Salt-Induced Transformations of Hybrid Micelles Formed by Anionic Surfactant and Poly(4-vinylpyridine)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
2.4. Small-Angle Neutron Scattering (SANS)
2.5. Dynamic Light Scattering (DLS)
2.6. Two-Dimensional 1H Overhauser NMR-Spectroscopy
3. Results and Discussion
3.1. Structural Transitions of Polymer-Free Micelles upon Addition of Salt
3.2. Structural Transitions of Hybrid Micelles of Polymer-Surfactant Complexes upon Addition of Salt
3.3. Polymer Inside the Hybrid Micelles of Surfactant-Polymer Complex: Conformation and Localization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: San Diego, CA, USA, 2011. [Google Scholar]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of Self-Assembly of Hydrocarbon Amphiphiles into Micelles and Bilayers. J. Chem. Soc. Faraday Trans. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Tanford, C. Thermodynamics of Micelle Formation: Prediction of Micelle Size and Size Distribution. Proc. Natl. Acad. Sci. USA 1974, 71, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R. Micellization, Mixed Micellization and Solubilization: The Role of Interfacial Interactions. Adv. Colloid Interface Sci. 1986, 26, 205–264. [Google Scholar] [CrossRef]
- Falbe, J. Surfactants in Consumer Products: Theory, Technology and Application; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Philippova, O.E.; Khokhlov, A.R. Smart Polymers for Oil Production. Pet. Chem. 2010, 50, 266–270. [Google Scholar] [CrossRef]
- Feng, Y.; Chu, Z.; Dreiss, C.A. Smart Wormlike Micelles: Design, Characteristics and Applications; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783662459492. [Google Scholar]
- Zana, R.; Kaler, E.W. Giant Micelles: Properties and Applications; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-8493-7308-4. [Google Scholar]
- Dreiss, C.A.; Feng, Y. Wormlike Micelles: Advances in Systems, Characterisation and Applications; The Royal Society of Chemistry: London, UK, 2017; ISBN 9781782629788. [Google Scholar]
- Le Garrec, D.; Ranger, M.; Leroux, J.C. Micelles in Anticancer Drug Delivery. Am. J. Drug Deliv. 2004, 2, 15–42. [Google Scholar] [CrossRef]
- Kim, J.H.; Domach, M.M.; Tilton, R.D. Pyrene Micropartitioning and Solubilization by Sodium Dodecyl Sulfate Complexes with Poly(Ethylene Glycol). J. Phys. Chem. B 1999, 103, 10582–10590. [Google Scholar] [CrossRef]
- Chu, Z.; Dreiss, C.A.; Feng, Y. Smart Wormlike Micelles. Chem. Soc. Rev. 2013, 42, 7174–7203. [Google Scholar] [CrossRef]
- Flood, C.; Dreiss, C.A.; Croce, V.; Cosgrove, T.; Karlsson, G.G. Wormlike Micelles Mediated by Polyelectrolyte. Langmuir 2005, 21, 7646–7652. [Google Scholar] [CrossRef]
- Croce, V.; Cosgrove, T.; Dreiss, C.A.; King, S.; Maitland, G.; Hughes, T. Giant Micellar Worms under Shear: A Rheological Study Using SANS. Langmuir 2005, 21, 6762–6768. [Google Scholar] [CrossRef]
- Kwiatkowski, A.L.; Molchanov, V.S.; Orekhov, A.S.; Vasiliev, A.L.; Philippova, O.E. Impact of Salt Co- and Counterions on Rheological Properties and Structure of Wormlike Micellar Solutions. J. Phys. Chem. B 2016, 120, 12547–12556. [Google Scholar] [CrossRef]
- Kuperkar, K.; Abezgauz, L.; Danino, D.; Verma, G.; Hassan, P.A.; Aswal, V.K.; Varade, D.; Bahadur, P. Viscoelastic Micellar Water/CTAB/NaNO3 Solutions: Rheology, SANS and Cryo-TEM Analysis. J. Colloid Interface Sci. 2008, 323, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Varade, D.; Joshi, T.; Aswal, V.K.; Goyal, P.S.; Hassan, P.A.; Bahadur, P. Effect of Salt on the Micelles of Cetyl Pyridinium Chloride. Colloids Surf. A Physicochem. Eng. Asp. 2005, 259, 95–101. [Google Scholar] [CrossRef]
- Hassan, P.A.; Raghavan, S.R.; Kaler, E.W. Microstructural Changes in SDS Micelles Induced by Hydrotropic Salt. Langmuir 2002, 18, 2543–2548. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, S.; Mou, J.; Wu, D.; Zheng, S. Research Progress on the Collaborative Drag Reduction Effect of Polymers and Surfactants. Materials 2020, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Elbing, B.R.; Solomon, M.J.; Perlin, M.; Dowling, D.R.; Ceccio, S.L. Flow-Induced Degradation of Drag-Reducing Polymer Solutions within a High-Reynolds-Number Turbulent Boundary Layer. J. Fluid Mech. 2011, 670, 337–364. [Google Scholar] [CrossRef]
- Tam, K.C.; Wyn-Jones, E. Insights on Polymer Surfactant Complex Structures during the Binding of Surfactants to Polymers as Measured by Equilibrium and Structural Techniques. Chem. Soc. Rev. 2006, 35, 693. [Google Scholar] [CrossRef]
- Cavallaro, G.; Micciulla, S.; Chiappisi, L.; Lazzara, G. Chitosan-Based Smart Hybrid Materials: A Physico-Chemical Perspective. J. Mater. Chem. B 2021, 9, 594–611. [Google Scholar] [CrossRef]
- Hamza, M.F.; Sinnathambi, C.M.; Merican, Z.M.A. Recent Advancement of Hybrid Materials Used in Chemical Enhanced Oil Recovery (CEOR): A Review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012007. [Google Scholar] [CrossRef]
- Bronich, T.K.; Nehls, A.; Eisenberg, A.; Kabanov, V.A.; Kabanov, A.V. Novel Drug Delivery Systems Based on the Complexes of Block Ionomers and Surfactants of Opposite Charge. Colloids Surf. B Biointerfaces 1999, 16, 243–251. [Google Scholar] [CrossRef]
- Nagarajan, R. Thermodynamics of Nonionic Polymer—Micelle Association. Colloids Surf. 1985, 13, 1–17. [Google Scholar] [CrossRef]
- Piculell, L.; Norrman, J.; Svensson, A.V.; Lynch, I.; Bernardes, J.S.; Loh, W. Ionic Surfactants with Polymeric Counterions. Adv. Colloid Interface Sci. 2009, 147–148, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Plazzotta, B.; Fegyver, E.; Mészáros, R.; Pedersen, J.S. Anisometric Polyelectrolyte/Mixed Surfactant Nanoassemblies Formed by the Association of Poly(Diallyldimethylammonium Chloride) with Sodium Dodecyl Sulfate and Dodecyl Maltoside. Langmuir 2015, 31, 7242–7250. [Google Scholar] [CrossRef] [PubMed]
- Artykulnyi, O.P.; Shibaev, A.V.; Avdeev, M.M.; Ivankov, O.I.; Bulavin, L.A.; Petrenko, V.I.; Philippova, O.E. Structural Investigations of Poly(Ethylene Glycol)-Dodecylbenzenesulfonic Acid Complexes in Aqueous Solutions. J. Mol. Liq. 2020, 308, 113045. [Google Scholar] [CrossRef]
- Cabane, B.; Duplessix, R. Decoration of Semidilute Polymer Solutions with Surfactant Micelles. J. Phys. 1987, 48, 651–662. [Google Scholar] [CrossRef]
- Cabane, B. Structure of Some Polymer-Detergent Aggregates in Water. J. Phys. Chem. 1977, 81, 1639–1645. [Google Scholar] [CrossRef]
- Chari, K.; Kowalczyk, J.; Lal, J. Conformation of Poly(Ethylene Oxide) in Polymer−Surfactant Aggregates. J. Phys. Chem. B 2004, 108, 2857–2861. [Google Scholar] [CrossRef]
- Wang, G.; Olofsson, G. Titration Calorimetric Study of the Interaction between Ionic Surfactants and Uncharged Polymers in Aqueous Solution. J. Phys. Chem. B 1998, 102, 9276–9283. [Google Scholar] [CrossRef]
- Magny, B.; Iliopoulos, I.; Zana, R.; Audebert, R. Mixed Micelles Formed by Cationic Surfactants and Anionic Hydrophobically Modified Polyelectrolytes. Langmuir 2002, 10, 3180–3187. [Google Scholar] [CrossRef]
- Del Sorbo, G.R.; Cristiglio, V.; Clemens, D.; Hoffmann, I.; Schneck, E. Influence of the Surfactant Tail Length on the Viscosity of Oppositely Charged Polyelectrolyte/Surfactant Complexes. Macromolecules 2021, 54, 2529–2540. [Google Scholar] [CrossRef]
- Nakamura, K.; Shikata, T. Formation and Physicochemical Features of Hybrid Threadlike Micelles in Aqueous Solution. ChemPhysChem 2007, 8, 2568–2574. [Google Scholar] [CrossRef]
- Thuresson, K.; Söderman, O.; Hansson, P.; Wang, G. Binding of SDS to Ethyl(Hydroxyethyl)Cellulose. Effect of Hydrophobic Modification of the Polymer. J. Phys. Chem. 1996, 100, 4909–4918. [Google Scholar] [CrossRef]
- Wittgren, B.; Stefansson, M.; Porsch, B. Interactions between Sodium Dodecyl Sulphate and Non-Ionic Cellulose Derivatives Studied by Size Exclusion Chromatography with Online Multi-Angle Light Scattering and Refractometric Detection. J. Chromatogr. A 2005, 1082, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R. Polymer—Surfactant Interactions. In New Horizons: Detergents for the New Millennium Conference Invited Papers; American Oil Chemisits Society and Consumer Specialty Products Association: Fort Myers, FL, USA, 2001. [Google Scholar]
- Nakamura, K.; Shikata, T. Viscoelastic Behavior of Aqueous Hybrid Threadlike Micellar Solution. Macromolecules 2004, 37, 8381–8388. [Google Scholar] [CrossRef]
- Khan, N.; Brettmann, B. Intermolecular Interactions in Polyelectrolyte and Surfactant Complexes in Solution. Polymers 2018, 11, 51. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamanaka, K.; Shikata, T. Hybrid Threadlike Micelle Formation between a Surfactant and Polymer in Aqueous Solution. Langmuir 2003, 19, 8654–8660. [Google Scholar] [CrossRef]
- Kuntz, D.M.; Walker, L.M. Solution Behavior of Rod-Like Polyelectrolyte-Surfactant Aggregates Polymerized from Wormlike Micelles. J. Phys. Chem. B 2007, 111, 6417–6424. [Google Scholar] [CrossRef]
- Diehl, A.; Kuhn, P.S. Effect of Monovalent Salt on the Conformation of Polyelectrolyte-Surfactant Complexes. Phys. Rev. E 2009, 79, 011805. [Google Scholar] [CrossRef]
- Chen, S. Conformation and Solubility of Poly(Ethylene Oxide) in Polymer-Surfactant Complex at High-Temperature and High-Salinity Conditions. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 581, 123811. [Google Scholar] [CrossRef]
- Kwiatkowski, A.L.; Molchanov, V.S.; Kuklin, A.I.; Orekhov, A.S.; Arkharova, N.A.; Philippova, O.E. Structural Transformations of Charged Spherical Surfactant Micelles upon Solubilization of Water-Insoluble Polymer Chains in Salt-Free Aqueous Solutions. J. Mol. Liq. 2022, 347, 118326. [Google Scholar] [CrossRef]
- Kwiatkowski, A.L.; Sharma, H.; Molchanov, V.S.; Orekhov, A.S.; Vasiliev, A.L.; Dormidontova, E.E.; Philippova, O.E. Wormlike Surfactant Micelles with Embedded Polymer Chains. Macromolecules 2017, 50, 7299–7308. [Google Scholar] [CrossRef]
- Iancu, C.V.; Tivol, W.F.; Schooler, J.B.; Dias, D.P.; Henderson, G.P.; Murphy, G.E.; Wright, E.R.; Li, Z.; Yu, Z.; Briegel, A.; et al. Electron Cryotomography Sample Preparation Using the Vitrobot. Nat. Protoc. 2007, 1, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Tegunov, D.; Cramer, P. Real-Time Cryo-Electron Microscopy Data Preprocessing with Warp. Nat. Methods 2019, 16, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Kuklin, A.I.; Soloviov, D.V.; Rogachev, A.V.; Utrobin, P.K.; Kovalev, Y.S.; Balasoiu, M.; Ivankov, O.I.; Sirotin, A.P.; Murugova, T.N.; Petukhova, T.B.; et al. New Opportunities Provided by Modernized Small-Angle Neutron Scattering Two-Detector System Instrument (YuMO). J. Phys. Conf. Ser. 2011, 291, 1–7. [Google Scholar] [CrossRef]
- Soloviev, A.G.; Solovjeva, T.M.; Ivankov, O.I.; Soloviov, D.V.; Rogachev, A.V.; Kuklin, A.I. SAS Program for Two-Detector System: Seamless Curve from Both Detectors. J. Phys. Conf. Ser. 2017, 848, 012020. [Google Scholar] [CrossRef]
- Korchagina, E.V.; Philippova, O.E. Multichain Aggregates in Dilute Solutions of Associating Polyelectrolyte Keeping a Constant Size at the Increase in the Chain Length of Individual Macromolecules. Biomacromolecules 2010, 11, 3457–3466. [Google Scholar] [CrossRef]
- González, Y.I.; Kaler, E.W. Cryo-TEM Studies of Worm-like Micellar Solutions. Curr. Opin. Colloid Interface Sci. 2005, 10, 256–260. [Google Scholar] [CrossRef]
- Lin, Z. Branched Worm-like Micelles and Their Networks. Langmuir 1996, 12, 1729–1737. [Google Scholar] [CrossRef]
- Almgren, M.; Edwards, K.; Karlsson, G. Cryo Transmission Electron Microscopy of Liposomes and Related Structures. Colloids Surf. A Physicochem. Eng. Asp. 2000, 174, 3–21. [Google Scholar] [CrossRef]
- Croce, V.; Cosgrove, T.; Maitland, G.; Hughes, T. Rheology, Cryogenic Transmission Electron Spectroscopy, and Small-Angle Neutron Scattering of Highly Viscoelastic Wormlike Micellar Solutions. Langmuir 2003, 19, 8536–8541. [Google Scholar] [CrossRef]
- Ospennikov, A.S.; Gavrilov, A.A.; Artykulnyi, O.P.; Kuklin, A.I.; Novikov, V.V.; Shibaev, A.V.; Philippova, O.E. Transformations of Wormlike Surfactant Micelles Induced by a Water-Soluble Monomer. J. Colloid Interface Sci. 2021, 602, 590–601. [Google Scholar] [CrossRef]
- Chodankar, S.; Aswal, V.K.; Hassan, P.A.; Wagh, A.G. Structure of Protein-Surfactant Complexes as Studied by Small-Angle Neutron Scattering and Dynamic Light Scattering. Phys. B 2007, 398, 112–117. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Y. Applications of Small-Angle X-Ray Scattering/Small-Angle Neutron Scattering and Cryogenic Transmission Electron Microscopy to Understand Self-Assembly of Surfactants. Curr. Opin. Colloid Interface Sci. 2019, 42, 1–16. [Google Scholar] [CrossRef]
- Sommer, C.; Pedersen, J.S.; Egelhaaf, S.U.; Cannavacciuolo, L.; Kohlbrecher, J.; Schurtenberger, P. Wormlike Micelles as “Equilibrium Polyelectrolytes”: Light and Neutron Scattering Experiments. Langmuir 2002, 18, 2495–2505. [Google Scholar] [CrossRef]
- Svergun, D.; Feigin, L. Structure Analysis by SANS and SAXS; Plenum Press: New York, NY, USA; London, UK, 1987. [Google Scholar]
- Zaroslov, Y.D.; Gordeliy, V.I.; Kuklin, A.I.; Islamov, A.H.; Philippova, O.E.; Khokhlov, A.R.; Wegner, G. Self-Assembly of Polyelectrolyte Rods in Polymer Gel and in Solution: Small-Angle Neutron Scattering Study. Macromolecules 2002, 35, 4466–4471. [Google Scholar] [CrossRef]
- Dreiss, C.A. Wormlike Micelles: Where Do We Stand? Recent Developments, Linear Rheology and Scattering Techniques. Soft Matter 2007, 3, 956–970. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; Agrawal, N.R.; Raghavan, S.R. Wormlike Micelles versus Water-Soluble Polymers as Rheology-Modifiers: Similarities and Differences. Phys. Chem. Chem. Phys. 2017, 19, 24458–24466. [Google Scholar] [CrossRef] [PubMed]
- Philippova, O.E.; Molchanov, V.S. Polymer-Surfactant Networks Highly Responsive to Hydrocarbons. Macromol. Symp. 2010, 291–292, 137–143. [Google Scholar] [CrossRef]
- Kwiatkowski, A.L.; Molchanov, V.S.; Sharma, H.; Kuklin, A.I.; Dormidontova, E.E.; Philippova, O.E. Growth of Wormlike Micelles of Surfactant Induced by Embedded Polymer: Role of Polymer Chain Length. Soft Matter 2018, 14, 4792–4804. [Google Scholar] [CrossRef]
- Beaucage, G. Determination of Branch Fraction and Minimum Dimension of Mass-Fractal Aggregates. Phys. Rev. E 2004, 70, 10. [Google Scholar] [CrossRef]
- Beaucage, G.; Rane, S.; Sukumaran, S.; Satkowski, M.M.; Schechtman, L.A.; Doi, Y. Persistence Length of Isotactic Poly(Hydroxy Butyrate). Macromolecules 1997, 30, 4158–4162. [Google Scholar] [CrossRef]
- Melnichenko, Y.B.; Wignall, G.D. Small-Angle Neutron Scattering in Materials Science: Recent Practical Applications. J. Appl. Phys. 2007, 102, 021101. [Google Scholar] [CrossRef]
- Schaefer, D.W. Polymers, Fractals, and Ceramic Materials. Science 1989, 4, 1023–1027. [Google Scholar] [CrossRef]
- Ramachandran, R.; Beaucage, G.; Kulkarni, A.S.; McFaddin, D.; Merrick-Mack, J.; Galiatsatos, V. Persistence Length of Short-Chain Branched Polyethylene. Macromolecules 2008, 41, 9802–9806. [Google Scholar] [CrossRef]
- Beaucage, G. Toward Resolution of Ambiguity for the Unfolded State. Biophys. J. 2008, 95, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Stepanek, M.; Matejicek, P.; Prochazka, K.; Filippov, S.K.; Angelov, B.; Mountrichas, G.; Pispas, S. Polyelectrolyte-Surfactant Complexes Formed by Poly-Block-Poly ( Ethylene Oxide ) and Sodium Dodecyl Sulfate in Aqueous Solutions. Langmuir 2011, 27, 5275–5281. [Google Scholar] [CrossRef]
- Buchold, P.; Schweins, R.; Di, Z.; Gradzielski, M. Structural Behaviour of Sodium Hyaluronate in Concentrated Oppositely Charged Surfactant Solutions. Soft Matter 2017, 13, 2253–2263. [Google Scholar] [CrossRef]
- Santos, S.F.; Zanette, D.; Fischer, H.; Itri, R. A Systematic Study of Bovine Serum Albumin (BSA) and Sodium Dodecyl Sulfate (SDS) Interactions by Surface Tension and Small Angle X-Ray Scattering. J. Colloid Interface Sci. 2003, 262, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Abdullahi, W.; Dalgliesh, R.; Crossman, M.; Griffiths, P.C. Charge Modification as a Mechanism for Tunable Properties in Polymer–Surfactant Complexes. Polymers 2021, 13, 2800. [Google Scholar] [CrossRef]
- Schurtenberger, P.; Magid, L.J.; King, S.M.; Lindner, P. Cylindrical Structure and Flexibility of Polymerlike Lecithin Reverse Micelles. J. Phys. Chem. 1991, 95, 4173–4176. [Google Scholar] [CrossRef]
- Boue, F.; Lindner, P. Semi-Dilute Polymer Solutions under Shear. Eur. Lett. 1994, 25, 421–427. [Google Scholar] [CrossRef]
- Groot, R.D. Electrostatic Interactions in Dissipative Particle Dynamics-Simulation of Polyelectrolytes and Anionic Surfactants. J. Chem. Phys. 2003, 118, 11265–11277. [Google Scholar] [CrossRef]
- Landry, J.M.; Marangoni, D.G.; Arden, D.A.; MacLennan, I.J.; Kwak, J.C.T. A 1D-and 2D-NMR Study of an Anionic Surfactant/Neutral Polymer Complex. J. Surfactants Deterg. 2009, 12, 155–164. [Google Scholar] [CrossRef]
- Tzeng, J.K.; Hou, S.S. Interactions between Poly(N-Vinylformamide) and Sodium Dodecyl Sulfate as Studied by Fluorescence and Two-Dimensional NOE NMR Spectroscopy. Macromolecules 2008, 41, 1281–1288. [Google Scholar] [CrossRef]
- Ortona, O.; Roscigno, P.; Paduano, L.; Asaro, F.; Pellizer, G. Complex Formation between Poly(Vinylpyrrolidone) and Sodium Decyl Sulfate Studied through NMR. Langmuir 2003, 19, 9638–9644. [Google Scholar] [CrossRef]
- Li, J.; Ngai, T.; Wu, C. The slow relaxation mode: From solutions to gel networks. Polym. J. 2010, 42, 609–625. [Google Scholar] [CrossRef]
- Hassan, P.A.; Rana, S.; Verma, G. Making sense of brownian motion: Colloid characterization by dynamic light scattering. Langmuir 2015, 31, 3–12. [Google Scholar] [CrossRef]
- VMolchanov, S.; Philippova, O.E.; Khokhlov, A.R.; Kovalev, Y.A.; Kuklin, A.I. Self-assembled networks highly responsive to hydrocarbons. Langmuir 2007, 23, 105–111. [Google Scholar] [CrossRef]

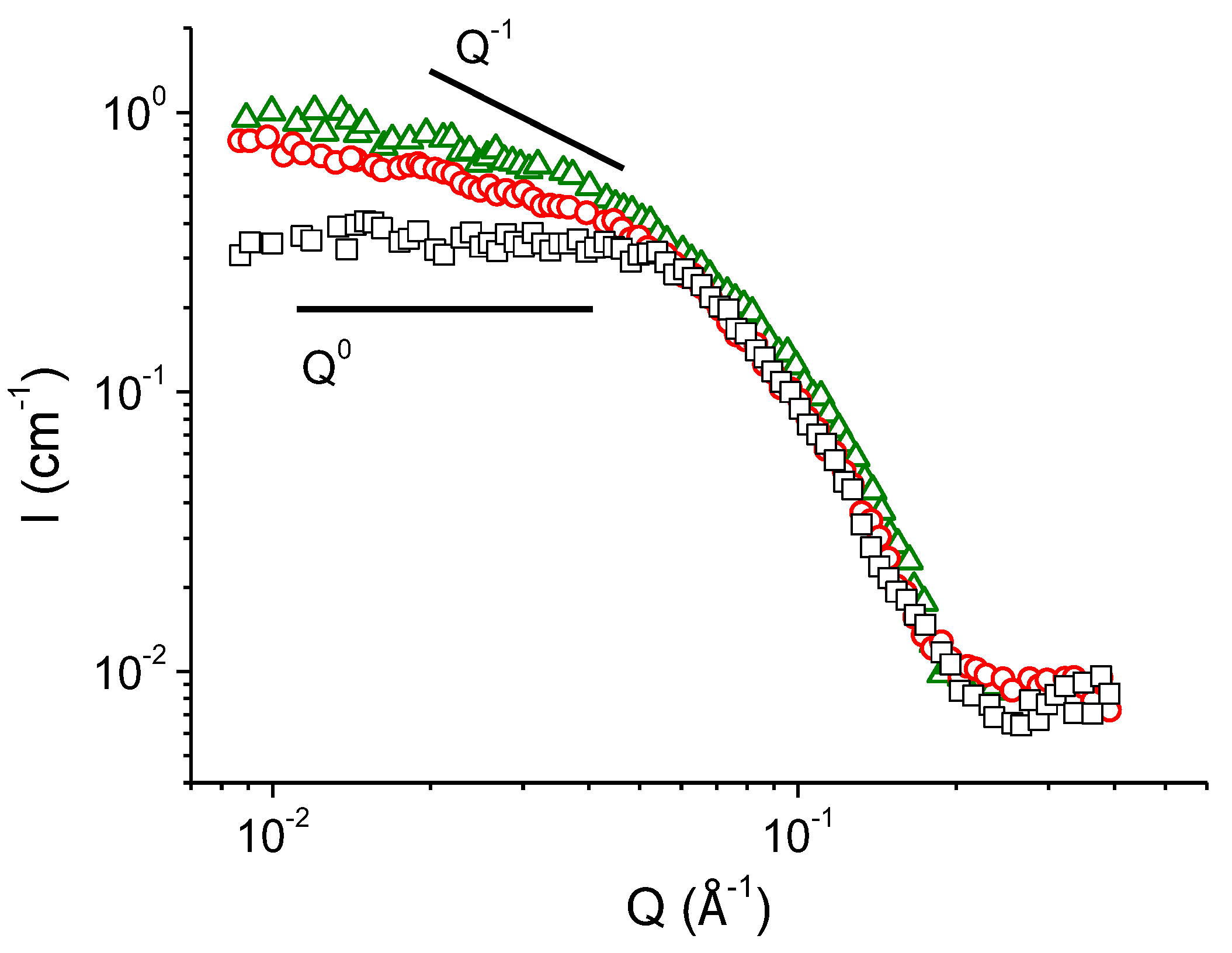


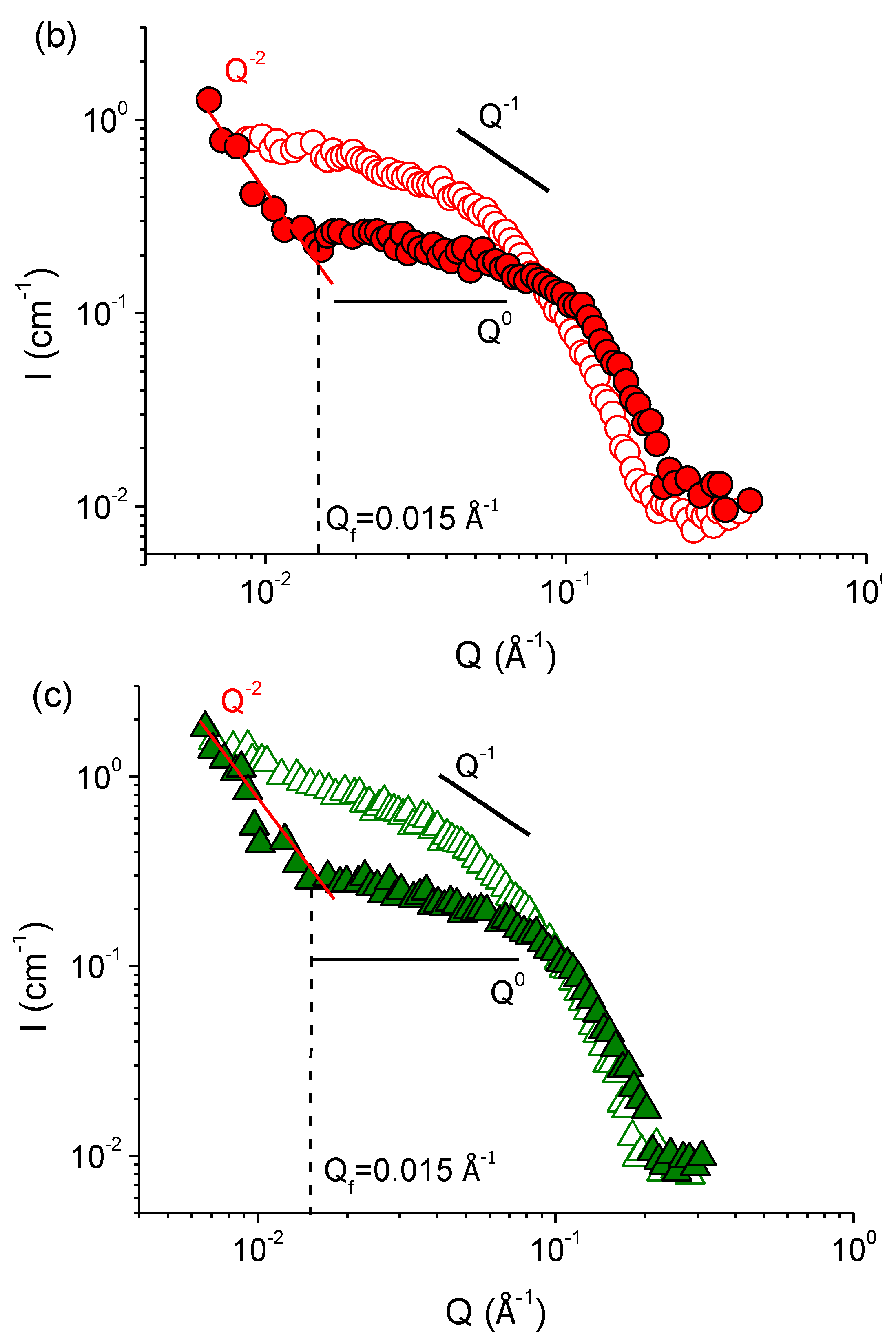
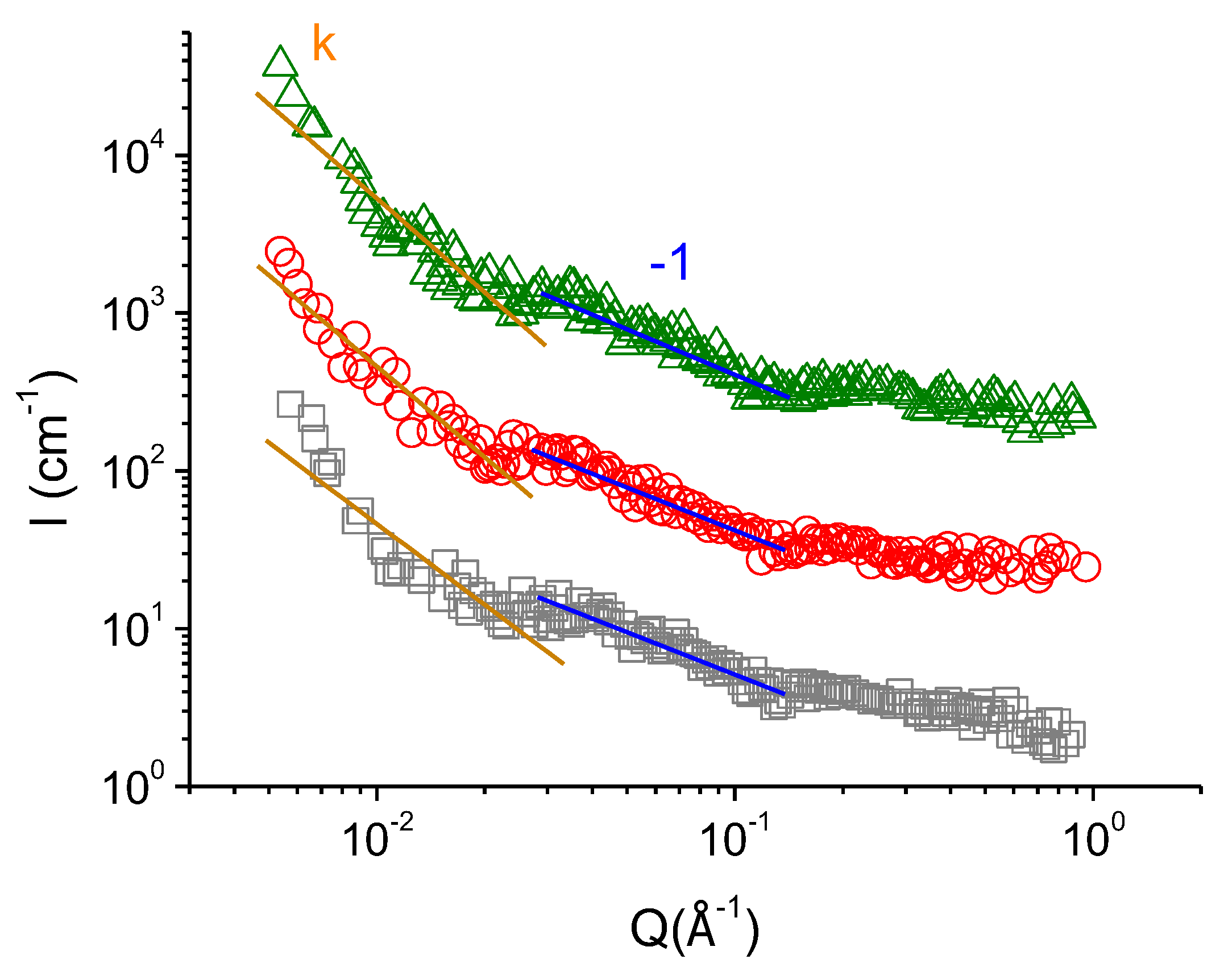
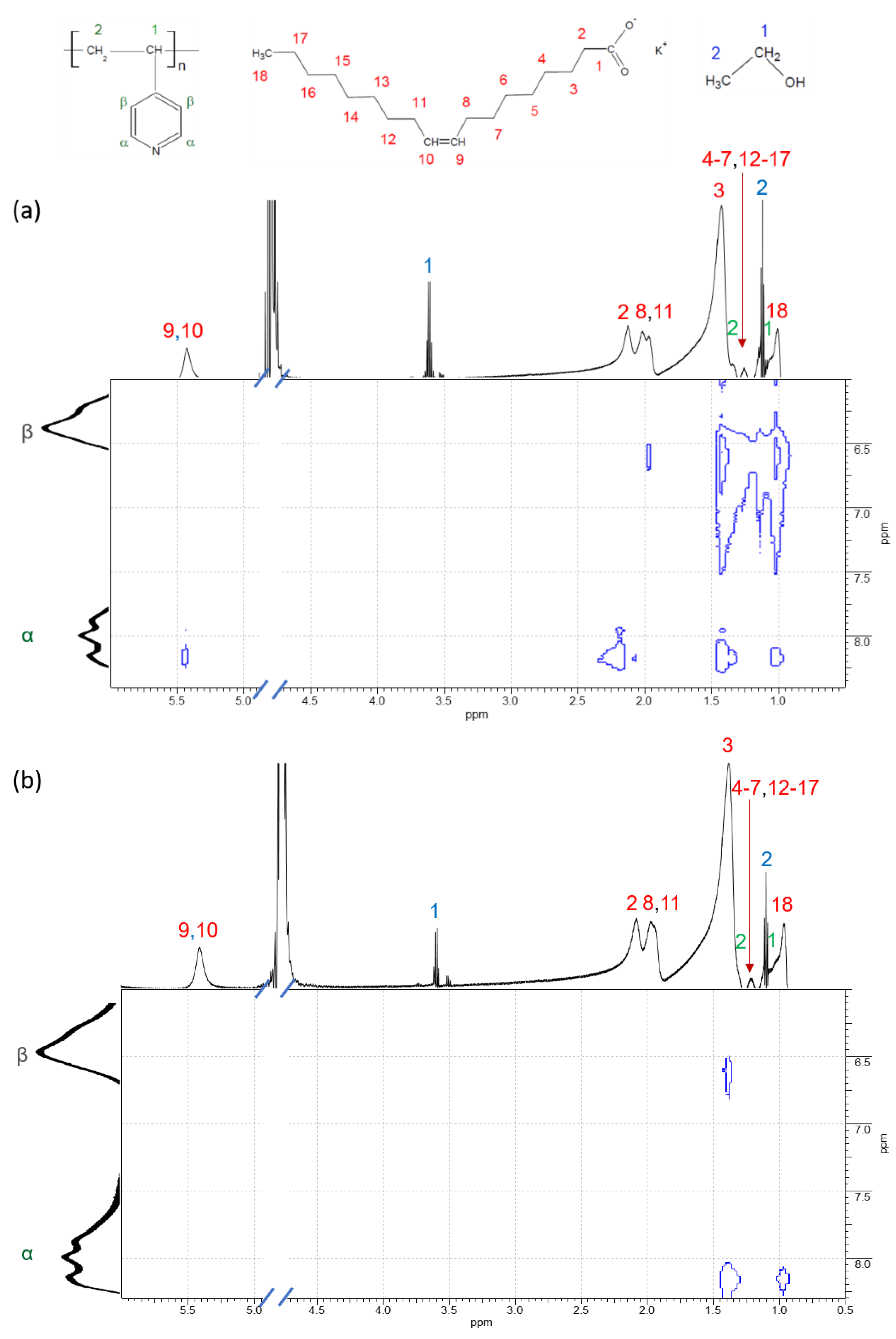

| Concentration of KCl, mM | Fractal Dimension df | Characteristic Scale of the Mass-Fractal Scattering ξf, nm |
|---|---|---|
| 67 | 1.1 ± 0.1 | 37 |
| 110 | 2.2 ± 0.2 | 40 |
| 160 | 2.2 ± 0.2 | 40 |
| Concentration of KCl, mM | k |
|---|---|
| 67 | 1.7 ± 0.1 |
| 110 | 1.9 ± 0.1 |
| 160 | 2.0 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowski, A.L.; Molchanov, V.S.; Kuklin, A.I.; Chesnokov, Y.M.; Philippova, O.E. Salt-Induced Transformations of Hybrid Micelles Formed by Anionic Surfactant and Poly(4-vinylpyridine). Polymers 2022, 14, 5086. https://doi.org/10.3390/polym14235086
Kwiatkowski AL, Molchanov VS, Kuklin AI, Chesnokov YM, Philippova OE. Salt-Induced Transformations of Hybrid Micelles Formed by Anionic Surfactant and Poly(4-vinylpyridine). Polymers. 2022; 14(23):5086. https://doi.org/10.3390/polym14235086
Chicago/Turabian StyleKwiatkowski, Alexander L., Vyacheslav S. Molchanov, Alexander I. Kuklin, Yuri M. Chesnokov, and Olga E. Philippova. 2022. "Salt-Induced Transformations of Hybrid Micelles Formed by Anionic Surfactant and Poly(4-vinylpyridine)" Polymers 14, no. 23: 5086. https://doi.org/10.3390/polym14235086
APA StyleKwiatkowski, A. L., Molchanov, V. S., Kuklin, A. I., Chesnokov, Y. M., & Philippova, O. E. (2022). Salt-Induced Transformations of Hybrid Micelles Formed by Anionic Surfactant and Poly(4-vinylpyridine). Polymers, 14(23), 5086. https://doi.org/10.3390/polym14235086







