Abstract
A polycondensation aromatic polyester with an oxygen spacer was synthesized and used as a macroinitiator for the grafting of linear poly(2-isopropyl-2-oxazoline) (PiPrOx) by the cationic polymerization method. The length of the thermosensitive side chains was varied by the initiator:monomer ratio. Using methods of molecular hydrodynamics, light scattering and turbidimetry, the copolymers were studied in organic solvents and in water. The molecular characteristics of the main chain and graft copolymers, the polymerization degree of side chains and their grafting density have been determined. The equilibrium rigidity of the macroinitiator and the conformations of grafted macromolecules were evaluated. In selective solvents, they take on a star-like conformation or aggregate depending on the degree of shielding of the main chain by side chains. The thermoresponsiveness of graft copolymers in aqueous solutions was studied, and their LCST were estimated. The results are compared with data for graft copolymers composed of PiPrOx side chains and flexible or rigid chain backbones of aromatic polyester type.
1. Introduction
Grafted polymers, the so-called cylindrical polymer brushes, are a class of polymers consisting of a long main chain and relatively short side chains grafted to it [1]. The physicochemical properties of molecular brushes in solution and in block are largely determined by their architectural parameters, such as the degree of polymerization of the main and side chains, the grafting density of side chains, etc. [1,2,3,4,5,6,7,8]. Due to the peculiarities of molecular architecture, cylindrical brushes are widely used to design new nanoobjects of various morphologies that cannot be obtained on the basis of linear polymers [9,10,11].
Research into molecular brushes began at the end of the 20th century due to the development of controlled synthesis of polymers of various architectures [2,3,12,13]. As a result, the main regularities of the behavior of graft copolymers in solutions, such as the effect of the length of the main and side chains and the grafting density of the latter, on the hydrodynamic characteristics and conformation of their macromolecules have been established [5,6,7,8,9,14,15,16,17,18,19,20].
The properties of grafted block-copolymers can depend on the interaction of chemically dissimilar side and main chains and on the solvent affinity to different blocks of the graft copolymer [4,13,21,22,23,24,25,26]. Accordingly, one of the ways to control the self-organization of molecular brushes in selective solvents is the targeted synthesis of brushes with different chemical structures of the main and side chains and molecular architecture parameters. The self-organization of amphiphilic polymer brushes differs significantly from the behavior of linear copolymers in solutions [27,28,29,30]. For example, amphiphilic polymer brushes in selective solvents make it possible to obtain monomolecular micellar core–shell structures, which can serve for the transport and isolation of inorganic particles in solution. For solutions of amphiphilic aromatic polyimides grafted onto vinyl polymers such as polystyrene, poly(methyl-methacrylate), poly(tert-butyl-acrylates) and their copolymers with poly(methacrylic acid), it has been shown that copolymers with a low grafting density form aggregates due to contacts with polyimide backbones. At high grafting density and longer side chains, brush conformations change from spherical in theta solvents to rod-like in good solvents [24,31,32,33].
One of the promising trends in the development of nanostructured polymeric materials is the synthesis of complex polymeric associated systems capable of reversibly changing the hydrophilic-hydrophobic balance under external impacts, such as temperature, pH, irradiation, etc. Under conditions, such “smart” systems can modify their intra- and intermolecular organization, as well as complex-forming properties. The stimuli-responsive properties can be controlled by varying the chemical structure, molecular architecture, and composition of the graft copolymer [34,35,36,37,38]. For example, for graft copolymers with poly(N-isopropylacrylamide) side chains, a significant effect of the end group structure and side chain length on the behavior of aqueous solutions upon heating was revealed [36]. An important role in the formation of the properties of molecular brushes in solutions is also played by the rigidity of their structural elements, main and side chains. The presence of rigid chain fragments in side chains can lead to their orientational ordering in solution [4]. On the other hand, grafting of long, flexible chains onto a rigid aromatic backbone prevents aggregation when the grafting is sufficiently dense [39].
To obtain reversibly thermosensitive systems, polymers based on poly(N-isopropylacrylamide), dimethyl-amino-ethyl-acrylates, oligo(ethylene-oxide), and poly(2-alkyloxazolines) (PAlOx) are used [40]. PAlOx are especially in demand for medical applications due to their biocompatibility and rather low LCST (i.e., the lower critical solution temperature) [41]. Tertiary amide groups in PAlOx ensure their stability in biological media [42,43,44,45]. Complexes of PAlOx and their copolymers with low molecular weight compounds and metal ions are promising for drug and DNA delivery [46], for the design of biocompatible composite structures [47] and other applications. PAlOx graft copolymers have been shown to be the most promising matrices for drug delivery [48].
To date, the effect of the PAlOx copolymer architecture, namely, their star-shaped, grafted or block structure, the chemical nature of the end groups and the hydrophobic-hydrophilic balance, on the temperature dependence of the polymer solubility has been studied [49,50,51,52,53,54,55]. For methacrylic acid copolymers or polystyrene derivatives as a backbone, it has been shown that the cloud point Tcp decreases with increasing molecular weight and backbone length [50]. Graft copolymers with methacrylate backbone and poly(2-ethyl-2-oxazoline) (PEtOx) side chains demonstrated the coexistence of aggregates and macromolecules at low temperatures [49].
Amphiphilic thermoresponsive PEtOx block copolymers with an aromatic polyester (APE) backbone have been studied to a lesser extent. Such copolymers can be obtained by a polymerization-polycondensation reaction, in which a main chain is formed as a result of polycondensation, followed by grafting from polymerization of side chains [56,57,58]. Most solvents for such macromolecules are selective. The authors of [59] proposed the synthesis of a polycondensation APE macroinitiator containing functional groups suitable for initiating the cationic polymerization of 2-alkyl-2-oxazolines to obtain graft copolymers. Using the polymerization-polycondensation approach, stimulus-sensitive graft copolymers (APE6) with an aromatic polyester backbone flexible main chain containing a –(CH2)6– as a spacer and PEtOx side chains (APE6-g-PEtOx) were synthesized [60,61]. For APE6-g-PEtOx, it was shown that the side chain grafting density z determines the mechanism of macromolecules self-organization, namely, compaction or aggregation, and causes a change in the phase separation temperature, which increases with z. The replacement of side chains with more hydrophobic poly(2-isopropyl-2oxazoline) (PiPrOx) leads to a decrease in phase separation temperatures [62]. Note that dehydration of 2-ethyl-2-oxazoline units begins at 50 °C, and that of 2-isopropyl-2-oxazoline at about 20 °C [63]. Cloud points observed for PiPrOx are generally higher than for the 2-isopropyl-2-oxazoline monomer; the thermal transition temperature of PiPrOx is affected by polymer concentration and molar mass, as well as terminal functional groups. When varying these parameters, Tcp have been observed by various studies at temperatures from 36 to 63 °C. Molecular architecture can also influence Tcp: for example, a cyclic PiPrOx exhibited Tcp around 10 °C higher than a linear PiPrOx of the same molar mass. [40,41,64]. It has been shown that, in a selective solvent, APE8-g-PiPrOx polymerization-polycondensation molecular brush with short PiPrOx chains and a backbone containing sufficiently long alkylene fragments –(CH2)8– in the monomer unit forms unimolecular micelles of a star-like conformation at a low z ~0.5. On the contrary, molecular brushes with PAlOx side chains and the rigid main chain of aromatic APE (APEr.ch.-g-PAlOx) at a low z and short side chains aggregate into large supramolecular structures, and they are molecularly dispersed only at sufficiently long side chains [65]. In this case, the LCST is affected by the chemical structure of the side chains.
Thus, the chemical structure and equilibrium rigidity of the main chain essentially determine the mechanism of self-organization of a molecular brush in solution. It seems important to continue the series of copolymers by varying the structure of the main chain, passing to a main chain with an intermediate equilibrium rigidity. For this purpose, an aromatic polyester type macroinitiator with an oxygen atom as a spacer was synthesized (APEO) and graft copolymers with PiPrOx side chains of various lengths were obtained (Figure 1). Thus, the main objectives of this work were to determine the hydrodynamic and conformational characteristics of the APEO macroinitiator and its block copolymers with grafted PiPrOx chains, and to study the effect of the side chain length on the conformational characteristics of APEO-g-PiPrOx copolymers in selective solvents and on thermal responsiveness in aqueous solutions.
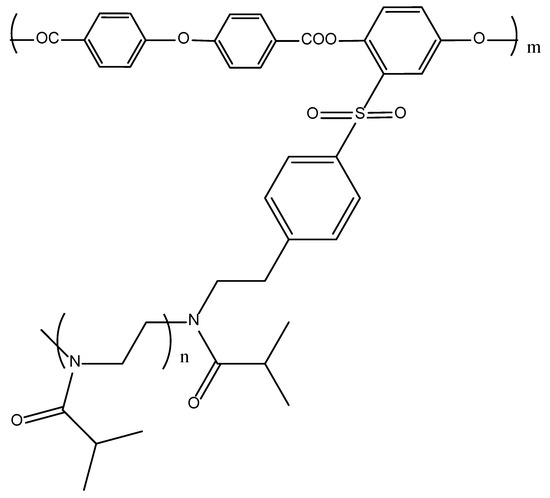
Figure 1.
Chemical structure of APEO-g-PiPrOx.
2. Experimental
2.1. Materials and Instruments
2-[4-(2-Br-ethyl)]phenylsulfonyl hydroquinone [66], 4,4′-oxydibenzoyldicloride [67], 2-isopropyl-2-oxazoline [68] were obtained according to the known procedures. Diphenyl oxide, 1-chloronaphalene and 1,1,2,2-tetrachloroethane (Aldrich) were dried over calcium hydride and distilled. NMR spectra of solutions of samples in CDCl3 were recorded using a Bruker AC 400 instrument (400 MHz). Dialysis was performed with the use of dialysis bags (CellaSep, Orange Scientific) with MWCO 3500 D. Chromatographic analysis was performed with the use of a Shimadzu LC-20AD chromatograph equipped with a TSKgel G5000HHR column (5 μm, 7.8 mm × 300 mm, Tosoh Bioscience) and a refractometric detector. Solution of LiBr in DMF (0.1 mol/L) at 60 °C was used as a mobile phase. Calibration was performed with the use of poly(ethylene glycol) standards (Mw = 6 × 102–4 × 104).
2.2. Synthesis of Poly(2-[4-(2-Br-ethyl)phenylsulfonyl]-1,4-phenylene-4′,4″-oxydibenzoate
A flask equipped with a stirrer and a gas-supplying tube was charged with 2-[4-(2-Br-ethyl)]phenylsulfonyl hydroquinone (4.23 g, 0.01 mol), 4,4′-oxydibenzoyl chloride (2.95 g, 0.01 mol), and diphenyl oxide (30 mL). The obtained mixture was purged with dry argon and heated up to 200 °C. The reaction mixture was kept at 200 °C for 2 h. The polymer was precipitated with hexane, filtered off, reprecipitated from chloroform into hexane, and dried to a constant mass. The yield was 5.9 g (92%). 1H NMR (CDCl3): 3.22 (d, ArCH2CH2Br), 3.56 (d, ArCH2CH2Br), 7.11–8.43 (m, Ar–H) ppm. [η] = 0.24 dL/g (CHCl3, 25 °C); Mw = 23,000.
2.3. Synthesis of Polyester-g-poly-2-isopropyl-2-oxazoline Copolymers
Solution of the initiator (30 wt.%) and the monomer in 1,1,2,2-tetrachloroethane (feed ratio of about 10% from the desired value) was heated in sealed tube at 100 °C for 3 h. Then, the rest of the monomer was added to reaction mixture and polymerization was allowed to proceed at 120 °C for 24 h. After completion of polymerization and removal of volatile compounds, the reaction mixture was diluted with ethanol, dialyzed against water for 48 hrs and lyophilized.
2.4. Determination of the Molecular Characteristics of Polymers
The weight-average molar masses Mw of the macroinitiator and copolymers and the hydrodynamic radii Rh of their macromolecules were determined in dilute solutions in organic solvents (chloroform, nitropropane) by static (LS) and dynamic (DLS) light scattering methods. Since there was practically no asymmetry in the scattered light intensity, Mw was determined by the Debye method. The equation [69]:
where I is the intensity of light scattering measured at 90°, A2 is the second virial coefficient, and H is the optical constant,
NA being the Avogadro’s number was applied. The refractive index increment dn/dc was measured on a Refractometer RA-620/600 (KEM, Kyoto, Japan), with LED Na-D Line light source. The LS and DLS measurements were carried out in a Photocor Complex set-up (Photocor Instrument, Moscow, Russia), which was equipped with a Photocor-PC2 correlator with 288 channels, and a Photocor-PD detector for measuring the transmitted light intensity. The light source was a Photocor-DL semiconductor laser with a wavelength of λ0 = 659.1 nm. Calibration was carried out with toluene, the absolute scattering intensity being Rv = 1.38 × 10−5 cm−1. Before measurements, the solutions were filtered into dust-free cells using Chromafil polyamide filters (Macherey-Nagel GmbH & Co. KG) with a pore size of 0.45 μm.
The hydrodynamic radius Rh was determined by the regularization method which is used as a part of the Photocor Complex software. The measurements were taken at an angle of 90 degrees.
Intrinsic viscosity [η] was measured in Ostwald type Cannon-Manning capillary viscometers (Cannon Instrument Company, State College, PA, USA) and an LOIP LT-100 temperature control unit (LOIP, St.-Petersburg, Russia). Viscosity data were analyzed using the Huggins equation
where k′ is the Huggins constant characterizing the polymer–solvent hydrodynamic and thermodynamic interactions in solutions and ηsp = (τ/τ0 − 1), τ being the solution flow time [70,71]. The solvent efflux time was τ0 = 116.0 s for chloroform, 105.4 s for nitropropane and 57.2 s for DMF.
ηsp/c = [η] + k′[η]2c,
2.5. Study of Thermoresponsiveness of Copolymers in Solutions upon Heating
The thermoresponsiveness of copolymers was studied by LS, DLS and turbidimetry in aqueous solutions using the Photocor instrument described above. The temperature T was controlled with an accuracy of 0.1 °C, changing it discretely with a step of 1.0 °C near the phase separation point T1 and with a larger interval up to 5.0–6.0 °C at temperatures far from T1.
Scattered light intensity I and transmitted light intensity I* were measured as functions of T upon heating. The temperatures T1 and T*1 of the onset of phase separation were determined from the dependences I(T) and I*(T), taking for T1 and T*1 the temperatures after which I began to increase or I* decreased, respectively. The hydrodynamic radii of scattering species Ri and their contribution Si to the total scattering intensity S, i being associated with the type of scattering specie, were also measured at various T. The Si value was estimated as the area under the corresponding peak of I(R) distribution obtained by DLS. It should be noted that measurements of all parameters were carried out after the solution reached an equilibrium state, that is, when the I and I* reached constant values after an abrupt change in T.
3. Results and Discussion
3.1. Synthesis of Multicenter Oligoester Initiator
In the synthesis of the macroinitiator, high-temperature solution polycondensation without acceptor was used [72]; this method allows synthesize polyesters over a wide range of molecular masses with polydispersity index of 2.1–2.3. Synthesis of the APEO macroinitiator was conducted in accordance with that described in [59]. The conditions for the polycondensation were the following: 1-chloronaphthalene as the solvent; temperature of 200 °C, monomer concentration 25 wt.%, reaction time 2 h.
3.2. Synthesis of APEO-g-PiPrOx with the Use of APEO Macrointiator
Methods and approaches to the preparation of APEO-g-PiPrOx block copolymers are described in [59]. Graft copolymers were synthesized via CROP of 2-isopropyl-2-oxazoline according to the following scheme (Figure 2):
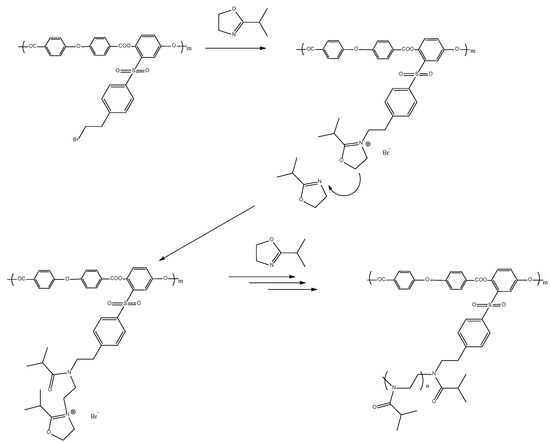
Figure 2.
Synthesis of APEO-g-PiPrOx graft copolymers.
Grafting of PiPrOx chains was carried out in tetrachloroethane solution at 120 °C to improve solubility of the macroinitiator monomer, since thermodynamic quality of a solvent becomes higher with increasing temperature. The process was carried out in two stages. In the first stage, 10% from the calculated amount of the monomer was introduced into polymerization mixture, and the mixture was heated at 100 °C for 3 h. The addition of the remaining amount of oxazoline made it possible to post-polymerize the side chains due to the “living” character of the process. When loading the reaction mixture, the initiator/monomer ratio was 1/40 (sample 1), 1/100 (sample 2) and 1/120 (sample 3), which made it possible to obtain three samples of the graft copolymer with PiPrOx side chains of various lengths. GPC data confirm unimodality in their size distribution (Figure 3). The amount of grafted PiPrOx was estimated using 1H NMR data by the ratio of signal intensity of the terminal methyl group of 4-methylpiperidine (at about 0.9 ppm) and signal intensity of poly(ethylene imine) fragments (at about 3.4 ppm). The average degree of polymerization of monomer units Ns in grafted oligo(oxazoline) chains was 30, when the initial initiator/monomer ratio was equal to 1/40; this value was 50 at a ratio of 1/100 and 60 at a ratio of 1/120. The fact that degree of polymerization of grafted chains is not proportional to initial initiator/monomer ratio is caused by chain transfer to monomer. This issue was analyzed by us earlier [59]. The observed decrease in initiation efficiency in the case of multicenter macroinitiators may be due to higher growth rate of side chains than the initiation rate; therefore, steric hindrances of side chains impede the process. Indeed, the asymmetric shape of the chromatogram obtained for graft copolymers (Figure 3) indicates some inhomogeneity in distribution of grafted fragments alongside the backbone.
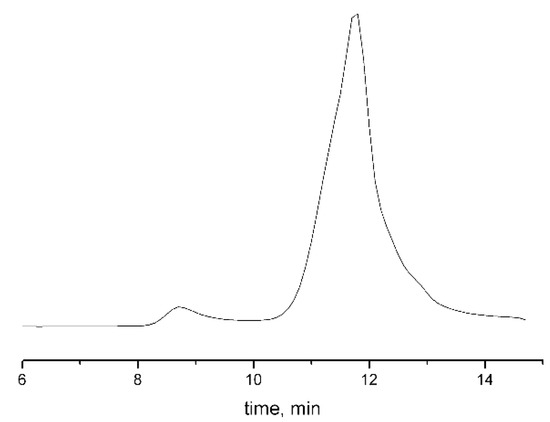
Figure 3.
Eluogram for APEO-g-PiPrOx sample obtained after grafting at 1/40 initiator/monomer ratio.
3.3. Molecular and Conformational Properties of APEO and APEO-g-PiPrOx
The molecular and hydrodynamic characteristics of the macroinitiator were determined in chloroform. Graphs for their determination are presented in Figure 4; Table 1 presents the results. The values of the second virial coefficient A2 and the Huggins constant k′ calculated from plots of Figure 4a,c (Equations (1)–(3)) testify to the good thermodynamic quality of CHCl3 as a solvent for APEO. The Rh was obtained by extrapolating the hydrodynamic radii determined at finite concentrations to c = 0 (Figure 4b). The viscometric hydrodynamic radius Rη = 5.7 nm for APEO, calculated from the formula
significantly exceeds Rh, which may testify to an extended (asymmetric) conformation of the macroinitiator. The polymerization degree of the macroinitiator calculated from the Mw value (Table 1) is N0 = 38, since the molar mass of the APEO monomer unit is M0 = 580 g/mol. To estimate the length of the monomer unit of the macroinitiator L0, it was assumed that the length of valence bonds is 0.14 nm and the bond angles are tetrahedral; then, in accordance with the APEO structural formula, the sum of its bond lengths along the chain direction is L0 = 1.6 nm (without deformation of the bond angles) and the macromolecular chain length is L = 60.7 nm.
Rη = (3M[η]/10πNA)1/3,
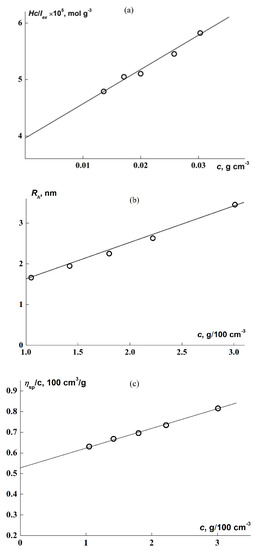
Figure 4.
Concentration dependences of cH/Iex (a), Rh (b), and ηsp/c (c) for APEO solutions in CHCl3.

Table 1.
Molecular characteristics for APEO and APEO-g-PiPrOx.
The equilibrium rigidity, which is measured by the Kuhn segment length A, for an APEO can be estimated by comparing its characteristics with the A value for a polymer of close chemical structure, for example, semi-rigid poly(m-phenylene isophthalamide) (PMPhIPhA), studied in [73]. For this polymer, the values A ≈ 4–4.7 nm were obtained, which are increased, compared to flexible-chain polymers, due to aromatic fragments in the meta position. Although the comparison of structures of APEO and PMPhIPhA is quite approximate, it can be assumed that the equilibrium rigidity of APEO should not be greater than that of PMPhIPhA, since the –O– spacer present in the structure of APEO allows rotation of the chain around the valence bond; thus, Kuhn segment length for APEO should be 2 nm < A < 4 nm, where A = 2 nm was obtained for APE macroinitiators with flexible alkylene spacers [74]. Then the APEO chain consists of more than 15 Kuhn segments, therefore it should be sufficiently coiled in solution. Thus, a worm-shaped cylinder with a contour length of 60.7 nm can serve as a model for the APEO macroinitiator chain in solution. The increased equilibrium rigidity of APEO and, consequently, the increased permeability of its macromolecules in NP probably cause their low Rh (Table 1).
Mw and hydrodynamic characteristics of APEO-g-PiPrOx graft copolymers were determined in nitropropane (NP) (Table 1). Debye plots for determining Mw are shown in Figure S1. The A2 values are positive and decrease with increasing iPrOx monomer content indicating that the solvent is worsening for the copolymers upon the initiator/monomer ratio. The hydrodynamic radii obtained by the DLS method did not depend on the concentration, and Table 1 presents the concentration-averaged Rh values. The hydrodynamic radii of APEO-g-PiPrOx exceed the Rh obtained for the macroinitiator (Table 1). This increase is conditioned by significant contribution of side chains to the friction coefficient of the macromolecule in the solvent. However, the values of both Rh and intrinsic viscosities [η] are very small at sufficiently large Mw, which can be seen from the Figure 5. Here, the points corresponding to the data for samples 1 and 2 in NP lie well below the logarithmic dependence [η](M) carried out for linear PEtOx according to the equation [η] = 5.0 × 10−2 M0.63 from the work [75], and also lower than for APE8-g-PiPrOx in NP [62]. This testifies to a high intramolecular density of APEO-g-PiPrOx macromolecules. As was shown in [32,62], the grafted block-copolymer can adopt a star-like conformation, in case of low grafting degree of side chains and short grafted blocks; here the arms are the PiPrOx side chains and the core is the backbone macromolecule collapsed in a nonsolvent. The [η] and Rh values of sample 3 were measured in DMF (Table 1). They noticeably exceed [η] and Rh obtained for APEO-g-PiPrOx in NP (Figure 5), which, at close Mw of all copolymers, could be caused by swelling of PiPrOx side chains in this solvent. However, the increase in Rh by more than 2 times in DMF rather attributes to intermolecular aggregation, what is indicated by a negative k’ value. This issue will be discussed below.
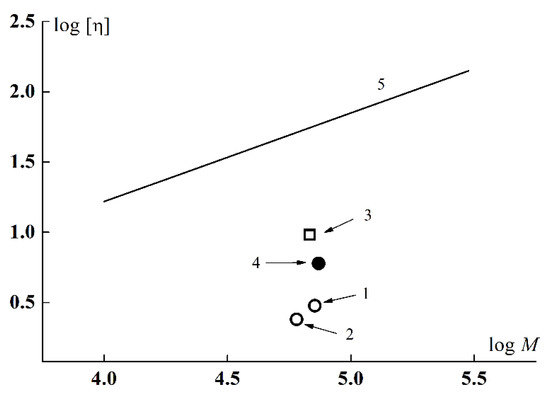
Figure 5.
Intrinsic viscosity [η] vs. Mw for APEO-g-PiPrOx sample 1 (1) and sample 2 (2) in nitropropane, APEO-g-PiPrOx sample 3 in DMF (3), APE8-g-PiPrOx [62] (4), and Mark–Kuhn–Houwink–Sakurada dependence [η] = 5.0 × 10−2M0.63 given for linear PEtOx [75] (5).
Based on the experimentally measured values of Mw for APEO (MAPE) and the copolymers, as well as the mass of the side chain Ms, the architecture parameters of the APEO-g-PiPrOx graft copolymer were calculated, which are given in Table 2. The side chain length in the extended conformation without deformation of bond angles was calculated using the formula Ls = λ × Ns, since the length of the projection of the alkylene unit onto the direction of the PiPrOx chain is λ = 0.252 nm. Accordingly, molar masses Ms of side chains are equal to Ms = Ns × M0s, where the mass of the PiPrOx monomer unit, M0s = 113 g/mol. Then the degree of functionalization of the macroinitiator (or grafting density of side chains) is z = m/(n + m) = m/N0, where m is the number of the backbone monomer units containing side chains (i.e., the number of side chains) and n is the number of the monomer units of the macroinitiator which do not contain side chains [59,60]; m was calculated using the formula m = (Mw − MAPE)/Ms.

Table 2.
Structural parameters for APEO-g-PiPrOx.
It can be seen from Table 2 that in all cases the value of z is small, i.e., APEO-g-PiPrOx macromolecules are brushes with rather loosely attached side chains. Less than half of the monomer units in the main chain carry the side chains. The value of z decreases upon an increase in Ls, and for sample 1 this is about two times greater than for samples 2 and 3. At the same time, the side chains are 2.2–2.7 times longer than the distance between the grafting points, calculated as ∆L = L0/z (Table 2). The parameter Ls/∆L makes it possible to compare the distance between the grafting points of the side chains and their length, which is maximum for sample 1.
The degree of shielding of the molecular brush backbone by side chains and its accessibility for contacts can be quantitatively characterized by the parameter δ that takes into account the size of the side chain folded into a molecular coil [24]. This parameter is conveniently calculated in terms of the number of bonds in the side chains and in the fragments of the main chain between their grafting points, δ = [(NA × Ns)]0.5/(N0 × z), where the number of bonds in the Kuhn segment of the side chain NA = 3 for PiPrOx [74], and N0 × z characterizes the number of bonds in the backbone of the brush between adjacent side chains. Characteristically, the degree of shielding calculated for the studied brushes is always low (Table 2). However, the parameter δ obtained for brush 1 significantly exceeds this parameter for brushes 2 and 3 with longer side chains, but with a lower side chain grafting density.
3.4. Composition of Scattering Species in Solution and Their Hydrodynamic Size at Room Temperature
At room temperatures (T < 27 °C), two modes associated with scattering species with hydrodynamic radii Rf and Rs, were registered in DLS experiments in water solutions. For all samples, species of large hydrodynamic size, which are aggregates, were observed. Their averaged hydrodynamic radii Rs were 300 nm for brush 1, 40 nm for brush 2 and 170 nm for brush 3 (Table 1). It can be noted that Rs tends to increase with concentration despite a general significant scatter of points (Figure 6). The proportion of light scattered by aggregates, Ss, in the total light scattering always exceeds 98.5%. However, it should be understood that a high contribution from large species to scattering intensity does not mean their high concentration in solution. Indeed, for a particle of type i, the relation Ii ~ ciMi is true, where Mi and ci are the molar mass of the specie and its content in the solution. In this case, Mi ~ Rix, where the parameter x depends on the particle shape. For example, x = 1, 2 and 3 for the long rod, the macromolecular coil, and for the sphere, respectively. Then the partial concentration for spheres is ci ~ Ii/Ri3. It can be estimated that for spherical particles, whose sizes differ by 1–2 orders, even at a high contribution of the scattering intensity, the concentration ci can be very small. However, since the shape and density of aggregates are a priori unknown, we cannot obtain their content in solution. Though measurements at a large scattering angle could be advantageous when using DLS to determine the relative concentration of nanoparticles in the samples with bimodal size distributions [76], the question of the physical and topological properties of supramolecular structures does not allow us to carry out a quantitative analysis of their content in solution. Nevertheless, we can trace the change in the contribution of various species to light scattering with a change in the external parameters of the medium, such as concentration, temperature, etc.
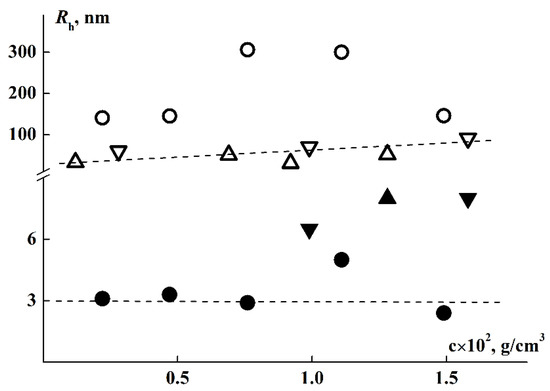
Figure 6.
Concentration dependence of Rf (solid) and Rs (open) for copolymers 1 (circle), 2 (triangles), and 3 (inverse triangles) in water at T = 21 °C.
From Table 1 it follows that the size of the fast mode Rf, obtained for aqueous solutions, exceed the radii of macromolecules Rh, determined in organic solvents. On the one hand, this may indicate increased size of macromolecules caused by swelling of PiPrOx chains in water, which apparently occurs in the case of brush 1. On the other hand, with a loose attachment of side chains, it is more likely that even far from the phase separation temperatures, the macromolecules form small supramolecular structures. As was shown above, the degree of shielding of the main chain by side chains is low for the studied brushes, especially in copolymers 2 and 3, which gives preconditions for hydrophobic interactions of their main chains and hence their intermolecular aggregation.
3.5. Thermoresponsiveness of APEO-g-PiPrOx in Water While Heated
Upon heating APEO-g-PiPrOx aqueous solutions, a significant increase in I and, respectively, a decrease in optical transmission I* are observed, indicating a phase separation (Figure 7). For copolymer 1, the increase in I was quite sharp. However, a smooth appearance in I/I0(T) and I*/I*0(T) dependencies, where I0 and I*0 are initial scattering intensity and optical transmission at T = 15 °C, respectively, for brush solutions 2 and 3 were obtained. Similarly, a smooth augmentation in I was noted in the study of the PEtOx graft copolymer synthesized on a flexible-chain aromatic macroinitiator of a similar structure APE6-g-PEtOx containing an alkylene spacer [60,61]. The temperature T1 was determined from the beginning of the drop in the optical transmission I* (Figure 7).
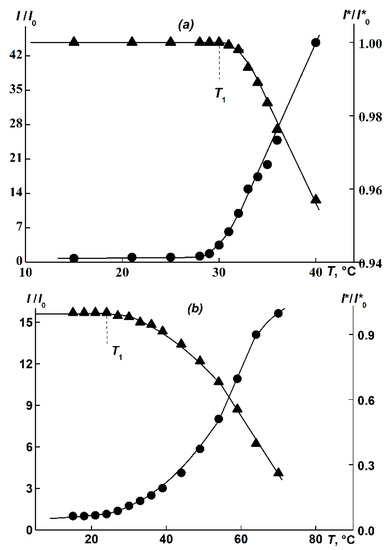
Figure 7.
I/I0(T) and I*/I*0(T) for sample 1 (a) at c = 0.0022 g/cm3 and sample 3 (b) at c = 0.016 g/cm3.
The hydrodynamic dimensions of dissolved species of both types, Rf and Rs, in solutions of all samples increased upon heating (Figure 8a and Figure 9a), augmentation beginning in the vicinity of T1. The increase in size takes place due to attaching of macromolecules to aggregates and to coupling of existing supramolecular structures. Thus, the fast mode in the sample 1 aqueous solutions transforms from macromolecules to small aggregates. The aggregate formation upon heating is conditioned by the decrease of PiPrOx solubility due to dehydration of 2-isopropyloxazoline units, which begins already at T ≈ 20 °C [63]. Note that the relative rate of change in the size of small aggregates Rf is noticeably higher than the rate of increase in Rs. Thus, Rf increases almost 20 times when heated to T ≈ 80 °C, while Rs changes by approximately 30%. Accordingly, the contribution of large aggregates to light scattering Ss at temperatures above T1 decreases, while Sf increases (Figure 8b and Figure 9b).
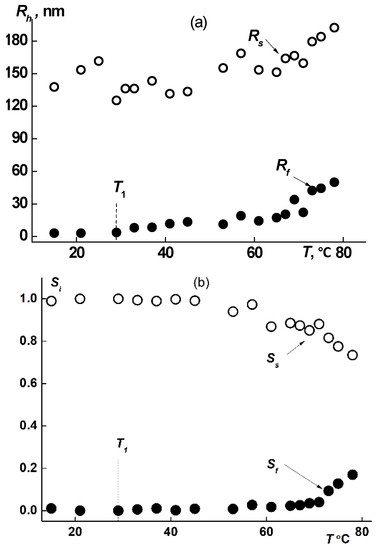
Figure 8.
Progress of hydrodynamic size (a) and Si (b) (i is f or s) upon heating for fast and slow modes in sample 1 aqueous solution at c = 0.0047 g/cm3.
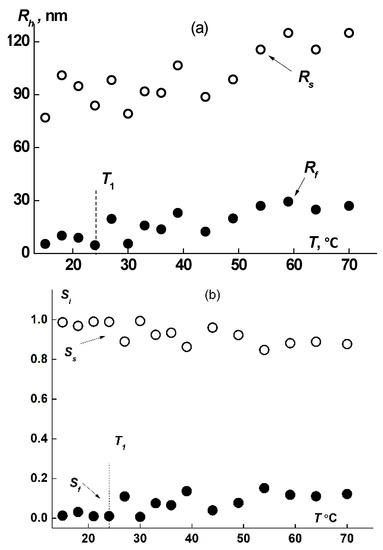
Figure 9.
Progress of hydrodynamic size (a) and Si (b) (i is f or s) upon heating for fast and slow modes in sample 3 aqueous solution at c = 0.016 g/cm3.
3.6. Concentration Dependence of the Phase Separation Temperatures
The T1 values obtained for APEO-g-PiPrOx at various concentrations are generally in the same region (Figure 10a,b). The T1 for brush 1 with shorter side chains and higher z is practically independent of concentration, and the concentration-averaged temperature of the phase transition is <T1> = (30 ± 1) °C. As for brushes 2 and 3, despite the rather large scatter of experimental points, they fit into the general dependence T1(c), demonstrating a tendency for T1 to decrease from 35 to 24 °C with an increase in c within a more than 10-fold concentration range. This trend is consistent with the previously obtained dependence for PiPrOx molecular brushes with flexible and rigid main chains [62,65]. In this case, in general, the region of the onset of phase separation T1 for the studied APEO-g-PiPrOx lies above the entire corresponding temperature range 20–25 °C for the phase separation of graft copolymer with a flexible backbone APE8-g-PiPrOx [62].
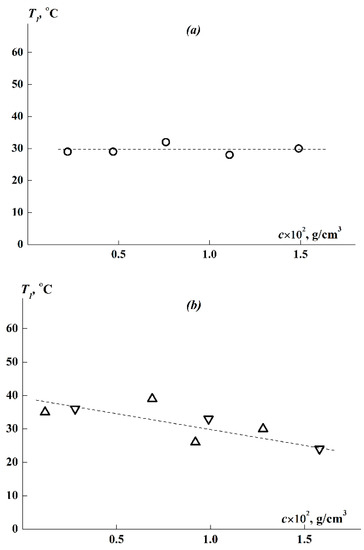
Figure 10.
Concentrational dependence of phase separation temperatures for (a) sample 1 and (b) samples 2 (triangles) and 3 (inverted triangles) in APEO-g-PiPrOx aqueous solutions.
The above results show similar behavior of copolymers with longer side chains but lower z in solutions at room temperature and upon heating: the I/I0(c) profile, the composition and size of the scattering particles, and the phase separation temperatures as functions of concentration are similar for samples 2 and 3. This can undoubtedly be due to their similar molecular properties, which can be described using architectural parameters, such as z, Ls, δ and so on.
4. Discussion and Conclusions
The studied APEO-g-PiPrOx copolymers with blocks that differ in affinity to the solvents, at low z exist in the regime of strong intra- and intermolecular interactions. For side chains of different length and similar backbone, their behavior can be controlled by the parameter δ, which is a parameter to compare the distance between the grafting points on the macromolecule backbone (and hence, z) and the model dimensions of the side chains, taking into account the size and rigidity of their structural units. The studied copolymers exhibiting similar molecular and thermoresponsive properties, namely samples 2 and 3, are characterized by a close value of δ, which differs by about 1.5 times from the δ value for sample 1 (Table 2). Due to the ability of the semirigid APEO to change the conformation, the main chain is always folded to avoid the solvent and is surrounded by swollen side chains. Schematically, the considered macromolecules with the same length of the main chain but different side chain length and different low grafting degree can be represented as star-shaped structures, with a core of collapsed APEO chain, and loosely grafted PiPrOx arms which are in a coiled conformation. In the case of sample 1, where the degree of shielding is δ = 0.21, the macromolecules are soluble, similarly to that for APE8-g-PiPrOx with flexible backbone [62] (Figure 11a). At δ ≤ 0.14 (samples 2 and 3), molecular coils of a soluble PiPrOx do not shield the core sufficiently, which creates the preconditions for the aggregation of copolymers due to hydrophobic interactions of the main chains (Figure 11b). For comparison, for the brush APE8-g-PiPrOx with long flexible –(CH2)8– spacer in the main chain and rather short PiPrOx arms (Ns = 37), the calculated value of δ = 0.18 is close to that of sample 1 (Table 3).
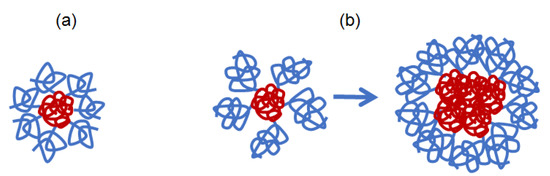
Figure 11.
Model conformations for APEO-g-PiPrOx samples 1 (a) and 2 and 3 (b) in water.

Table 3.
Comparison of PiPrOx bottle brushes with different rigidity of the APE main chains.
Since we determined the transition temperatures T1 over a wide, more than 10-fold concentration range from 0.0012 to 0.016 g/cm3 (see Figure 10), we attempted to estimate the LCST for the APEO-g-PiPrOx samples. For sample 1, whose solutions did not demonstrate a noticeable dependence T1(c), we can apparently assume LCST ≈ <T1> ≈ 30 °C. For sufficiently hydrophobic samples 2 and 3, the value of T1 decreases monotonically with increasing concentration. Judging by our observations of the dissolution of the samples, it is unlikely that, with a further increase in c above the measurement interval, the solubility of the polymer will improve, and hence T1 will increase. Therefore, LCST < 24 °C for samples 2 and 3 can be estimated. It should be understood that the LCST estimation performed for APEO-g-PiPrOx is rather rough, but it is helpful for comparing the thermosensitivity of polyoxazoline brushes with aromatic polyester backbones of different rigidities (Table 3).
Table 3 represents parameters for PiPrOx grafted copolymers with APE main chain of different rigidity. Due to the high proportion of the hydrophobic component ω = M/Mw in the composition of APEO-g-PiPrOx, where M = Mw − MAPE (Taблe 3), its hydrophilic–hydrophobic balance in water solutions is shifted to the temperature below the LCST detected for linear PiPrOx. As discussed in the Introduction, the cloud points for PiPrOx were observed at 36 < T < 63 °C depending on concentration, molar mass and architecture [40,41,64]. For block copolymers that are identical in chemical structure, but with a difference in architectural parameters, at close values of δ, a similar behavior of the APEO copolymers upon heating is observed; at higher δ, the LCST of the polymer is higher. However, upon transition to a rigid aromatic polyester main chain, the thermoresponsiveness range is significantly lower, than it is for APEO-g-PiPrOx at close structural parameters. Thus, the ability of the main chain of the grafted block copolymer to undergo intramolecular transformations plays a significant role in the formation of its properties in solutions at room temperature and upon heating.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym14245354/s1, Figure S1: Dependences cH/Iex(c) for APEO-g-PiPrOx solutions of samples 1 (a), 2 (b) and 3 (c) in nitropropane.
Author Contributions
Conceptualization, A.T. and A.F.; Formal analysis, A.K. and M.K.; Investigation, A.K. and M.K.; Data curation, A.K.; Writing – original draft, E.T. and A.T.; Writing – review & editing, E.T. and A.F.; Supervision, E.T. and A.T.; Project administration, A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (State Registration no.122012100166-4).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| APEO | aromatic polyester with—O—spacer |
| APE6 | aromatic polyester with—(CH2)6—spacer |
| APE8 | aromatic polyester with—(CH2)8—spacer |
| APEr.ch. | rigid chain aromatic polyester |
| Mw | weight-average molar mass |
| M0 | the mass of the macroinitiator monomer unit |
| MAPE | the macroinitiator molar mass |
| N0 | the degree of polymerization of the macroinitiator |
| L0 | the length of the monomer unit of the macroinitiator |
| L | the length of the macroinitiator macromolecule |
| A | the Kuhn segment length |
| Ms | the mass of the side chain |
| Ns | the degree of polymerization side chains |
| M0s | the mass of the side chain monomer unit |
| λ | the length of the projection of the alkylene bond onto the direction of the PiPrOx chain |
| n | the number of side chains |
| z | the density of side chains grafting |
| ΔL | the distance between the grafting points along the main chain |
| δ | the degree of shielding of the backbone by side chains |
References
- Zhang, B.; Grohn, F.; Pedersen, J.S.; Fischer, K.; Schmidt, M. Conformation of Cylindrical Brushes in solution: Effect of side chain length. Macromolecules 2006, 39, 8440–8450. [Google Scholar] [CrossRef]
- Zhang, M.; Müller, A.H.E. Cylindrical polymer brushes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3461–3481. [Google Scholar] [CrossRef]
- Sheiko, S.S.; Sumerlin, B.S.; Matyjaszewski, M. Cylindrical molecular brushes: Synthesis, characterization, and properties. Prog. Polym. Sci. 2008, 33, 759–785. [Google Scholar] [CrossRef]
- Nakamura, Y. Stiffness parameter of brush-like polymers with rod-like side chains. J. Chem. Phys. 2016, 145, 014903. [Google Scholar] [CrossRef]
- Dutta, S.; Wade, M.A.; Walsh, D.J.; Guironnet, D.; Rogers, S.A.; Sing, C.E. Dilute solution structure of bottlebrush polymers. Soft Matter. 2019, 15, 2928–2941. [Google Scholar] [CrossRef] [PubMed]
- Birshtein, T.M.; Borisov, O.V.; Zhulina, Y.B.; Khokhlov, A.R.; Yurasova, T.A. Conformations of comb-like macromolecules. Polym. Sci. U.S.S.R. 1987, 29, 1293–1300. [Google Scholar] [CrossRef]
- Wintermantel, M.; Schmidt, M.; Tsukahara, Y.; Kajiwara, K.; Kohjiya, S. Rodlike combs. Macromol. Rapid Commun. 1994, 15, 279–284. [Google Scholar] [CrossRef]
- Terao, K.; Hokajo, T.; Nakamura, Y.; Norisuye, T. Solution Properties of polymacromonomers consisting of polystyrene. 3. Viscosity behavior in cyclohexane and toluene. Macromolecules 1999, 32, 3690–3694. [Google Scholar] [CrossRef]
- Rzayev, J. Molecular bottlebrushes: New opportunities in nanomaterials fabrication. Macro Lett. 2012, 1, 1146–1149. [Google Scholar] [CrossRef]
- Liu, X.; Claesson, P.M. Bioinspired Bottlebrush Polymers for Aqueous Boundary Lubrication. Polymers 2022, 14, 2724. [Google Scholar] [CrossRef]
- Li, Z.; Tang, M.; Liang, S.; Zhang, M.; Gill, M.B.; He, Y.; Hao, S.-M.; Choi, W.; Liu, Y.; Peng, J.; et al. Bottlebrush polymers: From controlled synthesis, self-assembly, properties to applications. Prog. Polym. Sci. 2021, 116, 101387. [Google Scholar] [CrossRef]
- Wintermantel, M.; Gerle, M.; Fischer, K.; Schmidt, M.; Wataoka, I.; Urakawa, H.; Kajiwara, K.; Tsukahara, Y. Molecular bottlebrushes. Macromolecules 1996, 29, 978–983. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with complex architecture by living anionic polymerization. Chem. Rev. 2010, 101, 3747–3792. [Google Scholar] [CrossRef]
- Filippov, A.; Kozlov, A.; Tarabukina, E.; Obrezkova, M.; Muzafarov, A. Solution properties of comb-like polymers consisting of dimethylsiloxane monomer units. Polym. Int. 2016, 65, 393–399. [Google Scholar] [CrossRef]
- Potemkin, I.I.; Palyulin, V.V. Comb-like macromolecules. Polym. Sci. Ser. A 2009, 51, 123–149. [Google Scholar] [CrossRef]
- Yilmaz, G.; Toiserkani, H.; Demirkol, D.O.; Sakarya, S.; Timur, S.; Yagci, Y.; Torun, L. Modification of polysulfones by click chemistry: Amphiphilic graft copolymers and their protein adsorption and cell adhesion properties. J. Polym. Sci. A 2011, 49, 110–117. [Google Scholar] [CrossRef]
- Pesek, S.L.; Li, X.; Hammouda, B.; Hong, K.; Verduzco, R. Small-angle neutron scattering analysis of bottlebrush polymers prepared via grafting-through polymerization. Macromolecules 2013, 46, 6998–7005. [Google Scholar] [CrossRef]
- Ilgach, D.M.; Meleshko, T.K.; Yakimansky, A.V. Methods of controlled radical polymerization for the synthesis of polymer brushes. Polym. Sci. Ser. C 2015, 57, 3–19. [Google Scholar] [CrossRef]
- Dalsin, S.J.; Rions-Maehren, T.G.; Beam, M.D.; Bates, F.S.; Hillmyer, M.A.; Matsen, M.W. Bottlebrush block polymers: Quantitative theory and experiments. ACS Nano 2015, 9, 12233–12245. [Google Scholar] [CrossRef]
- Tarabukina, E.B.; Tarasova, E.V.; Filippov, A.P. Molecular properties of comb-shaped maleimide copolymers in dilute solutions: Effect of alkyl side chains. Polym. Sci. Ser. A 2022, 64, 261–269. [Google Scholar] [CrossRef]
- Ishizu, K.; Tsubaki, K.; Mori, A.; Uchida, S. Architecture of nanostructured polymers. Progr. Polym. Sci. 2003, 28, 27–54. [Google Scholar] [CrossRef]
- Borisov, O.V.; Zhulina, E.B. Amphiphilic graft copolymer in a selective solvent: Intramolecular structures and conformational transitions. Macromolecules 2005, 38, 2506–2514. [Google Scholar] [CrossRef]
- Wataoka, I.; Urakawa, H.; Kobayashi, K.; Akaike, T.; Schmidt, M.; Kajiwara, K. Structural characterization of glycoconjugate polystyrene in aqueous solution. Macromolecules 1999, 32, 1816–1821. [Google Scholar] [CrossRef]
- Filippov, A.P.; Krasova, A.S.; Tarabukina, E.B.; Kashina, A.V.; Meleshko, T.K.; Yakimansky, A.V. The effect of side chain length on hydrodynamic and conformational characteristics of polyimide-graft-polymethylmethacrylate copolymers in thermodynamically good solutions. J. Polym. Res. 2016, 23, 219–228. [Google Scholar] [CrossRef]
- Theodorakis, P.E.; Paul, W.; Binder, K. Microphase separation in bottlebrush polymers under poor-solvent conditions. Europhys. Lett. 2009, 88, 63002. [Google Scholar] [CrossRef][Green Version]
- Liu, W.; Liu, Y.; Hao, X.; Zeng, G.; Wang, W.; Liu, R.; Huang, Y. Backbone-collapsed intra- and inter-molecular self-assembly of cellulose-based dense graft copolymer. Carbohydr. Polym. 2012, 88, 290–298. [Google Scholar] [CrossRef]
- Djalali, R.; Li, S.Y.; Schmidt, M. Amphipolar core-shell cylindrical brushes as templates for the formation of gold clusters and nanowires. Macromolecules 2002, 35, 4282–4288. [Google Scholar] [CrossRef]
- Zhang, M.; Estournes, C.; Bietsch, W.; Mueller, A.H.E. Superparamagnetic hybrid nanocylinders. Adv. Funct. Mater. 2004, 14, 871–882. [Google Scholar] [CrossRef]
- Zhang, M.F.; Drechsler, M.; Mueller, A.H.E. Template-controlled synthesis of wire-like cadmium sulfide nanoparticle assemblies within core-shell cylindrical polymer brushes. Chem. Mater. 2004, 16, 537–543. [Google Scholar] [CrossRef]
- Yuan, J.Y.; Xu, Y.Y.; Walther, A.; Bolisetty, S.; Schumacher, M.; Mueller, A.H.E. Water-soluble organo-silica hybrid nanowires. Nat. Mater. 2008, 7, 718–722. [Google Scholar] [CrossRef]
- Meleshko, T.; Il’gach, D.; Bogorad, N.; Kukarkina, N.; Yakimansky, A. Synthesis of graft copolyimides via controlled radical polymerization of methacrylates with a polyimide macroinitiators. Polym. Sci. Ser. B 2014, 56, 118–126. [Google Scholar] [CrossRef]
- Filippov, A.P.; Belyaeva, E.V.; Krasova, A.S.; Simonova, M.A.; Meleshko, T.K.; Ilgach, D.M.; Bogorad, N.N.; Yakimansky, A.V.; Larin, S.V.; Darinskii, A.A. Conformations of molecular brushes based on polyimide and poly(methyl methacrylate) in selective solvents: Experiment and computer simulation. Polym. Sci. Ser. A 2014, 56, 393–404. [Google Scholar] [CrossRef]
- Filippov, A.P.; Krasova, A.S.; Tarabukina, E.B.; Meleshko, T.K.; Yakimansky, A.V.; Sheiko, S.S. Behavior of amphiphilic molecular brushes with polyimide main chain and side chains of polymethylmethacrylate and polystyrene in the vicinity of θ-point. Polym. Sci. Ser. C 2018, 60, 219–227. [Google Scholar] [CrossRef]
- Lee, H.; Pietrasik, J.; Sheiko, S.S.; Matyjaszewski, K. Stimuli-responsive molecular brushes. Progr. Polym. Sci. 2010, 35, 24–44. [Google Scholar] [CrossRef]
- Sui, K.; Zhao, X.; Wu, Z.; Xia, Y.; Liang, H.; Li, Y. Synthesis, rapid responsive thickening, and self-assembly of brush copolymer poly(ethylene oxide)-graft-poly(n,n-dimethylaminoethyl methacrylate) in aqueous solutions. Langmuir 2012, 28, 153–160. [Google Scholar] [CrossRef]
- Li, X.; ShamsiJazeyi, H.; Pesek, S.L.; Agrawal, A.; Hammouda, B.; Verduzco, R. Thermoresponsive PNIPAAM bottlebrush polymers with tailored side-chain length and end-group structure. Soft Matter. 2014, 10, 2008–2015. [Google Scholar] [CrossRef]
- Wessels, M.G.; Jayaraman, A. Molecular dynamics simulation study of linear, bottlebrush, and star-like amphiphilic block polymer assembly in solution. Soft Matter. 2019, 15, 3987–3998. [Google Scholar] [CrossRef]
- Xu, Y.; Bolisetty, S.; Drechsler, M.; Jiayin, B.; Matthias, Y.; Axel, B.; Müller, H.E. pH and salt responsive poly(N,N-dimethylaminoethyl methacrylate) cylindrical brushes and their quaternized derivatives. Polymer 2008, 49, 3957–3964. [Google Scholar] [CrossRef]
- Song, H.H.; Dotrong, M.; Price, G.E.; Dotrong, M.H.; Vakil, M.; Santhosh, U.; Evers, R.C. Rod aggregation in graft rigid-rod copolymers for single-component molecular composites. Polym. Pap. 1994, 35, 675–680. [Google Scholar] [CrossRef]
- Aseyev, V.; Tenhu, H.; Winnik, F.M. Non-Ionic Thermoresponsive Polymers in Water. Adv. Polym. Sci. 2010, 57, 29–89. [Google Scholar]
- Hoogenboom, R.; Thijs, H.M.L.; Jochems, M.; van Lankvelt, B.M.; Fijten, M.; Schubert, U.S. Tuning the LCST of poly(2-oxazoline)s by varying composition and molecular weight: Alternatives to poly(N-isopropylacrylamide). Chem. Commun. 2008, 30, 5758–5760. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, R. Poly(2-oxazoline)s: A polymer class with numerous potential applications. Angew. Chem. Int. Ed. 2009, 48, 7978–7994. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, R.; Schlaad, H. Thermoresponsive poly(2-oxazoline)s, polypeptoids, and polypeptides. Polym. Chem. 2017, 8, 24–40. [Google Scholar] [CrossRef]
- De la Rosa, V.R. Poly(2-oxazoline)s as materials for biomedical applications. J. Mater. Sci. Mater. Med. 2014, 25, 1211–1225. [Google Scholar] [CrossRef]
- Rossegger, E.; Schenk, V.; Wiesbrock, F. Design strategies for functionalized poly(2-oxazoline)s and derived materials. Polymers 2013, 5, 956–1011. [Google Scholar] [CrossRef]
- Hsiue, G.H.; Chiang, H.Z.; Wang, C.H.; Juang, T.M. Nonviral gene carriers based on diblock copolymers of poly(2-ethyl-2-oxazoline) and linear polyethylenimine. Bioconj. Chem. 2006, 17, 781–786. [Google Scholar] [CrossRef]
- Sauer, M.; Haefele, T.; Graff, A.; Nardin, C.; Meier, W. Ion-carrier controlled precipitation of calcium phosphate in giant ABA triblock copolymer vesicles. Chem. Commun. 2001, 23, 2452–2453. [Google Scholar] [CrossRef]
- Schlaad, H.; Diehl, C.; Gress, A.; Meyer, M.; Demirel, A.L.; Nur, Y.; Bertin, A. Poly (2-oxazoline)s as smart bioinspired polymers. Macromol. Rapid Commun. 2010, 31, 511–525. [Google Scholar] [CrossRef]
- Weber, C.; Rogers, S.; Vollrath, A.; Hoeppener, S.; Rudolph, T.; Fritz, N.; Hoogenboom, R.; Schubert, U.S. Aqueous solution behavior of comb-shaped poly(2-ethyl-2-oxazoline). J. Polym. Sci. Part A Polym. Chem. 2013, 51, 139–148. [Google Scholar] [CrossRef]
- Zhang, N.; Luxenhofer, R.; Jordan, R. Thermoresponsive poly(2-oxazoline) molecular brushes by living ionic polymerization: Kinetic investigations of pendant chain grafting and cloud point modulation by backbone and side chain length variation. Macromol. Chem. Phys. 2012, 213, 973–981. [Google Scholar] [CrossRef]
- Bühler, J.; Muth, S.; Fischer, K.; Schmidt, M. Collapse of cylindrical brushes with 2-isopropyloxazoline side chains close to the phase boundary. Macromol. Rapid Commun. 2013, 34, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Wagner, M.; Baykal, D.; Hoeppener, S.; Paulus, R.M.; Festag, G.; Altuntas, E.; Schacher, F.H.; Schubert, U.S. Easy access to amphiphilic heterografted poly(2-oxazoline) comb copolymers. Macromolecules 2013, 46, 5107–5116. [Google Scholar] [CrossRef]
- Alvaradejo, G.G.; Nguyen, H.V.-T.; Harvey, P.; Gallagher, N.M.; Le, D.; Ottaviani, M.F.; Jasanoff, A.; Delaittre, G.; Johnson, J.A. Polyoxazoline-based bottlebrush and brush-arm star polymers via ROMP: Syntheses and applications as organic radical contrast agents. ACS Macro Lett. 2019, 8, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Luxenhofer, R.; Bezen, M.; Jordan, R. Kinetic investigations on the polymerization of 2-oxazolines using pluritriate initators. Macromol. Rapid Commun. 2008, 29, 1509–1513. [Google Scholar] [CrossRef]
- Zhang, N.; Huber, S.; Schulz, A.; Luxenhofer, R.; Jordan, R. Cylindrical Molecular Brushes of Poly(2-oxazoline)s from 2-Isopropenyl-2-oxazoline. Macromolecules 2009, 42, 2215–2221. [Google Scholar] [CrossRef]
- Liang, M.; Jhuang, Y.-J.; Zhang, C.-F.; Tsai, W.-J.; Feng, H.-C. Synthesis and characterization of poly(phenylene oxide) graft copolymers by atom transfer radical polymerizations. Eur. Polym. J. 2009, 45, 2348–2357. [Google Scholar] [CrossRef]
- Fu, G.D.; Kang, E.T.; Neoh, K.G.; Lin, C.C.; Liaw, D.J. Rigid Fluorinated Polyimides with Well-Defined Polystyrene/Poly(pentafluorostyrene) Side Chains from Atom Transfer Radical Polymerization. Macromolecules 2005, 38, 7593–7600. [Google Scholar] [CrossRef]
- Meleshko, T.K.; Ilgach, D.M.; Bogorad, N.N.; Kukarkina, N.V.; Vlasova, E.N.; Dobrodumov, A.V.; Malakhova, I.I.; Gorshkov, N.I.; Krasikov, V.D.; Yakimanskii, A.V. Synthesis of multicentered polyimide initiators for the preparation of regular graft copolymers via controlled radical polymerization. Polym. Sci. Ser. B 2010, 52, 589–599. [Google Scholar] [CrossRef]
- Kurlykin, M.; Bursian, A.; Filippov, A.; Dudkina, M.; Tenkovtsev, A. Multicenter polyester initiators for the preparation of graft copolymers with oligo(2-oxazoline) side chains. Macromol. Symp. 2017, 375, 1600162. [Google Scholar] [CrossRef]
- Kudryavtseva, A.A.; Kurlykin, M.P.; Tarabukina, E.B.; Tenkovtsev, A.V.; Filippov, A.P. Behavior of thermosensitive graft-copolymers with aromatic polyester backbone and poly-2-ethyl-2-oxazoline side chains in aqueous solutions. Int. J. Polym. Anal. Char. 2017, 22, 526–533. [Google Scholar] [CrossRef]
- Filippov, A.; Tarabukina, E.; Kudryavtseva, A.; Fatullaev, E.; Kurlykin, M.; Tenkovtsev, A. Molecular brushes with poly-2-ethyl-2-oxazoline side chains and aromatic polyester backbone manifesting double stimuli responsiveness. Coll. Polym. Sci. 2019, 297, 1445–1454. [Google Scholar] [CrossRef]
- Tarabukina, E.; Fatullaev, E.; Krasova, A.; Kurlykin, M.; Tenkovtsev, A.; Sheiko, S.S.; Filippov, A. Synthesis, structure, hydrodynamics and thermoresponsiveness of graft copolymer with aromatic polyester backbone at poly-2-isopropyl-2-oxazoline side chains. Polymers 2020, 12, 2643. [Google Scholar] [CrossRef] [PubMed]
- Katsumoto, Y.; Tsuchiizu, A.; Qiu, X.P.; Winnik, F.M. Dissecting the mechanism of the heat-induced phase separation and crystallization of poly(2-isopropyl-2-oxazoline) in water through vibrational spectroscopy and molecular orbital calculations. Macromolecules 2012, 45, 3531–3541. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, J.-H.; Jang, W.-D. Linear and cyclic poly(2-isopropyl-2-oxazoline)s for fine control of thermoresponsiveness. Eur. Polym. J. 2017, 88, 605–612. [Google Scholar] [CrossRef]
- Tarabukina, E.; Fatullaev, E.; Krasova, A.; Sokolova, M.; Kurlykin, M.; Neelov, I.; Tenkovtsev, A.; Filippov, A. Thermoresponsive molecular brushes with a rigid-chain aromatic polyester backbone and poly-2-alkylyl-2-oxazoline side chains. Int. J. Mol. Sci. 2021, 22, 12265. [Google Scholar] [CrossRef]
- Spinner, I.H.; Yannopoulis, J.; Metamonski, W. Oxidation–reduction polymers: I. Synthesis of monomers. Can. J. Chem. 1961, 39, 2529–2535. [Google Scholar] [CrossRef]
- Damaceanu, M.-D.; Rusu, R.-D.; Nicolescu, A.; Bruma, M. Blue fluorescent polyamides containing naphthalene and oxadiazole rings. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 893–906. [Google Scholar] [CrossRef]
- Witte, H.; Seeliger, W. Justus Liebigs Annalen der Chemie; Plenum: Berlin, Germany, 1974; p. 996. [Google Scholar]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions; Plenum: Berlin, Germany, 2007; p. 205. [Google Scholar]
- Tsvetkov, V.N.; Eskin, V.E.; Frenkel, Y.S. Structure of Macromolecules in Solution; Plenum: London, UK, 1970. [Google Scholar]
- Yamakawa, H. Modern Theory of Polymer Solutions; Plenum: Berlin, Germany, 1971; p. 434. [Google Scholar]
- Bilibin, A.Y.; Tenkovtsev, A.V.; Piraner, O.N.; Pashkovsky, E.E.; Skorokhodov, S.S. Thermotropic polyesters 2. Synthesis of regular polyesters. Makromol. Chem. 1985, 186, 1575–1591. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Lavrenko, P.N.; Pavlov, G.M.; Bushin, S.V.; Astapenko, E.P.; Boikov, A.A.; Shil’dyayeva, N.A.; Didenko, S.A.; Malichenko, B.F. Sedimentation-diffusion and viscometric studies poly-m-phenylene isophthalamide and the equilibrium rigidity of its molecules. Polym. Sci. U.S.S.R. 1982, 24, 2689–2700. [Google Scholar] [CrossRef]
- Kurlykin, M.P.; Bursian, A.E.; Golub, O.V.; Filippov, A.P.; Tenkovtsev, A.V. Multicenter polyester initiators for the synthesis of graft copolymers with oligo(2-ethyl-2-oxazoline) side chains. Polym. Sci. Ser. B 2016, 58, 421–427. [Google Scholar] [CrossRef]
- Grube, M.; Leiske, M.N.; Schubert, U.S.; Nischang, I. POx as an alternative to PEG? A hydrodynamic and light scattering study. Macromolecules 2018, 51, 1905–1916. [Google Scholar] [CrossRef]
- Kato, H.; Nakamura, A.; Kinugasa, S. Effects of Angular Dependency of Particulate Light Scattering Intensity on Determination of Samples with Bimodal Size Distributions Using Dynamic Light Scattering Methods. Nanomaterials 2018, 8, 708. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).