Uricase Crowding via Polyelectrolyte Layers Coacervation for Carbon Fiber-Based Electrochemical Detection of Uric Acid
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, C.; Saurabh, S.; Bruchez, M.P.; Schwartz, R.; LeDuc, P. Molecular crowding shapes gene expression in synthetic cellular nanosystems. Nat. Nanotechnol. 2013, 8, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.S.; Klimov, D.; Thirumalai, D. Molecular crowding enhances native state stability and refolding rates of globular proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 4753–4758. [Google Scholar] [CrossRef] [PubMed]
- Akabayov, B.; Akabayov, S.R.; Lee, S.-J.; Wagner, G.; Richardson, C.C. Impact of molecular crowding on DNA replication. Nat. Commun. 2013, 4, 1615. [Google Scholar] [CrossRef] [PubMed]
- Weilandt, D.R.; Hatzimanikatis, V. Particle-based simulation reveals macromolecular crowding effects on the Michaelis-Menten mechanism. Biophys. J. 2019, 117, 355–368. [Google Scholar] [CrossRef]
- Pavlovic, M.; Plucinski, A.; Zhang, J.; Antonietti, M.; Zeininger, L.; Schmidt, B.V.K.J. Cascade kinetics in an enzyme-loaded aqueous two-phase system. Langmuir 2020, 36, 1401–1408. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, T.; Wei, X.; Su, F.; Li, A.; Tai, Y.; Wei, T.; Zhang, Q.; Kong, D.; Zhang, C. Bioinspired enzymatic compartments constructed by spatiotemporally confined in situ self-assembly of catalytic peptide. Commun. Chem. 2022, 5, 81. [Google Scholar] [CrossRef]
- Rodrigues, R.T.; Siqueira, J.R., Jr.; Caseli, L. Structural and viscoelastic properties of floating monolayers of a pectinolytic enzyme and their influence on the catalytic properties. J. Colloid Interface Sci. 2021, 589, 568–577. [Google Scholar] [CrossRef]
- Muravev, A.A.; Solovieva, S.E.; Kochetkov, E.N.; Mel’nikova, N.B.; Safiullin, R.A.; Kadirov, M.K.; Latypov, S.K.; Antipin, I.S.; Konovalov, A.I. Thiacalix[4]monocrowns substituted by sulfur-containing anchoring groups: New ligands for gold surface modification. Macroheterocycles 2013, 6, 302–307. [Google Scholar] [CrossRef]
- Van der Meeren, L.; Verduijn, J.; Li, J.; Verwee, E.; Krysko, D.V.; Parakhonskiy, B.V.; Skirtach, A.G. Encapsulation of cells in gold nanoparticle functionalized hybrid Layer-by-Layer (LbL) hybrid shells—Remote effect of laser light. Appl. Surf. Sci. 2021, 5, 100111. [Google Scholar] [CrossRef]
- Skorb, E.V.; Andreeva, D.V. Layer-by-layer approaches for formation of smart self-healing materials. Polym. Chem. 2013, 4, 4834–4845. [Google Scholar] [CrossRef]
- Kienle, D.F.; Chaparro Sosa, A.F.; Kaar, J.L.; Schwartz, D.K. Polyelectrolyte multilayers enhance the dry storage and pH stability of physically entrapped enzymes. ACS Appl. Mater. Interfaces 2020, 12, 22640–22649. [Google Scholar] [CrossRef] [PubMed]
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer- and hybrid-based biomaterials for interstitial, connective, vascular, nerve, visceral and musculoskeletal tissue engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Prokopovic, V.Z.; Vikulina, A.S.; Sustr, D.; Shchukina, E.M.; Shchukin, D.G.; Volodkin, D.V. Binding mechanism of the model charged dye carboxyfluorescein to hyaluronan/polylysine multilayers. ACS Appl. Mater. Interfaces 2017, 9, 38908–38918. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Gao, G.; Watanabe, J.; Liu, H.; Shen, H. Hydrophilic polyelectrolyte multilayers improve the ELISA system: Antibody enrichment and blocking free. Polymers 2017, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Stekolshchikova, A.A.; Radaev, A.V.; Orlova, O.Y.; Nikolaev, K.G.; Skorb, E.V. Thin and flexible ion sensors based on polyelectrolyte multilayers assembled onto the carbon adhesive tape. ACS Omega 2019, 4, 15421–15427. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Tiyyagura, H.R.; Pottathara, Y.B.; Sadasivuni, K.K.; Ponnamma, D.; Douglas, T.E.L.; Skirtach, A.G.; Mohan, M.K. Surface functionalization of chitosan as a coating material for orthopaedic applications: A comprehensive review. Carbohydr. Polym. 2021, 255, 117487. [Google Scholar] [CrossRef]
- Qian, B.; Zheng, Z.; Michailids, M.; Fleck, N.; Bilton, M.; Song, Y.; Li, G.; Shchukin, D. Mussel-inspired self-healing coatings based on polydopamine-coated nanocontainers for corrosion protection. ACS Appl. Mater. Interfaces 2019, 11, 10283–10291. [Google Scholar] [CrossRef]
- Johnston, A.R.; Minckler, E.D.; Shockley, M.C.J.; Matsushima, L.N.; Perry, S.L.; Ayzner, A.L. Conjugated polyelectrolyte-based complex fluids as aqueous exciton transport networks. Angew. Chem. Int. Ed. 2022, 134, e202117759. [Google Scholar] [CrossRef]
- Park, S.; Barnes, R.; Lin, Y.; Jeon, B.-J.; Najafi, S.; Delaney, K.T.; Fredrickson, G.H.; Shea, J.-E.; Hwang, D.S.; Han, S. Dehydration entropy drives liquid-liquid phase separation by molecular crowding. Commun. Chem. 2020, 3, 83. [Google Scholar] [CrossRef]
- Wilcox, X.E.; Ariola, A.; Jackson, J.R.; Slade, K.M. Overlap concentration and the effect of macromolecular crowding on citrate synthase activity. Biochemistry 2020, 59, 1737–1746. [Google Scholar] [CrossRef]
- Hata, Y.; Kojima, T.; Koizumi, T.; Okura, H.; Sakai, T.; Sawada, T.; Serizawa, T. Enzymatic synthesis of cellulose oligomer hydrogels composed of crystalline nanoribbon networks under macromolecular crowding conditions. ACS Macro Lett. 2017, 6, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.S.; Nikolaev, K.G.; Stekolshchikova, A.A.; Tesfatsion, W.T.; Yurchenko, S.O.; Novoselov, K.S.; Andreeva, D.V.; Rubtsova, M.Y.; Vorovitch, M.F.; Ishmukhametov, A.A.; et al. Tick-borne Encephalitis electrochemical detection by multilayer perceptron on liquid–metal interface. ACS Appl. Bio Mater. 2020, 3, 7352–7356. [Google Scholar] [CrossRef] [PubMed]
- Baldina, A.A.; Nikolaev, K.G.; Ivanov, A.S.; Nikitina, A.A.; Rubtsova, M.Y.; Vorovitch, M.F.; Ishmukhametov, A.A.; Egorov, A.M.; Skorb, E.V. Immunochemical biosensor for single virus particle detection based on molecular crowding polyelectrolyte system. J. Appl. Polym. Sci. 2022, 139, 52360. [Google Scholar] [CrossRef]
- Lavrentev, F.V.; Rumyantsev, I.S.; Ivanov, A.S.; Shilovskikh, V.V.; Orlova, O.Y.; Nikolaev, K.G.; Andreeva, D.V.; Skorb, E.V. Soft hydrogel actuator for fast machine-learning-assisted bacteria detection. ACS Appl. Mater. Interfaces 2022, 14, 7321–7328. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Muthukumar, M. Topologically frustrated dynamics of crowded charged macromolecules in charged hydrogels. Nat. Commun. 2018, 9, 2248. [Google Scholar] [CrossRef]
- Testa, A.; Dindo, M.; Rebane, A.A.; Nasouri, B.; Style, R.W.; Golestanian, R.; Dufresne, E.R.; Laurino, P. Sustained enzymatic activity and flow in crowded protein droplets. Nat. Commun. 2021, 12, 6293. [Google Scholar] [CrossRef]
- Ferreira, C.; Pinto, M.F.; Macedo-Ribeiro, S.; Pereira, P.J.B.; Rocha, F.A.; Martins, P.M. Protein crystals as a key for deciphering macromolecular crowding effects on biological reactions. Phys. Chem. Chem. Phys. 2020, 22, 16143–16149. [Google Scholar] [CrossRef]
- Li, G.; Wu, X.; Zhou, C.-l.; Wang, Y.-m.; Song, B.; Cheng, X.-b.; Dong, Q.-f.; Wang, L.-l.; You, S.-s.; Ba, Y.-m. Uric acid as a prognostic factor and critical marker of COVID-19. Sci. Rep. 2021, 11, 17791. [Google Scholar] [CrossRef]
- Arora, K.; Sumana, G.; Saxena, V.; Gupta, R.K.; Gupta, S.K.; Yakhmi, J.V.; Pandey, M.K.; Chand, S.; Malhotra, B.D. Improved performance of polyaniline-uricase biosensor. Anal. Chim. Acta 2007, 594, 17–23. [Google Scholar] [CrossRef]
- Singh, A.; Ahmed, A.; Sharma, A.; Arya, S. Graphene and its derivatives: Synthesis and application in the electrochemical detection of analytes in sweat. Biosensors 2022, 12, 910. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Arya, S. Human sweat-based wearable glucose sensor on cotton fabric for real-time monitoring. J. Anal. Sci. Technol. 2022, 13, 11. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Arya, S. Highly selective and efficient electrochemical sensing of ascorbic acid via CuO/rGO nanocomposites deposited on conductive fabric. Appl. Phys. A 2022, 128, 262. [Google Scholar] [CrossRef]
- Lim, G.N.; Ross, A.E. Purine functional group type and placement modulate the interaction with carbon-fiber microelectrodes. ACS Sens. 2019, 4, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Farajikhah, S.; Innis, P.C.; Paull, B.; Wallace, G.G.; Harris, A.R. Facile development of a fiber-based electrode for highly selective and sensitive detection of dopamine. ACS Sens. 2019, 4, 2599–2604. [Google Scholar] [CrossRef] [PubMed]
- Sweilam, M.N.; Varcoe, J.R.; Crean, C. Fabrication and optimization of fiber-based lithium sensor: A step toward wearable sensors for lithium drug monitoring in interstitial fluid. ACS Sens. 2018, 3, 1802–1810. [Google Scholar] [CrossRef]
- Li, W.; Chen, R.; Qi, W.; Cai, L.; Sun, Y.; Sun, M.; Li, C.; Yang, X.; Xiang, L.; Xie, D.; et al. Reduced graphene oxide/mesoporous ZnO NSs hybrid fibers for flexible, stretchable, twisted, and wearable NO2 e-textile gas sensor. ACS Sens. 2019, 4, 2809–2818. [Google Scholar] [CrossRef]
- Nikolaev, K.G.; Kalmykov, E.V.; Shavronskaya, D.O.; Nikitina, A.A.; Stekolshchikova, A.A.; Kosareva, E.A.; Zenkin, A.A.; Pantiukhin, I.S.; Orlova, O.Y.; Skalny, A.V.; et al. ElectroSens platform with a polyelectrolyte-based carbon fiber sensor for point-of-care analysis of Zn in blood and urine. ACS Omega 2020, 5, 18987–18994. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; O’Neill, R.D.; González-Mora, J. Biosensors based on Prussian Blue modified carbon fibers electrodes for monitoring lactate in the extracellular space of brain tissue. Int. J. Electrochem. Sci. 2012, 7, 5910–5926. [Google Scholar]
- Piermarini, S.; Migliorelli, D.; Volpe, G.; Massoud, R.; Pierantozzi, A.; Cortese, C.; Palleschi, G. Uricase biosensor based on a screen-printed electrode modified with Prussian blue for detection of uric acid in human blood serum. Sens. Actuators B Chem. 2013, 179, 170–174. [Google Scholar] [CrossRef]
- Skorb, E.V.; Shchukin, D.G.; Möhwald, H.; Sviridov, D.V. Photocatalytically-active and photocontrollable coatings based on titania-loaded hybrid sol–gel films. J. Mater. Chem. 2009, 19, 4931–4937. [Google Scholar] [CrossRef]
- Bohmer, M.R.; Evers, O.A.; Scheutjens, J.M.H.M. Weak polyelectrolytes between two surfaces: Adsorption and stabilization. Macromolecules 1990, 23, 2288–2301. [Google Scholar] [CrossRef]
- van der Straeten, A.; Bratek-Skicki, A.; Jonas, A.M.; Fustin, C.-A.; Dupont-Gillain, C. Integrating proteins in layer-by-layer assemblies independently of their electrical charge. ACS Nano 2018, 12, 8372–8381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, Y.; Song, J.; Yang, J.; Pan, C.; Xu, T.; Zhang, L. Novel balanced charged alginate/PEI polyelectrolyte hydrogel that resists foreign-body reaction. ACS Appl. Mater. Interfaces 2018, 10, 6879–6886. [Google Scholar] [CrossRef] [PubMed]
- Achazi, K.; Haag, R.; Ballauff, M.; Dernedde, J.; Kizhakkedathu, J.N.; Maysinger, D.; Multhaup, D. Understanding the interaction of polyelectrolyte architectures with proteins and biosystems. Angew. Chem. Int. Ed. 2021, 60, 3882–3904. [Google Scholar] [CrossRef] [PubMed]
- Mauser, T.; Déjugnat, C.; Möhwald, H.; Sukhorukov, G.B. Microcapsules made of weak polyelectrolytes: templating and stimuli-responsive properties. Langmuir 2006, 22, 5888–5893. [Google Scholar] [CrossRef]
- Delcea, M.; Möhwald, H.; Skirtach, A.G. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef]
- Cheng, C.I.; Chang, Y.-P.; Chu, Y.-H. Biomolecular interactions and tools for their recognition: Focus on the quartz crystal microbalance and its diverse surface chemistries and applications. Chem. Soc. Rev. 2012, 41, 1947–1971. [Google Scholar] [CrossRef]
- Dutta, P.; Sharma, V.; Bhardwaj, H.; Agrawal, V.V.; Sumana, R.; Sumana, G. Fabrication of electrochemical biosensor using zinc oxide nanoflowers for the detection of uric acid. MAPAN 2022, 37, 585–595. [Google Scholar] [CrossRef]
- Nagal, V.; Kumar, V.; Khan, M.; AlOmar, S.Y.; Tripathy, N.; Singh, K.; Khosla, A.; Ahmad, N.; Hafiz, A.K.; Ahmad, R. A highly sensitive uric acid biosensor based on vertically arranged ZnO nanorods on a ZnO nanoparticle-seeded electrode. New J. Chem. 2021, 45, 18863–18870. [Google Scholar] [CrossRef]
- Thakur, B.; Sawant, S.N. Polyaniline/Prussian-Blue-based amperometric biosensor for detection of uric acid. ChemPlusChem 2013, 78, 166–174. [Google Scholar] [CrossRef]
- Cancelliere, R.; Tinno, A.D.; Cataldo, A.; Bellucci, S.; Micheli, L. Powerful electron-transfer screen-printed platforms as biosensing tools: The case of uric acid biosensor. Biosensors 2021, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Muguruma, H.; Iwasa, H.; Tanaka, T.; Hiratsuka, A.; Shimizu, T.; Tsuji, K.; Kishimoto, T. Electrochemical determination of uric acid in urine and serum with uricase/carbon nanotube /carboxymethylcellulose electrode. Anal. Biochem. 2020, 590, 113533. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, H.; Liu, P.; Cheng, J. A 3D electrochemical biosensor based on Super-Aligned Carbon NanoTube array for point-of-care uric acid monitoring. Biosens. Bioelectron. 2021, 179, 113082. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Saiki, H.; Anzai, J.-i. Amperometric uric acid sensors based on polyelectrolyte multilayer films. Talanta 2003, 61, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhi, N.; Yang, L.; Xu, G.; Feng, Q.; Zhang, Q.; Sun, S. A highly sensitive uric acid electrochemical biosensor based on a nano-cube cuprous oxide/ferrocene/uricase modified glassy carbon electrode. Sci. Rep. 2020, 10, 10607. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Arya, S.; Verma, S.; Singh, A.; Sharma, A.; Singh, B.; Prerna; Sharma, R. Performance of template-assisted electrodeposited Copper/Cobalt bilayered nanowires as an efficient glucose and Uric acid senor. Mater. Chem. Phys. 2019, 238, 121969. [Google Scholar] [CrossRef]
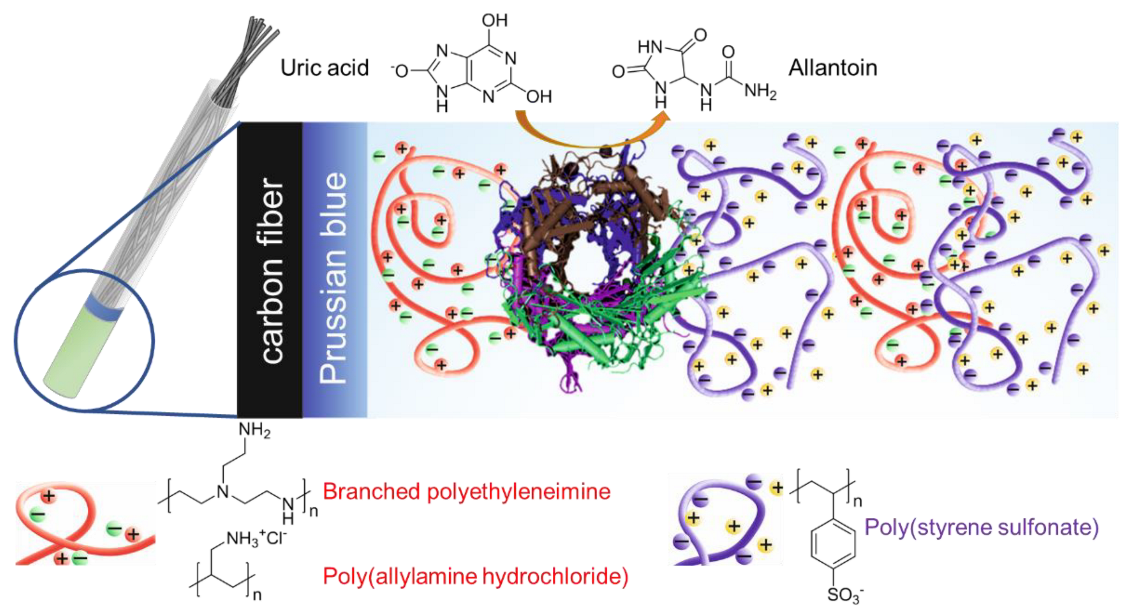
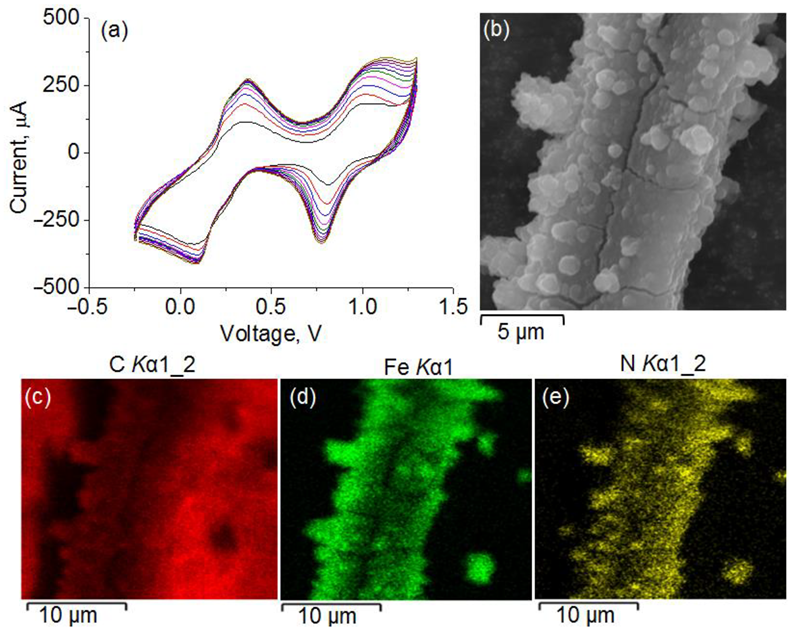
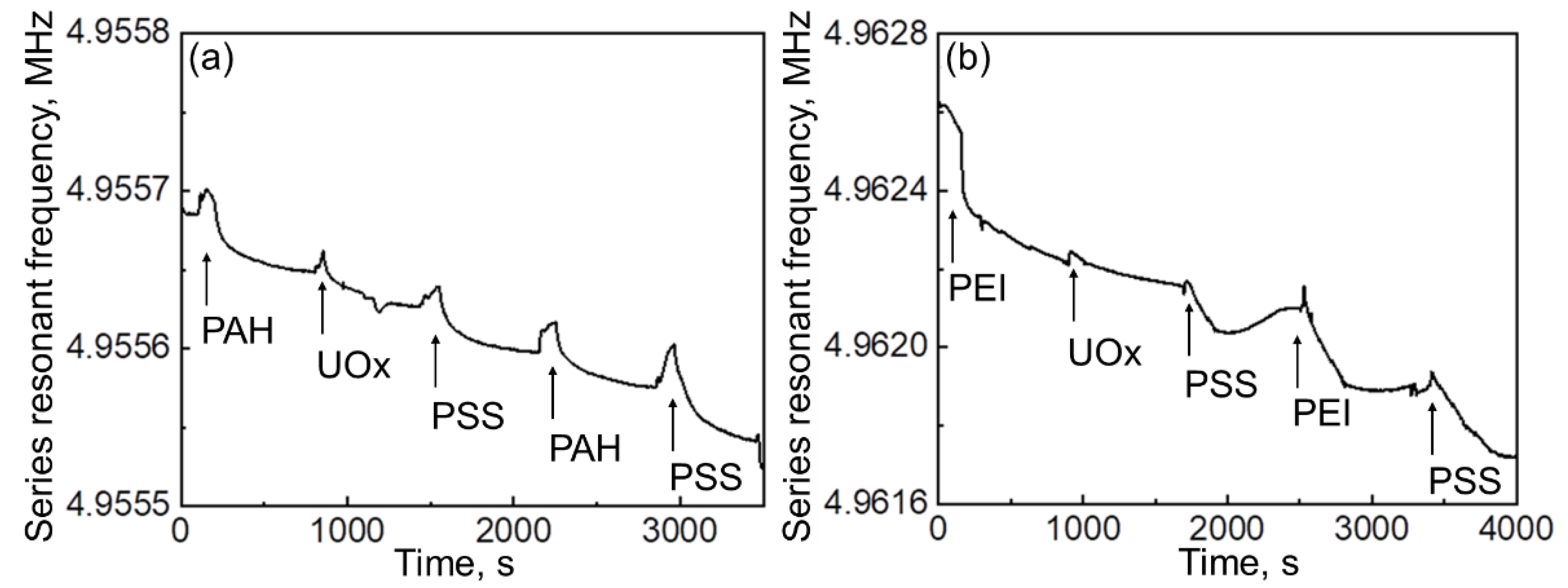

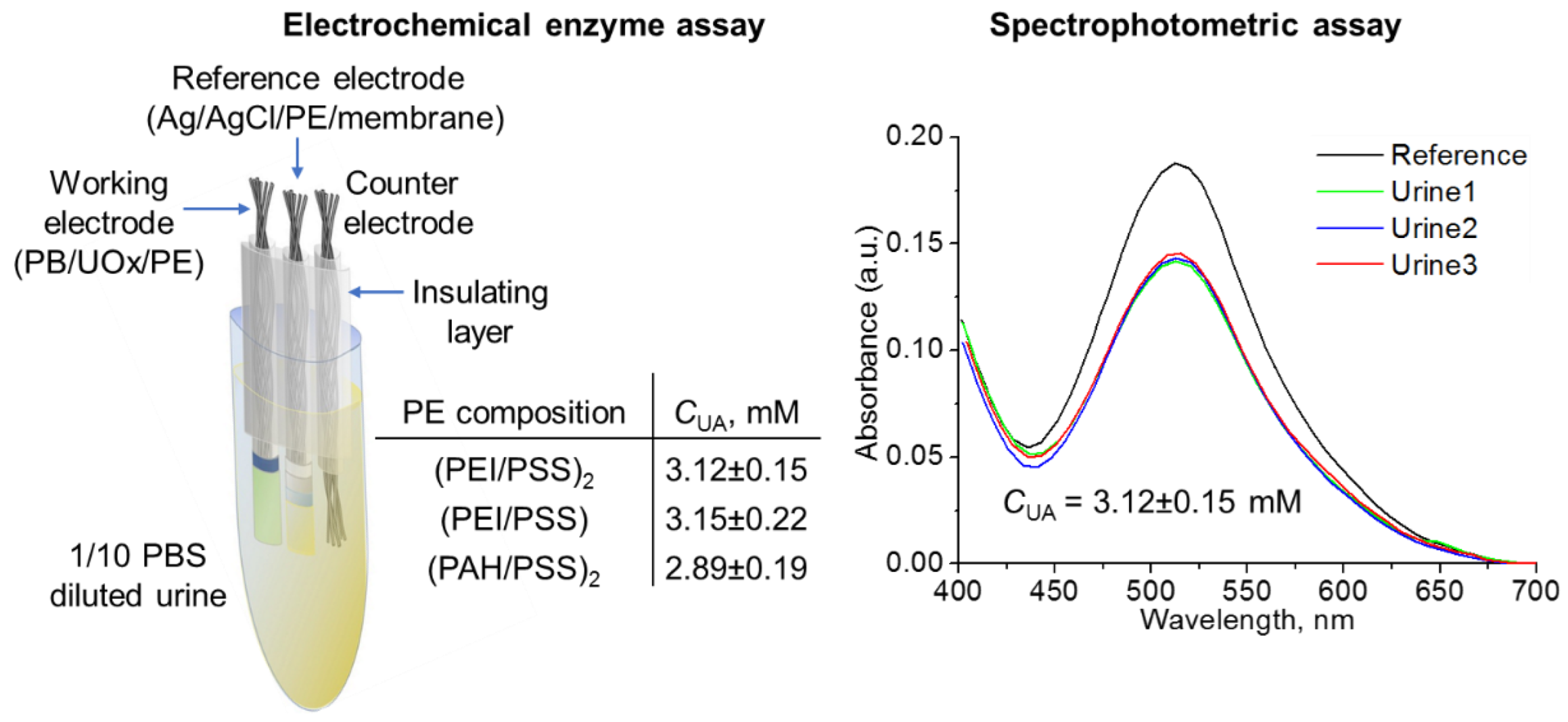
| Polyelectrolyte System | Layer Composition | Added Mass, μg | Final Mass, μg |
|---|---|---|---|
| PAH/UOx/PSS/PAH/PSS | PAH | 0.1468 | 0.1468 |
| PAH/UOx | 0.0544 | 0.2012 | |
| PAH/UOx/PSS | 0.0739 | 0.2751 | |
| PAH/UOx/PSS/PAH | 0.0645 | 0.3396 | |
| PAH/UOx/PSS/PAH/PSS | 0.0890 | 0.4286 | |
| PEI/UOx/PSS/PEI/PSS | PEI | 0.3839 | 0.3839 |
| PEI/UOx | 0.1637 | 0.5476 | |
| PEI/UOx/PSS | 0.1547 | 0.7023 | |
| PEI/UOx/PSS/PEI | 0.5686 | 1.2709 | |
| PEI/UOx/PSS/PEI/PSS | 0.4201 | 1.6910 |
| Sensor | Linear Range, μM | LOD, μM | KM, μM | Sensitivity, μA/μM |
|---|---|---|---|---|
| UOx/ZnO/ITO [48] | 5–750 | 130 | n/a | 0.010 |
| Nafion/UOx/ZnO/FTO [49] | 10–1500 | 2.5 | n/a | 0.345 |
| PANI–UOx/ITO [29] | 10–600 | 10 | 5.1 | 0.016–0.047 |
| UOx/PANI–PB/Pt [50] | 10–160 | 2.6 | 34.13 | 0.160 |
| UOx/MWCNT/SPE [51] | 0.0001–1 | 0.0005 | 0.04 | 418 |
| UOx/MWCNT-CMC/Au [52] | 20–5000 | 2.8 | 630 | 0.233 |
| GA/UOx/Chitosan/SACNT/Pt [53] | 100–1000 | 1.0 | n/a | 0.518 |
| (UOx/PAA)9UOx/(PAA/PVS)2PAA/Pt [54] | 1–1000 | n/a | n/a | n/a |
| UOx/Fc/Cu2O/GCE [55] | 0.1–1000 | 0.06 | 34.74 | 0.002 |
| This work | 1–70 | 0.81 ± 0.07 | 2 | 10.64 ± 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldina, A.A.; Pershina, L.V.; Noskova, U.V.; Nikitina, A.A.; Muravev, A.A.; Skorb, E.V.; Nikolaev, K.G. Uricase Crowding via Polyelectrolyte Layers Coacervation for Carbon Fiber-Based Electrochemical Detection of Uric Acid. Polymers 2022, 14, 5145. https://doi.org/10.3390/polym14235145
Baldina AA, Pershina LV, Noskova UV, Nikitina AA, Muravev AA, Skorb EV, Nikolaev KG. Uricase Crowding via Polyelectrolyte Layers Coacervation for Carbon Fiber-Based Electrochemical Detection of Uric Acid. Polymers. 2022; 14(23):5145. https://doi.org/10.3390/polym14235145
Chicago/Turabian StyleBaldina, Anna A., Liubov V. Pershina, Ulyana V. Noskova, Anna A. Nikitina, Anton A. Muravev, Ekaterina V. Skorb, and Konstantin G. Nikolaev. 2022. "Uricase Crowding via Polyelectrolyte Layers Coacervation for Carbon Fiber-Based Electrochemical Detection of Uric Acid" Polymers 14, no. 23: 5145. https://doi.org/10.3390/polym14235145
APA StyleBaldina, A. A., Pershina, L. V., Noskova, U. V., Nikitina, A. A., Muravev, A. A., Skorb, E. V., & Nikolaev, K. G. (2022). Uricase Crowding via Polyelectrolyte Layers Coacervation for Carbon Fiber-Based Electrochemical Detection of Uric Acid. Polymers, 14(23), 5145. https://doi.org/10.3390/polym14235145






