Chemical Recycling of High-Molecular-Weight Organosilicon Compounds in Supercritical Fluids
Abstract
1. Introduction
2. General Approaches to the Chemical Recycling of Polysiloxanes
2.1. Thermal Decomposition of Siloxanes
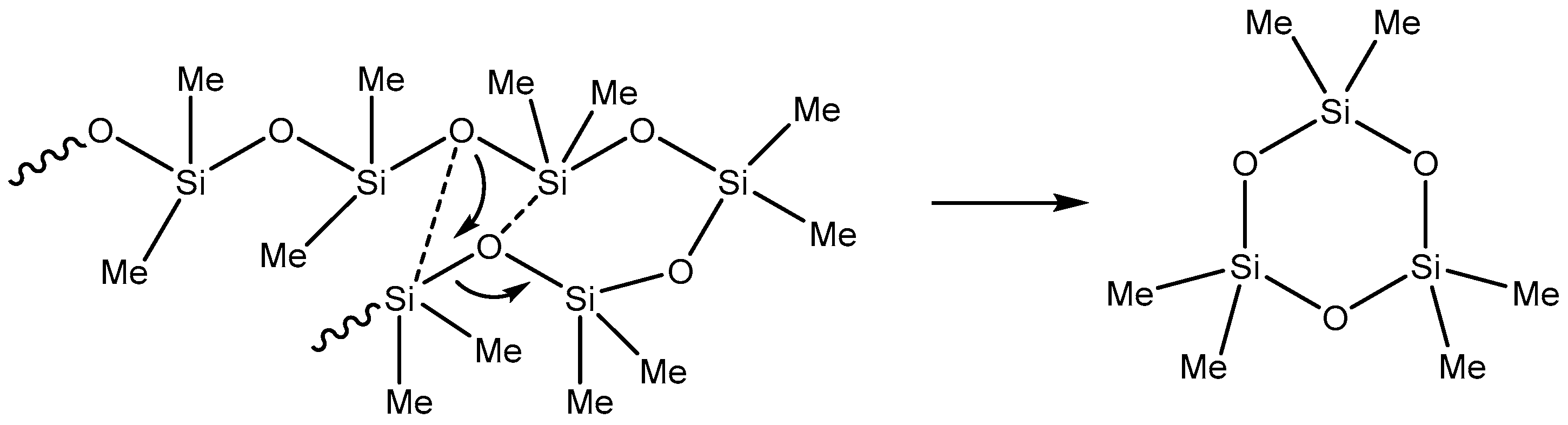
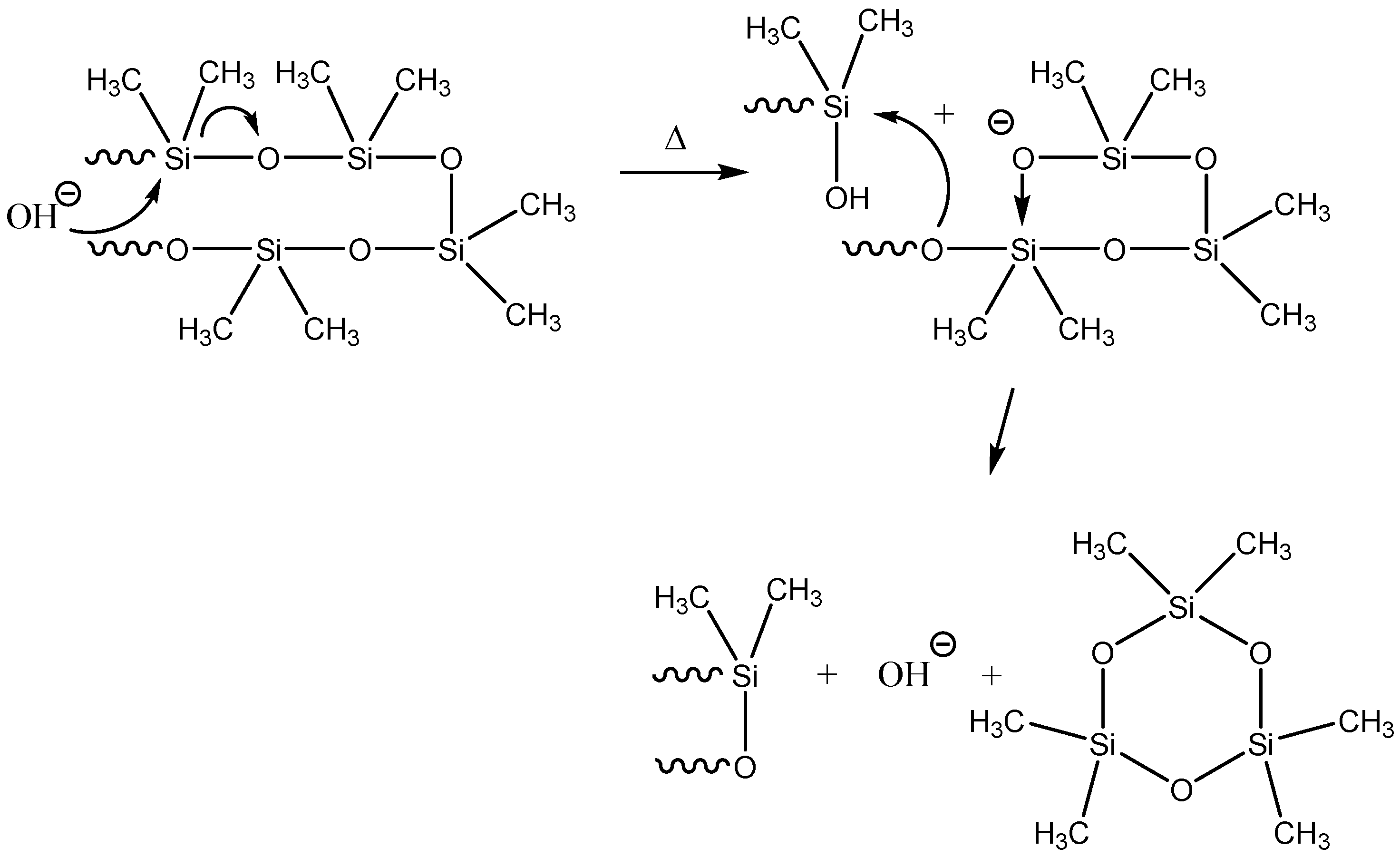
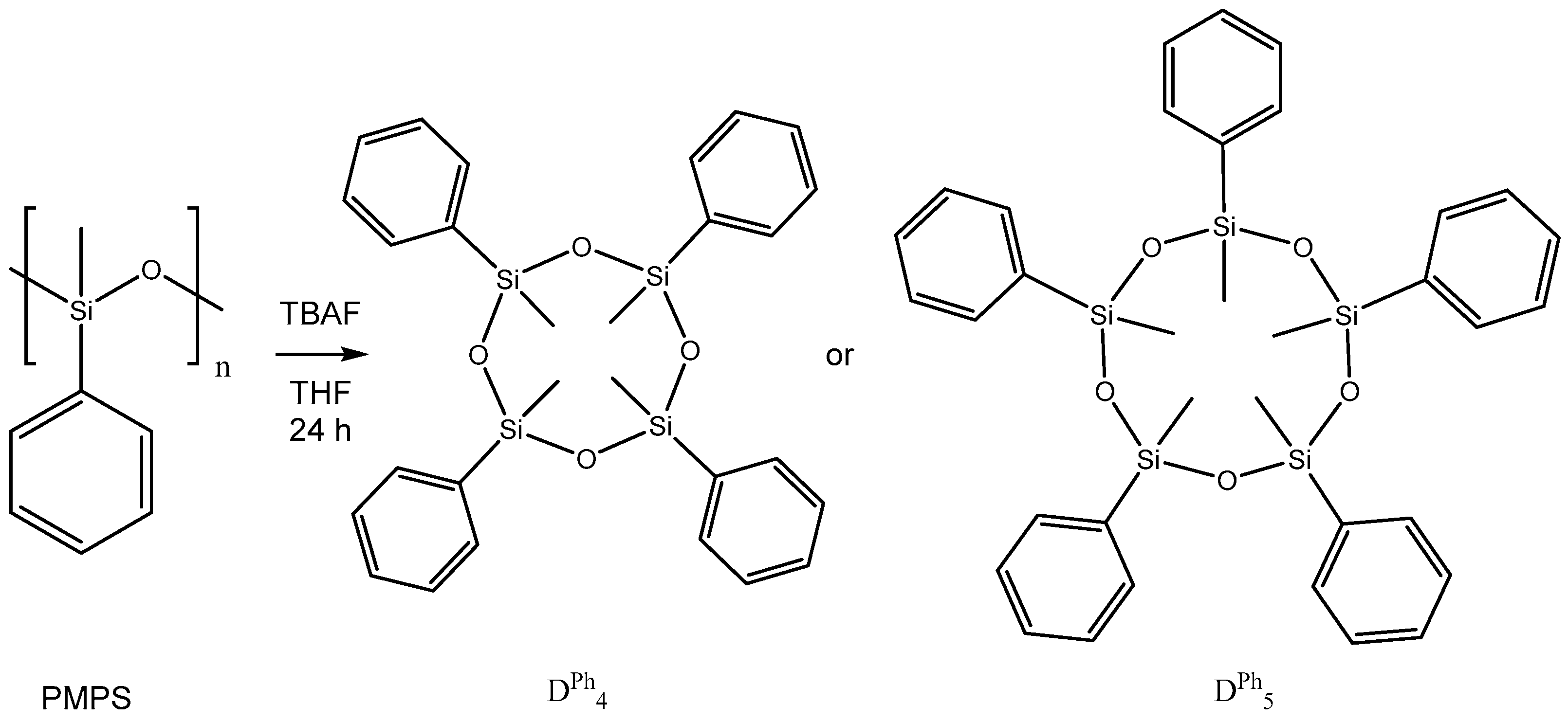
2.2. Catalytic Decomposition of Siloxanes
3. Recycling of Polysiloxanes in Supercritical Media
3.1. Plastic Waste Recycling in Supercritical Media
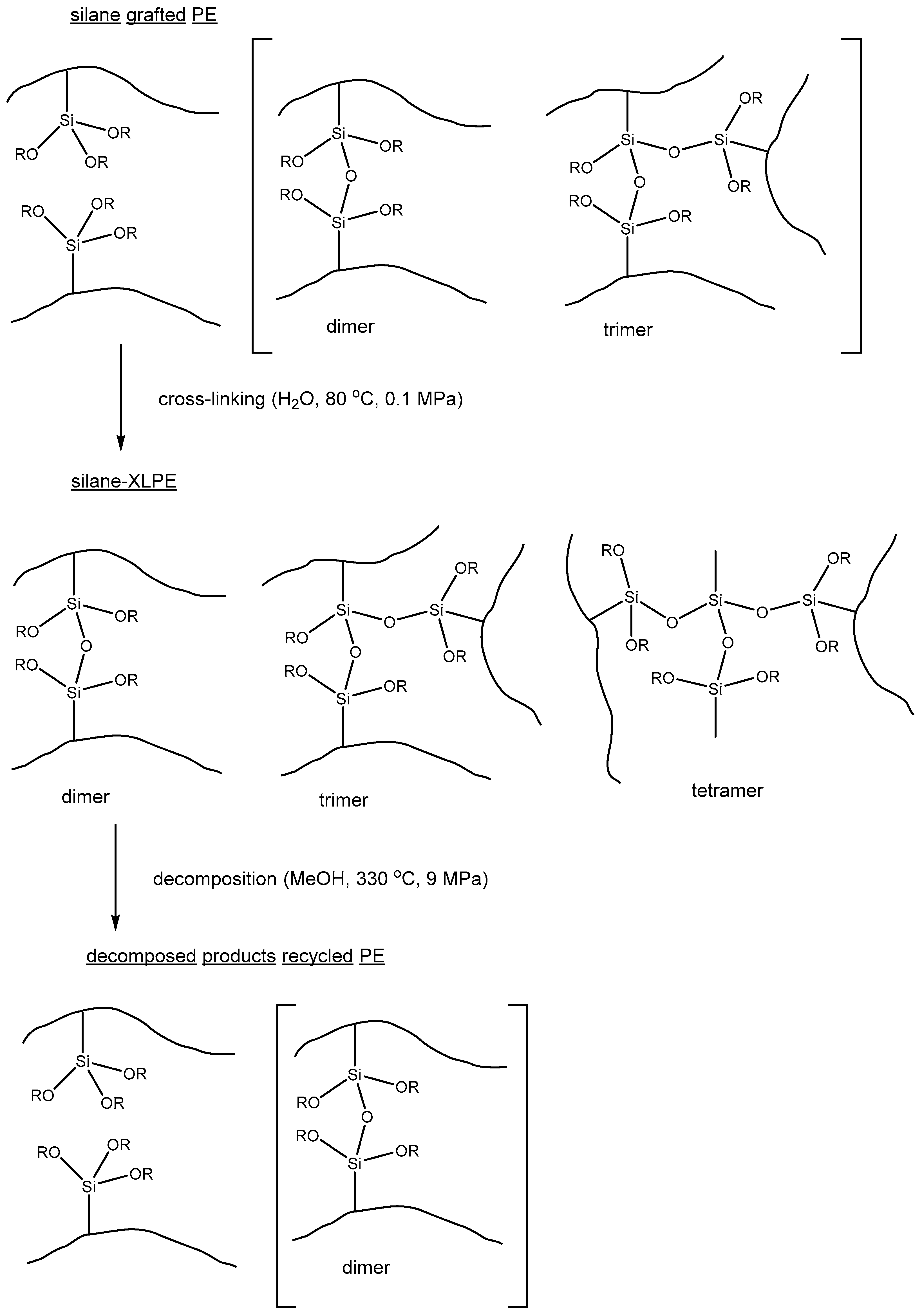
3.2. Recycling of Silane-Crosslinked Polyethylene in SCW and sc Alcohols
4. The Prospect of Chemical Recycling of Polysiloxanes in sc CO2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griessbach, E.F.C.; Lehmann, R.G. Degradation of Polydimethylsiloxane Fluids in the Environment—A Review. Chemosphere 1999, 38, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.C.; Cella, J.A.; Dorn, S.B. Study of the Degradation of Polydimethylsiloxanes on Soil. Environ. Sci. Technol. 1995, 29, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, C.L.; Carpenter, J.C.; Leib, T.K.; Spivack, J.L. Biodegradation of Dimethylsilanediol in Soils. Appl. Environ. Microbiol. 1996, 62, 4352–4360. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, F.; Alves, A.; Homem, V. A Review of Bioaccumulation of Volatile Methylsiloxanes in Aquatic Ecosystems. Sci. Total Environ. 2022, 824, 153821. [Google Scholar] [CrossRef] [PubMed]
- Voronkov, M.G.; Lukevics, E. Biologically Active Compounds of Silicon. Russ. Chem. Rev. 1969, 38, 975–986. [Google Scholar] [CrossRef]
- Graiver, D.; Farminer, K.W.; Narayan, R. A Review of the Fate and Effects of Silicones in the Environment. J. Polym. Environ. 2003, 11, 129–136. [Google Scholar] [CrossRef]
- Fackler, P.H.; Dionne, E.; Hartley, D.A.; Hamelink, J.L. Bioconcentration by Fish of a Highly Volatile Silicone Compound in a Totally Enclosed Aquatic Exposure System. Environ. Toxicol. Chem. 1995, 14, 1649–1656. [Google Scholar] [CrossRef]
- Gobas, F.A.P.C.; Powell, D.E.; Woodburn, K.B.; Springer, T.; Huggett, D.B. Bioaccumulation of Decamethylpentacyclosiloxane (D5): A Review. Environ. Toxicol. Chem. 2015, 34, 2703–2714. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X. The Recycle and Cracking Reutilization for the Waste Silicone Products. Silicone Mater. 2005, 19, 23–24. [Google Scholar]
- Grassie, N.; Macfarlane, I.G. The Thermal Degradation of Polysiloxanes-I. Poly(Dimethylsiloxane). Eur. Polym. J. 1978, 14, 875–884. [Google Scholar] [CrossRef]
- Camino, C.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane Thermal Degradation Part 1. Kinetic Aspects. Polymer 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Thomas, T.H.; Kendrick, T.C. Thermal Analysis of Polydimethylsiloxanes. I. Thermal Degradation in Controlled Atmospheres. J. Polym. Sci. Part A-2 Polym. Phys. 1969, 7, 537–549. [Google Scholar] [CrossRef]
- Aleksandrova, Y.A.; Kotleva, N.S.; Sorokina, R.S.; Bakhurina, L.M.; Nikitina, T.S.; Panov, Y.M.; Pravednikov, A.N. Hydrolytic and Thermal Degradation Study of Some Model and Polymeric Siloxane Compounds. Polym. Sci. USSR 1969, 11, 2808–2813. [Google Scholar] [CrossRef]
- Andrianov, K.A.; Papkov, V.S.; Slonimskii, G.L.; Zhdanov, A.A.; Yakushkina, S.Y. Thermogravimetric Study of the Vacuum Depolymerization of Polydimethylsiloxane. Polym. Sci. USSR 1969, 11, 2313–2329. [Google Scholar] [CrossRef]
- Svistunov, V.S.; Papkov, V.S.; Zhdanov, A.A. The High-Temperature Equilibrium of the System Octamethylcyclotetrasiloxane-Polydimethyl-Siloxane. Polym. Sci. USSR 1980, 22, 1445–1449. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lageard, M. Thermal Polydimethylsiloxane Degradation. Part 2. The Degradation Mechanisms. Polymer 2002, 43, 2011–2015. [Google Scholar] [CrossRef]
- Papkov, V.S.; Bulkin, A.F.; Zhadanov, A.A.; Slonimskii, G.L.; Andrianov, K.A. Investigation of the Thermal Oxidation of Polydimethylsiloxanes. Polym. Sci. USSR 1977, 19, 962–976. [Google Scholar] [CrossRef]
- Bulkin, A.F.; Papkov, V.S.; Zhdanov, A.A.; Slonimskii, G.L. The Uninhibited Oxidation of Polydiethylsiloxane. Polym. Sci. USSR 1986, 28, 591–599. [Google Scholar] [CrossRef]
- Papkov, V.S.; Bulkin, A.F.; Slonimskii, G.L.; Zhdanov, A.A.; Andrianov, K.A. Inhibited Oxidation of Polydimethylsiloxane in the Presence of Transition Metal Compounds. Polym. Sci. USSR 1978, 20, 1186–1196. [Google Scholar] [CrossRef]
- Deshpande, G.; Rezac, M.E. Kinetic Aspects of the Thermal Degradation of Poly(Dimethyl Siloxane) and Poly(Dimethyl Diphenyl Siloxane). Polym. Degrad. Stab. 2002, 76, 17–24. [Google Scholar] [CrossRef]
- Dvornic, P.R. Thermal Properties of Polysiloxanes. In Silicon-Containing Polymers; Springer: Dordrecht, The Netherlands, 2000; pp. 185–212. [Google Scholar]
- Enthaler, S. Zinc-Catalyzed Depolymerization of End-of-Life Polysiloxanes. Angew. Chem. 2014, 126, 2754–2759. [Google Scholar] [CrossRef]
- Enthaler, S. Iron-Catalyzed Depolymerization of Polysiloxanes to Produce Dichlorodimethylsilane, Diacetoxydimethylsilane, or Dimethoxydimethylsilane. J. Appl. Polym. Sci. 2015, 132, 41287. [Google Scholar] [CrossRef]
- Weidauer, M.; Heyder, B.; Woelki, D.; Tschiersch, M.; Köhler-Krützfeldt, A.; Enthaler, S. Iron-Catalyzed Depolymerizations of End-of-Life Silicones with Fatty Alcohols. Resour. Technol. 2015, 1, 73–79. [Google Scholar] [CrossRef]
- Döhlert, P.; Enthaler, S. Depolymerization Protocol for Linear, Branched, and Crosslinked End-of-Life Silicones with Boron Trifluoride Diethyl Etherate as the Depolymerization Reagent. J. Appl. Polym. Sci. 2015, 132, 42814. [Google Scholar] [CrossRef]
- Döhlert, P.; Pfrommer, J.; Enthaler, S. Recycling Concept for End-of-Life Silicones: Boron Trifluoride Diethyl Etherate as Depolymerization Reagent to Produce Difluorodimethylsilane as Useful Commodity. ACS Sustain. Chem. Eng. 2015, 3, 163–169. [Google Scholar] [CrossRef]
- Galaburda, M.V.; Klonos, P.; Gun’ko, V.M.; Bogatyrov, V.M.; Borysenko, M.V.; Pissis, P. Dielectric Properties and Thermal Destruction of Poly(Dimethylsiloxane)/Fe2O3/SiO2 Nanocomposites. Appl. Surf. Sci. 2014, 305, 67–76. [Google Scholar] [CrossRef]
- Bogatyr’ov, V.M.; Borysenko, M.V. Thermal Destruction of Polydimethylsiloxane on a Phosphorus-Containing Silica Surface. J. Therm. Anal. Calorim. 2000, 62, 335–344. [Google Scholar] [CrossRef]
- Rupasinghe, B.; Furgal, J.C. Full Circle Recycling of Polysiloxanes via Room-Temperature Fluoride-Catalyzed Depolymerization to Repolymerizable Cyclics. ACS Appl. Polym. Mater. 2021, 3, 1828–1839. [Google Scholar] [CrossRef]
- Krug, D.J.; Asuncion, M.Z.; Laine, R.M. Facile Approach to Recycling Highly Cross-Linked Thermoset Silicone Resins under Ambient Conditions. ACS Omega 2019, 4, 3782–3789. [Google Scholar] [CrossRef]
- Buch, R.R.; Ingebrigtson, D.N. Rearrangement of Poly (Dimethylsiloxane) Fluids on Soil. Environ. Sci. Technol. 1979, 13, 676–679. [Google Scholar] [CrossRef]
- Lehmann, R.G.; Varaprath, S.; Frye, C.L. Degradation of Silicone Polymers in Soil. Environ. Toxicol. Chem. 1994, 13, 1061–1064. [Google Scholar] [CrossRef]
- Xu, S.; Lehmann, R.G.; Miller, J.R.; Chandra, G. Degradation of Polydimethylsiloxanes (Silicones) as Influenced by Clay Minerals. Environ. Sci. Technol. 1998, 32, 1199–1206. [Google Scholar] [CrossRef]
- Rücker, C.; Kümmerer, K. Environmental Chemistry of Organosiloxanes. Chem. Rev. 2015, 115, 466–524. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, B.; Furgal, J.C. Degradation of Silicone-Based Materials as a Driving Force for Recyclability. Polym. Int. 2022, 71, 521–531. [Google Scholar] [CrossRef]
- Bettenhausen, C. Eco USA Expands Silicone Recycling Plant. C EN 2021, 99, 14–15. [Google Scholar] [CrossRef]
- Muzafarov, A.M.; Bystrova, A.V.; Vasilenko, N.G.; Ignat’eva, G.M. New Approaches in Silicon Production and Recycling for Sustainable Future. Russ. Chem. Rev. 2013, 82, 635–647. [Google Scholar] [CrossRef]
- Furgal, J.C.; Lenora, C.U. Green Routes to Silicon-Based Materials and Their Environmental Implications. Phys. Sci. Rev. 2020, 5, 20190024. [Google Scholar] [CrossRef]
- Goto, M.; Sasaki, M.; Hirose, T. Reactions of Polymers in Supercritical Fluids for Chemical Recycling of Waste Plastics. J. Mater. Sci. 2006, 41, 1509–1515. [Google Scholar] [CrossRef]
- Goto, M. Chemical Recycling of Plastics Using Sub- and Supercritical Fluids. J. Supercrit. Fluids 2009, 47, 500–507. [Google Scholar] [CrossRef]
- Goto, M. Subcritical and Supercritical Fluid Technology for Recycling Waste Plastics. J. Jpn. Pet. Inst. 2016, 59, 254–258. [Google Scholar] [CrossRef]
- Queiroz, A.; Pedroso, G.B.; Kuriyama, S.N.; Fidalgo-Neto, A.A. Subcritical and Supercritical Water for Chemical Recycling of Plastic Waste. Curr. Opin. Green Sustain. Chem. 2020, 25, 100364. [Google Scholar] [CrossRef]
- Wang, W.; Meng, L.; Huang, Y. Hydrolytic Degradation of Monomer Casting Nylon in Subcritical Water. Polym. Degrad. Stab. 2014, 110, 312–317. [Google Scholar] [CrossRef]
- Mishra, S.; Zope, V.S.; Kulkarni, R.D. Kinetics and Thermodynamics of Hydrolytic Depolymerization of Polyurethane Foam at Higher Temperature and Pressure. Polym.-Plast. Technol. Eng. 2004, 43, 1001–1011. [Google Scholar] [CrossRef]
- Kao, C.Y.; Wan, B.Z.; Cheng, W.H. Kinetics of Hydrolytic Depolymerization of Melt Poly(Ethylene Terephthalate). Ind. Eng. Chem. Res. 1998, 37, 1228–1234. [Google Scholar] [CrossRef]
- Okajima, I.; Okamoto, H.; Sako, T. Recycling of Aramid Fiber Using Subcritical and Supercritical Water. Polym. Degrad. Stab. 2019, 162, 22–28. [Google Scholar] [CrossRef]
- Ciuffi, B.; Chiaramonti, D.; Rizzo, A.M.; Frediani, M.; Rosi, L. A Critical Review of SCWG in the Context of Available Gasification Technologies for Plastic Waste. Appl. Sci. 2020, 10, 6307. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal Degradation of PVC: A Review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Honma, R.; Hori, H.; Da Cunha, F.R.; Horiike, N.; Steinbach, L.; Ameduri, B. Permanganate-Induced Efficient Mineralization of Poly(Vinylidene Fluoride) and Vinylidene-Fluoride Based Copolymers in Low-Temperature Subcritical Water. Ind. Eng. Chem. Res. 2019, 58, 13030–13040. [Google Scholar] [CrossRef]
- Hori, H.; Tanaka, H.; Watanabe, K.; Tsuge, T.; Sakamoto, T.; Manseri, A.; Ameduri, B. Hydrogen Peroxide Induced Efficient Mineralization of Poly(Vinylidene Fluoride) and Related Copolymers in Subcritical Water. Ind. Eng. Chem. Res. 2015, 54, 8650–8658. [Google Scholar] [CrossRef]
- Adschiri, T.; Lee, Y.W.; Goto, M.; Takami, S. Green Materials Synthesis with Supercritical Water. Green Chem. 2011, 13, 1380–1390. [Google Scholar] [CrossRef]
- Andanson, J.M.; Kazarian, S.G. In Situ ATR-FTIR Spectroscopy of Poly(Ethylene Terephthalate) Subjected to High-Temperature Methanol. Macromol. Symp. 2008, 265, 195–204. [Google Scholar] [CrossRef]
- Suhaimi, N.A.S.; Muhamad, F.; Abd Razak, N.A.; Zeimaran, E. Recycling of Polyethylene Terephthalate Wastes: A Review of Technologies, Routes, and Applications. Polym. Eng. Sci. 2022, 62, 2355–2375. [Google Scholar] [CrossRef]
- Tagaya, H.; Suzuki, Y.I.; Asou, T.; Kadokawa, J.I.; Chiba, K. Reaction of Model Compounds of Phenol Resin and Molding Materials of Phenol Resin in Supercritical Water for Chemical Recylcing of Polymer Waste. Chem. Lett. 1998, 27, 937–938. [Google Scholar] [CrossRef]
- Nanda, S.; Reddy, S.N.; Hunter, H.N.; Vo, D.V.N.; Kozinski, J.A.; Gökalp, I. Catalytic Subcritical and Supercritical Water Gasification as a Resource Recovery Approach from Waste Tires for Hydrogen-Rich Syngas Production. J. Supercrit. Fluids 2019, 154, 104627. [Google Scholar] [CrossRef]
- Ozaki, J.I.; Djaja, S.K.I.; Oya, A. Chemical Recycling of Phenol Resin by Supercritical Methanol. Ind. Eng. Chem. Res. 2000, 39, 245–249. [Google Scholar] [CrossRef]
- Yan, H.; Lu, C.X.; Jing, D.Q.; Hou, X.L. Chemical Degradation of Amine-Cured DGEBA Epoxy Resin in Supercritical 1-Propanol for Recycling Carbon Fiber from Composites. Chin. J. Polym. Sci. 2014, 32, 1550–1563. [Google Scholar] [CrossRef]
- Asahi, N.; Sakai, K.; Kumagai, N.; Nakanishi, T.; Hata, K.; Katoh, S.; Moriyoshi, T. Methanolysis Investigation of Commercially Available Polyurethane Foam. Polym. Degrad. Stab. 2004, 86, 147–151. [Google Scholar] [CrossRef]
- Liu, L.; Tang, L.; Zhu, Z.; Ni, Y.; Wu, Y. Performance of Supercritical Methanol in Polyurethane Degradation. MATEC Web Conf. 2016, 64, 4–9. [Google Scholar] [CrossRef]
- Morin, C.; Loppinet-Serani, A.; Cansell, F.; Aymonier, C. Near- and Supercritical Solvolysis of Carbon Fibre Reinforced Polymers (CFRPs) for Recycling Carbon Fibers as a Valuable Resource: State of the Art. J. Supercrit. Fluids 2012, 66, 232–240. [Google Scholar] [CrossRef]
- Okajima, I.; Watanabe, K.; Haramiishi, S.; Nakamura, M.; Shimamura, Y.; Sako, T. Recycling of Carbon Fiber Reinforced Plastic Containing Amine-Cured Epoxy Resin Using Supercritical and Subcritical Fluids. J. Supercrit. Fluids 2017, 119, 44–51. [Google Scholar] [CrossRef]
- Gharde, S.; Kandasubramanian, B. Mechanothermal and Chemical Recycling Methodologies for the Fibre Reinforced Plastic (FRP). Environ. Technol. Innov. 2019, 14, 100311. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Karl, C.W.; Gagani, A.I.; Jørgensen, J.K. Composite Material Recycling Technology—State-of-the-Art and Sustainable Development for the 2020s. J. Compos. Sci. 2021, 5, 28. [Google Scholar] [CrossRef]
- Goto, T.; Yamazaki, T.; Sugeta, T.; Okajima, I.; Sako, T. Selective Decomposition of the Siloxane Bond Constituting the Crosslinking Element of Silane- Crosslinked Polyethylene by Supercritical Alcohol. J. Appl. Polym. Sci. 2008, 109, 144–151. [Google Scholar] [CrossRef]
- Okajima, I.; Katsuzaki, A.; Goto, T.; Yamazaki, T.; Sako, T. Decomposition of Silane-Crosslinked Polyethylene with Supercritical Alcohol. J. Chem. Eng. Jpn. 2010, 43, 231–237. [Google Scholar] [CrossRef]
- Goto, T.; Ashihara, S.; Kato, M.; Okajima, I.; Sako, T. Use of Single-Screw Extruder for Continuous Silane Cross-Linked Polyethylene Recycling Process Using Supercritical Alcohol. Ind. Eng. Chem. Res. 2012, 51, 6967–6971. [Google Scholar] [CrossRef]
- Goto, T.; Ashihara, S.; Yamazaki, T.; Okajima, I.; Sako, T.; Iwamoto, Y.; Ishibashi, M.; Sugeta, T. Continuous Process for Recycling Silane Cross-Linked Polyethylene Using Supercritical Alcohol and Extruders. Ind. Eng. Chem. Res. 2011, 50, 5661–5666. [Google Scholar] [CrossRef]
- Baek, B.K.; Shin, J.W.; Jung, J.Y.; Hong, S.M.; Nam, G.J.; Han, H.; Koo, C.M. Continuous Supercritical Decrosslinking Extrusion Process for Recycling of Crosslinked Polyethylene Waste. J. Appl. Polym. Sci. 2015, 132, 41442. [Google Scholar] [CrossRef]
- Hong, G.; Hong, S.M.; Koo, C.M.; Baek, B.K.; Lee, H.; Lee, Y. A Kinetic Study on the De-Crosslinking and Decomposition of Silane-Crosslinked Polyethylene in Supercritical Methanol. Ind. Eng. Chem. Res. 2015, 54, 11961–11965. [Google Scholar] [CrossRef]
- Baek, B.K.; La, Y.H.; Na, W.J.; Lee, S.H.; Hong, S.M.; Han, H.; Lee, Y.W.; Nam, G.J.; Koo, C.M. A Kinetic Study on the Supercritical Decrosslinking Reaction of Silane-Crosslinked Polyethylene in a Continuous Process. Polym. Degrad. Stab. 2016, 126, 75–80. [Google Scholar] [CrossRef]
- Baek, B.K.; Lo, Y.H.; Lee, A.S.; Han, H.; Kim, S.H.; Hong, S.M.; Koo, C.M. Decrosslinking Reaction Kinetics of Silane-Crosslinked Polyethylene in Sub- and Supercritical Fluids. Polym. Degrad. Stab. 2016, 130, 103–108. [Google Scholar] [CrossRef]
- Vandenburg, H.J.; Clifford, A.A.; Bartle, K.D.; Carroll, J.; Newton, I.; Garden, L.M.; Dean, J.R.; Costley, C.T. Analytical Extraction of Additives from Polymers. Analyst 1997, 122, 101–115. [Google Scholar] [CrossRef]
- Garde, J.A.; Catalá, R.; Gavara, R. Analysis of Antioxidants Extracted from Polypropylene by Supercritical Fluid Extraction. Food Addit. Contam. 1998, 15, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chau, E.; Azimi, G. Supercritical Fluid Extraction for Purification of Waxes Derived from Polyethylene and Polypropylene Plastics. Waste Manag. 2019, 97, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Elmanovich, I.V.; Stakhanov, A.I.; Kravchenko, E.I.; Stakhanova, S.V.; Pavlov, A.A.; Ilyin, M.M.; Kharitonova, E.P.; Gallyamov, M.O.; Khokhlov, A.R. Chemical Recycling of Polyethylene in Oxygen-Enriched Supercritical CO2. J. Supercrit. Fluids 2021, 181, 105503. [Google Scholar] [CrossRef]
- Elmanovich, I.V.; Stakhanov, A.I.; Zefirov, V.V.; Pavlov, A.A.; Lokshin, B.V.; Gallyamov, M.O. Thermal Oxidation of Polypropylene Catalyzed by Manganese Oxide Aerogel in Oxygen-Enriched Supercritical Carbon Dioxide. J. Supercrit. Fluids 2020, 158, 104744. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Lee, S.H.; Kim, J.K.; Zhang, S.L.; Xin, Z.X. Preparation and Characterization of Polypropylene/Waste Ground Rubber Tire Powder Microcellular Composites by Supercritical Carbon Dioxide. Macromol. Res. 2008, 16, 404–410. [Google Scholar] [CrossRef]
- Engineering, A.M.; Korea, S.; National, K.; Korea, S. Microcellular Structure of PP/Waste Rubber Powder Blends with Supercritical CO2 by Foam Extrusion Process. J. Cell. Plast. 2009, 45, 499–514. [Google Scholar] [CrossRef]
- Kwon, D.E.; Park, B.K.; Lee, Y.W. Solid-State Foaming of Acrylonitrile-Butadiene- Styrene/Recycled Polyethylene Terephthalate Using Carbon Dioxide as a Blowing Agent. Polymers 2019, 11, 291. [Google Scholar] [CrossRef]
- Xiong, Y.; Kiran, E. Miscibility, Density and Viscosity of Poly (Dimethylsiloxane) in Supercritical Carbon Dioxide. Polymer 1995, 36, 4817–4826. [Google Scholar] [CrossRef]
- Lee, J.J.; Cummings, S.D.; Beckman, E.J.; Enick, R.M.; Burgess, W.A.; Doherty, M.D.; Brien, M.J.O.; Perry, R.J. The Solubility of Low Molecular Weight Poly (Dimethyl Siloxane) in Dense CO2 and Its Use as a CO2-Philic Segment. J. Supercrit. Fluids 2017, 119, 17–25. [Google Scholar] [CrossRef]
- Domingo, C.; Loste, E.; Fraile, J. Grafting of Trialkoxysilane on the Surface of Nanoparticles by Conventional Wet Alcoholic and Supercritical Carbon Dioxide Deposition Methods. J. Supercrit. Fluids 2006, 37, 72–86. [Google Scholar] [CrossRef]
- Loste, B.E.; Fraile, J.; Fanovich, M.A.; Woerlee, G.F.; Domingo, C. Anhydrous Supercritical Carbon Dioxide Method for the Controlled Silanization of Inorganic Nanoparticles. Adv. Mater. 2004, 16, 739–744. [Google Scholar] [CrossRef]
- Vyhmeister, E.; Muscat, A.J.; Suleiman, D.; Estévez, L.A. High-Pressure Phase Equilibria for Chlorosilane + Carbon Dioxide Mixtures. Fluid Phase Equilibria 2008, 270, 121–128. [Google Scholar] [CrossRef]
- Pigaleva, M.A.; Elmanovich, I.V.; Temnikov, M.N.; Gallyamov, M.O.; Muzafarov, A.M. Organosilicon Compounds in Supercritical Carbon Dioxide: Synthesis, Polymerization, Modification, and Production of New Materials. Polym. Sci.-Ser. B 2016, 58, 235–270. [Google Scholar] [CrossRef]
- Elmanovich, I.V.; Zefirov, V.V.; Sizov, V.E.; Kondratenko, M.S.; Gallyamov, M.O. Polymer–Inorganic Composites Based on Celgard Matrices Obtained from Solutions of (Aminopropyl)Triethoxysilane in Supercritical Carbon Dioxide. Dokl. Phys. Chem. 2019, 485, 53–57. [Google Scholar] [CrossRef]
- Simonov, A.S.; Kondratenko, M.S.; Elmanovich, I.V.; Sizov, V.E.; Kharitonova, E.P.; Abramchuk, S.S.; Nikolaev, A.Y.; Fedosov, D.A.; Gallyamov, M.O.; Khokhlov, A.R. Modification of Nafion with Silica Nanoparticles in Supercritical Carbon Dioxide for Electrochemical Applications. J. Membr. Sci. 2018, 564, 106–114. [Google Scholar] [CrossRef]
- Loy, D.A.; Russick, E.M.; Yamanaka, S.A.; Baugher, B.M.; Shea, K.J. Direct Formation of Aerogels by Sol-Gel Polymerizations of Alkoxysilanes in Supercritical Carbon Dioxide. Chem. Mater. 1997, 4756, 2264–2268. [Google Scholar] [CrossRef]
- Zou, F.; Peng, L.; Fu, W.; Zhang, J.; Li, Z. Flexible Superhydrophobic Polysiloxane Aerogels for Oil–Water Separation via One-Pot Synthesis in Supercritical CO2. RSC Adv. 2015, 5, 76346–76351. [Google Scholar] [CrossRef]
- Elmanovich, I.V.; Pryakhina, T.A.; Vasil’ev, V.G.; Gallyamov, M.O.; Muzafarov, A.M. A Study of the Hydrosilylation Approach to a One-Pot Synthesis of Silicone Aerogels in Supercritical CO2. J. Supercrit. Fluids 2018, 133, 512–518. [Google Scholar] [CrossRef]
- Elmanovich, I.V.; Pryakhina, T.A.; Gallyamov, M.O.; Migulin, D.A.; Meshkov, I.B.; Vasil’ev, V.G.; Muzafarov, A.M. Silicone Aerogels with Tunable Mechanical Properties Obtained via Hydrosilylation Reaction in Supercritical CO2. J. Supercrit. Fluids 2019, 149, 120–126. [Google Scholar] [CrossRef]
- Qiang, W.; Zhao, L.; Gao, X.; Liu, T.; Liu, Z.; Yuan, W. The Journal of Supercritical Fluids Dual Role of PDMS on Improving Supercritical CO2 Foaming of Polypropylene: CO2-Philic Additive and Crystallization Nucleating Agent. J. Supercrit. Fluids 2020, 163, 104888. [Google Scholar] [CrossRef]
- Pal, N.; Zhang, X.; Ali, M.; Mandal, A.; Hoteit, H. Carbon Dioxide Thickening: A Review of Technological Aspects, Advances and Challenges for Oilfield Application. Fuel 2022, 315, 122947. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmanovich, I.V.; Sizov, V.E.; Zefirov, V.V.; Kalinina, A.A.; Gallyamov, M.O.; Papkov, V.S.; Muzafarov, A.M. Chemical Recycling of High-Molecular-Weight Organosilicon Compounds in Supercritical Fluids. Polymers 2022, 14, 5170. https://doi.org/10.3390/polym14235170
Elmanovich IV, Sizov VE, Zefirov VV, Kalinina AA, Gallyamov MO, Papkov VS, Muzafarov AM. Chemical Recycling of High-Molecular-Weight Organosilicon Compounds in Supercritical Fluids. Polymers. 2022; 14(23):5170. https://doi.org/10.3390/polym14235170
Chicago/Turabian StyleElmanovich, Igor V., Victor E. Sizov, Vadim V. Zefirov, Alexandra A. Kalinina, Marat O. Gallyamov, Vladimir S. Papkov, and Aziz M. Muzafarov. 2022. "Chemical Recycling of High-Molecular-Weight Organosilicon Compounds in Supercritical Fluids" Polymers 14, no. 23: 5170. https://doi.org/10.3390/polym14235170
APA StyleElmanovich, I. V., Sizov, V. E., Zefirov, V. V., Kalinina, A. A., Gallyamov, M. O., Papkov, V. S., & Muzafarov, A. M. (2022). Chemical Recycling of High-Molecular-Weight Organosilicon Compounds in Supercritical Fluids. Polymers, 14(23), 5170. https://doi.org/10.3390/polym14235170