Characterization of an In Vitro/Ex Vivo Mucoadhesiveness Measurement Method of PVA Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Mucoadhesive Films
2.2.2. Preparation of Biomimetic Gels
2.2.3. Preparation of Animal Tissue
2.2.4. Adhesion Measurements with the Texture Analyzer
- Investigation of the influence of the test equipment parameters on the maximum detachment force as well as the work of adhesion on agar/mucin gels;
- Characterization of further test parameters such as the area of the film used, wetting the gels with phosphate buffer pH 7.4 and storage of the agar/mucin gels;
- Comparative measurements of porcine intestinal tissue.
Influence of Instrument Parameters
Influence of Further Test Parameters
Comparative Measurements on Porcine Tissue
3. Results and Discussion
3.1. Influence of Test Equipment Parameters on the Adhesiveness of Films on Biomimetic Gels
3.1.1. Influence of the Sample Area
3.1.2. Influence of the Contact Force
3.1.3. Influence of Contact Time
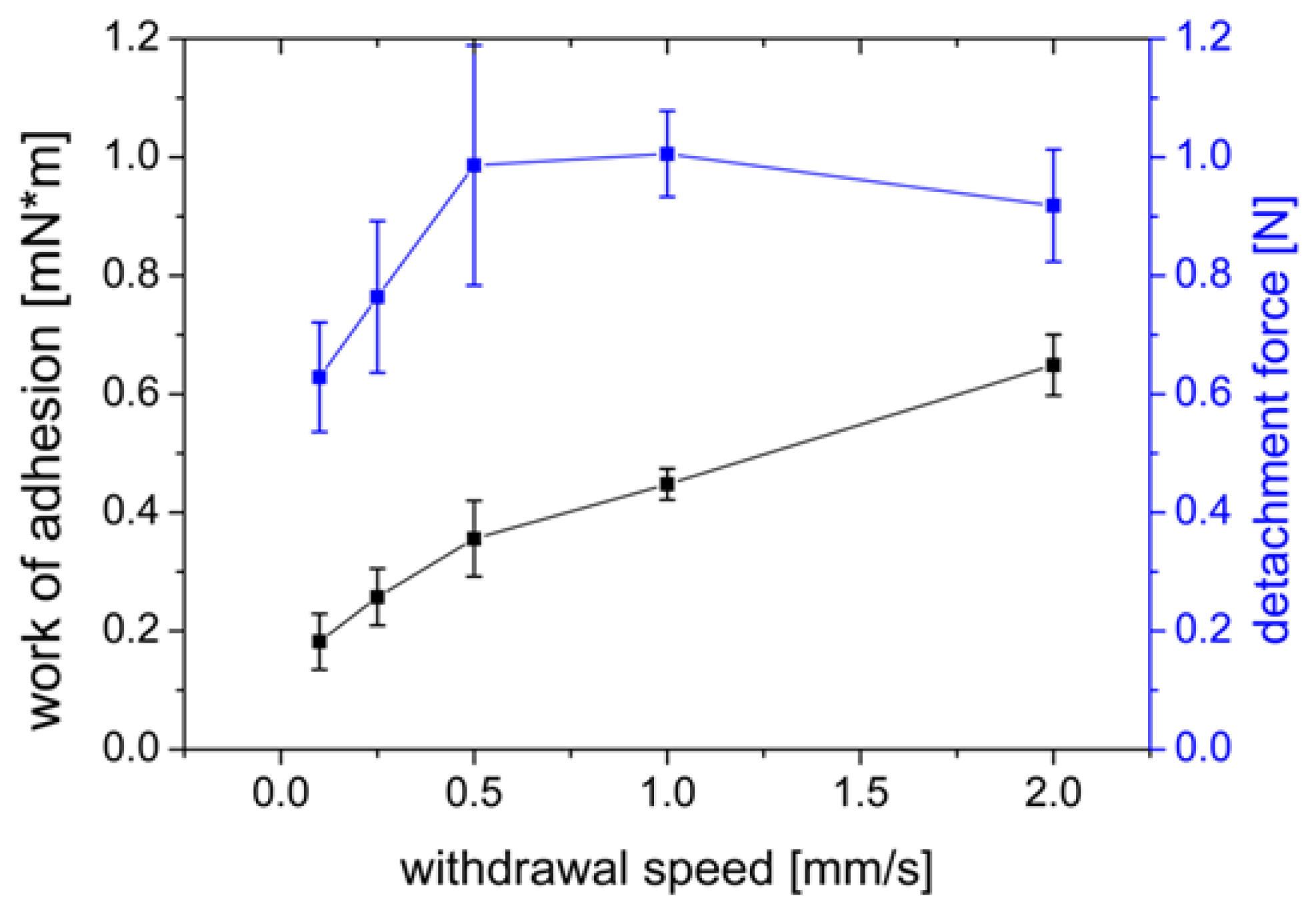
3.1.4. Influence of the Withdrawal Speed
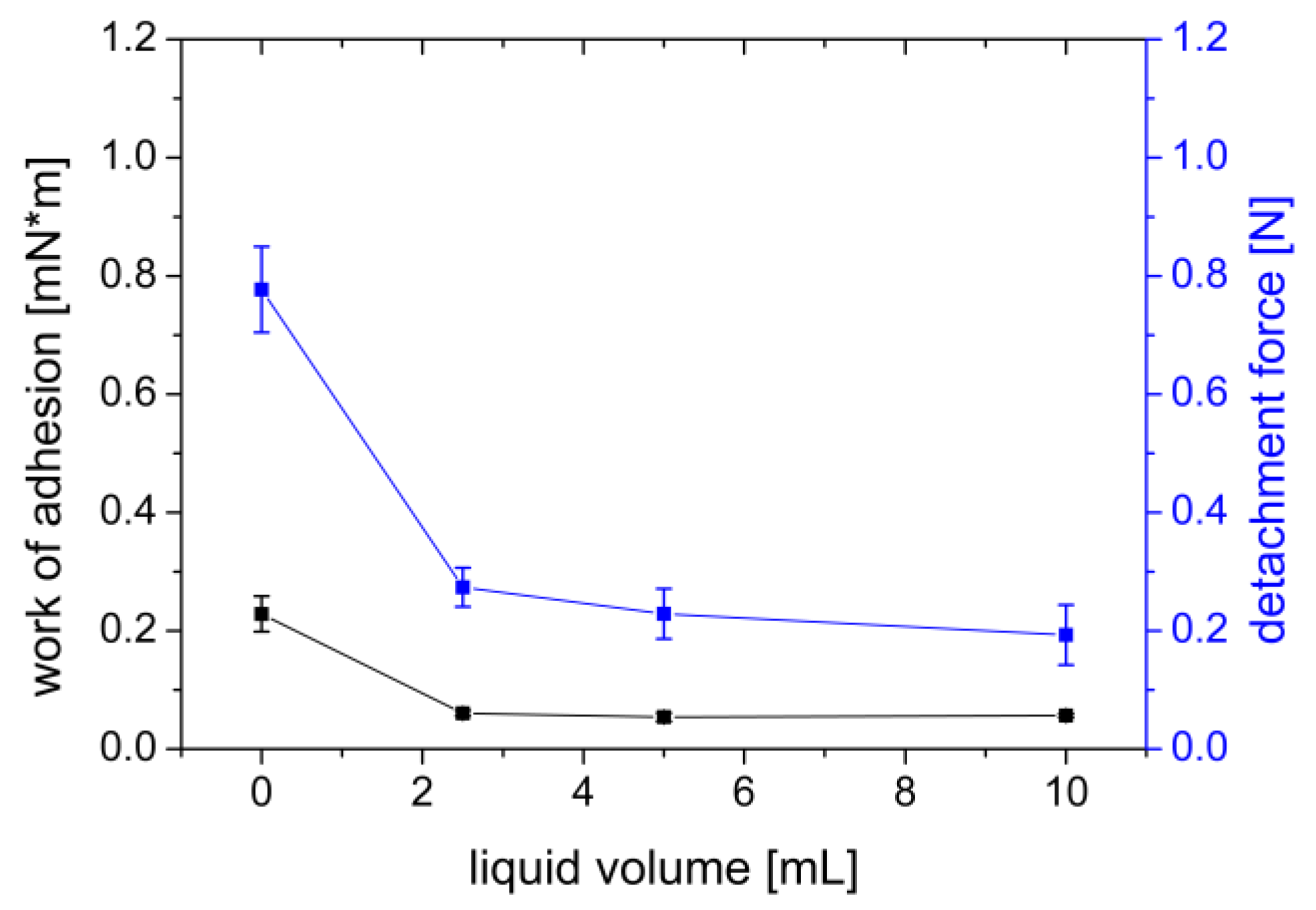
3.1.5. Addition of Wetting Liquid
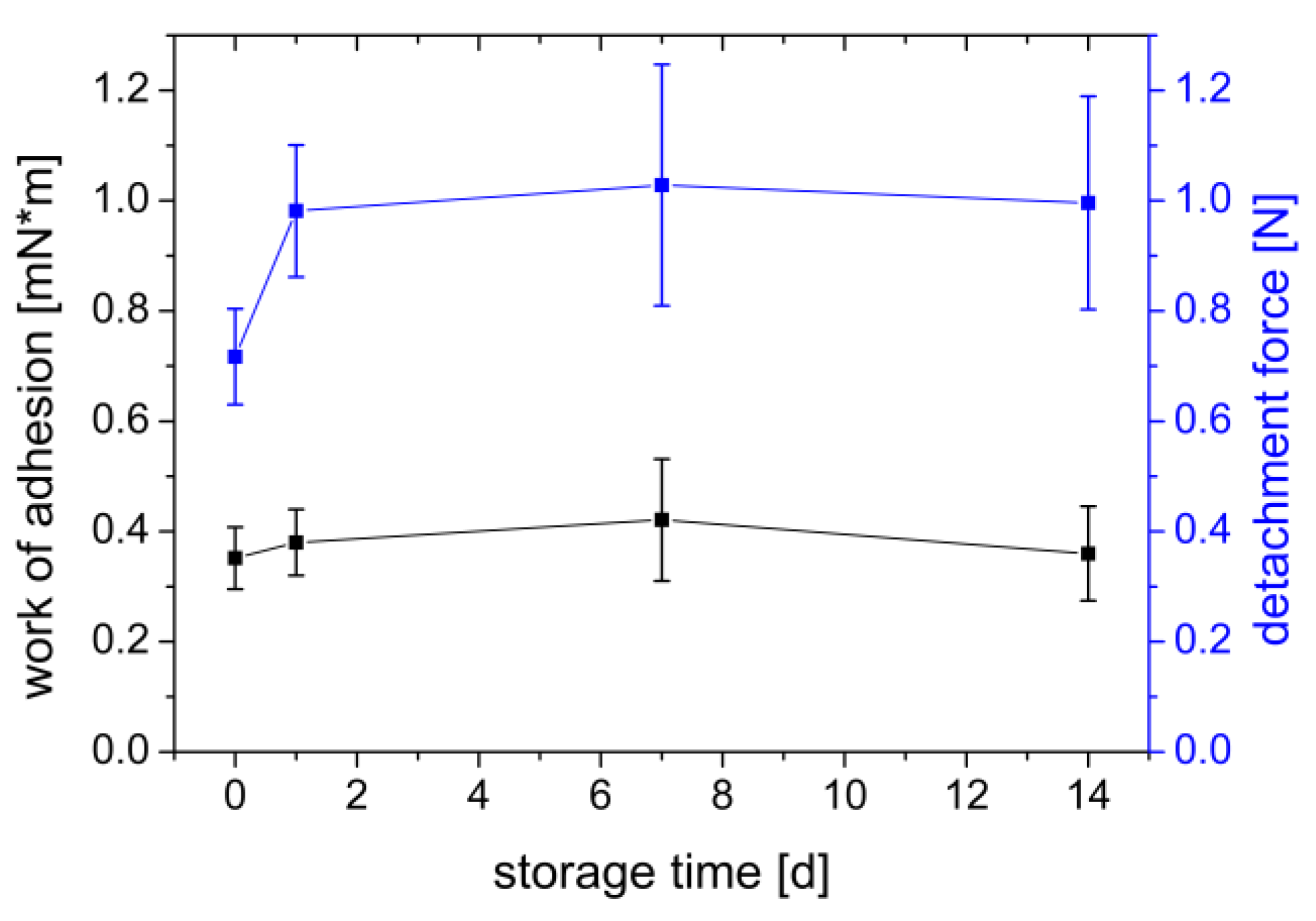
3.1.6. Influence of Gel Storage
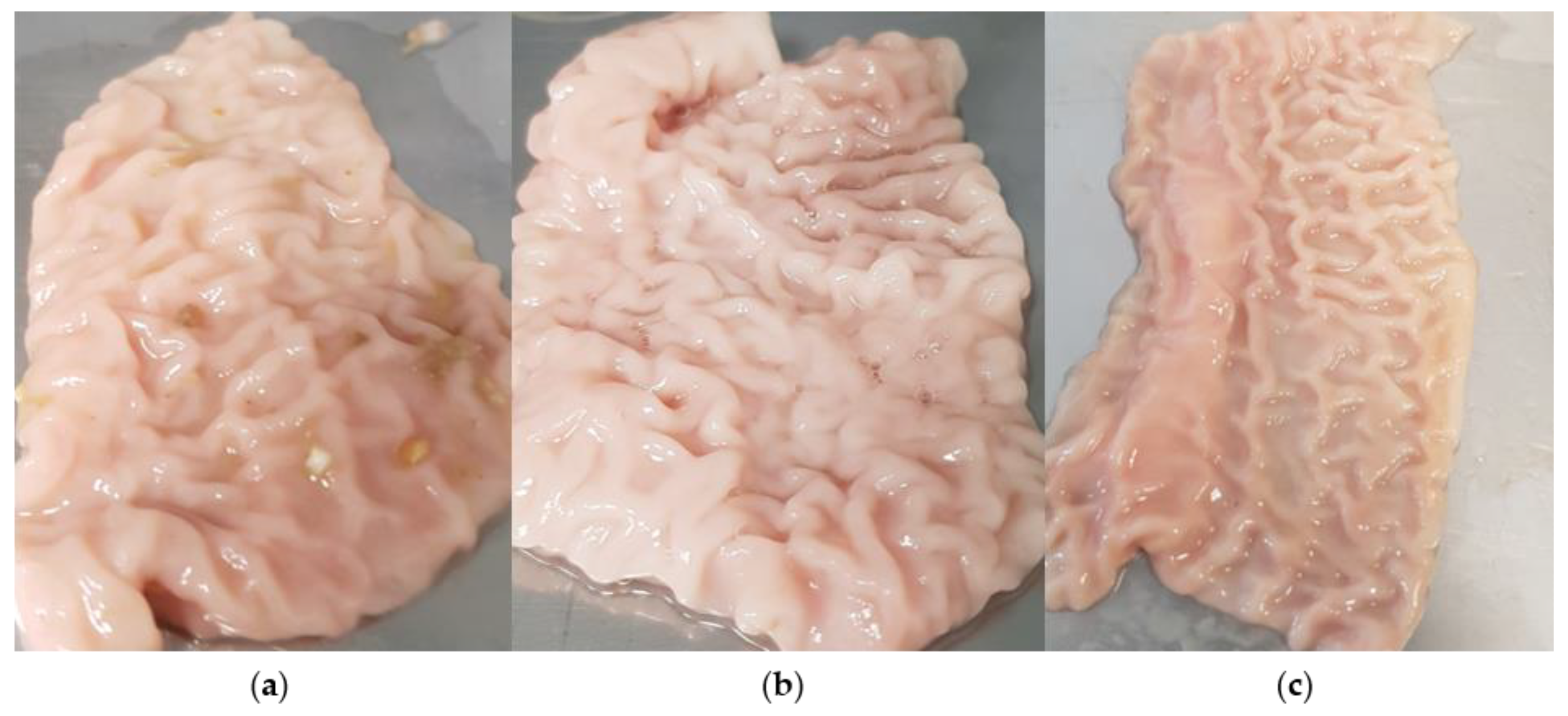
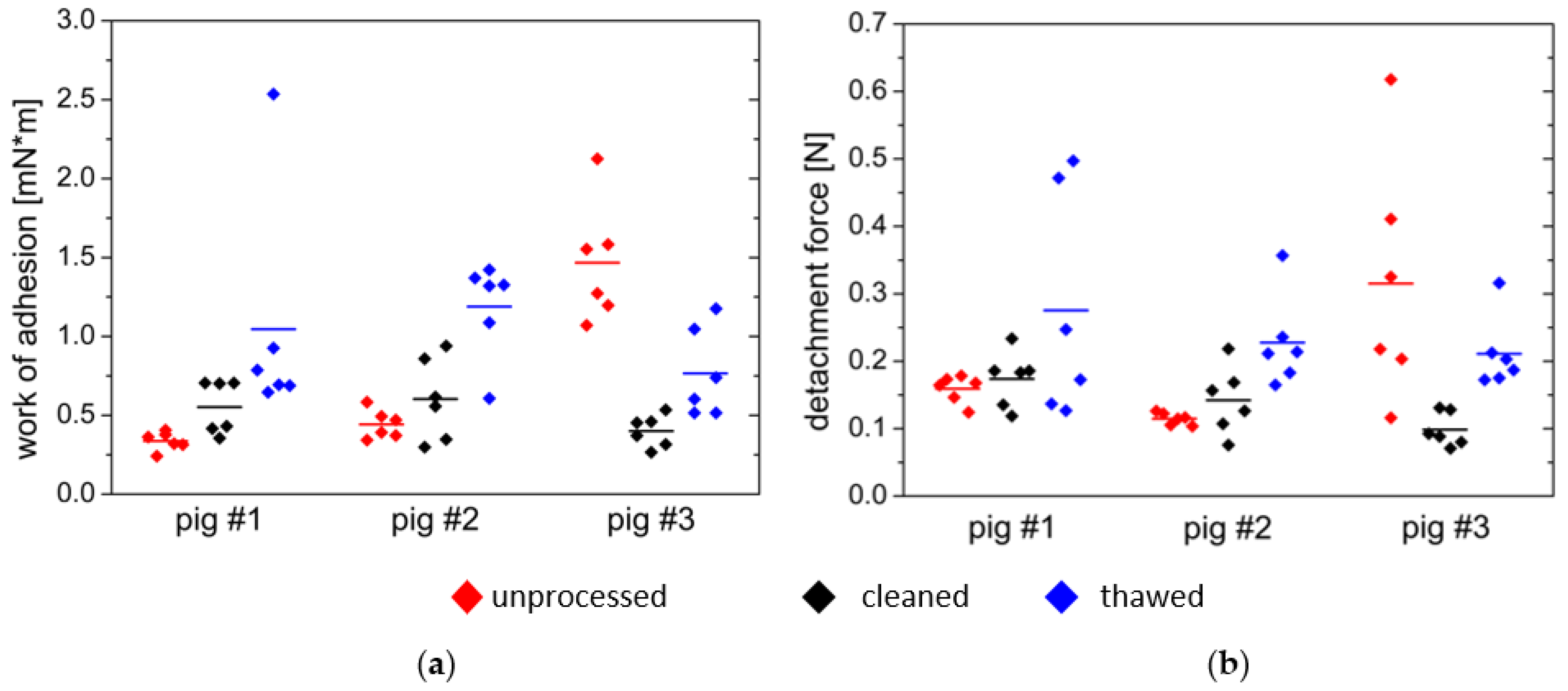
3.2. Comparative Measurements on Porcine Small Intestine Tissue
- First, the tissue was used in fresh and uncleaned condition;
- Second series of measurements were performed on fresh, cleaned tissue;
- Third series of measurements consisted of cleaned tissue frozen for seven days.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-del Rio, L.; Diaz-Rodriguez, P.; Landin, M. Design of Novel Orotransmucosal Vaccine-Delivery Platforms Using Artificial Intelligence. Eur. J. Pharm. Biopharm. 2021, 159, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Bandi, S.P.; Venuganti, V.V.K. Functionalized Polymeric Patch for Localized Oxaliplatin Delivery to Treat Gastric Cancer. Mater. Sci. Eng. C 2021, 128, 112302. [Google Scholar] [CrossRef] [PubMed]
- Paris, A.L.; Caridade, S.; Colomb, E.; Bellina, M.; Boucard, E.; Verrier, B.; Monge, C. Sublingual Protein Delivery by a Mucoadhesive Patch Made of Natural Polymers. Acta Biomater. 2021, 128, 222–235. [Google Scholar] [CrossRef]
- Kast, C.E.; Guggi, D.; Langoth, N.; Bernkop-Schnürch, A. Development and in Vivo Evaluation of an Oral Delivery System for Low Molecular Weight Heparin Based on Thiolated Polycarbophil. Pharm. Res. 2003, 20, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.L.J.; Billa, N. Improved Bioavailability of Poorly Soluble Drugs through Gastrointestinal Muco-Adhesion of Lipid Nanoparticles. Pharmaceutics 2021, 13, 1817. [Google Scholar] [CrossRef]
- Ngwuluka, N.C.; Choonara, Y.E.; Modi, G.; du Toit, L.C.; Kumar, P.; Ndesendo, V.M.K.; Pillay, V. Design of an Interpolyelectrolyte Gastroretentive Matrix for the Site-Specific Zero-Order Delivery of Levodopa in Parkinson’s Disease. AAPS PharmSciTech 2013, 14, 605–619. [Google Scholar] [CrossRef]
- Ige, P.P.; Gattani, S.G. Design and in Vitro and in Vivo Characterization of Mucoadhesive Matrix Pellets of Metformin Hydrochloride for Oral Controlled Release: A Technical Note. Arch. Pharm. Res. 2012, 35, 487–498. [Google Scholar] [CrossRef]
- Ruiz-Caro, R.; Gago-Guillan, M.; Otero-Espinar, F.J.; Veiga, M.D. Mucoadhesive Tablets for Controlled Release of Acyclovir. Chem. Pharm. Bull. 2012, 60, 1249–1257. [Google Scholar] [CrossRef]
- Kumar, A.; Bharkatiya, M. A Recent Update on Formulation and Development of Gastro-Retentive Drug Delivery Systems. Int. J. Pharm. Sci. Nanotechnol. 2021, 14, 5257–5270. [Google Scholar] [CrossRef]
- Subramanian, P. Mucoadhesive Delivery System: A Smart Way to Improve Bioavailability of Nutraceuticals. Foods 2021, 10, 1362. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci 2011, 11, 748–764. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.; Rosenbaum, C.; Grimm, M.; Rump, A.; Keßler, R.; Hosten, N.; Weitschies, W. The EsoCap-System—An Innovative Platform to Drug Targeting in the Esophagus. J. Control. Release 2020, 327, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Verma, A.; Saraf, S.; Jain, A.; Tiwari, A.; Panda, P.K.; Jain, S.K. Mucoadhesive Gastroretentive Microparticulate System for Programmed Delivery of Famotidine and Clarithromycin. J. Microencapsul. 2021, 38, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, W.; Qian, L.; Zhang, X.; Zeng, P.; Pan, J. In Vitro and in Vivo Studies on Mucoadhesive Microspheres of Amoxicillin. J. Control. Release 2005, 102, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, A.; Vavia, P.R. Bioadhesive Ranitidine Hydrochloride for Gastroretention with Controlled Microenvironmental PH. Drug Dev. Ind. Pharm. 2008, 34, 860–869. [Google Scholar] [CrossRef]
- Mumuni, M.A.; Kenechukwu, F.C.; Ofokansi, K.C.; Attama, A.A.; Díaz, D.D. Insulin-Loaded Mucoadhesive Nanoparticles Based on Mucin-Chitosan Complexes for Oral Delivery and Diabetes Treatment. Carbohydr. Polym. 2020, 229, 115506. [Google Scholar] [CrossRef]
- Banerjee, A.; Lee, J.; Mitragotri, S. Intestinal Mucoadhesive Devices for Oral Delivery of Insulin. Bioeng. Transl. Med. 2016, 1, 338–346. [Google Scholar] [CrossRef]
- Déat-Lainé, E.; Hoffart, V.; Garrait, G.; Jarrige, J.F.; Cardot, J.M.; Subirade, M.; Beyssac, E. Efficacy of Mucoadhesive Hydrogel Microparticles of Whey Protein and Alginate for Oral Insulin Delivery. Pharm. Res. 2013, 30, 721–734. [Google Scholar] [CrossRef]
- Amaral, M.; Martins, A.S.; Catarino, J.; Faísca, P.; Kumar, P.; Pinto, J.F.; Pinto, R.; Correia, I.; Ascensão, L.; Afonso, R.A.; et al. How Can Biomolecules Improve Mucoadhesion of Oral Insulin? A Comprehensive Insight Using Ex-Vivo, in Silico, and in Vivo Models. Biomolecules 2020, 10, 675. [Google Scholar] [CrossRef]
- Banerjee, A.; Mitragotri, S. Intestinal Patch Systems for Oral Drug Delivery. Curr. Opin. Pharm. 2017, 36, 58–65. [Google Scholar] [CrossRef]
- Smart, J.D. Theories of Mucoadhesion. Mucoadhesive Mater. Drug Deliv. Syst. 2014, 9781119941, 159–174. [Google Scholar] [CrossRef]
- Singh, S.; Govind, M.; Bothara, S.B. A Review on in Vitro—In Vivo Mucoadhesive Strength Assessment. PhaTechMed 2013, 2, 221–229. [Google Scholar]
- Schattling, P.; Taipaleenmäki, E.; Zhang, Y.; Städler, B. A Polymer Chemistry Point of View on Mucoadhesion and Mucopenetration. Macromol. Biosci. 2017, 17, 1700060. [Google Scholar] [CrossRef] [PubMed]
- Bagan, J.; Paderni, C.; Termine, N.; Campisi, G.; lo Russo, L.; Compilato, D.; di Fede, O. Mucoadhesive Polymers for Oral Transmucosal Drug Delivery: A Review. Curr. Pharm. Des. 2012, 18, 5497–5514. [Google Scholar] [CrossRef]
- Smart, J.D. The Basics and Underlying Mechanisms of Mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Woertz, C.; Preis, M.; Breitkreutz, J.; Kleinebudde, P. Assessment of Test Methods Evaluating Mucoadhesive Polymers and Dosage Forms: An Overview. Eur. J. Pharm. Biopharm. 2013, 85, 843–853. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Bianco-Peled, H. Mucoadhesion: A Review of Characterization Techniques. Expert Opin. Drug Deliv. 2010, 7, 259–271. [Google Scholar] [CrossRef]
- Alaei, S.; Omidian, H. Mucoadhesion and Mechanical Assessment of Oral Films. Eur. J. Pharm. Sci. 2021, 159, 105727. [Google Scholar] [CrossRef]
- Drumond, N.; Stegemann, S. Polymer Adhesion Predictions for Oral Dosage Forms to Enhance Drug Administration Safety. Part 3: Review of in Vitro and in Vivo Methods Used to Predict Esophageal Adhesion and Transit Time. Colloids Surf. B Biointerfaces 2018, 165, 303–314. [Google Scholar] [CrossRef]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In Vitro Techniques to Evaluate Buccal Films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef]
- Hall, D.J.; Khutoryanskaya, O.V.; Khutoryanskiy, V.V. Developing Synthetic Mucosa-Mimetic Hydrogels to Replace Animal Experimentation in Characterisation of Mucoadhesive Drug Delivery Systems. Soft Matter 2011, 7, 9620–9623. [Google Scholar] [CrossRef]
- Alaei, S.; Omidi, Y.; Omidian, H. In Vitro Evaluation of Adhesion and Mechanical Properties of Oral Thin Films. Eur. J. Pharm. Sci. 2021, 166, 105965. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.B.; Khutoryanskiy, V.V.; Bruschi, M.L.; Cook, M.T. A Mucosa-Mimetic Material for the Mucoadhesion Testing of Thermogelling Semi-Solids. Int. J. Pharm. 2017, 528, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Smith, S.L.; Khutoryanskiy, V.V. Novel Glycopolymer Hydrogels as Mucosa-Mimetic Materials to Reduce Animal Testing. Chem. Commun. 2015, 51, 14447–14450. [Google Scholar] [CrossRef]
- Cook, M.T.; Khutoryanskiy, V.V. Mucoadhesion and Mucosa-Mimetic Materials—A Mini-Review. Int. J. Pharm. 2015, 495, 991–998. [Google Scholar] [CrossRef]
- Hägerström, H.; Edsman, K. Interpretation of Mucoadhesive Properties of Polymer. J. Pharm. Pharmacol. 2001, 53, 1589–1599. [Google Scholar] [CrossRef]
- Varum, F.J.O.; Veiga, F.; Sousa, J.S.; Basit, A.W. An Investigation into the Role of Mucus Thickness on Mucoadhesion in the Gastrointestinal Tract of Pig. Eur. J. Pharm. Sci. 2010, 40, 335–341. [Google Scholar] [CrossRef]
- do Couto, R.O.; Cubayachi, C.; Calefi, P.L.; Lopez, R.F.V.; Pedrazzi, V.; de Gaitani, C.M.; de Freitas, O. Combining Amino Amide Salts in Mucoadhesive Films Enhances Needle-Free Buccal Anesthesia in Adults. J. Control. Release 2017, 266, 205–215. [Google Scholar] [CrossRef]
- Gupta, V.; Hwang, B.H.; Lee, J.; Anselmo, A.C.; Doshi, N.; Mitragotri, S. Mucoadhesive Intestinal Devices for Oral Delivery of Salmon Calcitonin. J. Control. Release 2013, 172, 753–762. [Google Scholar] [CrossRef]
- Rohani Shirvan, A.; Hemmatinejad, N.; Bahrami, S.H.; Bashari, A. Fabrication of Multifunctional Mucoadhesive Buccal Patch for Drug Delivery Applications. J. Biomed. Mater. Res. A 2021, 109, 2640–2656. [Google Scholar] [CrossRef]
- Baus, R.A.; Haug, M.F.; Leichner, C.; Jelkmann, M.; Bernkop-Schnürch, A. In Vitro-in Vivo Correlation of Mucoadhesion Studies on Buccal Mucosa. Mol. Pharm. 2019, 16, 2719–2727. [Google Scholar] [CrossRef] [PubMed]
- Smart, J.D. An in Vitro Assessment of Some Mucosa-Adhesive Dosage Forms. Int. J. Pharm. 1991, 73, 69–74. [Google Scholar] [CrossRef]
- Park, H.; Robinson, J.R. Physico-Chemical Properties of Water Insoluble Polymers Important to Mucin/Epithelial Adhesion. J. Control. Release 1985, 2, 47–57. [Google Scholar] [CrossRef]
- Thirawong, N.; Nunthanid, J.; Puttipipatkhachorn, S.; Sriamornsak, P. Mucoadhesive Properties of Various Pectins on Gastrointestinal Mucosa: An in Vitro Evaluation Using Texture Analyzer. Eur. J. Pharm. Biopharm. 2007, 67, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, D.; Wahlgren, M. Comparison of in Vitro Methods of Measuring Mucoadhesion: Ellipsometry, Tensile Strength and Rheological Measurements. Colloids Surf. B Biointerfaces 2012, 92, 353–359. [Google Scholar] [CrossRef]
- Wong, C.F.; Yuen, K.H.; Peh, K.K. Formulation and Evaluation of Controlled Release Eudragit Buccal Patches. Int J Pharm 1999, 178, 11–22. [Google Scholar] [CrossRef]
- Göbel, A.; da Silva, J.B.; Cook, M.; Breitkreutz, J. Development of Buccal Film Formulations and Their Mucoadhesive Performance in Biomimetic Models. Int. J. Pharm. 2021, 610, 121233. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.F.; Yuen, K.H.; Peh, K.K. An In-Vitro Method for Buccal Adhesion Studies: Importance of Instrument Variables. Int. J. Pharm. 1999, 180, 47–57. [Google Scholar] [CrossRef]
- Estrellas, K.M.; Fiecas, M.; Azagury, A.; Laulicht, B.; Cho, D.Y.; Mancini, A.; Reineke, J.; Furtado, S.; Mathiowitz, E. Time-Dependent Mucoadhesion of Conjugated Bioadhesive Polymers. Colloids Surf. B Biointerfaces 2019, 173, 454–469. [Google Scholar] [CrossRef]
- Grillet, A.M.; Wyatt, N.B.; Gloe, L.M. Polymer Gel Rheology and Adhesion. In Rheology; InTech: London, UK, 2012. [Google Scholar]
- Shojaei, A.H.; Paulson, J.; Honary, S. Evaluation of Poly(Acrylic Acid-Co-Ethylhexyl Acrylate) Films for Mucoadhesive Transbuccal Drug Delivery: Factors Affecting the Force of Mucoadhesion. J. Control. Release 2000, 67, 223–232. [Google Scholar] [CrossRef]
- Divoux, T.; Mao, B.; Snabre, P. Syneresis and Delayed Detachment in Agar Plates. Soft Matter 2015, 11, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Dillard, D.A.; Pocius, A.v. (Eds.) The Mechanics of Adhesion; Elsevier Science B. V.: Amsterdam, The Netherlands, 2002; ISBN 0912659998. [Google Scholar]
- Dahlgren, D.; Venczel, M.; Ridoux, J.P.; Skjöld, C.; Müllertz, A.; Holm, R.; Augustijns, P.; Hellström, P.M.; Lennernäs, H. Fasted and Fed State Human Duodenal Fluids: Characterization, Drug Solubility, and Comparison to Simulated Fluids and with Human Bioavailability. Eur. J. Pharm. Biopharm. 2021, 163, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Baraibar, M.A.; Schoning, P. Effects of Freezing and Frozen Storage on Histological Characteristics of Canine Tissues. J. Forensic. Sci. 1985, 30, 11823J. [Google Scholar] [CrossRef]
- Mortazavi, S.A.; Smart, J.D. An Investigation of Some Factors Influencing the in Vitro Assessment of Mucoadhesion. Int. J. Pharm. 1995, 116, 223–230. [Google Scholar] [CrossRef]
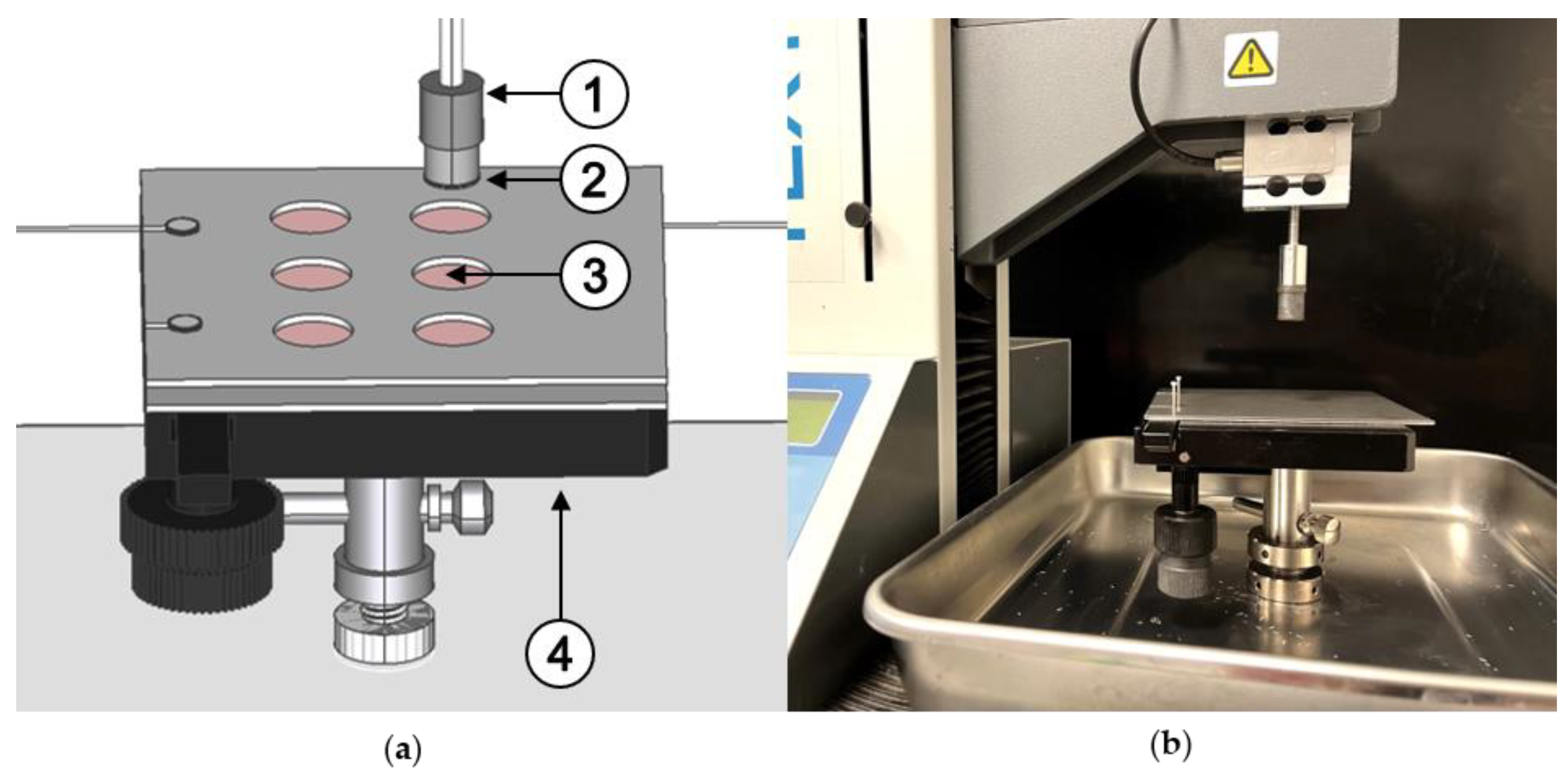
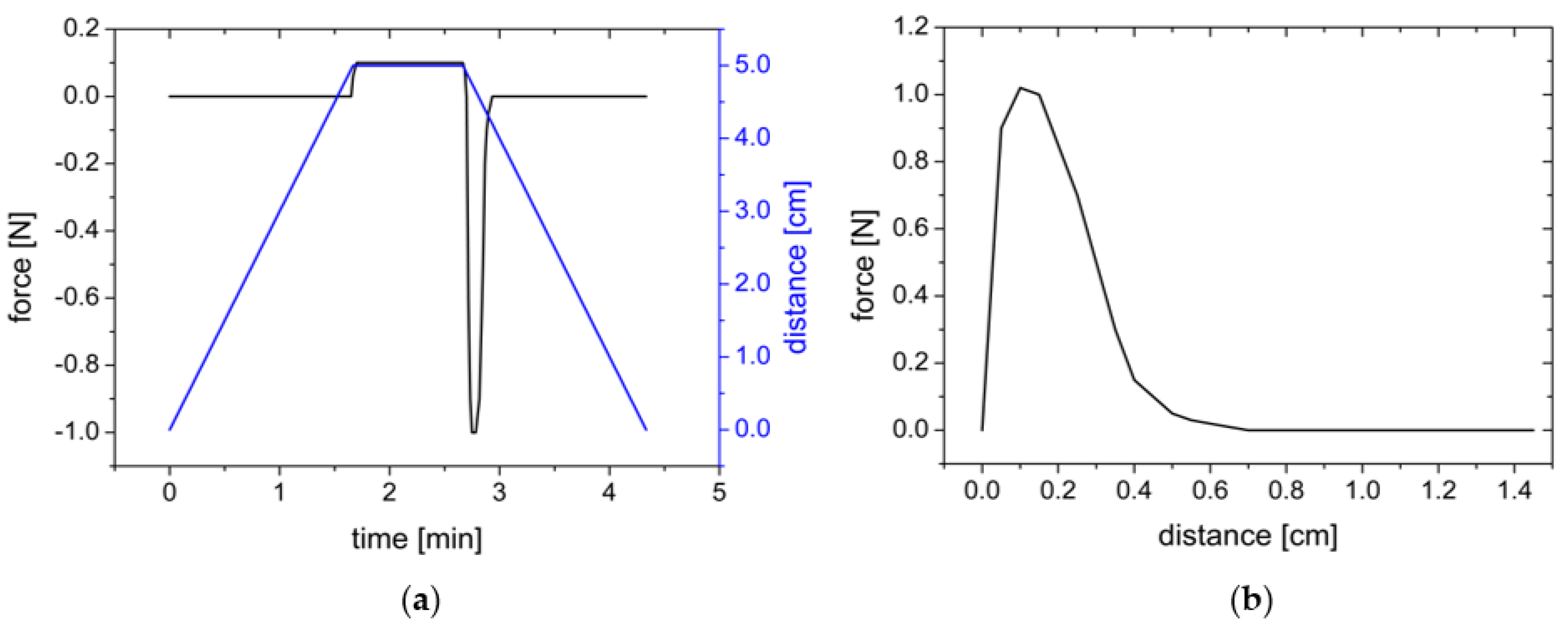

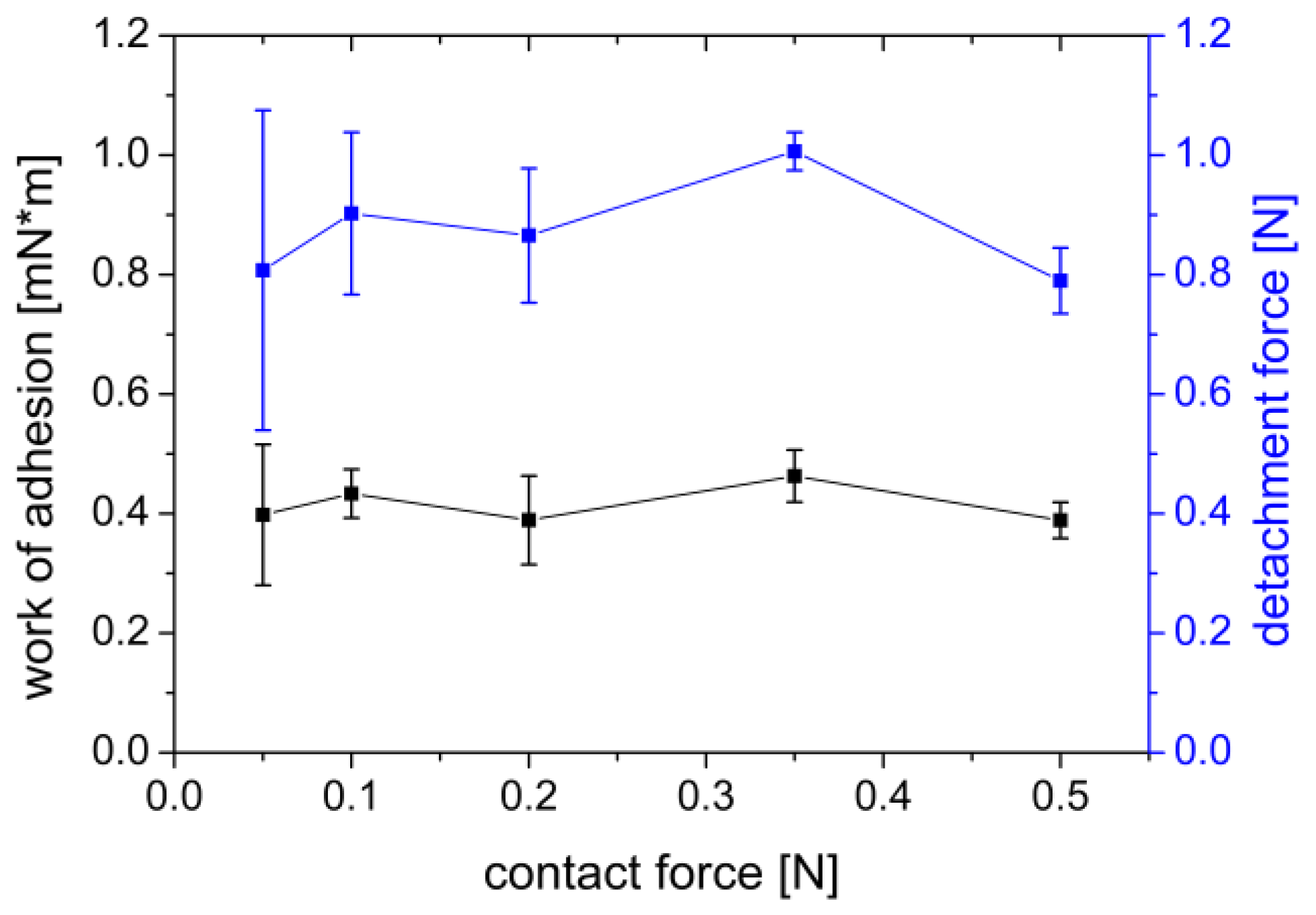
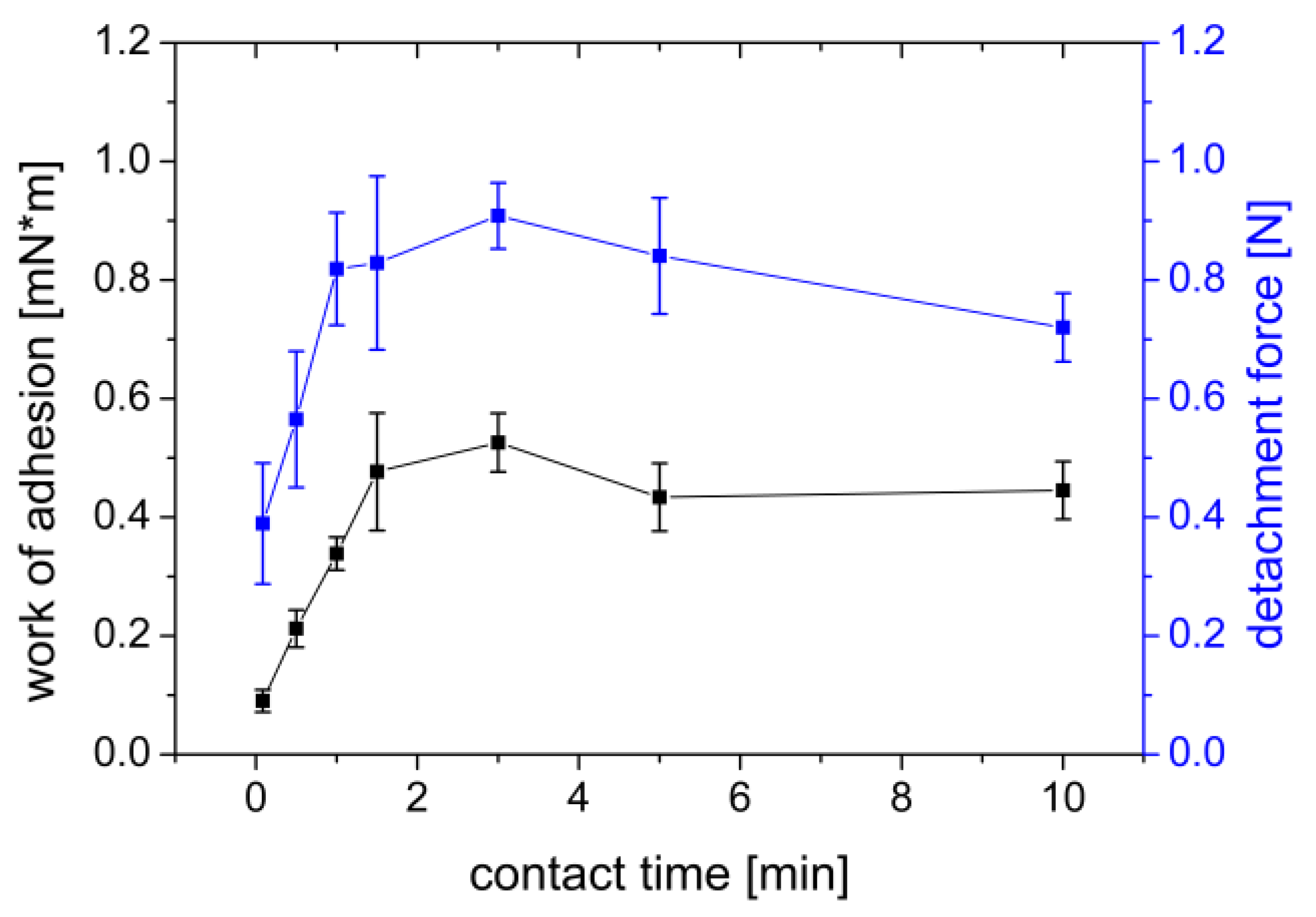

| Sample | Settings | Contact Force | Contact Time | Withdrawal Speed | Sample Area | Liquid | Storage Time | Processing |
|---|---|---|---|---|---|---|---|---|
| N | s | mm/s | mm2 | mL | d | |||
| gel | standard | 0.10 | 60 | 0.50 | 153.94 | - | - | - |
| f1 | 0.05 | 60 | 0.50 | 153.94 | - | - | - | |
| f2 | 0.10 | |||||||
| f3 | 0.20 | |||||||
| f4 | 0.35 | |||||||
| f5 | 0.50 | |||||||
| t1 | 0.10 | 5 | 0.50 | 153.94 | - | - | - | |
| t2 | 30 | |||||||
| t3 | 60 | |||||||
| t4 | 90 | |||||||
| t5 | 180 | |||||||
| t6 | 300 | |||||||
| t7 | 600 | |||||||
| w1 | 0.10 | 60 | 0.10 | 153.94 | - | - | - | |
| w2 | 0.25 | |||||||
| w3 | 0.50 | |||||||
| w4 | 1.00 | |||||||
| w5 | 2.00 | |||||||
| a1 | 0.10 | 60 | 0.50 | 38.48 | - | - | - | |
| a2 | 78.54 | |||||||
| a3 | 153.94 | |||||||
| l1 | 0.10 | 60 | 0.50 | 153.94 | 0.00 | - | - | |
| l2 | 2.50 | |||||||
| l3 | 5.00 | |||||||
| l4 | 10.00 | |||||||
| s1 | 0.10 | 60 | 0.50 | 153.94 | - | - | - | |
| s2 | 1 | |||||||
| s3 | 7 | |||||||
| s4 | 14 | |||||||
| optimized * | 0.35 | 180 | 1.00 | 153.94 | - | - | - | |
| tissue | optimized1 * | 0.35 | 180 | 1.00 | 153.94 | - | - | unprocessed |
| optimized2 * | - | cleaned | ||||||
| optimized3 * | 7 | thawed |
| Wad | Fmax | ||
|---|---|---|---|
| mN×m | N | ||
| pig #1 | unprocessed | 0.337 ± 0.058 | 0.147 ± 0.020 |
| cleaned | 0.553 ± 0.168 | 0.144 ± 0.041 | |
| Thawed | 1.046 ± 0.737 | 0.217 ± 0.168 | |
| pig #2 | unprocessed | 0.443 ± 0.090 | 0.111 ± 0.009 |
| cleaned | 0.599 ± 0.260 | 0.121 ± 0.050 | |
| thawed | 1.190 ± 0.307 | 0.190 ± 0.068 | |
| pig #3 | unprocessed | 1.467 ± 0.380 | 0.292 ± 0.180 |
| cleaned | 0.402 ± 0.101 | 0.098 ± 0.025 | |
| thawed | 0.766 ± 0.283 | 0.165 ± 0.054 | |
| agar/mucin gel | 0.730 ± 0.122 | 1.104 ± 0.060 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, L.; Rosenbaum, C.; Krause, J.; Weitschies, W. Characterization of an In Vitro/Ex Vivo Mucoadhesiveness Measurement Method of PVA Films. Polymers 2022, 14, 5146. https://doi.org/10.3390/polym14235146
Müller L, Rosenbaum C, Krause J, Weitschies W. Characterization of an In Vitro/Ex Vivo Mucoadhesiveness Measurement Method of PVA Films. Polymers. 2022; 14(23):5146. https://doi.org/10.3390/polym14235146
Chicago/Turabian StyleMüller, Laura, Christoph Rosenbaum, Julius Krause, and Werner Weitschies. 2022. "Characterization of an In Vitro/Ex Vivo Mucoadhesiveness Measurement Method of PVA Films" Polymers 14, no. 23: 5146. https://doi.org/10.3390/polym14235146
APA StyleMüller, L., Rosenbaum, C., Krause, J., & Weitschies, W. (2022). Characterization of an In Vitro/Ex Vivo Mucoadhesiveness Measurement Method of PVA Films. Polymers, 14(23), 5146. https://doi.org/10.3390/polym14235146











