Zero–Waste Recycling of Fiber/Epoxy from Scrap Wind Turbine Blades for Effective Resource Utilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Pyrolysis of Fibesr/Epoxy
2.3. Preparation of the Flexible Composite
2.4. Preparation of Flexible Composite Granules
2.5. Characterization
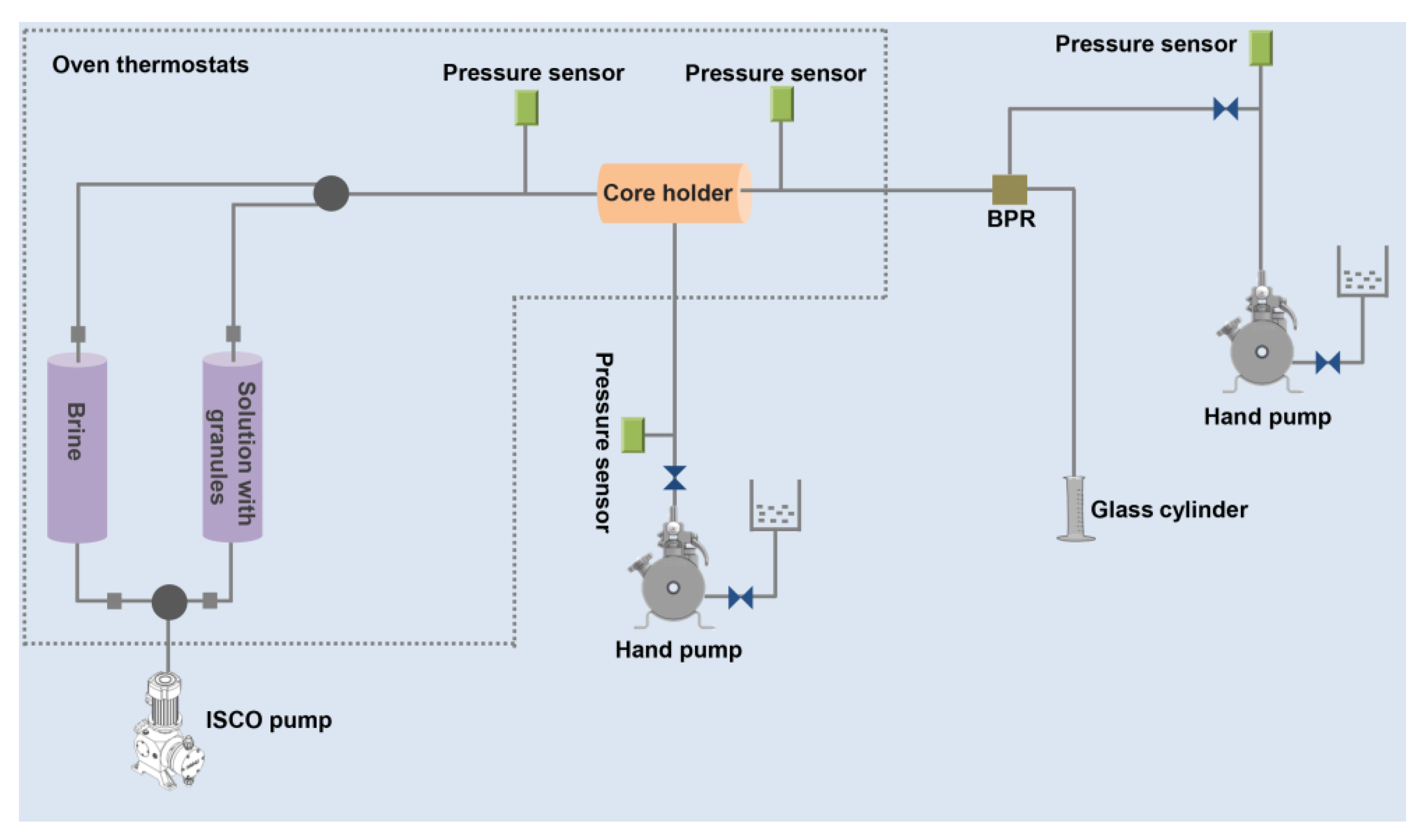
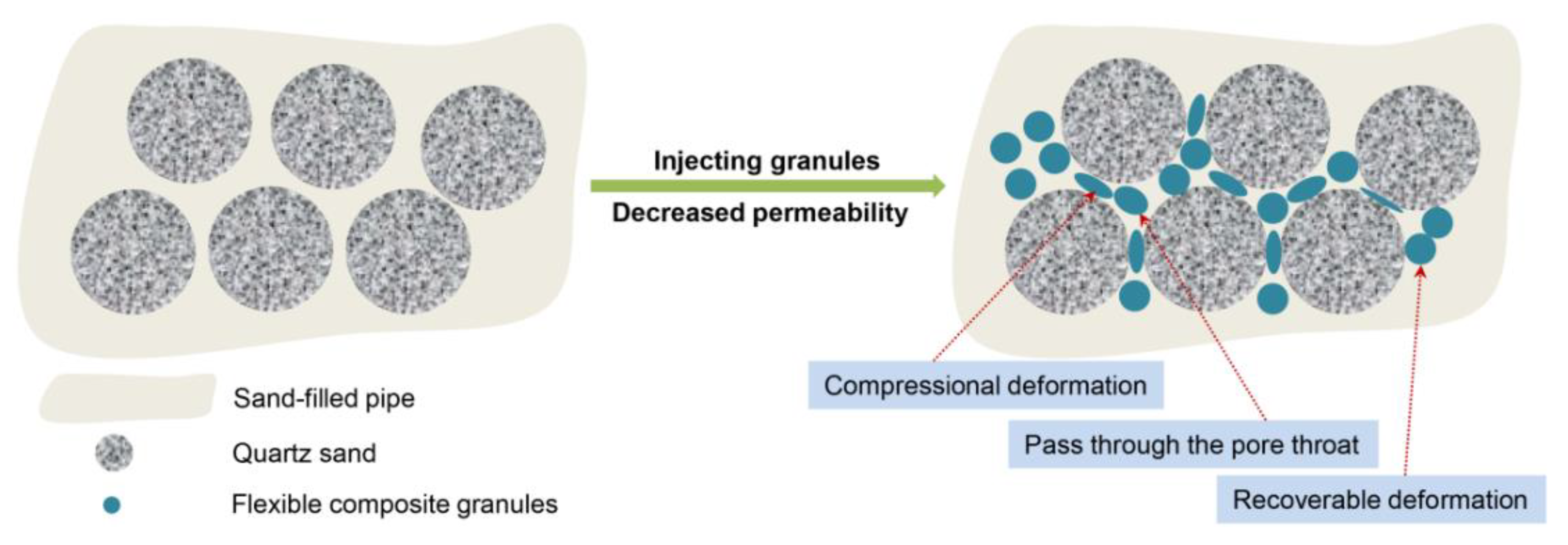
2.6. Plugging Experiment
3. Results and Discussion
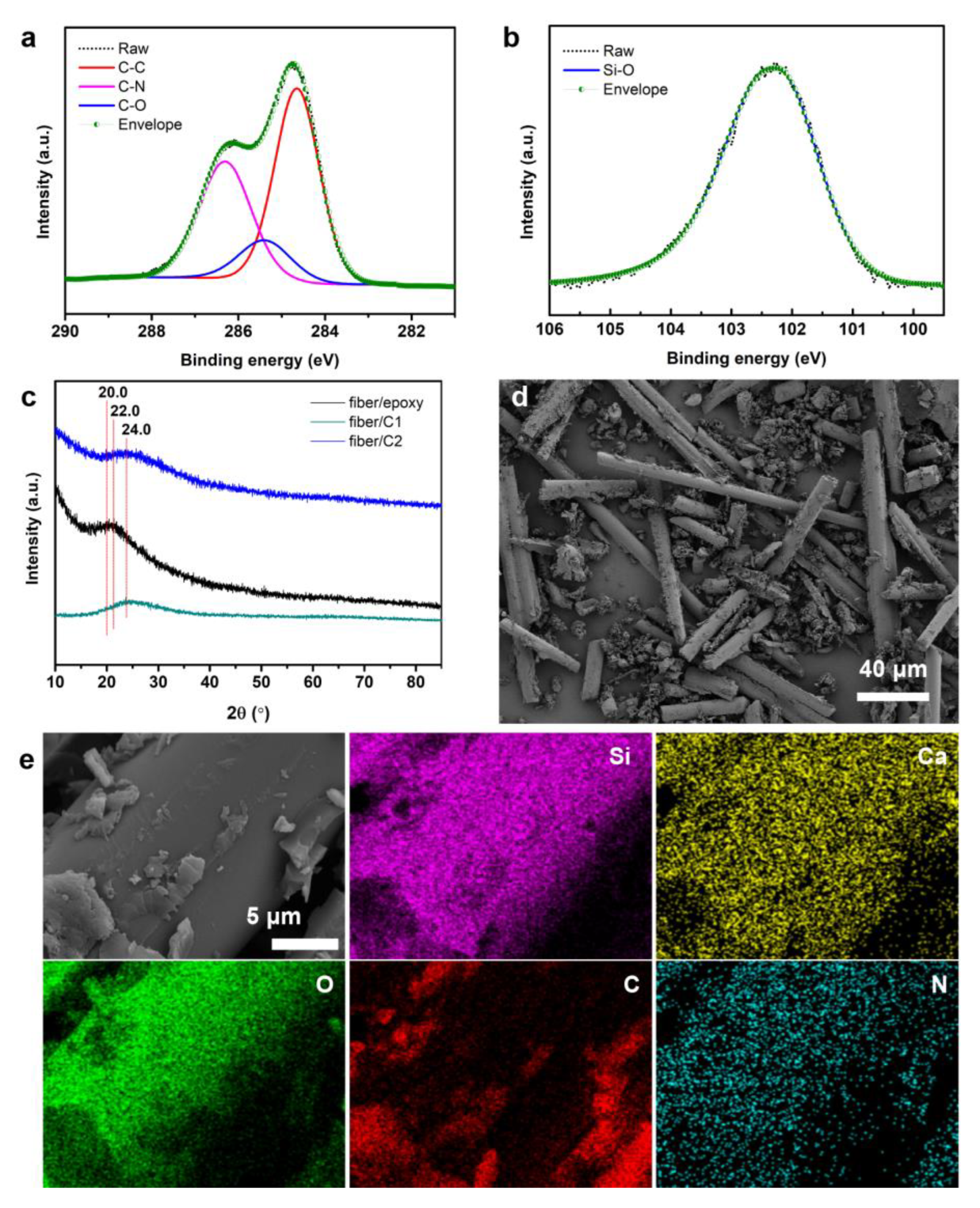
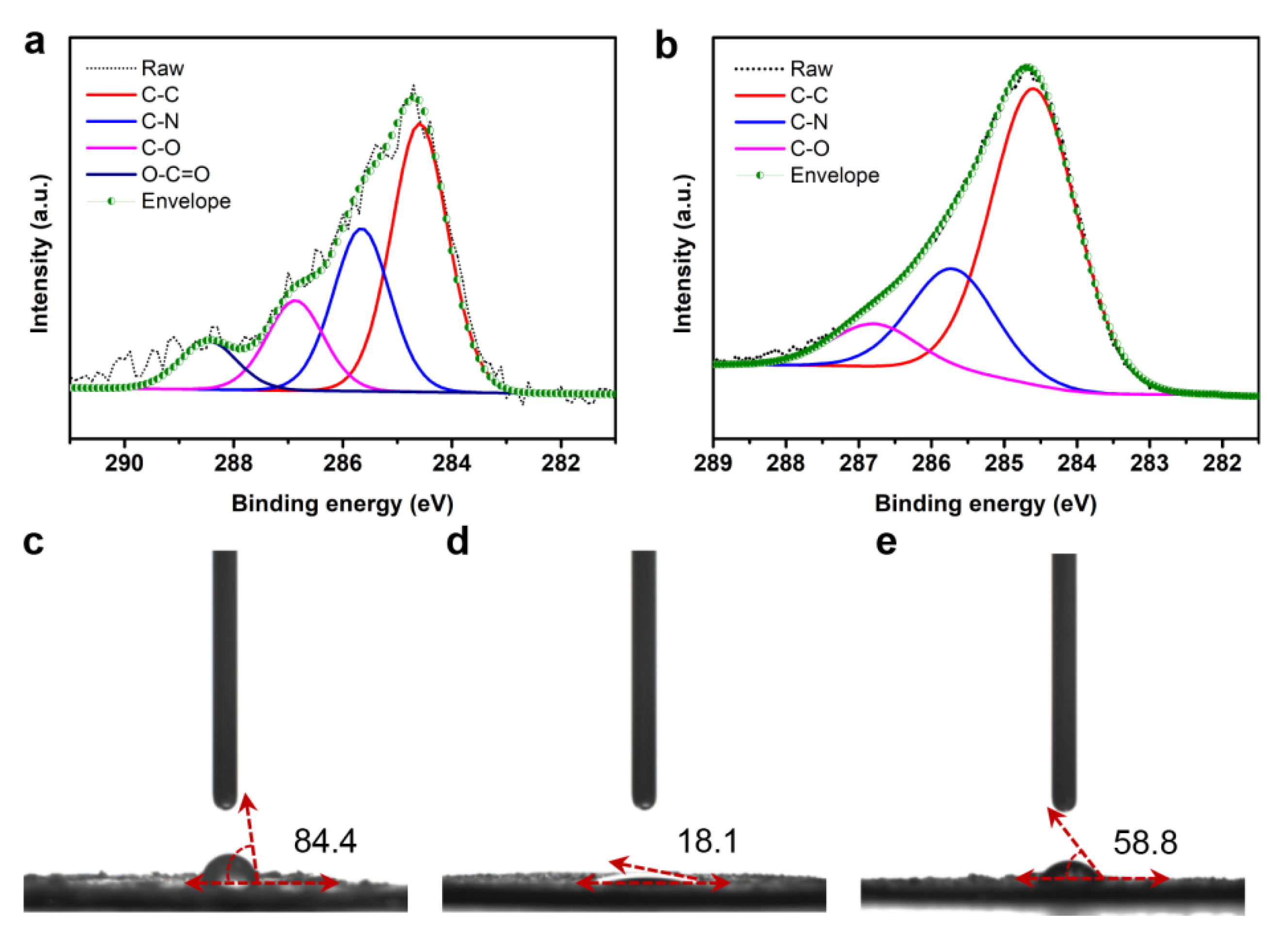
3.1. Structural and Compositional Analysis of the Fibers/Epoxy
3.2. TG Experiments of Fiber/Epoxy
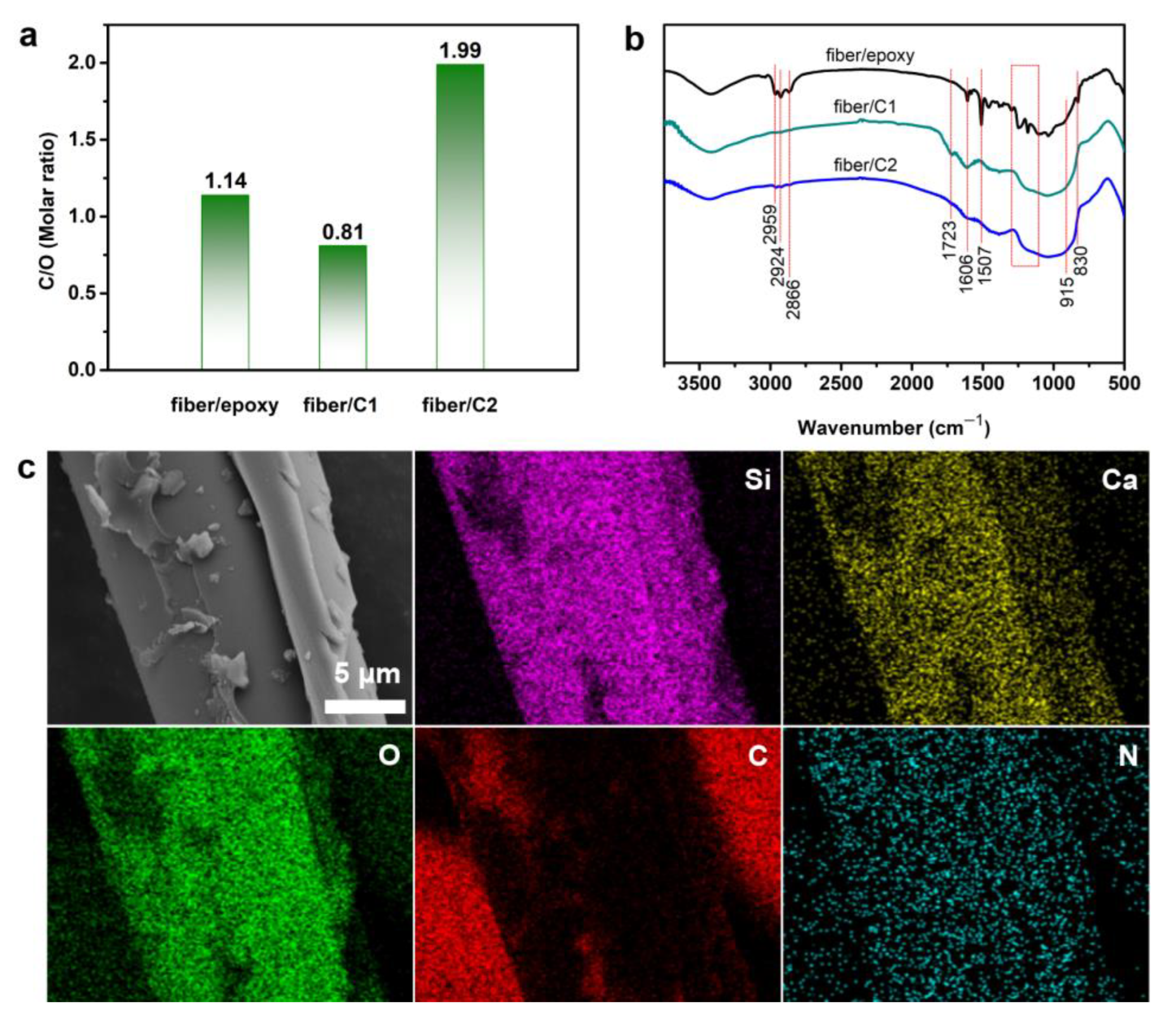
3.3. Characterization of the Fibers/Epoxy under Different Conditions
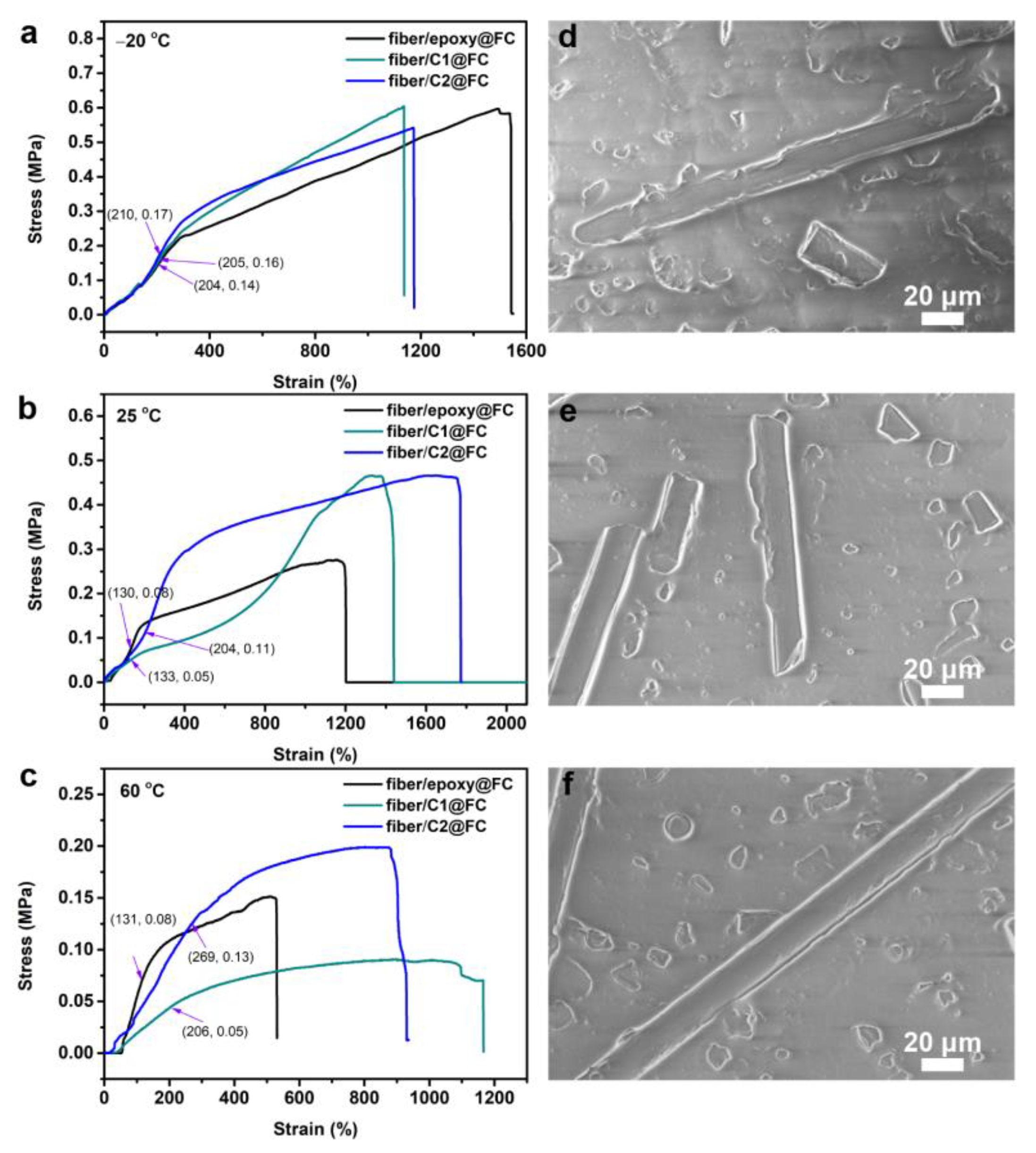
3.4. Elastic Deformation
3.5. A Feasible Route towards Industrial Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vargas, S.A.; Esteves, G.R.T.; Maçaira, P.M.; Bastos, Q.B.; Oliveira, F.L.C.; Souza, R.C. Wind power generation: A review and a research agenda. J. Clean. Prod. 2019, 218, 850–870. [Google Scholar] [CrossRef]
- Grams, C.M.; Beerli, R.; Pfenninger, S.; Staffell, I.; Wernli, H. Balancing Europe’s wind–power output through spatial deployment informed by weather regimes. Nat. Clim. Chang. 2017, 7, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K. Recycling wind turbine blades. Renew. Energ. Focus 2009, 9, 70–73. [Google Scholar] [CrossRef]
- Cooperman, A.; Eberle, A.; Lantz, E. Wind turbine blade material in the United States: Quantities, costs, and end–of–life options. Resour. Conserv. Recy. 2021, 168, 105439. [Google Scholar] [CrossRef]
- Jani, H.K.; Kachhwaha, S.S.; Nagababu, G.; Das, A. A brief review on recycling and reuse of wind turbine blade materials. Mater. Today Proc. 2022, 62, 7124–7130. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Ni, A. Recycling and reuse of composite materials for wind turbine blades: An overview. J. Reinf. Plast. Comp. 2019, 38, 567–577. [Google Scholar] [CrossRef]
- Rani, M.; Choudhary, P.; Krishnan, V.; Zafar, S. Development of sustainable microwave–based approach to recover glass fibers for wind turbine blades composite waste. Resour. Conserv. Recy. 2022, 179, 106107. [Google Scholar] [CrossRef]
- Ajam, A.; Tehrani–Bagha, A.; Mustapha, S.; Harb, M. Zero–waste recycling of shelf–cured pre–impregnated carbon fiber reinforced epoxy laminae. Appl. Compos. Mater. 2020, 27, 357–373. [Google Scholar] [CrossRef]
- Giorgini, L.; Benelli, T.; Brancolini, G.; Mazzocchetti, L. Recycling of carbon fiber reinforced composite waste to close their life cycle in a cradle–to–cradle approach. Curr. Opin. Green Sust. 2020, 26, 100368. [Google Scholar] [CrossRef]
- Kuang, X.; Zhou, Y.; Shi, Q.; Wang, T.; Qi, H.J. Recycling of epoxy thermoset and composites via good solvent assisted and small molecules participated exchange reactions. ACS Sustain. Chem. Eng. 2018, 6, 9189–9197. [Google Scholar] [CrossRef]
- Gong, H.; Wu, J.; Zhao, Z.; Guo, Z.; Gao, L.; Zhang, B.; Li, M.; Hu, J. Recyclable high–performance glass–fiber/epoxy composites with UV–shielding and intrinsic damage self–reporting properties. Chem. Eng. J. 2022, 446, 137392. [Google Scholar] [CrossRef]
- Cousins, D.; Suzuki, Y.; Murray, R.; Samaniuk, J.; Stebner, A. Recycling glass fiber thermoplastic composites from wind turbine blades. J. Clean. Prod. 2019, 209, 1252–1263. [Google Scholar] [CrossRef]
- Verma, S.; Balasubramaniam, B.; Gupta, R.K. Recycling, reclamation and re–manufacturing of carbon fibres. Curr. Opin. Green Sust. 2018, 13, 86–90. [Google Scholar] [CrossRef]
- Jiang, J.; Deng, G.; Chen, X.; Gao, X.; Guo, Q.; Xu, C.; Zhou, L. On the successful chemical recycling of carbon fiber/epoxy resin composites under the mild condition. Compos. Sci. Technol. 2017, 151, 243–251. [Google Scholar] [CrossRef]
- Castaldo, R.; Falco, F.; Avolio, R.; Bossanne, E.; Fernandes, F.; Cocca, M.; Pace, E.; Errico, M.; Gentile, G.; Jasinski, D.; et al. Critical factors for the recycling of different end–of–life materials: Wood wastes, automotive shredded residues, and dismantled wind turbine blades. Polymers 2019, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sánchez–Rodríguez, D.; Kamo, T. A comprehensive study on the oxidative pyrolysis of epoxy resin from fiber/epoxy composites: Product characteristics and kinetics. J. Hazard. Mater. 2021, 412, 125329. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, H.M.; An, J.H.; Chung, D.C.; An, K.H.; Kim, B.J. Recycling and characterization of carbon fibers from carbon fiber reinforced epoxy matrix composites by a novel super–heated–steam method. J. Environ. Manag. 2017, 203, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Régnier, N.; Fontaine, S. Determination of the thermal degradation kinetic parameters of carbon fibre reinforced epoxy using TG. J. Therm. Anal. Calorim. 2001, 64, 789–799. [Google Scholar] [CrossRef]
- Pickering, S.J.; Kelly, R.M.; Kennerley, J.R.; Rudd, C.D.; Fenwick, N.J. A fluidised–bed process for the recovery of glass fibres from scrap thermoset composites. Compos. Sci. Technol. 2000, 60, 509–523. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Prabhakara, H.M.; Bramer, E.A.; Dierkes, W.; Akkerman, R.; Brem, G. A critical review on recycling of end–of–life carbon fibre/glass fibre reinforced composites waste using pyrolysis towards a circular economy. Resour. Conserv. Recy. 2018, 136, 118–129. [Google Scholar] [CrossRef]
- Guo, L.; Xu, L.; Ren, Y.; Shen, Z.; Fu, R.; Xiao, H.; Liu, J. Research on a two–step pyrolysis–oxidation process of carbon fiber–reinforced epoxy resin–based composites and analysis of product properties. J. Environ Chem. Eng. 2022, 10, 107510. [Google Scholar] [CrossRef]
- Biswas, S.; Satapathy, A. A comparative study on erosion characteristics of red mud filled bamboo–epoxy and glass–epoxy composites. Mater. Design 2010, 31, 1752–1767. [Google Scholar] [CrossRef]
- Mishnaevsky, L. Sustainable end–of–life management of wind turbine blades: Overview of current and coming solutions. Materials 2021, 14, 1124. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Barlow, C.Y. Wind turbine blade waste in 2050. Waste Manag. 2017, 62, 229–240. [Google Scholar] [CrossRef]
- Du, C.; Chang, Z.; Yu, H.; Zhu, Y.; Ma, Y.; Ma, G.; Yan, Y.; Wang, C.; Wang, W.; Cheng, Y. Magnetic quantum dots–stabilized foam fluid for enhanced oil recovery. Chem. Eng. J. 2022, 450, 138334. [Google Scholar] [CrossRef]
- Yin, H.; Yin, X.; Cao, R.; Zeng, P.; Wang, J.; Wu, D.; Luo, X.; Zhu, Y.; Zheng, Z.; Feng, Y. In situ crosslinked weak gels with ultralong and tunable gelation times for improving oil recovery. Chem. Eng. J. 2022, 432, 134350. [Google Scholar] [CrossRef]
- Gao, Q.; Zhong, C.; Han, P.; Cao, R.; Jiang, G. Synergistic effect of alkali–surfactant–polymer and preformed particle gel on profile control after polymer flooding in heterogeneous reservoirs. Energy Fuels 2020, 34, 15957–15968. [Google Scholar] [CrossRef]
- Garipov, T.T.; Hui, M.H. Discrete fracture modeling approach for simulating coupled thermo–hydro–mechanical effects in fractured reservoirs. Int. J. Rock. Mech. Min. 2019, 122, 104075. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Liu, Z.Y.; Zhang, J.; Chen, C.; Ma, M. Investigation on matching relationship and plugging mechanism of self–adaptive micro–gel (SMG) as a profile control and oil displacement agent. Powder Technol. 2020, 364, 774–784. [Google Scholar] [CrossRef]
- Yao, C.; Lei, G.; Li, L.; Gao, X. Selectivity of pore–scale elastic microspheres as a novel profile control and oil displacement agent. Energy Fuels 2012, 26, 5092–5101. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.; Chen, Z.; Wu, K.; Lu, N.; Zhang, Q. Enhanced oil recovery techniques for heavy oil and oilsands reservoirs after steam injection. Appl. Energ. 2019, 239, 1190–1211. [Google Scholar] [CrossRef]
- Zhu, D.; Hou, J.; Chen, Y.; Zhao, S.; Bai, B. In situ surface decorated polymer microsphere technology for enhanced oil recovery in high–temperature petroleum reservoirs. Energy Fuels 2018, 32, 3312–3321. [Google Scholar] [CrossRef]
- Ho, M.W.; Lam, C.K.; Lau, K.; Ng, D.H.L.; Hui, D. Mechanical properties of epoxy–based composites using nanoclays. Compos. Struct. 2006, 75, 415–421. [Google Scholar] [CrossRef]
- Hamad, M.A.; Khattab, I.A. Effect of the combustion process on the structure of rice hull silica. Thermochim. Acta 1981, 48, 343–349. [Google Scholar] [CrossRef]
- Wei, B.; Cao, H.; Song, S. Surface modification and characterization of basalt fibers with hybrid sizings. Compos. Part A 2011, 42, 22–29. [Google Scholar] [CrossRef]
- Du, C.; Zhang, N.; Ding, S.; Gao, X.; Guan, P.; Hu, X. Preparation of highly cross–linked raspberry–like nano/microspheres and surface tailoring for controlled immunostimulating peptide adsorption. Polym. Chem. 2016, 7, 4531–4541. [Google Scholar] [CrossRef]
- Meyer, L.O.; Schulte, K.; Grove–Nielsen, E. CFRP–recycling following a pyrolysis route: Process optimization and potentials. J. Compos. Mater. 2009, 43, 1121–1132. [Google Scholar] [CrossRef]
- Ma, C.; Sánchez–Rodríguez, D.; Kamo, T. Influence of thermal treatment on the properties of carbon fiber reinforced plastics under various conditions. Polym. Degrad. Stabil. 2020, 178, 109199. [Google Scholar] [CrossRef]
- Senneca, O.; Chirone, R.; Salatino, P. A thermogravimetric study of nonfossil solid fuels. 2. Oxidative pyrolysis and char combustion. Energy Fuels 2002, 16, 661–668. [Google Scholar] [CrossRef]
- Fan, L.Z.; He, H.; Nan, C.W. Tailoring inorganic–polymer composites for the mass production of solid–state batteries. Nat. Rev. Mater. 2021, 6, 1003–1019. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Chen, H.; Chen, J.; Zhu, R.; Ma, P.; Towers, A.; Lin, Y.; Gesquiere, A.J.; Wu, S.T.; et al. Ultrastable, highly luminescent organic–inorganic perovskite–polymer composite films. Adv. Mater. 2016, 28, 10710–10717. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, M.; Xiang, Y.; Zeng, T.; Yang, Z. Zr–induced thermostable polymeric nanospheres with double–cross–linked architectures for oil recovery. Energy Fuels 2019, 33, 10356–10364. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, G.; Hua, Z.; Zhao, Q.; Sun, F. Conformation and plugging properties of crosslinked polymer microspheres for profile control. Colloid Surf. A 2015, 477, 49–54. [Google Scholar] [CrossRef]
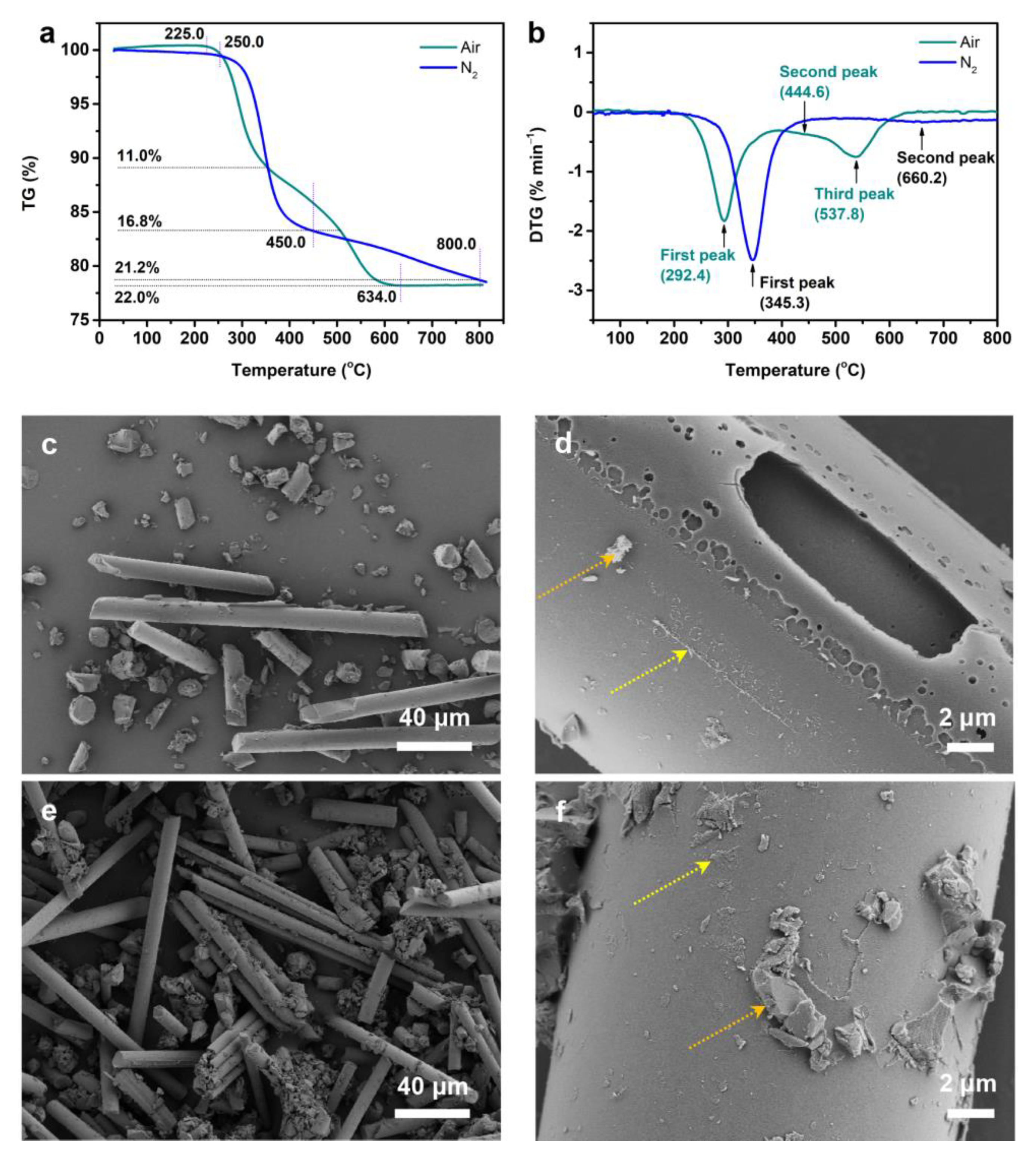
| Atmosphere | Temperature (°C) | (dm/dt)max (% min−1) | |||||
|---|---|---|---|---|---|---|---|
| T0.05 | T0.11 | T0.22 | Tmax1 | Tmax2 | Tmax1 | Tmax2 | |
| Air | 292.9 | 356.4 | 634.0 | 292.5 | 538.4 | 1.81 | 0.74 |
| N2 | 328.4 | 354.8 | – | 345.6 | 658.8 | 2.48 | 0.15 |
| PV | NaCl (g L−1) | Permeability (mD) | Plugging Rate (%) | |
|---|---|---|---|---|
| Before the Injection of Fiber/C2@FC Granules | After the Injection of Fiber/C2@FC Granules | |||
| 3.0 | 0 | 1987 | 653 | 67.1 |
| 4.0 | 0 | 1931 | 361 | 81.3 |
| 5.0 | 0 | 2022 | 354 | 82.5 |
| 5.0 | 50 | 2236 | 422 | 81.1 |
| 5.0 | 100 | 2214 | 440 | 80.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, C.; Jin, G.; Zhang, L.; Tong, B.; Wang, B.; Zhang, G.; Cheng, Y. Zero–Waste Recycling of Fiber/Epoxy from Scrap Wind Turbine Blades for Effective Resource Utilization. Polymers 2022, 14, 5408. https://doi.org/10.3390/polym14245408
Du C, Jin G, Zhang L, Tong B, Wang B, Zhang G, Cheng Y. Zero–Waste Recycling of Fiber/Epoxy from Scrap Wind Turbine Blades for Effective Resource Utilization. Polymers. 2022; 14(24):5408. https://doi.org/10.3390/polym14245408
Chicago/Turabian StyleDu, Chunbao, Ge Jin, Lihui Zhang, Bo Tong, Bingjia Wang, Gang Zhang, and Yuan Cheng. 2022. "Zero–Waste Recycling of Fiber/Epoxy from Scrap Wind Turbine Blades for Effective Resource Utilization" Polymers 14, no. 24: 5408. https://doi.org/10.3390/polym14245408
APA StyleDu, C., Jin, G., Zhang, L., Tong, B., Wang, B., Zhang, G., & Cheng, Y. (2022). Zero–Waste Recycling of Fiber/Epoxy from Scrap Wind Turbine Blades for Effective Resource Utilization. Polymers, 14(24), 5408. https://doi.org/10.3390/polym14245408









