.Q.; Wang, Y.H. Synthesis and mechanical properties of dopamine modified titanium dioxide/waterborne polyurethane composites. Polym. Compos. 2019, 40, 328–336. [Google Scholar] [CrossRef]
- Zhang, H.N.; Li, K.; Yao, C.C.; Gu, J.T.; Qin, X.H. Preparation of zinc oxide loaded polyurethane/polysulfone composite nanofiber membrane and study on its waterproof and moisture permeability properties. Colloids Surf. A 2021, 629, 127493. [Google Scholar] [CrossRef]
- Adak, B.; Butola, B.S.; Joshi, M. Effect of organoclay-type and clay-polyurethane interaction chemistry for tuning the morphology, gas barrier and mechanical properties of clay/polyurethane nanocomposites. Appl. Clay. Sci. 2018, 161, 343–353. [Google Scholar] [CrossRef]
- Kim, H.A.; Kim, B.K. Synthesis and properties of waterborne polyurethane/hydroxyapatite chemical hybrids. Prog. Org. Coat. 2019, 128, 69–74. [Google Scholar] [CrossRef]
- Chen, X.; Ye, X.M.; Lu, L.L.; Qian, Y.D.; Wang, L.N.; Bi, Y.H.; Wang, Z.F.; Cai, Z.H. Preparation of cross-linkable waterborne polyurethane-acrylate coating films with multifunctional properties. Coatings 2020, 10, 65. [Google Scholar] [CrossRef]
- Jiao, C.Y.; Sun, L.; Shao, Q.; Song, J.Y.; Hu, Q.; Naik, N.; Guo, Z.H. Advances in waterborne acrylic resins: Synthesis principle, modification strategies, and their applications. ACS. Omega. 2021, 6, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Kanny, M.K.; Mohanty, S. Highly transparent castor oil-derived polyurethane/silica nanocomposite coating synthesized by in situ polymerization. Polym. Advan. Technol. 2022, 33, 3605–3618. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, Y.Z.; Chen, W.S.; Liu, Y.X.; Wang, Q.W.; Li, J.; Yu, H.P. Homogeneous dispersion of cellulose nanofibers in waterborne acrylic coatings with improved properties and unreduced transparency. ACS Sustain. Chem. Eng. 2016, 4, 3766–3772. [Google Scholar] [CrossRef]
- Khan, A.A.; De Vera, M.A.T.; Mohamed, B.A.; Javed, R.; Al-Kheraif, A.A. Enhancing the physical properties of acrylic resilient denture liner using graphene oxide nanosheets. J. Vinyl. Addit. Techn. 2022, 28, 487–493. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Pei, X.B.; Yang, S.Y.; Qin, H.; Cai, H.; Hu, S.S.; Sui, L.; Wan, Q.B.; Wang, J. Graphene oxide/hydroxyapatite composite coatings fabricated by electrochemical deposition. Surf. Coat. Technol. 2016, 286, 72–79. [Google Scholar] [CrossRef]
- Le, T.D.H.; Tuan, H.N.A.; Trinh, K.S.; Van, K.T. Enhanced antibacterial property of zinc oxide nanoparticles by incorporation of graphene oxide. J. Sol-Gel Sci. Techn. 2022, 104, 246–257. [Google Scholar] [CrossRef]
- Li, M.N.; Chen, Z.F.; Yang, L.X.; Li, J.Y.; Xu, J.; Chen, C.; Wu, Q.; Yang, M.M.; Liu, T.L. Antibacterial activity and mechanism of GO/Cu2O/ZnO coating on ultrafine glass fiber. Nanomaterials 2022, 12, 1857. [Google Scholar] [CrossRef]
- Li, M.M.; Zou, D.N.; Zhao, B.F.; Chu, J.H.; Tong, L.B. Simultaneously improved corrosion/wear resistances of epoxy coating on Mg alloy via the coupled hybridization of GO and nano-SiO2. Diam. Relat. Mater. 2022, 128, 109224. [Google Scholar] [CrossRef]
- Sinh, L.H.; Luong, N.D.; Seppala, J. Enhanced mechanical and thermal properties of polyurethane/functionalised graphene oxide composites by in situ polymerization. Plast. Rubber. Compos. 2019, 48, 466–476. [Google Scholar] [CrossRef]
- Wan, T.; Chen, D.J. Mechanical enhancement of self-healing waterborne polyurethane by graphene oxide. Prog. Org. Coat. 2018, 121, 73–79. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A. Graphene-based composite materials. Nature 2006, 442, 282. [Google Scholar] [CrossRef] [PubMed]
- Farivar, F.; Pei, L.Y.; Karunagaran, R.U.; Losic, D. Thermogravimetric analysis (tga) of graphene materials: Effect of particle size of graphene, graphene oxide and graphite on thermal parameters. C-J. Carbon Res. 2021, 7, 41. [Google Scholar] [CrossRef]
- Safarpour, M.; Najjarizad-Peyvasti, S.; Khataee, A.; Karimi, A. Polyethersulfone ultrafiltration membranes incorporated with CeO2/GO nanocomposite for enhanced fouling resistance and dye separation. J. Environ. Chem. Eng. 2022, 10, 107533. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Binder, C.; Bendo, T.; Hammes, G.; Neves, G.O.; Binder, R.; de Mello, J.D.B.; Klein, A.N. Structure and properties of in situ-generated two-dimensional turbostratic graphite nodules. Carbon 2017, 124, 685–692. [Google Scholar] [CrossRef]
- Jiang, W.; Li, X.; Chen, Y.L.; Li, H.G.; Shen, L.D.; Chen, Y.; Tian, Z.J.; Lao, Y.X. Research on the wear and corrosion resistance of Ni-GO-TiC composite coating by scanning jet electrodeposition. Surf. Topogr. Metrol. Prop. 2022, 10, 015048. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Wang, S.; Liu, W.Q.; Shi, H.Y.; Liang, L.Y.; Liu, C.H.; Pi, K.; Zhang, W.C.; Zeng, J.J. Design on the corrosion protection of eco-friendly and multifunctional polyhedral oligomeric silsesquioxane functionalized graphene oxide reinforced waterborne polyurethane. Colloids Surf. A 2022, 64, 127718. [Google Scholar] [CrossRef]
- Fang, Z.; Huang, L.J.; Fu, J.J. Research status of graphene polyurethane composite coating. Coatings 2022, 12, 264. [Google Scholar] [CrossRef]
- Bai, T.; Liu, X.L. Effect of magnetic field on the tribological performance of waterborne polyurethane coatings with magnetized graphene oxide. Prog. Org. Coat. 2022, 167, 106839. [Google Scholar] [CrossRef]
- Dai, M.W.; Wang, J.Y.; Zhang, Y. Improving water resistance of waterborne polyurethane coating with high transparency and good mechanical properties. Colloids Surf. A 2020, 601, 124994. [Google Scholar] [CrossRef]
- Wei, Z.K.; Liu, Z.M.; Fu, X.W.; Wang, Y.C.; Yuan, A.Q.; Lei, J.X. Effect of crystalline structure on water resistance of waterborne polyurethane. Eur. Polym. J. 2021, 157, 110647. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Chen, H.X.; Hu, G.; Wang, J.; Xin, Y.F.; Xiang, C.X.; Zhou, Y. Excellent water resistance and mechanically robust waterborne polyurethane-acrylate based on dithiol post-chain extension. JCT. Research 2020, 17, 1065–1074. [Google Scholar] [CrossRef]
- Liu, H.S.; Shang, Q.S.B.; Wang, D.; Song, J. Properties of rosin-based waterborne polyurethanes/cellulose nanocrystals composites. Carbohyd. Polym. 2013, 96, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.S.; Ren, S.B.; Lu, X.N. Application of eco-friendly waterborne polyurethane composite coating incorporated with nano cellulose crystalline and silver nano particles on wood antibacterial board. Polymers 2020, 12, 407. [Google Scholar] [CrossRef]
- Zhang, J.N.; Zhang, J.; Liu, Q.; Ren, H.J.; Li, P.F.; Yang, K. Investigation of hybrid materials based on polyurethane modified with aliphatic side chains combined with nano-TiO2. Aust. J. Chem. 2018, 71, 47–57. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical properties of monolayer graphene oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, X.; Chen, P.; Wang, B.; Gu, J.; Wang, W.; Zhao, Y. Rationalized improvement of Tsai–Wu failure criterion considering different failure modes of composite materials. Compos. Struct. 2021, 256, 113120. [Google Scholar] [CrossRef]
- Wang, B.; Chen, X.; Wang, W.; Yang, J.; Zhang, R. Post-buckling failure analysis of composite stiffened panels considering the mode III fracture. J. Compos. Mater. 2022, 56, 3099–3111. [Google Scholar] [CrossRef]
- Kabir, H.; Aghdam, M.M. A robust Bézier based solution for nonlinear vibration and post-buckling of random checkerboard graphene nano-platelets reinforced composite beams. Compos. Struct. 2019, 212, 184–198. [Google Scholar] [CrossRef]
- Kabir, H.; Aghdam, M.M. A generalized 2D Bézier-based solution for stress analysis of notched epoxy resin plates reinforced with graphene nanoplatelets. Thin Wall. Struct. 2021, 169, 108484. [Google Scholar] [CrossRef]
- Chisty, A.H.; Mallik, A.K.; Robel, F.N.; Shahruzzaman, M.; Haque, P.; Hossain, K.S.; Khan, R.A.; Rahman, M.M. Enhanced epoxy/GO composites mechanical and thermal properties by removing air bubbles with shear mixing and ultrasonication. Chemistryselect 2019, 4, 11417–11425. [Google Scholar] [CrossRef]
- Wu, Y.; Song, N.N.; Wang, W.C.; Zhao, Y.P. Synthesis of graphene/epoxy resin composite via 1,8-diaminooctane by ultrasonication approach for corrosion protection. Ultrason Sonochem 2018, 42, 464–470. [Google Scholar] [CrossRef]
- Fu, X.K.; Cao, H.B.; An, Y.L.; Zhou, H.D.; Shi, Y.P.; Hou, G.L.; Ha, W. Bioinspired hydroxyapatite coating infiltrated with a graphene oxide hybrid supramolecular hydrogel orchestrates antibacterial and self-lubricating performance. ACS Appl. Mater. Interfaces 2022, 14, 31702–31714. [Google Scholar] [CrossRef] [PubMed]


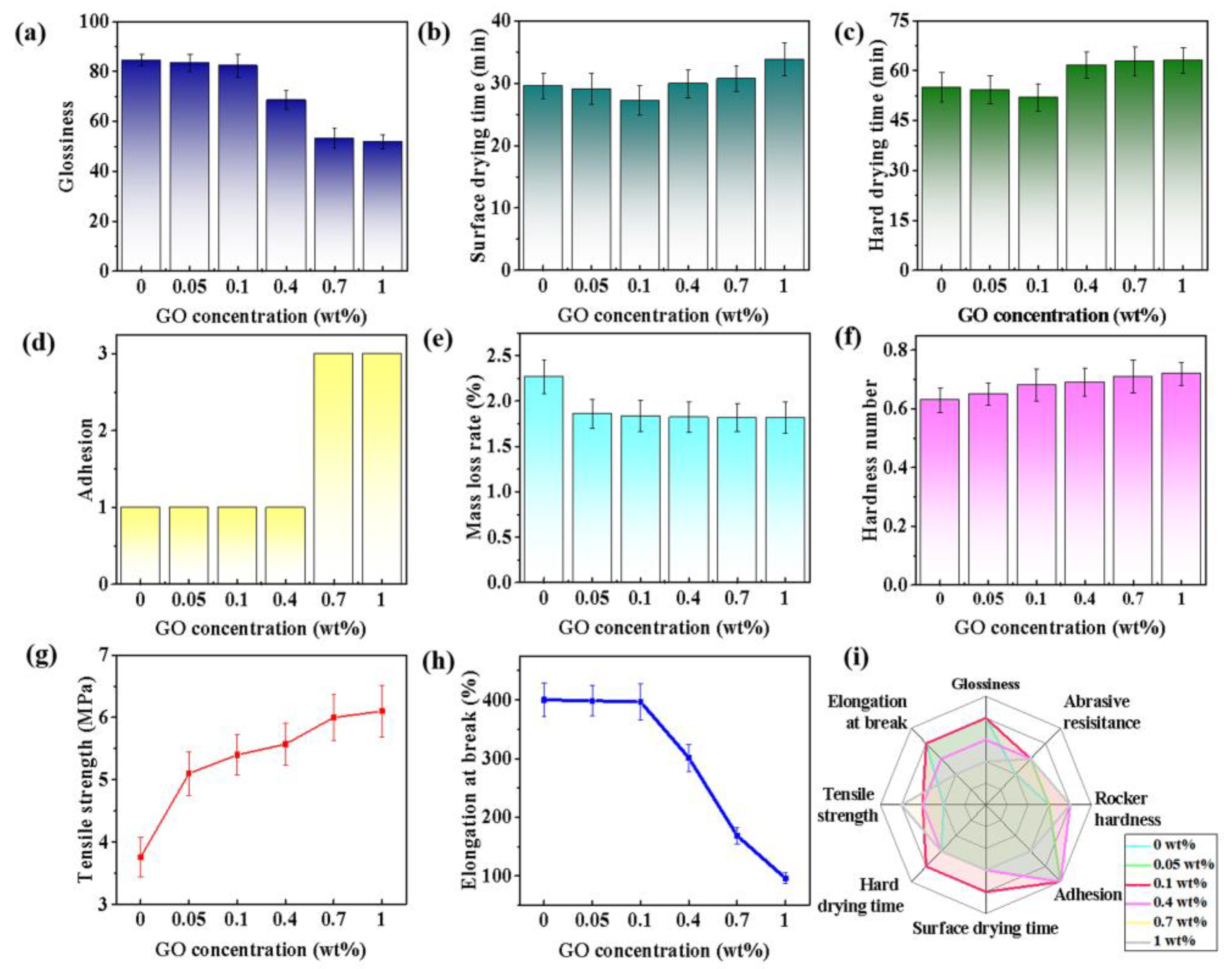

| Sample | 0 wt% | 0.05 wt% | 0.1 wt% | 0.4 wt% | 0.7 wt% | 1 wt% | |
|---|---|---|---|---|---|---|---|
| Property | |||||||
| Pencil hardness | 3B | 3B | 2B | 2B | 2B | 2B | |
| Burnish | Easy | Easy | Easy | Easy | Easy | Easy | |
| Hydrolytic resistance | 3 | 3 | 2 | 2 | 2 | 2 | |
| Alcohol resistance | 5 | 5 | 4 | 4 | 4 | 4 | |
| Alkali resistance | 1 | 1 | 1 | 1 | 1 | 1 | |
| Property | Glossiness | Abrasive Resistance (%) | Rocker Hardness | Surface/Hard Drying Time (min) | Tensile Strength (MPa) | |
|---|---|---|---|---|---|---|
| Sample | ||||||
| 0 | 84.53 | 2.27 | 0.63 | 29.6/55 | 3.76 | |
| 0.1 | 82.1 | 2 | 0.65 | 27.2/52 | 5.12 | |
| CF | 73.5 | 2.793 | 0.598 | 33.9/72.57 | 3.49 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Liang, G.; Qi, Y.; Gong, R.; Zhang, X.; Zhang, Y.; Liu, B.; Kong, L.; Dong, X.; Li, Y. Enhancing the Mechanical Properties of Waterborne Polyurethane Paint by Graphene Oxide for Wood Products. Polymers 2022, 14, 5456. https://doi.org/10.3390/polym14245456
Xu D, Liang G, Qi Y, Gong R, Zhang X, Zhang Y, Liu B, Kong L, Dong X, Li Y. Enhancing the Mechanical Properties of Waterborne Polyurethane Paint by Graphene Oxide for Wood Products. Polymers. 2022; 14(24):5456. https://doi.org/10.3390/polym14245456
Chicago/Turabian StyleXu, Dandan, Guotao Liang, Yanran Qi, Ruizhi Gong, Xingquan Zhang, Yumin Zhang, Baoxuan Liu, Linglong Kong, Xiaoying Dong, and Yongfeng Li. 2022. "Enhancing the Mechanical Properties of Waterborne Polyurethane Paint by Graphene Oxide for Wood Products" Polymers 14, no. 24: 5456. https://doi.org/10.3390/polym14245456
APA StyleXu, D., Liang, G., Qi, Y., Gong, R., Zhang, X., Zhang, Y., Liu, B., Kong, L., Dong, X., & Li, Y. (2022). Enhancing the Mechanical Properties of Waterborne Polyurethane Paint by Graphene Oxide for Wood Products. Polymers, 14(24), 5456. https://doi.org/10.3390/polym14245456