Antibacterial Properties of Honey Nanocomposite Fibrous Meshes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Honey
2.1.2. Chemical Analysis
2.1.3. Bacteria Strains and Media
2.1.4. Polymer Solution Preparation
2.2. Methods
2.2.1. Chemical Analysis
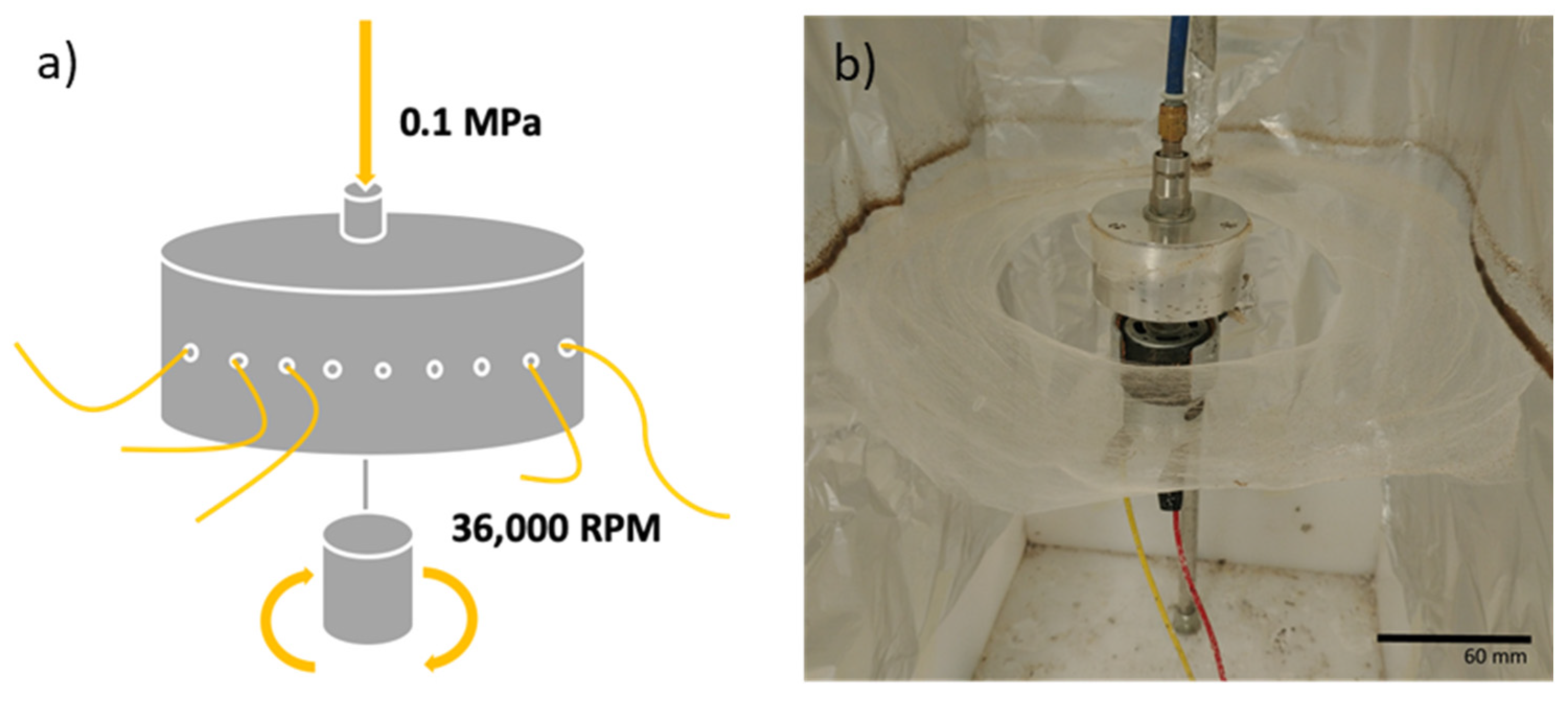
2.2.2. Mesh Production
2.2.3. Characterisation
Viscosity
Surface Tension
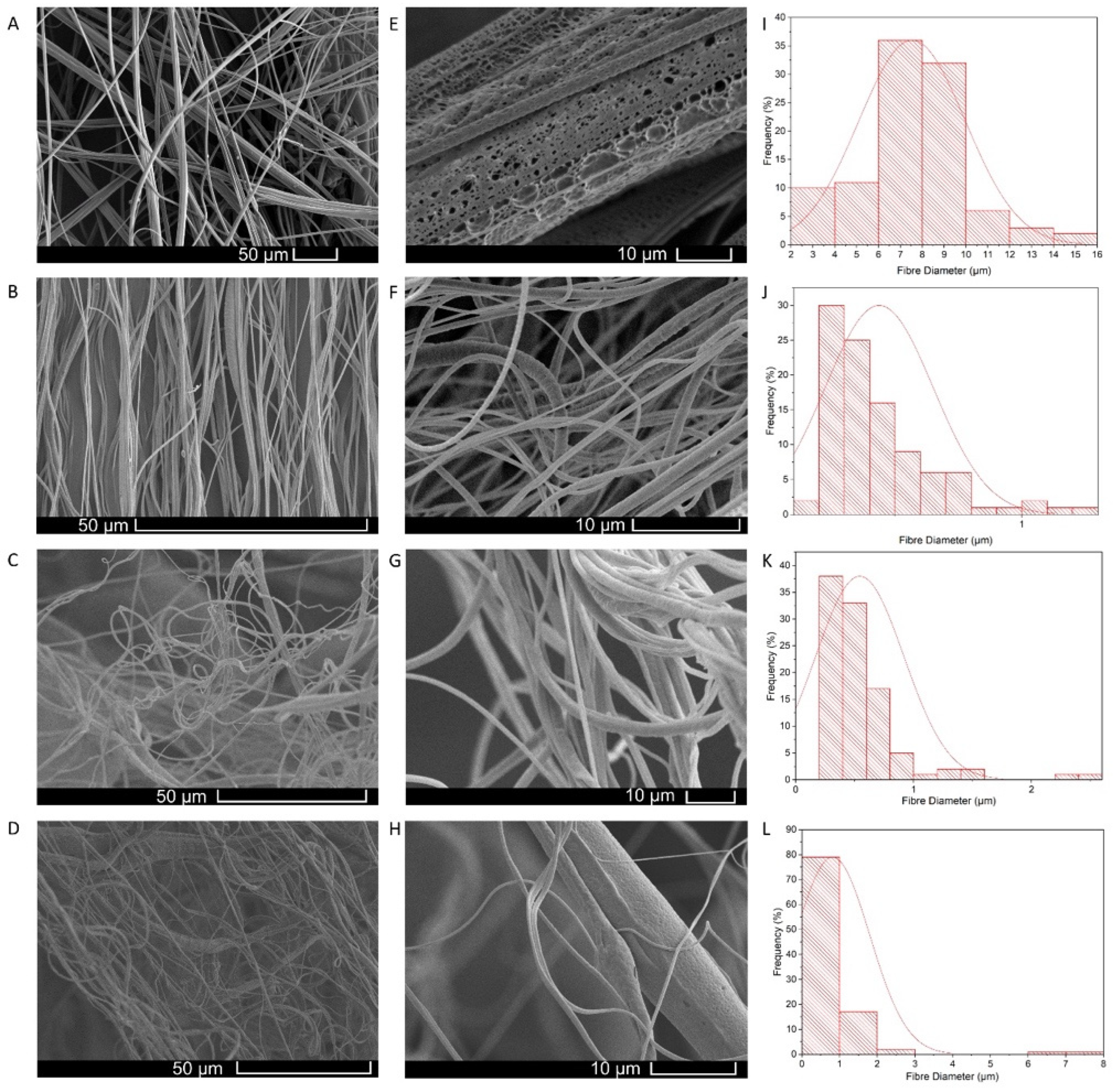
Fibre Morphology
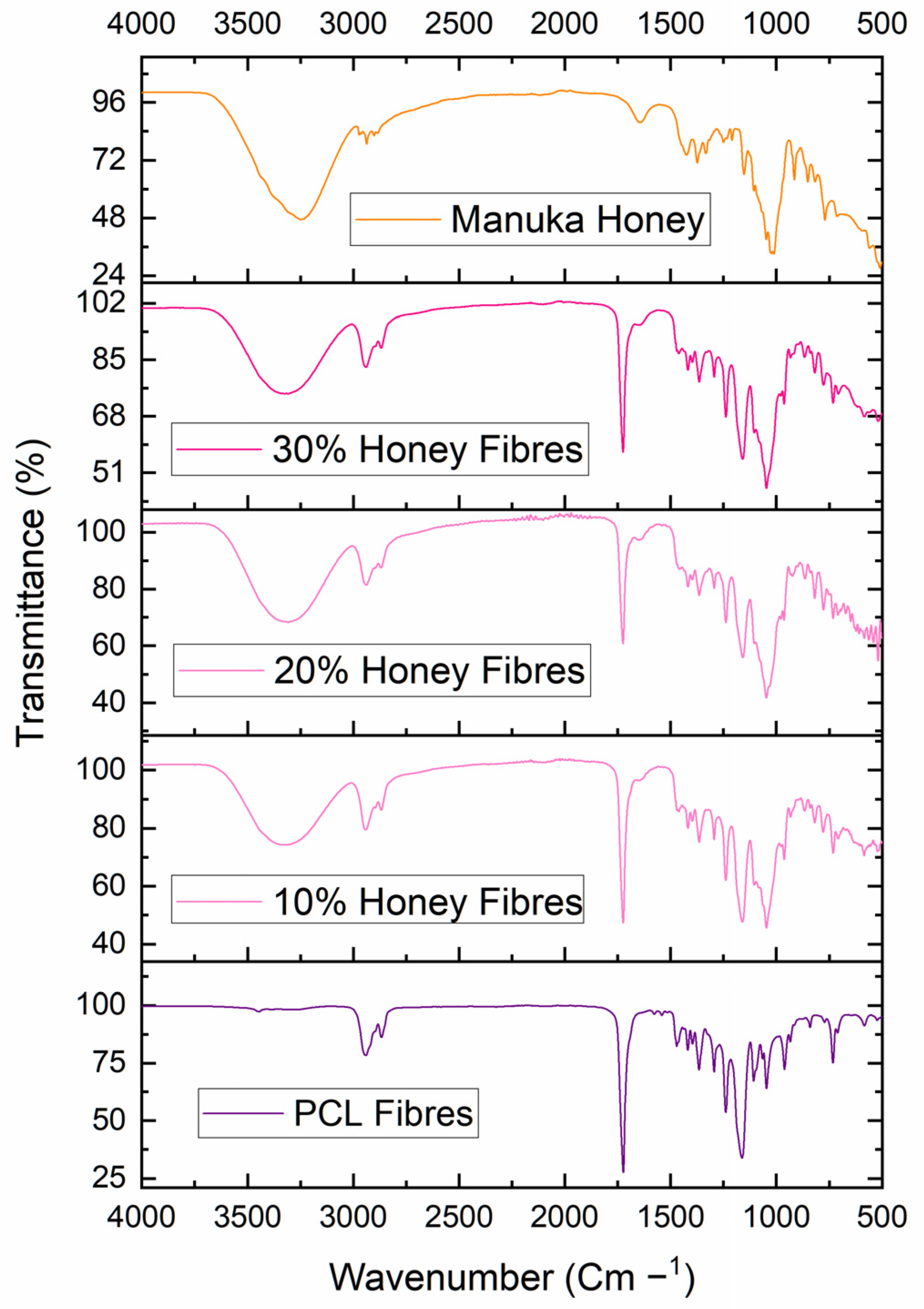
Fourier Transform Infrared Spectroscopy
2.2.4. Antibacterial Activity of Honey and Honey Fibre Meshes
2.2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis
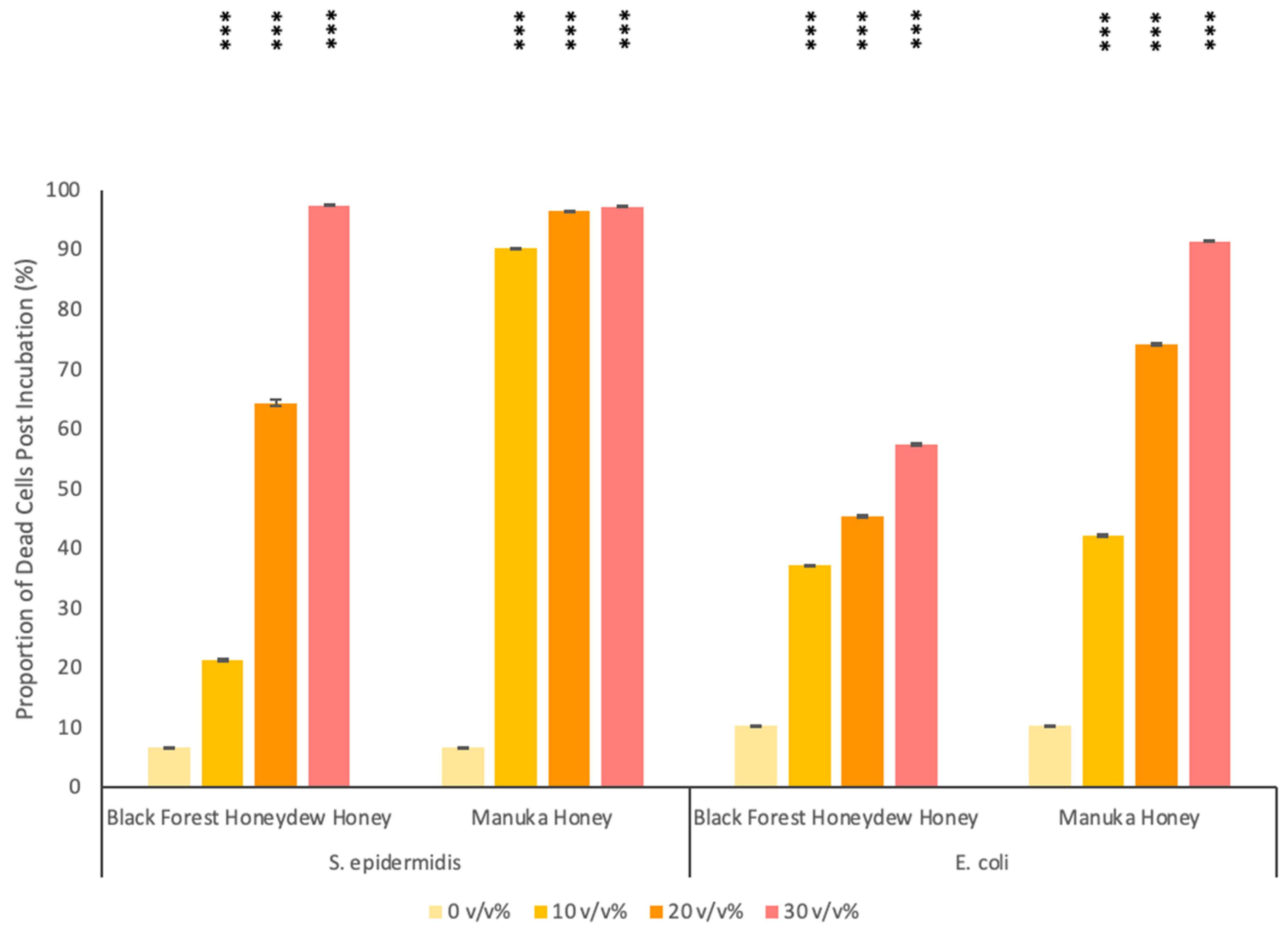
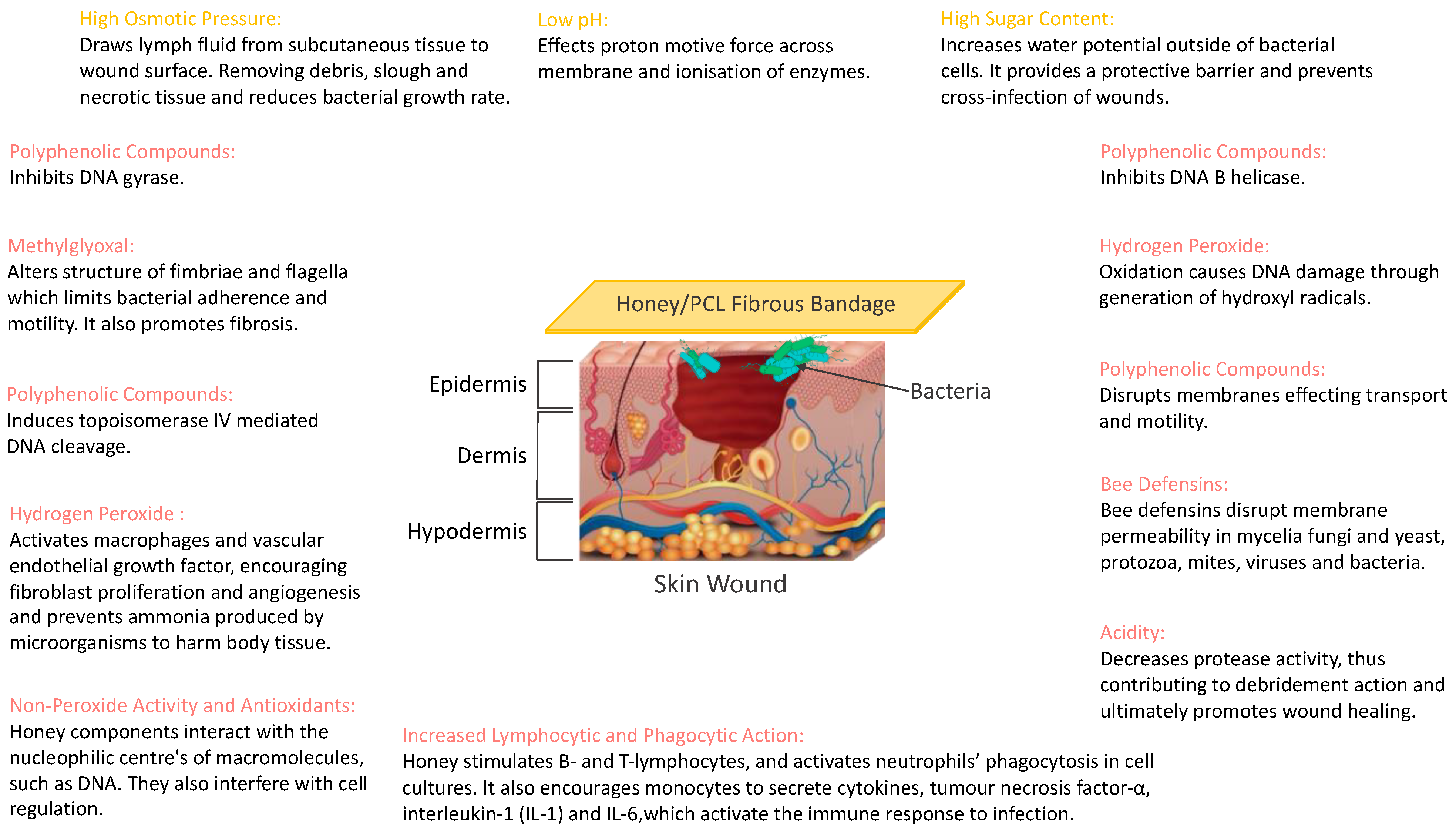
3.2. Antibacterial Activity of Honey
3.3. Honey Composite Fibrous Meshes
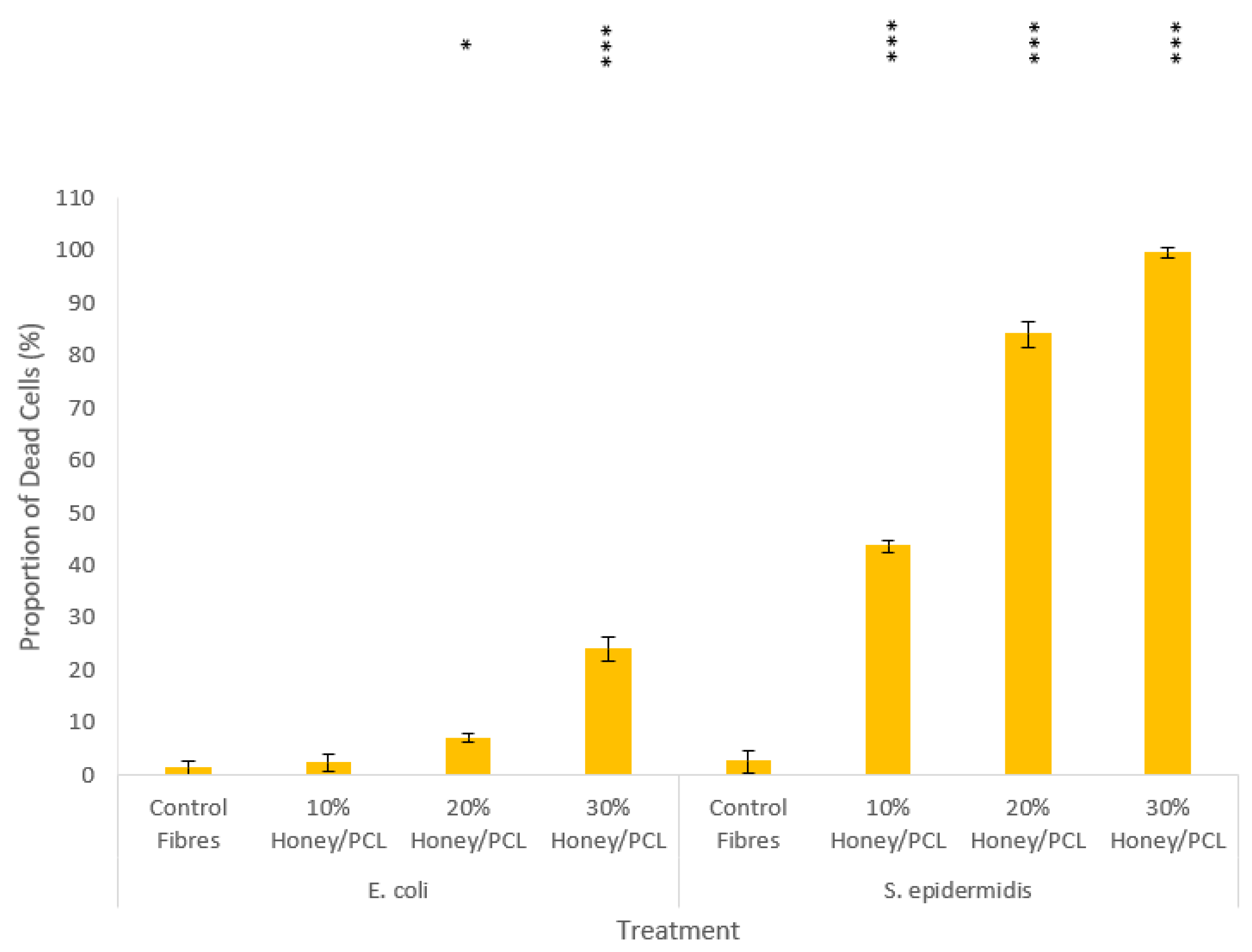
3.4. Antibacterial Studies of Honey Composite Fibres
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Posnett, J.; Franks, P.J. The Burden of Chronic Wounds in the UK. Nurs. Times 2008, 104, 2. [Google Scholar]
- Yang, L.; Liu, Y.; Wu, H.; Høiby, N.; Molin, S.; Song, Z.j. Current understanding of multi-species biofilms. Int. J. Oral Sci. 2011, 3, 74. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef]
- Coutts, P.; Sibbald, R.G. The effect of a silver-containing Hydrofiber dressing on superficial wound bed and bacterial balance of chronic wounds. Int. Wound J. 2005, 2, 348–356. [Google Scholar] [CrossRef]
- Ousey, K.; McIntosh, C. Topical antimicrobial agents for the treatment of chronic wounds. Br. J. Community Nurs. 2009, 14, S6, S8, S10 passim. [Google Scholar] [CrossRef]
- Boni, B.O.O.; Lamboni, L.; Bakadia, B.M.; Hussein, S.A.; Yang, G. Combining Silk Sericin and Surface Micropatterns in Bacterial Cellulose Dressings to Control Fibrosis and Enhance Wound Healing. Eng. Sci. 2020, 10, 68–77. [Google Scholar] [CrossRef]
- Brako, F.; Luo, C.; Matharu, R.K.; Ciric, L.; Harker, A.; Edirisinghe, M.; Craig, D.Q.M. A Portable Device for the Generation of Drug-Loaded Three-Compartmental Fibers Containing Metronidazole and Iodine for Topical Application. Pharmaceutics 2020, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Bowler, P.G.; Walker, M.; Parsons, D. Controlling wound bioburden with a novel silver-containing Hydrofiber dressing. Wound Repair Regen. 2004, 12, 288–294. [Google Scholar] [CrossRef]
- Cutting, K.; White, R.; Edmonds, M. The safety and efficacy of dressings with silver—Addressing clinical concerns. Int. Wound J. 2007, 4, 177–184. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J.; Dardik, R.; Eidelman, A.I.; Lavie, Y.; Grunfeld, Y.; Ikher, S.; Huszar, M.; Zatcoff, R.C.; Marikovsky, M. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. Wound Repair Regen. 2010, 18, 266–275. [Google Scholar] [CrossRef]
- Lansdown, A.B.; Mirastschijski, U.; Stubbs, N.; Scanlon, E.; Agren, M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007, 15, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Matharu, R.K.; Ciric, L.; Edirisinghe, M. Nanocomposites: Suitable alternatives as antimicrobial agents. Nanotechnology 2018, 29, 282001. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Honey, Wound Repair and Regenerative Medicine. J. Funct. Biomater. 2018, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jasim, A.; Zhao, W.; Fu, L.; Ullah, M.W.; Shi, Z.; Yang, G. Fabrication of pH-electroactive Bacterial Cellulose/Polyaniline Hydrogel for the Development of a Controlled Drug Release System. ES Mater. Manuf. 2018, 1, 41–49. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, X.; Walcott, M.P.; Lin, H. Applications of Cellulose Nanocrystals: A Review. Eng. Sci. 2018, 2, 4–16. [Google Scholar] [CrossRef]
- Nair, S.S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. Hydrogels Prepared from Cross-Linked Nanofibrillated Cellulose. ACS Sustain. Chem. Eng. 2014, 2, 772–780. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver nanoparticle/bacterial cellulose gel membranes for antibacterial wound dressing: Investigation in vitro and in vivo. Biomed. Mater. 2014, 9, 035005. [Google Scholar] [CrossRef]
- Rujitanaroj, P.-O.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Shalumon, K.; Anulekha, K.; Nair, S.V.; Nair, S.; Chennazhi, K.; Jayakumar, R. Sodium alginate/poly (vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef]
- Kumar, P.; Lakshmanan, V.-K.; Biswas, R.; Nair, S.V.; Jayakumar, R. Synthesis and biological evaluation of chitin hydrogel/nano ZnO composite bandage as antibacterial wound dressing. J. Biomed. Nanotechnol. 2012, 8, 891–900. [Google Scholar] [CrossRef]
- Li, Q.; Lu, F.; Zhou, G.; Yu, K.; Lu, B.; Xiao, Y.; Dai, F.; Wu, D.; Lan, G. Silver inlaid with gold nanoparticle/chitosan wound dressing enhances antibacterial activity and porosity, and promotes wound healing. Biomacromolecules 2017, 18, 3766–3775. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Ye, H.; Shang, S.; Xiong, Q.; Yu, K.; Li, Q.; Xiao, Y.; Dai, F.; Lan, G. Novel wound dressing with chitosan gold nanoparticles capped with a small molecule for effective treatment of multiantibiotic-resistant bacterial infections. Nanotechnology 2018, 29, 425603. [Google Scholar] [CrossRef] [PubMed]
- Baghaie, S.; Khorasani, M.T.; Zarrabi, A.; Moshtaghian, J. Wound healing properties of PVA/starch/chitosan hydrogel membranes with nano Zinc oxide as antibacterial wound dressing material. J. Biomater. Sci. Polym. Ed. 2017, 28, 2220–2241. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. Silver in Healthcare: Its Antimicrobial Efficacy and Safety in Use; Royal Society of Chemistry: Cambridge, UK, 2010; pp. 164–170. [Google Scholar]
- Ahmed, J.; Altun, E.; Aydogdu, M.O.; Gunduz, O.; Kerai, L.; Ren, G.; Edirisinghe, M. Anti-fungal bandages containing cinnamon extract. Int. Wound J. 2019, 16, 730–736. [Google Scholar] [CrossRef]
- Zumla, A.; Lulat, A. Honey—A remedy rediscovered. J. R. Soc. Med. 1989, 87, 384–385. [Google Scholar] [CrossRef]
- Al-Jabri, A.A. Honey, milk and antibiotics. Afr. J. Biotechnol. 2005, 4, 7. [Google Scholar]
- Bansal, V.; Medhi, B.; Pandhi, P. Honey—A remedy rediscovered and its therapeutic utility. Kathmandu Univ. Med. J. 2005, 3, 305–309. [Google Scholar]
- Efem, S.E. Clinical observations on the wound healing properties of honey. Br. J. Surg. 1988, 75, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Lusby, P.E.; Coombes, A.; Wilkinson, J.M. Honey: A potent agent for wound healing? J. Wound Ostomy Cont. Nurs. 2002, 29, 295–300. [Google Scholar] [CrossRef]
- Bergman, A.; Yanai, J.; Weiss, J.; Bell, D.; David, M.P. Acceleration of wound healing by topical application of honey. An animal model. Am. J. Surg. 1983, 145, 374–376. [Google Scholar] [CrossRef]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.A. Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. ScientificWorldJournal 2011, 11, 766–787. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Zaker, S.R. Effects of topical application of honey on cutaneous wound healing in rabbits. J. Vet. Med. Ser. A 1998, 45, 181–188. [Google Scholar] [CrossRef]
- Subrahmanyam, M. Topical application of honey in treatment of burns. Br. J. Surg. 1991, 78, 497–498. [Google Scholar] [CrossRef]
- Carreck, N.L. Special issue: Honey. J. Apic. Res. 2018, 57, 1–4. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, D.; Beazley, J.; Ostapowicz, F. Radical operation for carcinoma of the vulva. A new approach to wound healing. J. Obstet. Gynaecol. Br. Commonw. 1970, 77, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Ball, D.W. The Chemical Composition of Honey. J. Chem. Educ. 2007, 84, 1643. [Google Scholar] [CrossRef]
- Bizerra, F.C.; Da Silva, P.I., Jr.; Hayashi, M.A. Exploring the antibacterial properties of honey and its potential. Front. Microbiol. 2012, 3, 398. [Google Scholar] [CrossRef] [PubMed]
- Alsomal, N.A.; Coley, K.E.; Molan, P.C.; Hancock, B.M. Susceptibility of Helicobacter-Pylori to the Antibacterial Activity of Manuka Honey. J. R. Soc. Med. 1994, 87, 9–12. [Google Scholar]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef]
- Kumar, K.P.S.; Bhowmik, D.; Chiranjib Biswajit Chandira, M.R. Medicinal uses and health benefits of Honey: An Overview. J. Chem. Pharm. Res. 2010, 2, 385–395. [Google Scholar]
- Chirife, J.; Scarmato, G.; Herszage, L. Scientific basis for use of granulated sugar in treatment of infected wounds. Lancet 1982, 319, 560–561. [Google Scholar] [CrossRef]
- Khan, F.R.; Abadin, Z.U.; Rauf, N. Honey: Nutritional and medicinal value. Int. J. Clin. Pract. 2007, 61, 1705–1707. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G.T.; Cowman, S.; Conroy, R.M. The impact of Manuka honey dressings on the surface pH of chronic wounds. Int. Wound J. 2008, 5, 185–194, Reprinted in Int. Wound J. 2014, 11, 342. [Google Scholar] [CrossRef]
- Calvin, M. Cutaneous wound repair. Wounds 1998, 10, 12–32. [Google Scholar]
- Yoo, S.K.; Huttenlocher, A. Innate Immunity: Wounds Burst H2O2 Signals to Leukocytes. Curr. Biol. 2009, 19, R553–R555. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Hunt, T.K.; Hussain, M.Z. Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release. Am. J. Physiol.-Heart Circ. Physiol. 2001, 280, H2357–H2363. [Google Scholar] [CrossRef]
- Burdon, R.H. Superoxide and Hydrogen-Peroxide in Relation to Mammalian-Cell Proliferation. Free Radical. Bio. Med. 1995, 18, 775–794. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, T.; Artusa, S.; Offerman, C.; Barnes, L.; McIntosh, A. The Use of 100% Manuka Honey for Moist Wound Management and Promotion of Autolytic Debridement in an Inpatient Population with Wounds of Mixed Etiology. J. Wound Ostomy Cont. 2015, 42, S25. [Google Scholar]
- Santos-Buelga, C.; González-Paramás, A.M. Chemical Composition of Honey. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 43–82. [Google Scholar]
- Khan, S.U.; Anjum, S.I.; Rahman, K.; Ansari, M.J.; Khan, W.U.; Kamal, S.; Khattak, B.; Muhammad, A.; Khan, H.U. Honey: Single food stuff comprises many drugs. Saudi J. Biol. Sci. 2018, 25, 320–325. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef] [PubMed]
- Minden-Birkenmaier, B.A.; Neuhalfen, R.M.; Janowiak, B.E.; Sell, S.A. Preliminary Investigation and Characterization of Electrospun Polycaprolactone and Manuka Honey Scaffolds for Dermal Repair. J. Eng. Fiber Fabr. 2015, 10, 126–138. [Google Scholar] [CrossRef]
- Maleki, H.; Gharehaghaji, A.A.; Dijkstra, P.J. A novel honey-based nanofibrous scaffold for wound dressing application. J. Appl. Polym. Sci. 2013, 127, 4086–4092. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; McBride-Gagyi, S.H.; Janowiak, B.E.; Sell, S.A. A Comparison of Tissue Engineering Scaffolds Incorporated with Manuka Honey of Varying UMF. Biomed. Res. Int. 2017, 2017, 4843065. [Google Scholar] [CrossRef]
- Sarhan, W.A.; Azzazy, H.M.E. High concentration honey chitosan electrospun nanofibers: Biocompatibility and antibacterial effects. Carbohydr. Polym. 2015, 122, 135–143. [Google Scholar] [CrossRef]
- Sarhan, W.A.; Azzazy, H.M.E.; El-Sherbiny, I.M. Honey/Chitosan Nanofiber Wound Dressing Enriched with Allium sativum and Cleome droserifolia: Enhanced Antimicrobial and Wound Healing Activity. ACS Appl. Mater. Interfaces 2016, 8, 6379–6390. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Simsek, M.; Aldemir, S.D.; Kazaroglu, N.M.; Gumusderelioglu, M. Honey-based PET or PET/chitosan fibrous wound dressings: Effect of honey on electrospinning process. J. Biomat. Sci.-Polym. E 2014, 25, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, S.; Edirisinghe, M. Forming of Polymer Nanofibers by a Pressurised Gyration Process. Macromol. Rapid Comm. 2013, 34, 1134–1139. [Google Scholar] [CrossRef]
- Matharu, R.K.; Porwal, H.; Ciric, L.; Edirisinghe, M. The effect of graphene-poly(methyl methacrylate) fibres on microbial growth. Interface Focus 2018, 8, 20170058. [Google Scholar] [CrossRef] [PubMed]
- Matharu, R.K.; Charani, Z.; Ciric, L.; Illangakoon, U.E.; Edirisinghe, M. Antimicrobial activity of tellurium-loaded polymeric fiber meshes. J. Appl. Polym. Sci. 2018, 135, 46368. [Google Scholar] [CrossRef]
- Altun, E.; Aydogdu, M.O.; Koc, F.; Crabbe-Mann, M.; Brako, F.; Kaur-Matharu, R.; Ozen, G.; Kuruca, S.E.; Edirisinghe, U.; Gunduz, O.; et al. Novel Making of Bacterial Cellulose Blended Polymeric Fiber Bandages. Macromol. Mater. Eng. 2018, 303, 1700607. [Google Scholar] [CrossRef]
- Matharu, R.K.; Ciric, L.; Ren, G.; Edirisinghe, M. Comparative Study of the Antimicrobial Effects of Tungsten Nanoparticles and Tungsten Nanocomposite Fibres on Hospital Acquired Bacterial and Viral Pathogens. Nanomaterials 2020, 10, 1017. [Google Scholar] [CrossRef]
- Matharu, R.K.; Tabish, T.A.; Trakoolwilaiwan, T.; Mansfield, J.; Moger, J.; Wu, T.; Lourenço, C.; Chen, B.; Ciric, L.; Parkin, I.P.; et al. Microstructure and antibacterial efficacy of graphene oxide nanocomposite fibres. J. Colloid Interface Sci. 2020, 571, 239–252. [Google Scholar] [CrossRef]
- Matharu, R.K.; Porwal, H.; Chen, B.; Ciric, L.; Edirisinghe, M. Viral filtration using carbon-based materials. Med. Devices Sens. 2020, 3, e10107. [Google Scholar] [CrossRef] [PubMed]
- Mato, I.; Huidobro, J.F.; Simal-Lozano, J.; Sancho, M.T. Rapid determination of nonaromatic organic acids in honey by capillary zone electrophoresis with direct ultraviolet detection. J. Agric. Food Chem. 2006, 54, 1541–1550. [Google Scholar] [CrossRef]
- Boateng, J.; Diunase, K.N. Comparing the Antibacterial and Functional Properties of Cameroonian and Manuka Honeys for Potential Wound Healing-Have We Come Full Cycle in Dealing with Antibiotic Resistance? Molecules 2015, 20, 16068–16084. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Masoura, M.; Passaretti, P.; Overton, T.W.; Lund, P.A.; Gkatzionis, K. Use of a model to understand the synergies underlying the antibacterial mechanism of H2O2-producing honeys. Sci. Rep. 2020, 10, 17692. [Google Scholar] [CrossRef]
- Bang, L.M.; Buntting, C.; Molan, P. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J. Altern. Complement. Med. 2003, 9, 267–273. [Google Scholar] [CrossRef]
- Suto, M.; Kawashima, H.; Nakamura, Y. Determination of organic acids in honey by liquid chromatography with tandem mass spectrometry. Food Anal. Methods 2020, 13, 2249–2257. [Google Scholar] [CrossRef]
- Basualdo, C.; Sgroy, V.; Finola, M.S.; Marioli, J.M. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Vet. Microbiol. 2007, 124, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.T.; Rahman, R.A.; Gan, S.H.; Halim, A.S.; Hassan, S.A.; Sulaiman, S.A.; Bs, K.-K. The antibacterial properties of Malaysian tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complement. Altern. Med. 2009, 9, 34. [Google Scholar] [CrossRef]
- White, J.W.; Subers, M.H.; Schepartz, A.I. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta-Spec. Sect. Enzymol. Subj. 1963, 73, 57–70. [Google Scholar] [CrossRef]
- Bucekova, M.; Valachova, I.; Kohutova, L.; Prochazka, E.; Klaudiny, J.; Majtan, J. Honeybee glucose oxidase—Its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys. Naturwissenschaften 2014, 101, 661–670. [Google Scholar] [CrossRef] [PubMed]
- de Graft-Johnson, J.; Nowak, D. Effect of Selected Plant Phenolics on Fe2+-EDTA-H2O2 System Mediated Deoxyribose Oxidation: Molecular Structure-Derived Relationships of Anti- and Pro-Oxidant Actions. Molecules 2017, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Abubaker, K.; Miotto, D. Unraveling a mechanism of honey antibacterial action: Polyphenol/H2O2-induced oxidative effect on bacterial cell growth and on DNA degradation. Food Chem. 2012, 133, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Li, J.R.; Wang, Y.B.; Li, T.T.; Zhao, J.; Zhang, C.H. Green tea polyphenols function as prooxidants to inhibit Pseudomonas aeruginosa and induce the expression of oxidative stress-related genes. Folia Microbiol. 2013, 58, 211–217. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; Velde, A.A.T.; de Boer, L.; Vandenbroucke-Grauls, C.M.J.E.; Zaat, S.A.J. Two Major Medicinal Honeys Have Different Mechanisms of Bactericidal Activity. PLoS ONE 2011, 6, e17709. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Boult, C.H.; Deadman, B.J.; Farr, J.M.; Grainger, M.N.C.; Manley-Harris, M.; Snow, M.J. Isolation by HPLC and characterisation of the bioactive fraction of New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2008, 343, 651–659. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef]
- Krymkiewicz, N.; Dieguez, E.; Rekarte, U.D.; Zwaig, N. Properties and mode of action of a bactericidal compound (=methylglyoxal) produced by a mutant of Escherichia coli. J. Bacteriol. 1971, 108, 1338–1347. [Google Scholar] [CrossRef]
- Hayashi, K.; Fukushima, A.; Hayashi-Nishino, M.; Nishino, K. Effect of methylglyoxal on multidrug-resistant Pseudomonas aeruginosa. Front. Microbiol. 2014, 5, 180. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, M.P. The tandem of free radicals and methylglyoxal. Chem.-Biol. Interact. 2008, 171, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Atrott, J.; Henle, T. Methylglyoxal in Manuka Honey—Correlation with Antibacterial Properties. Czech J. Food Sci. 2009, 27, S163–S165. [Google Scholar] [CrossRef]
- Garcia, J.M.; Chambers, E.; Matta, Z.; Clark, M. Viscosity Measurements of Nectar- and Honey-thick Liquids: Product, Liquid, and Time Comparisons. Dysphagia 2005, 20, 325–335. [Google Scholar] [CrossRef]
- Ahmed, J.; Matharu, R.K.; Shams, T.; Illangakoon, U.E.; Edirisinghe, M. A Comparison of Electric-Field-Driven and Pressure-Driven Fiber Generation Methods for Drug Delivery. Macromol. Mater. Eng. 2018, 33, 1700577. [Google Scholar] [CrossRef]
- Fridrikh, S.V.; Yu, J.H.; Brenner, M.P.; Rutledge, G.C. Controlling the Fiber Diameter during Electrospinning. Phys. Rev. Lett. 2003, 90, 144502. [Google Scholar] [CrossRef]
- De Vrieze, S.; Van Camp, T.; Nelvig, A.; Hagström, B.; Westbroek, P.; De Clerck, K. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Mahalingam, S.; Pierin, G.; Colombo, P.; Edirisinghe, M. Facile one-pot formation of ceramic fibres from preceramic polymers by pressurised gyration. Ceram. Int. 2015, 41, 6067–6073. [Google Scholar] [CrossRef]
- Obregon, N.; Agubra, V.; Pokhrel, M.; Campos, H.; Flores, D.; De la Garza, D.; Mao, Y.; Macossay, J.; Alcoutlabi, M. Effect of Polymer Concentration, Rotational Speed, and Solvent Mixture on Fiber Formation Using Forcespinning®. Fibers 2016, 4, 20. [Google Scholar] [CrossRef]
- Husain, O.; Lau, W.; Edirisinghe, M.; Parhizkar, M. Investigating the particle to fibre transition threshold during electrohydrodynamic atomization of a polymer solution. Mater. Sci. Eng. C 2016, 65, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, Y. Centrifugal spinning: An alternative approach to fabricate nanofibers at high speed and low cost. Polym. Rev. 2014, 54, 677–701. [Google Scholar] [CrossRef]
- Altun, E.; Aydogdu, M.O.; Crabbe-Mann, M.; Ahmed, J.; Brako, F.; Karademir, B.; Aksu, B.; Sennaroglu, M.; Eroglu, M.S.; Ren, G.; et al. Co-Culture of Keratinocyte-Staphylococcus aureus on Cu-Ag-Zn/CuO and Cu-Ag-W Nanoparticle Loaded Bacterial Cellulose: PMMA Bandages. Macromol. Mater. Eng. 2018, 304, 1800537. [Google Scholar] [CrossRef]
- Croisier, F.; Duwez, A.S.; Jérôme, C.; Léonard, A.F.; van der Werf, K.O.; Dijkstra, P.J.; Bennink, M.L. Mechanical testing of electrospun PCL fibers. Acta Biomater. 2012, 8, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of Humidity and Solution Viscosity on Electrospun Fiber Morphology. Tissue Eng. Part C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Tonks, A.J.; Cooper, R.A.; Jones, K.P.; Blair, S.; Parton, J.; Tonks, A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003, 21, 242–247. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte–Fibroblast Interactions in Wound Healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound Healing—Aiming for Perfect Skin Regeneration. Science 1997, 276, 75. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Illangakoon, E.U.; Mahalingam, S.; Matharu, K.R.; Edirisinghe, M. Evolution of Surface Nanopores in Pressurised Gyrospun Polymeric Microfibers. Polymers 2017, 9, 508. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Inoue, Y. Novel FTIR method for determining the crystallinity of poly (ε-caprolactone). Polym. Int. 2000, 49, 623–626. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef]
- Azam, N.; Amin, K.A.M. Influence of Manuka Honey on Mechanical Performance and Swelling Behaviour of Alginate Hydrogel Film. Mater. Sci. Eng. 2018, 440, 012024. [Google Scholar] [CrossRef]
- Azam, N.; Amin, K. The physical and mechanical properties of gellan gum films incorporated manuka honey as wound dressing materials. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Iasi, Romania, 25–26 May 2017. [Google Scholar]
- Kasprzyk, I.; Depciuch, J.; Grabek-Lejko, D.; Parlinska-Wojtan, M. FTIR-ATR spectroscopy of pollen and honey as a tool for unifloral honey authentication. The case study of rape honey. Food Control. 2018, 84, 33–40. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Yang, E.; Shi, J.; Xue, Y. Influence of electric field interference on double nozzles electrospinning. J. Appl. Polym. Sci. 2010, 116, 3688–3692. [Google Scholar] [CrossRef]
- Bowler, P.; Jones, S.; Walker, M.; Parsons, D. Microbicidal properties of a silver-containing Hydrofiber® dressing against a variety of burn wound pathogens. J. Burn. Care Rehabil. 2004, 25, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.; Otto, M. Staphylococcus epidermidis infections. Microbes Infect. 2002, 4, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Schweitzer, O.; Gerke, C.; Vanittanakom, N.; Mack, D.; Götz, F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 1996, 20, 1083–1091. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis—The ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555. [Google Scholar] [CrossRef]
- Almasaudi, S.B.; El-Shitany, N.A.; Abbas, A.T.; Abdel-dayem, U.A.; Ali, S.S.; Al Jaouni, S.K.; Harakeh, S. Antioxidant, Anti-inflammatory, and Antiulcer Potential of Manuka Honey against Gastric Ulcer in Rats. Oxidative Med. Cell. Longev. 2016, 2016, 10. [Google Scholar] [CrossRef]
- Visavadia, B.G.; Honeysett, J.; Danford, M.H. Manuka honey dressing: An effective treatment for chronic wound infections. Br. J. Oral Maxillofac. Surg. 2008, 46, 55–56. [Google Scholar] [CrossRef]
- Jull, A.B.; Rodgers, A.; Walker, N. Honey as a topical treatment for wounds. Cochrane Database Syst. Rev. 2015, 2015, CD005083. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [PubMed]
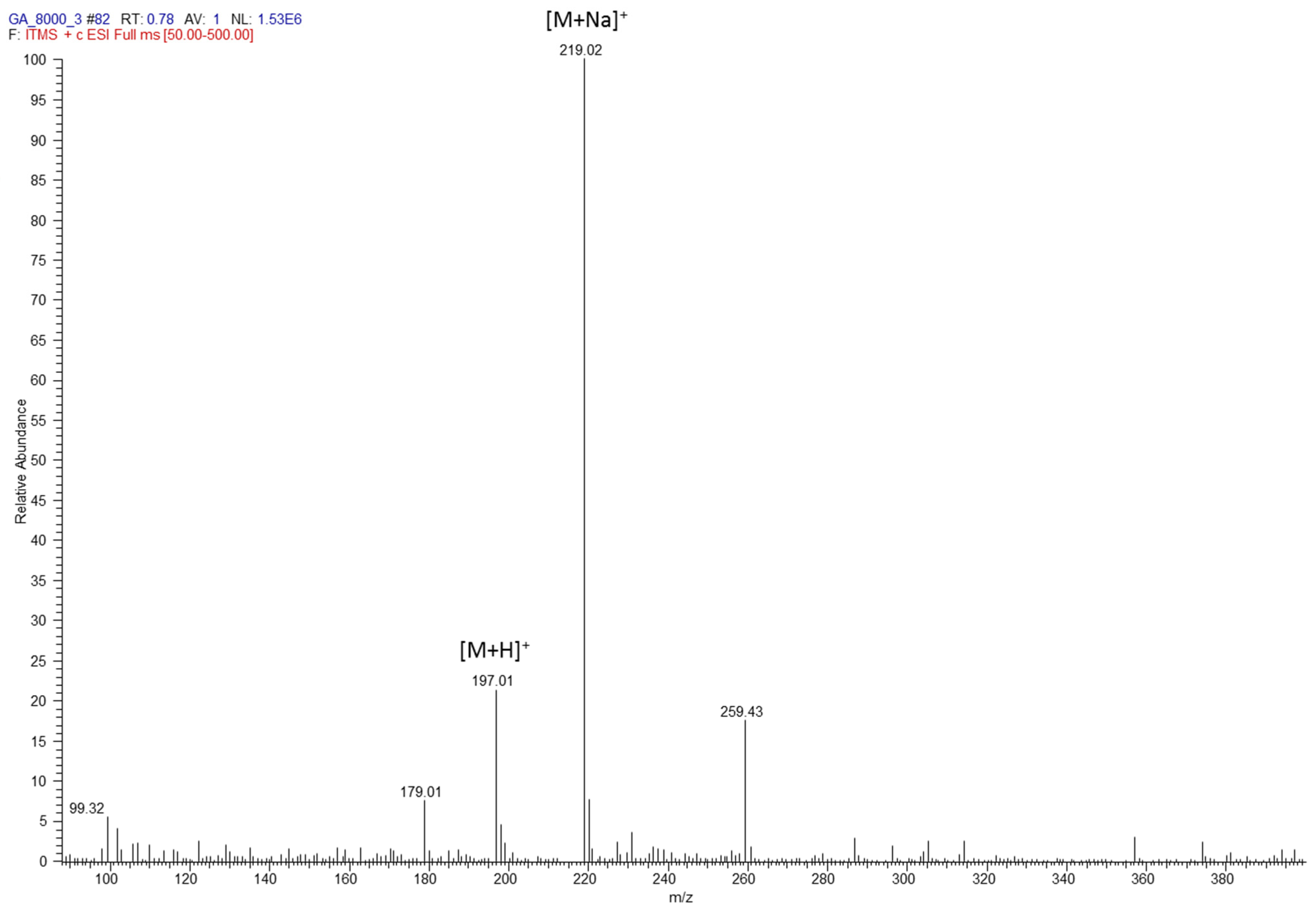
| Organic Acid | Linear Range (mg/kg) | Linear Equation | R2 | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|---|---|---|
| Gluconic acid | 1–20 | f(x) = 1387315x + 15875058 | 0.999 | 0.05 | 0.49 |
| Honey (Replicates) | Peak Area | Measured Concentration (mg/kg) | Average Concentration (mg/kg) | ±C.I. |
|---|---|---|---|---|
| Manuka honey (1) | 50,125,757 | 2468.8 | 2519.2 | 80.9 |
| Manuka honey (2) | 52,118,205 | 2612.5 | ||
| Manuka honey (3) | 50,227,315 | 2476.2 | ||
| Black forest honey (1) | 46,298,596 | 2193.0 | 2195.2 | 7.1 |
| Black forest honey (2) | 44,439,647 | 2059.0 | ||
| Black forest honey (3) | 46,251,523 | 2189.6 |
| Solution | Viscosity (mPa s) | Surface Tension (mN m−1) |
|---|---|---|
| 100% Virgin PCL | 7638 ± 128 | 33.1 ± 1.1 |
| 15% Virgin PCL | 7638 ± 128 | 33.1 ± 1.1 |
| 10% Honey/PCL | 44,091 ± 56 | 33.6 ± 1.1 |
| 20% Honey/PCL | 49,265 ± 89 | 34.4 ± 1.2 |
| 30% Honey/PCL | 54,096 ± 69 | 35.1 ± 0.8 |
| 100% Manukora Manuka Honey | >60,000 | 32.5 ± 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matharu, R.K.; Ahmed, J.; Seo, J.; Karu, K.; Golshan, M.A.; Edirisinghe, M.; Ciric, L. Antibacterial Properties of Honey Nanocomposite Fibrous Meshes. Polymers 2022, 14, 5155. https://doi.org/10.3390/polym14235155
Matharu RK, Ahmed J, Seo J, Karu K, Golshan MA, Edirisinghe M, Ciric L. Antibacterial Properties of Honey Nanocomposite Fibrous Meshes. Polymers. 2022; 14(23):5155. https://doi.org/10.3390/polym14235155
Chicago/Turabian StyleMatharu, Rupy Kaur, Jubair Ahmed, Jegak Seo, Kersti Karu, Mitra Ashrafi Golshan, Mohan Edirisinghe, and Lena Ciric. 2022. "Antibacterial Properties of Honey Nanocomposite Fibrous Meshes" Polymers 14, no. 23: 5155. https://doi.org/10.3390/polym14235155
APA StyleMatharu, R. K., Ahmed, J., Seo, J., Karu, K., Golshan, M. A., Edirisinghe, M., & Ciric, L. (2022). Antibacterial Properties of Honey Nanocomposite Fibrous Meshes. Polymers, 14(23), 5155. https://doi.org/10.3390/polym14235155








