Synthesis and Characterization of Chitosan-Containing ZnS/ZrO2/Graphene Oxide Nanocomposites and Their Application in Wound Dressing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Scaffold with Different Contents of Oxides
2.3. Characterizations
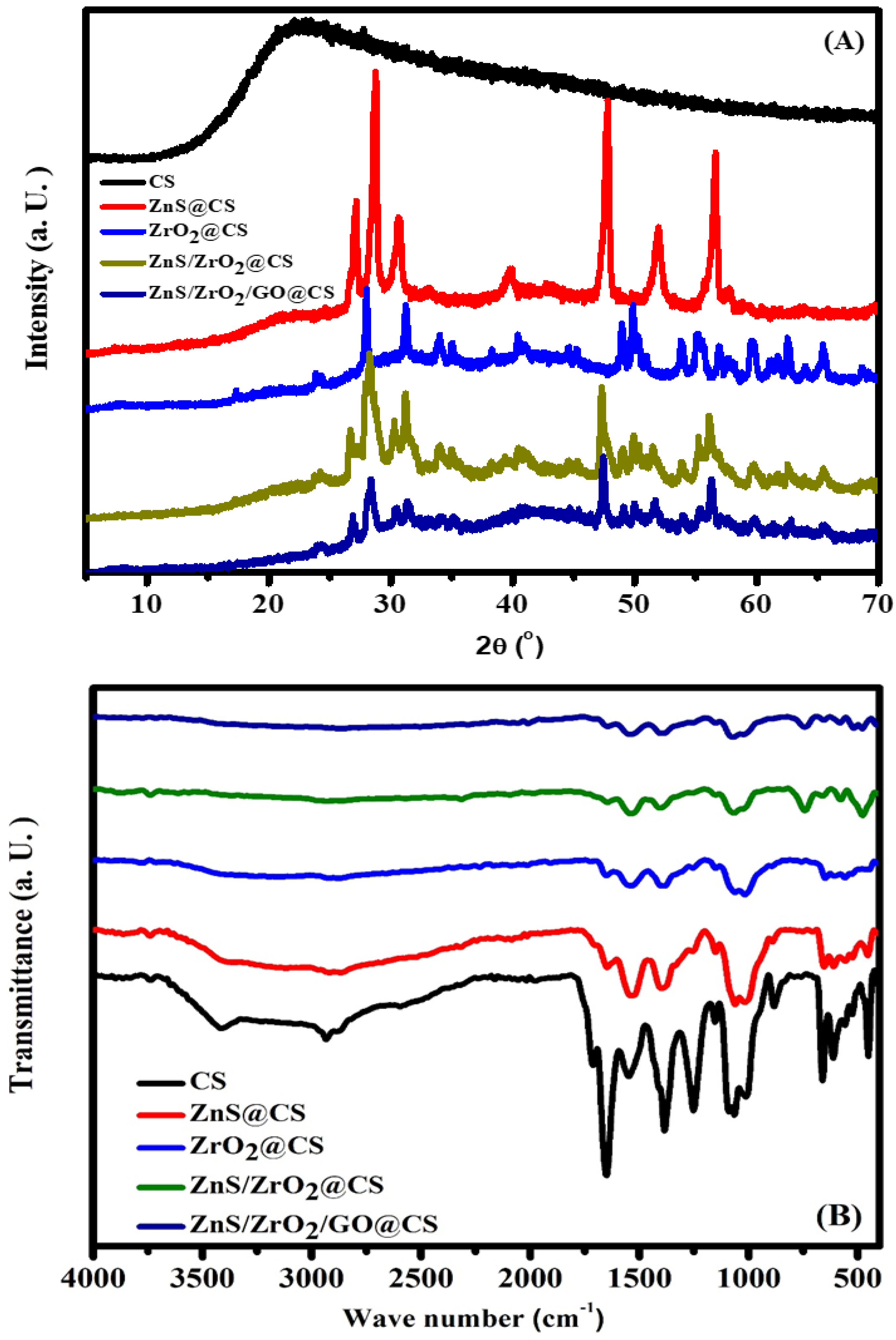
2.3.1. XRD Measurements
2.3.2. FTIR Measurements
2.3.3. Examination of Film Morphology and EDX
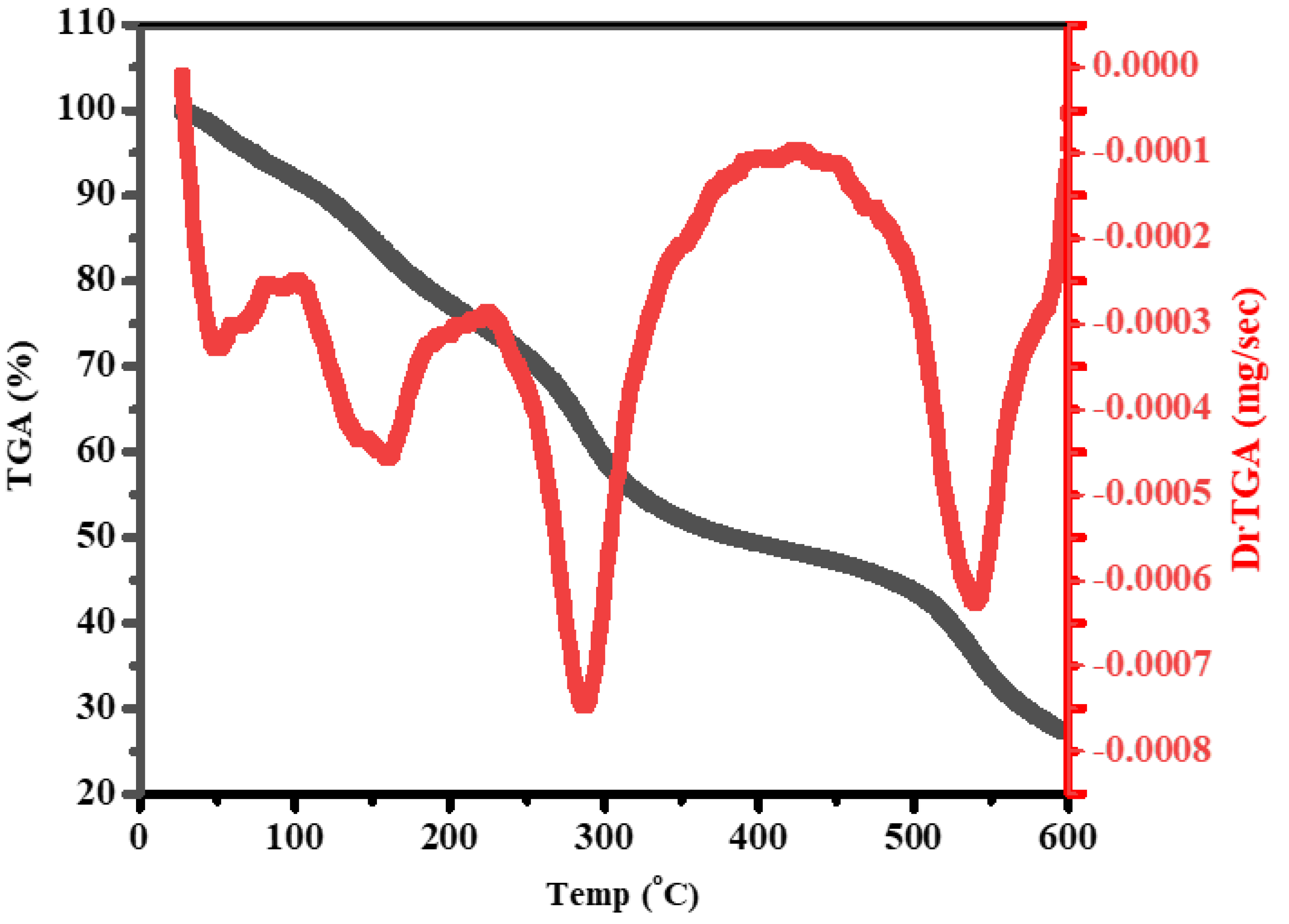
2.3.4. Thermogravimetric Analysis
2.3.5. UV Measurements
2.4. Contact Angle
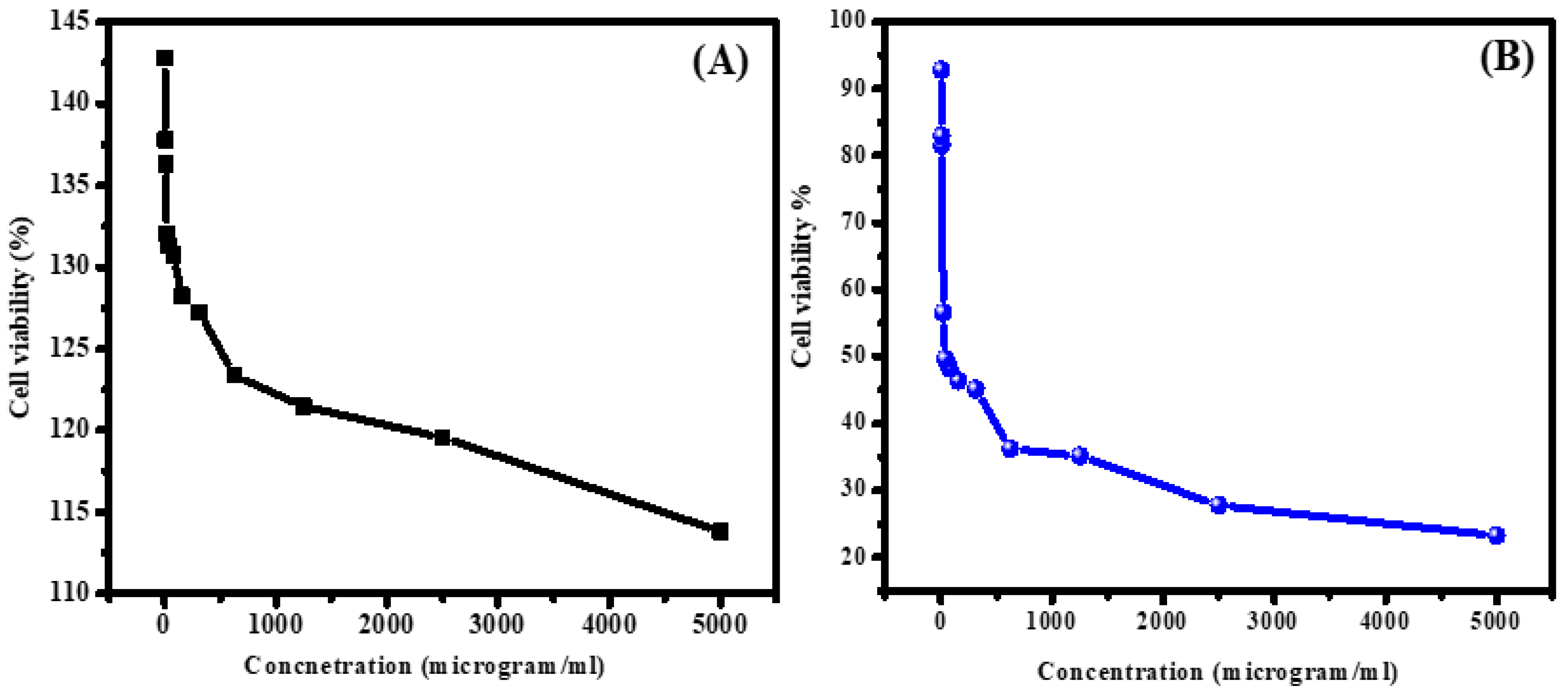
2.5. In Vitro Cell Viability Tests
3. Results and Discussion
3.1. Structural Investigation
3.2. EDX
3.3. Morphological Study
3.4. Wettability
3.5. Thermal Study
3.6. Optical Behavior
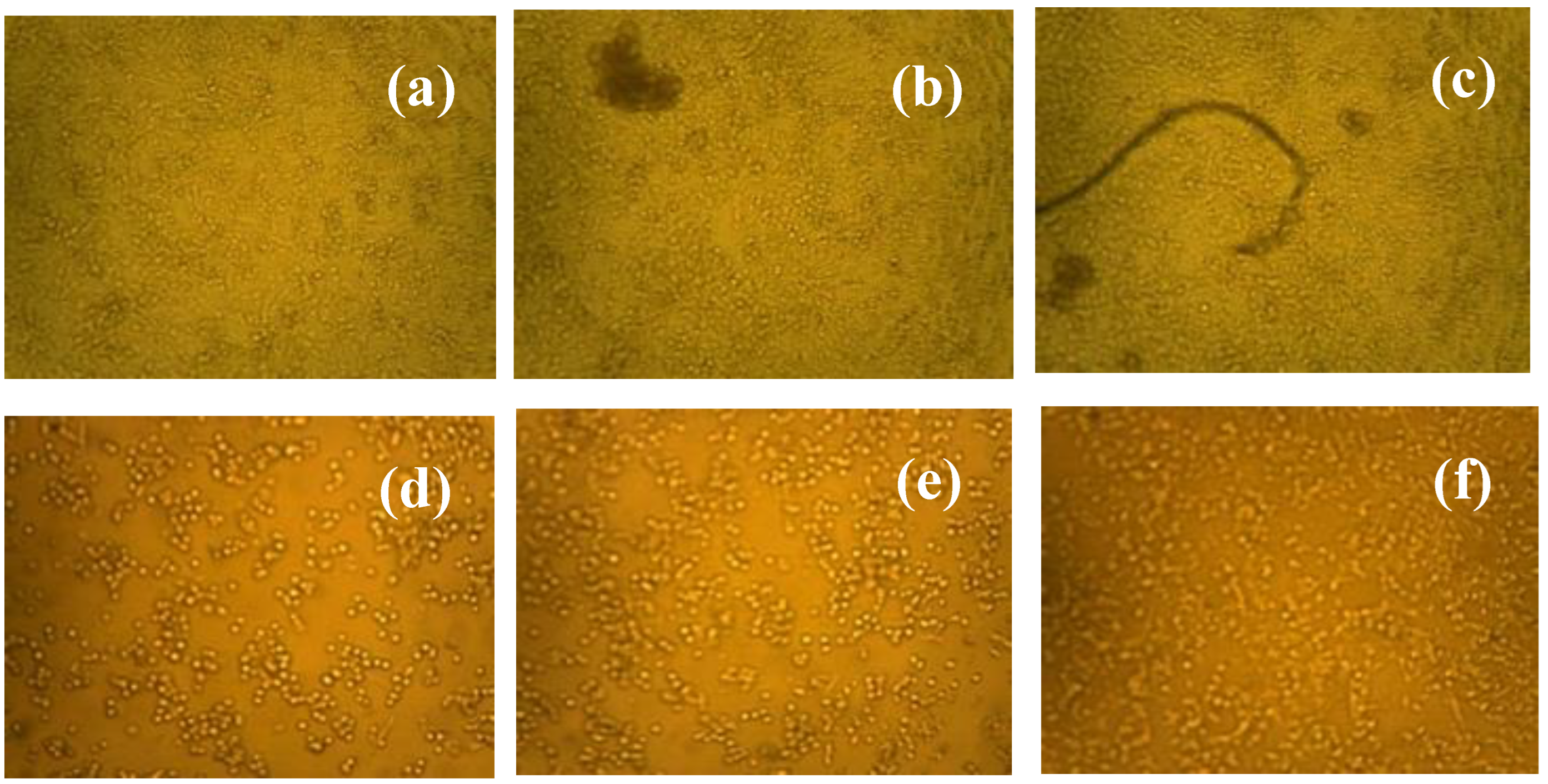
3.7. Selectivity Performance of ZnS/ZrO2/GO@CS in Reacting with Normal and Malignant Cell Lines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, R.; Xu, T.; Lei, X.; Wu, Q.; Xue, S. Novel design of porous hollow hydroxyapatite microspheres decorated by reduced graphene oxides with superior photocatalytic performance for tetracycline removal. Solid State Sci. 2020, 99, 106067. [Google Scholar] [CrossRef]
- Xie, Y.Y.; Hu, X.H.; Zhang, Y.W.; Wahid, F.; Chu, L.Q.; Jia, S.R.; Zhong, C. Development and antibacterial activities of bacterial cellulose/graphene oxide-CuO nanocomposite films. Carbohydr. Polym. 2020, 229, 115456. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, L.; Chen, B.; Shi, S.; Nie, J.; Ma, G. Functionalized chitosan electrospun nanofiber membranes for heavy-metal removal. Polymer 2019, 163, 74–85. [Google Scholar] [CrossRef]
- Xiao, F.; Cheng, J.; Cao, W.; Yang, C.; Chen, J.; Luo, Z. Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 2019, 540, 579–584. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Rasheed, M.A.; Abdullah, O.G.; Ahmed, H.M. Polymer Blending as a Novel Approach for Tuning the SPR Peaks of Silver Nanoparticles. Polymers 2017, 9, 486. [Google Scholar] [CrossRef]
- Venkatesh, G.; Suganesh, R.; Jayaprakash, J.; Srinivasan, M.; Prabu, K.M. Perovskite type BaSnO3-reduced graphene oxide nanocomposite for photocatalytic decolourization of organic dye pollutant. Chem. Phys. Lett. 2022, 787, 139237. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Graphene oxide/hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon 2014, 67, 185–197. [Google Scholar] [CrossRef]
- Kale, D.P.; Deshmukh, S.P.; Shirsath, S.R.; Bhanvase, B.A. Sonochemical preparation of multifunctional rGO-ZnS-TiO2 ternary nanocomposite and its application for CV dye removal. Optik 2020, 208, 164532. [Google Scholar] [CrossRef]
- Nanaki, S.; Barmpalexis, P.; Iatrou, A.; Christodoulou, E.; Kostoglou, M.; Bikiaris, D.N. Risperidone Controlled Release Microspheres Based on Poly(Lactic Acid)-Poly(Propylene Adipate) Novel Polymer Blends Appropriate for Long Acting Injectable Formulations. Pharmaceutics 2018, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Yahyazadehfar, M.; Ahmadi, S.A.; Sheikhhosseini, E.; Ghazanfari, D. High-yielding strategy for microwave-assisted synthesis of Cr2O3 nanocatalyst. J. Mater. Sci. Mater. Electron. 2020, 31, 11618–11623. [Google Scholar] [CrossRef]
- Schumacher, T.C.; Volkmann, E.; Yilmaz, R.; Wolf, A.; Treccani, L.; Rezwan, K. Mechanical evaluation of calcium-zirconium-silicate (baghdadite) obtained by a direct solid-state synthesis route. J. Mech. Behav. Biomed. Mater. 2014, 34, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Afifi, M.; El-Naggar, M.E.; Muhammad, S.; Alghamdi, N.A.; Wageh, S.; Abu-Saied, M.A.; El-Morsy, M.A.; Salem, W.M.; Mostafal, M.S.; Salem, R.M. Chemical stability, morphological behavior of Mg/Sr-hydroxyapatite@chitosan biocomposites for medical applications. J. Mater. Res. Technol. 2022, 18, 681–692. [Google Scholar] [CrossRef]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2020, 23, 100439. [Google Scholar] [CrossRef]
- Dahham, O.S.; Hamzah, R.; Noriman, N.Z.; Alakrach, A.M.; Syed-Idrus, S.Z.; Shayfull, Z.; Adam, T. The Influences of Zirconium Dioxide on ENR-25/ZrO2 Composites: FTIR and TGA Analysis. J. Phys. Conf. Ser. 2018, 1019, 012055. [Google Scholar] [CrossRef]
- Ghotekar, S.; Pansambal, S.; Bilal, M.; Pingale, S.S.; Oza, R. Environmentally friendly synthesis of Cr2O3 nanoparticles: Characterization, applications and future perspective—A review. Case Stud. Chem. Environ. Eng. 2021, 3, 100089. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Mariappan, A.; Neyvasagam, K.; Ayeshamariam, A.; Pandi, P.; Palanichamy, R.R.; Palanichamy, R.; Gopinathan, C.; Mola, G.T.; Maaza, M. Photocatalytic performance and antimicrobial activities of HAp-TiO2 nanocomposite thin films by sol-gel method. Surf. Interfaces 2017, 6, 247–255. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Noriega, S.E.; Subramanian, A. Consequences of Neutralization on the Proliferation and Cytoskeletal Organization of Chondrocytes on Chitosan-Based Matrices. Int. J. Carbohydr. Chem. 2011, 2011, 85200240. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Wiser, R.; Elder, S.H.; Jouett, R.; Yang, Y.; Ong, J.L. Contact angle, protein adsorption and osteoblast precursor cell attachment to chitosan coatings bonded to titanium. J. Biomater. Sci. Polym. Ed. 2003, 14, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Bhattacharaya, K.; Chattopadhyay, P. Nanostructured zirconium phosphate as ion exchanger: Synthesis, size dependent property and analytical application in radiochemical separation. Appl. Radiat. Isot. 2014, 85, 34–38. [Google Scholar] [CrossRef]
- Hiromoto, S.; Yamamoto, A. High corrosion resistance of magnesium coated with hydroxyapatite directly synthesized in an aqueous solution. Electrochim. Acta 2009, 54, 7085–7093. [Google Scholar] [CrossRef]
- Farivar, F.; Yap, P.L.; Karunagaran, R.U.; Losic, D. Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameters. C 2021, 7, 41. [Google Scholar] [CrossRef]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2013, 5, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, Y.A.; Zatsepin, A.F. Optical properties and energy parameters of Gd2O3 and Gd2O3, Er nanoparticles. J. Phys. Conf. Ser. 2017, 917, 062001. [Google Scholar] [CrossRef]
- Menazea, A.A.; Awwad, N.S.; Ibrahium, H.A.; Ahmed, M.K. Casted polymeric blends of carboxymethyl cellulose/polyvinyl alcohol doped with gold nanoparticles via pulsed laser ablation technique; morphological features, optical and electrical investigation. Radiat. Phys. Chem. 2020, 177, 109155. [Google Scholar] [CrossRef]
- Dawy, M.; Rifaat, H.M.; Menazea, A.A. Characterization of Ag Nanoparticles by Nanosecond Pulsed Laser Ablation Doped in Chitosan. Curr. Sci. Int. 2015, 4, 6. [Google Scholar]
- Menazea, A.A. One-Pot Pulsed Laser Ablation route assisted copper oxide nanoparticles doped in PEO/PVP blend for the electrical conductivity enhancement. J. Mater. Res. Technol. 2020, 9, 2412–2422. [Google Scholar] [CrossRef]
- El-dek, S.I.; Mansour, S.F.; Ahmed, M.A.; Ahmed, M.K. Microstructural features of flower like Fe brushite. Prog. Nat. Sci. Mater. Int. 2017, 27, 520–526. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Goh, Y.-F.; Akram, M.; Razali, I.R.; Abdul-Kadir, M.R.; Hussain, R. Microwave assisted synthesis of nano sized sulphate doped hydroxyapatite. Mater. Res. Bull. 2013, 48, 2106–2110. [Google Scholar] [CrossRef]
- Horti, N.C.; Kamatagi, M.D.; Nataraj, S.K.; Wari, M.N.; Inamdar, S.R. Structural and optical properties of zirconium oxide (ZrO2) nanoparticles: Effect of calcination temperature. Nano Express 2020, 1, 010022. [Google Scholar] [CrossRef]
- Han, B.; Fang, W.H.; Zhao, S.; Yang, Z.; Hoang, B.X. Zinc sulfide nanoparticles improve skin regeneration. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102263. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Yuan, P.; Cai, B.; Qiu, X.; Jin, R.; Liu, S.; Li, Y.; Chen, X. Stimuli-responsive scaffold for breast cancer treatment combining accurate photothermal therapy and adipose tissue regeneration. Adv. Funct. Mater. 2019, 29, 1904401. [Google Scholar] [CrossRef]
- Arjunan, N.; Singaravelu, C.M.; Kulanthaivel, J.; Kandasamy, J. A potential photocatalytic, antimicrobial and anticancer activity of chitosan-copper nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Lee, J.-Y.; Kang, D.S.; Anil, S.; Kim, S.-K.; Shim, M.S.; Kim, D.G. Antimicrobial and anticancer activities of porous chitosan-alginate biosynthesized silver nanoparticles. Int. J. Biol. Macromol. 2017, 98, 515–525. [Google Scholar] [CrossRef]
- Arjunan, N.; Kumari, H.L.J.; Singaravelu, C.M.; Kandasamy, R.; Kandasamy, J. Physicochemical investigations of biogenic chitosan-silver nanocomposite as antimicrobial and anticancer agent. Int. J. Biol. Macromol. 2016, 92, 77–87. [Google Scholar] [CrossRef]
- Salcedo, I.; Aguzzi, C.; Sandri, G.; Bonferoni, M.C.; Mori, M.; Cerezo, P.; Sánchez, R.; Viseras, C.; Caramella, C. In vitro biocompatibility and mucoadhesion of montmorillonite chitosan nanocomposite: A new drug delivery. Appl. Clay Sci. 2012, 55, 131–137. [Google Scholar] [CrossRef]
- Hsu, S.H.; Chang, Y.-B.; Tsai, C.-L.; Fu, K.-Y.; Wang, S.-H.; Tseng, H.-J. Characterization and biocompatibility of chitosan nanocomposites. Colloids Surf. B Biointerfaces 2011, 85, 198–206. [Google Scholar] [CrossRef]
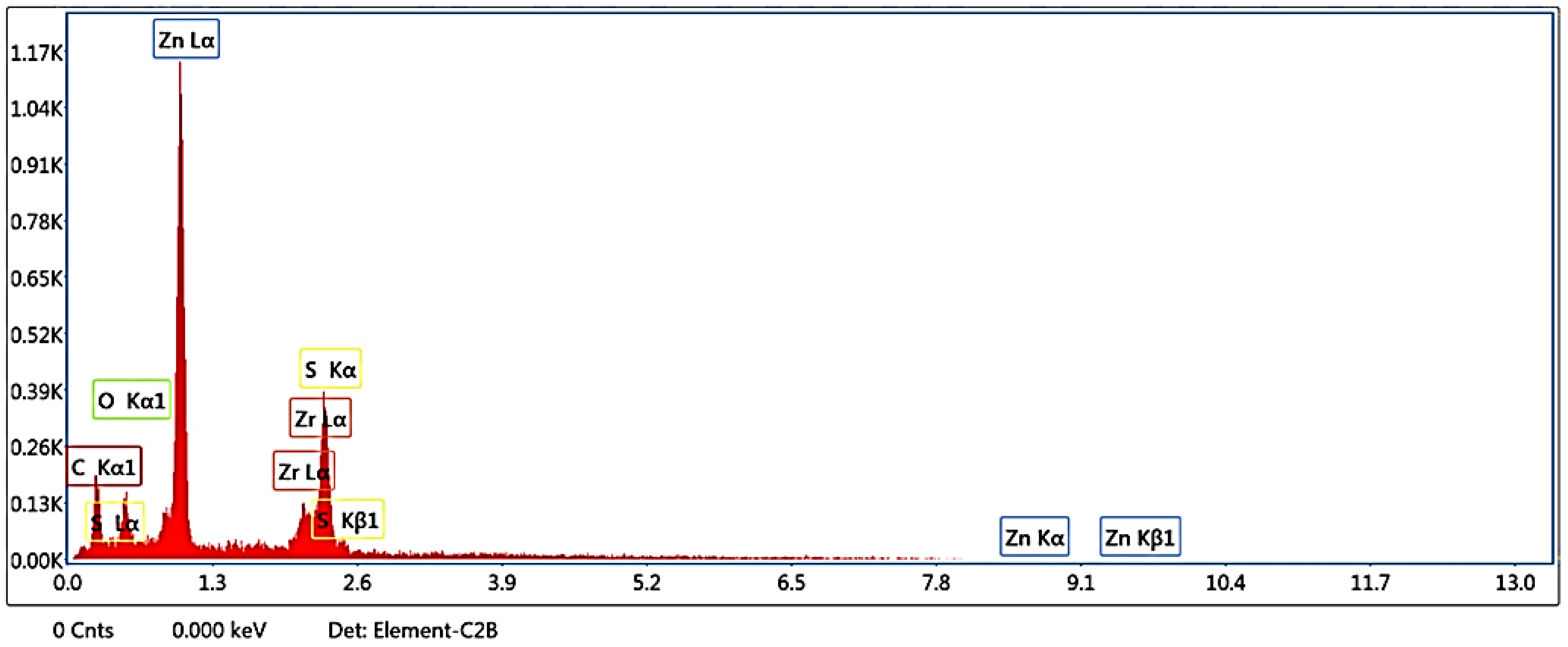



| Element | Error % | Weight % | Atomic % |
|---|---|---|---|
| C K | 13.66 | 29.74 | 59.44 |
| O K | 14.99 | 8.96 | 13.45 |
| ZnL | 3.59 | 46.47 | 17.07 |
| ZrL | 12.13 | 2.18 | 0.57 |
| S K | 5.56 | 12.65 | 9.47 |
| Composition | Thickness (mm) ± SD | Absorption Edge (eV) ± SD | Band-Gap (eV) | n ± SD | |
|---|---|---|---|---|---|
| Direct ± SD | Indirect ± SD | ||||
| CS | 0.66 ± 0.12 | 2.35 ± 0.11 | 2.35 ± 0.19 | 2.9 ± 0.23 | 3.577 ± 0.23 |
| ZnS@CS | 0.12 ± 0.07 | 1.5 ± 0.17 | 1.8 ± 0.10 | 2.4 ± 0.19 | 2.186 ± 0.21 |
| ZrO2@CS | 0.13 ± 0.09 | 1.55 ± 0.19 | 1.85 ± 0.21 | 2.4 ± 0.17 | 2.186 ± 0.19 |
| ZnS/ZrO2@CS | 0.06 ± 0.01 | 1.55 ± 0.13 | 1.85 ± 0.13 | 2.4 ± 0.22 | 2.186 ± 0.17 |
| ZnS/ZrO2/GO@CS | 0.17 ± 0.04 | 1.6 ± 0.09 | 1.9 ± 0.15 | 2.45 ± 0.09 | 2.171 ± 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghuwainem, Y.A.A.; Gouda, M.; Khalaf, M.M.; Elmushyakhi, A.; Abou Taleb, M.F.; El-Lateef, H.M.A. Synthesis and Characterization of Chitosan-Containing ZnS/ZrO2/Graphene Oxide Nanocomposites and Their Application in Wound Dressing. Polymers 2022, 14, 5195. https://doi.org/10.3390/polym14235195
Alghuwainem YAA, Gouda M, Khalaf MM, Elmushyakhi A, Abou Taleb MF, El-Lateef HMA. Synthesis and Characterization of Chitosan-Containing ZnS/ZrO2/Graphene Oxide Nanocomposites and Their Application in Wound Dressing. Polymers. 2022; 14(23):5195. https://doi.org/10.3390/polym14235195
Chicago/Turabian StyleAlghuwainem, Yousef A. A., Mohamed Gouda, Mai M. Khalaf, Abraham Elmushyakhi, Manal F. Abou Taleb, and Hany M. Abd El-Lateef. 2022. "Synthesis and Characterization of Chitosan-Containing ZnS/ZrO2/Graphene Oxide Nanocomposites and Their Application in Wound Dressing" Polymers 14, no. 23: 5195. https://doi.org/10.3390/polym14235195
APA StyleAlghuwainem, Y. A. A., Gouda, M., Khalaf, M. M., Elmushyakhi, A., Abou Taleb, M. F., & El-Lateef, H. M. A. (2022). Synthesis and Characterization of Chitosan-Containing ZnS/ZrO2/Graphene Oxide Nanocomposites and Their Application in Wound Dressing. Polymers, 14(23), 5195. https://doi.org/10.3390/polym14235195








