Active Films Based on Starch and Wheat Gluten (Triticum vulgare) for Shelf-Life Extension of Carrots
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
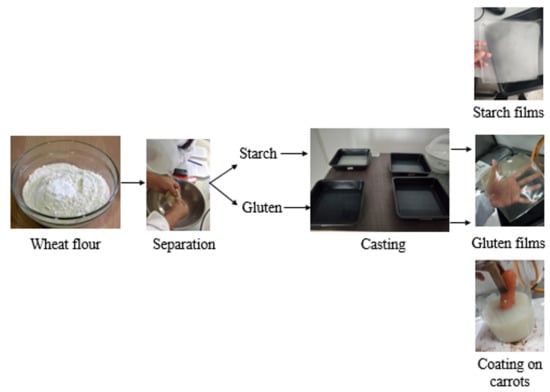
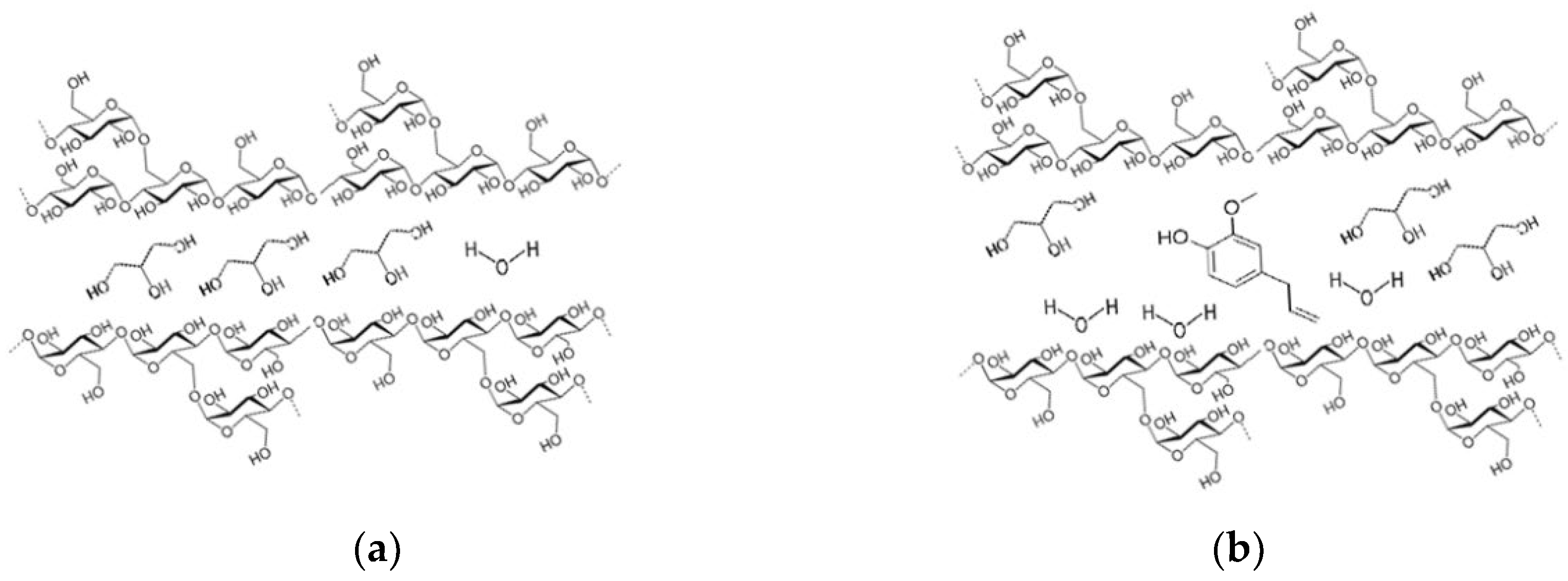
2.2. Wheat Starch and Gluten Extraction
2.3. Active Film Development
2.4. Film Characterization
2.4.1. Thickness
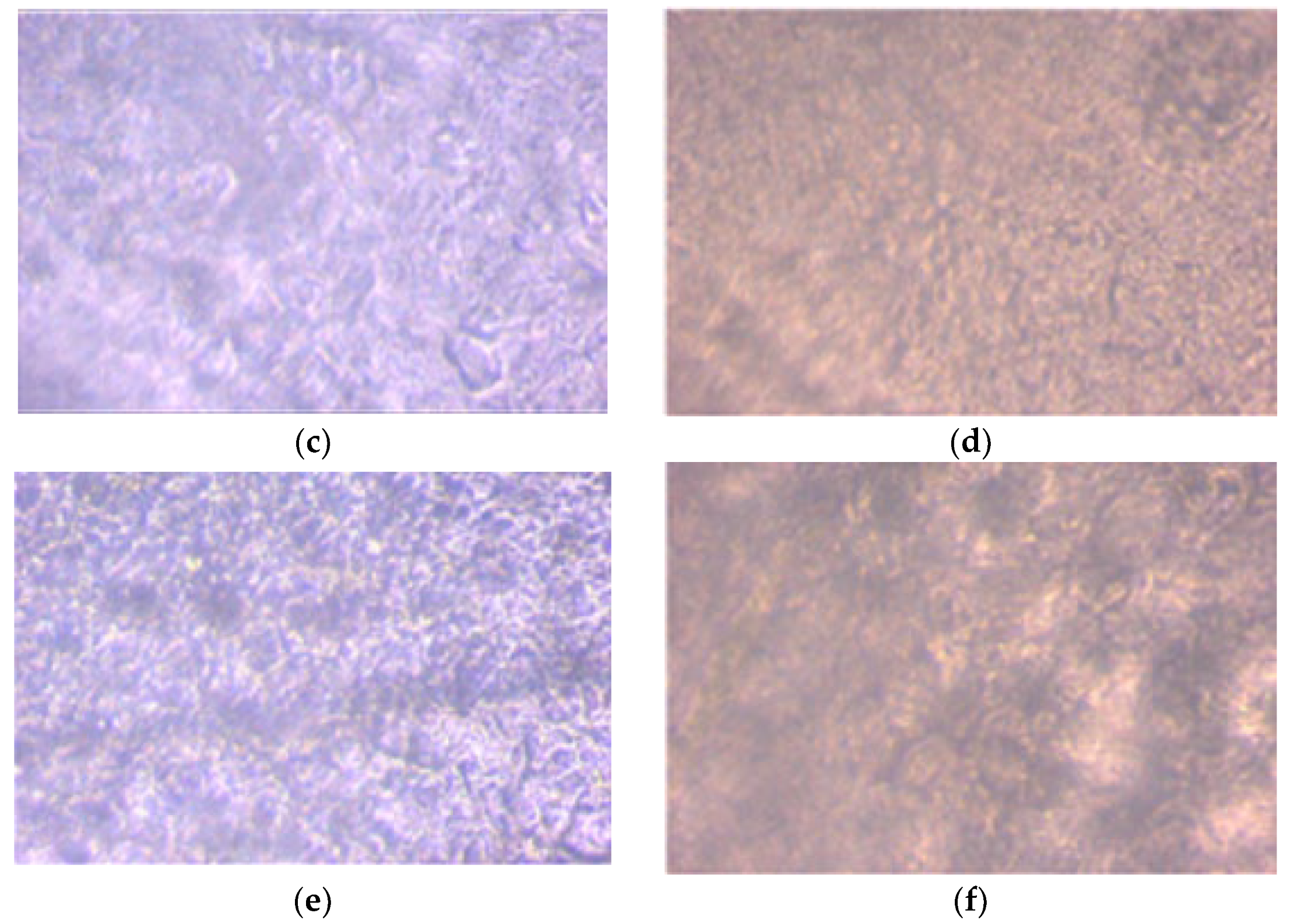
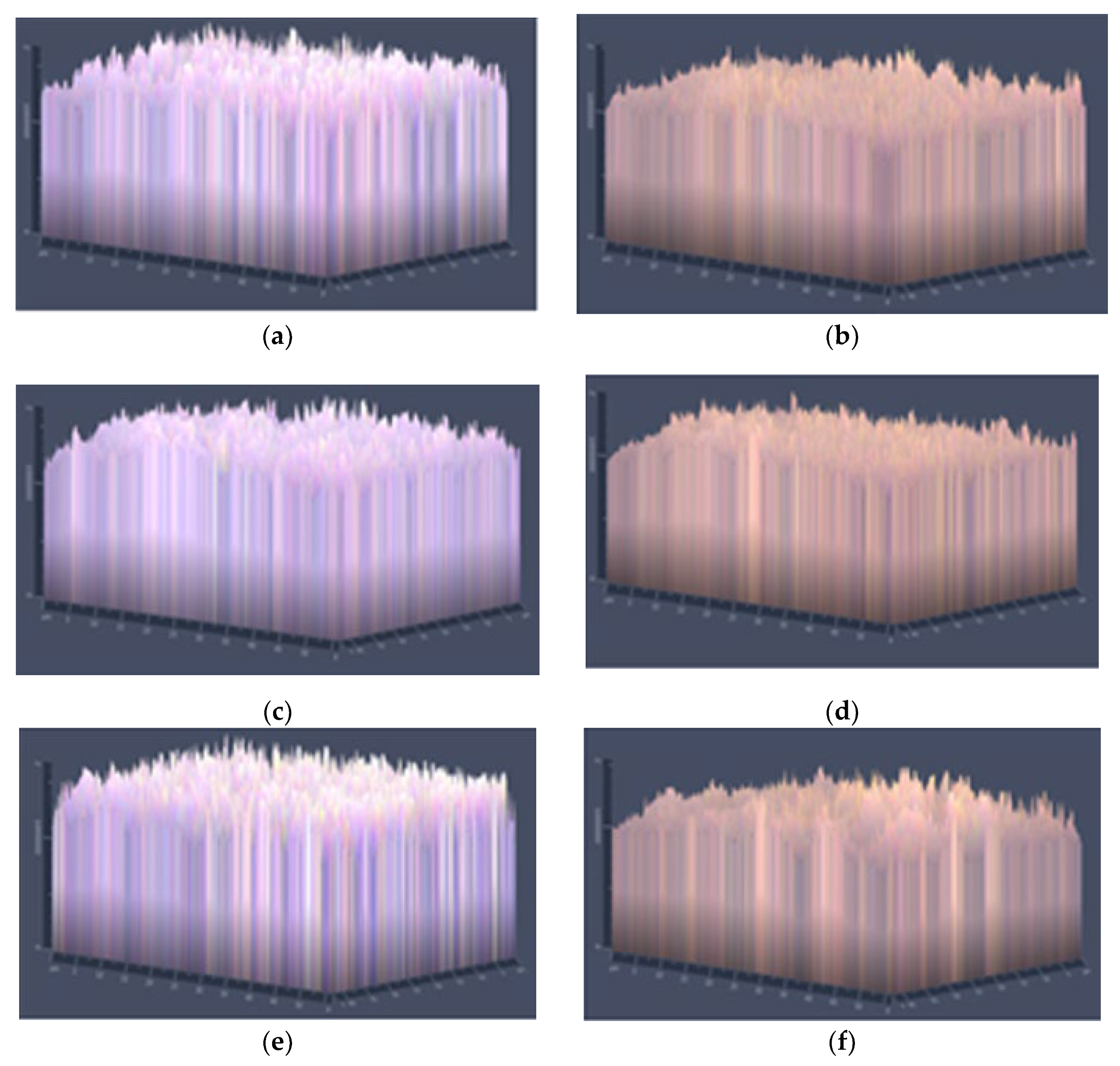
2.4.2. Morphology
2.4.3. Color
2.4.4. Moisture Content
2.4.5. Water Solubility
2.4.6. Water Absorption Capacity
2.4.7. Water Vapor Permeability (WVP)
2.4.8. Contact Angle
2.4.9. Thermal Properties
2.4.10. Antioxidant Activity
2.5. Application of Active Films on Baby Carrots
2.6. Statistical Analysis
3. Results
3.1. Active Films Characterization
3.1.1. Thickness
3.1.2. Morphology
3.1.3. Color Parameters
3.1.4. Moisture, Water Solubility and Water Absorption Capacity
3.1.5. Water Vapor Permeability
3.1.6. Contact Angle
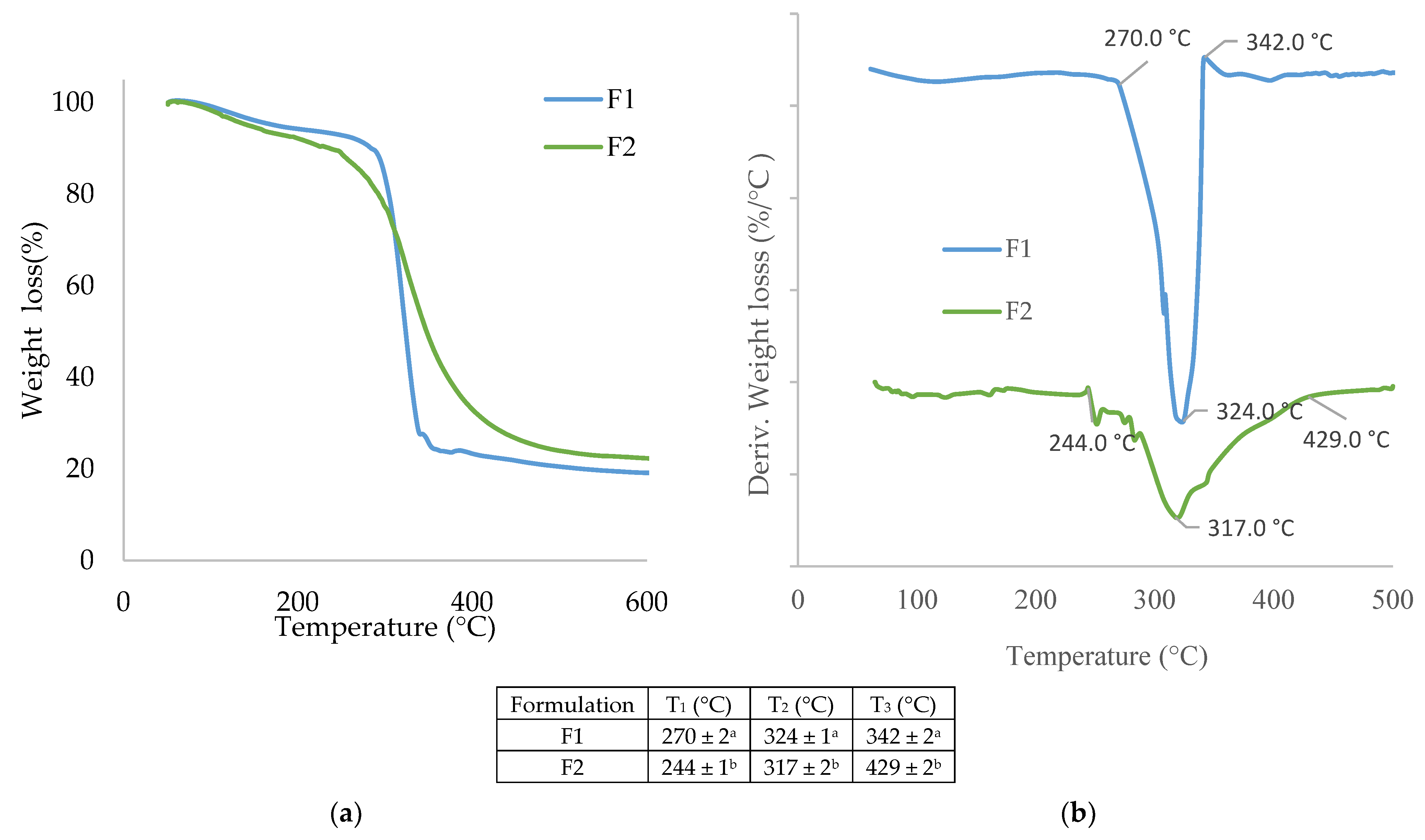
3.1.7. Thermal Properties
3.2. Active Properties
Antioxidant Activity
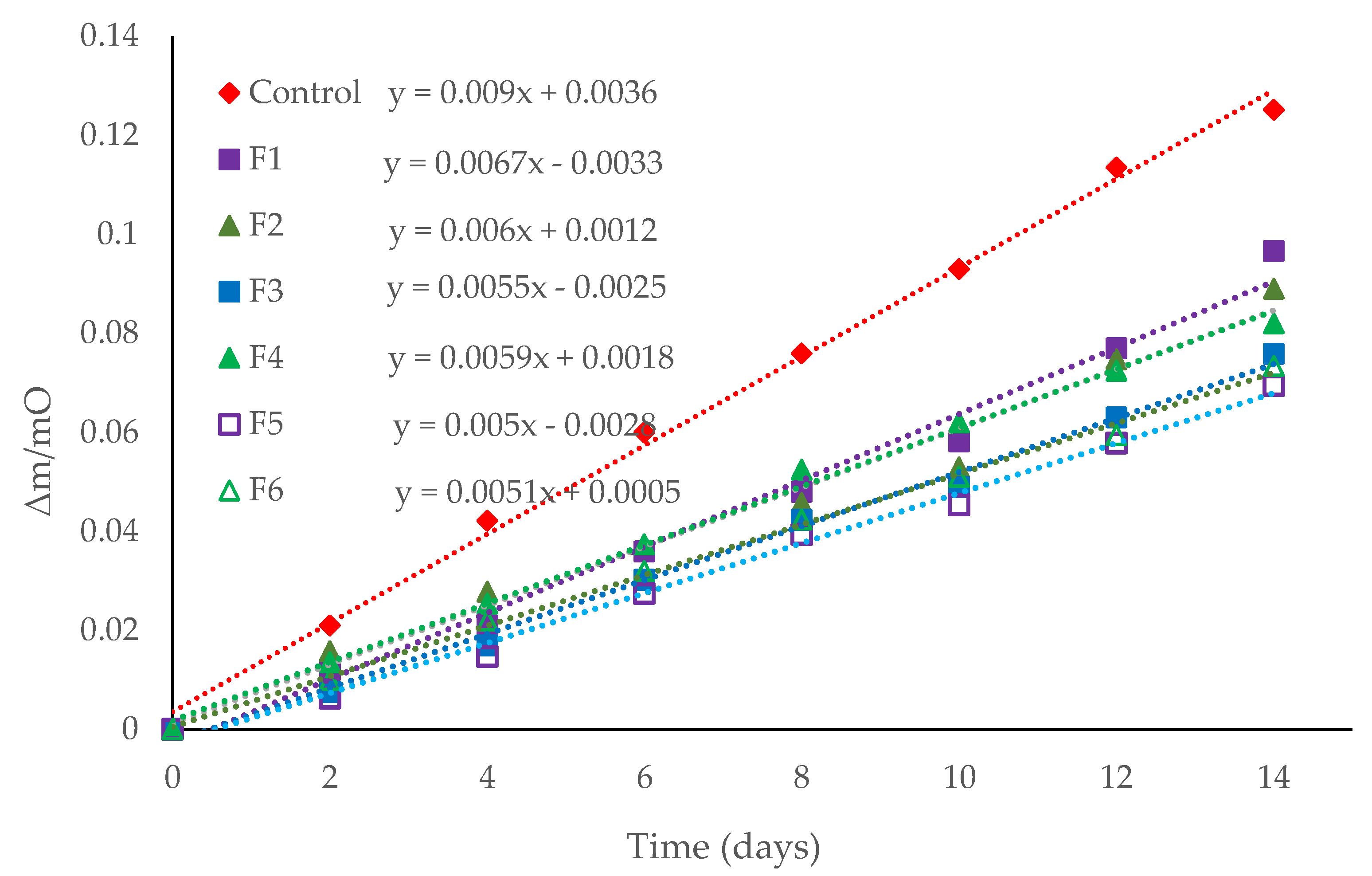
3.3. Coating Application on Baby Carrots
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lam, L.; Rovina, K.; Merillyn, J.; Matanjun, P.; Husna, K.; Nur, N.; Ling, W.; Funk, A. The Emergence of Edible and Food-Application Coatings for Food Packaging: A Review. Molecules 2022, 27, 5604. [Google Scholar] [CrossRef]
- Byun, Y.; Darby, D.; Cooksey, K.; Dawson, P.; Whiteside, S. Development of Oxygen Scavenging System Containing a Natural Free Radical Scavenger and a Transition Metal. Food Chem. 2011, 124, 615–619. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; López-Martínez, J.; Hernández-Fernández, J.; Arrieta, M.P. Films Based on Thermoplastic Starch Blended with Pine Resin Derivatives for Food Packaging. Foods 2021, 10, 1171. [Google Scholar] [CrossRef] [PubMed]
- Hatti-Kaul, R.; Nilsson, L.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol. 2019, 38, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.N. Biodegradable Films and Composite Coatings: Past, Present and Future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Pascuta, M.; Varvara, R.; Teleky, B.; Szabo, K.; Plamada, D.; Nemes, S.; Mitrea, L.; Martau, G.; Ciont, C.; Calinoiu, L.; et al. Polysaccharide-Based Edible Gels as Functional Ingredients: Characterization, Applicability, and Human Health Benefits. Gels 2022, 8, 524. [Google Scholar] [CrossRef]
- Moreno, O.; Atarés, L.; Chiralt, A. Effect of the Incorporation of Antimicrobial/Antioxidant Proteins on the Properties of Potato Starch Films. Carbohydr. Polym. 2015, 133, 353–364. [Google Scholar] [CrossRef]
- Shahbazi, M.; Majzoobi, M.; Farahnaky, A. Impact of Shear Force on Functional Properties of Native Starch and Resulting Gel and Film. J. Food Eng. 2018, 223, 10–21. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; Hernández-Fernández, J.; López-Martínez, J. Comparative Characterization of Gum Rosins for Their Use as Sustainable Additives in Polymeric Matrices. J. Appl. Polym. Sci. 2022, 139, 51734. [Google Scholar] [CrossRef]
- Joaquin, H.-F.; Juan, L.-M. Autocatalytic Influence of Different Levels of Arsine on the Thermal Stability and Pyrolysis of Polypropylene. J. Anal. Appl. Pyrolysis 2022, 161, 105385. [Google Scholar] [CrossRef]
- Rammak, T.; Boonsuk, P.; Kaewtatip, K. Mechanical and Barrier Properties of Starch Blend Films Enhanced with Kaolin for Application in Food Packaging. Int. J. Biol. Macromol. 2021, 192, 1013–1020. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Ashogbon, A.O.; Kumar, M. Recent Advances in Thermoplastic Starches for Food Packaging: A Review. Food Packag. Shelf Life 2021, 30, 100743. [Google Scholar] [CrossRef]
- Zubeldía, F.; Ansorena, M.R.; Marcovich, N.E. Wheat Gluten Films Obtained by Compression Molding. Polym. Test. 2015, 43, 68–77. [Google Scholar] [CrossRef]
- Rovera, C.; Türe, H.; Hedenqvist, M.S.; Farris, S. Water Vapor Barrier Properties of Wheat Gluten/Silica Hybrid Coatings on Paperboard for Food Packaging Applications. Food Packag. Shelf Life 2020, 26, 100561. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Torres-Giner, S.; Enescu, D.; Cabedo, L.; Cerqueira, M.A.; Pastrana, L.M.; Lagaron, J.M. Electrospun Active Biopapers of Food Waste Derived Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) with Short-Term and Long-Term Antimicrobial Performance. Nanomaterials 2020, 10, 506. [Google Scholar] [CrossRef]
- Gómez-Contreras, P.; Figueroa-Lopez, K.J.; Hernández-Fernández, J.; Cortés Rodríguez, M.; Ortega-Toro, R. Effect of Different Essential Oils on the Properties of Edible Coatings Based on Yam (Dioscorea rotundata L.) Starch and Its Application in Strawberry (Fragaria vesca L.) Preservation. Appl. Sci. 2021, 11, 11057. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Deans, S.G.; Ritchie, G. Antibacterial Properties of Plant Essential Oils. Int. J. Food Microbiol. 1987, 5, 165–180. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Encapsulation Systems for Antimicrobial Food Packaging Components: An Update. Molecules 2020, 25, 1134. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Madella, D.; Martinazzo, A.P.; de Aquino Garcia Moura, L.; de Melo, N.R.; Sanches-Silva, A. Revisiting an Ancient Spice with Medicinal Purposes: Cinnamon. Trends Food Sci. Technol. 2017, 62, 154–169. [Google Scholar] [CrossRef]
- da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical Composition, Extraction Sources and Action Mechanisms of Essential Oils: Natural Preservative and Limitations of Use in Meat Products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Lopez-Martinez, J.; Barceló, D. Quantification and Elimination of Substituted Synthetic Phenols and Volatile Organic Compounds in the Wastewater Treatment Plant during the Production of Industrial Scale Polypropylene. Chemosphere 2021, 263, 128027. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J. Quantification of Oxygenates, Sulphides, Thiols and Permanent Gases in Propylene. A Multiple Linear Regression Model to Predict the Loss of Efficiency in Polypropylene Production on an Industrial Scale. J. Chromatogr. A 2020, 1628, 461478. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial Mechanisms of Cinnamon and Its Constituents: A Review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological Activities of Curcuminoids, Other Biomolecules from Turmeric and Their Derivatives—A Review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.; Gimenez, E.; Lagaron, J. Morphology and barrier properties of solvent cast composites of thermoplastic biopolymers and purified cellulose fibers. Carbohydr. Polymers 2008, 71, 235–244. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film Formation and Deposition Methods of Edible Coating on Food Products: A Review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Mohanty, A.K.; Singh, S.P.; Ng, P.K.W. Effect of the Processing Methods on the Performance of Polylactide Films: Thermocompression versus Solvent Casting. J. Appl. Polym. Sci. 2006, 101, 3736–3742. [Google Scholar] [CrossRef]
- Chacon, H.; Cano, H.; Fernández, J.H.; Guerra, Y.; Puello-Polo, E.; Ríos-Rojas, J.F.; Ruiz, Y. Effect of Addition of Polyurea as an Aggregate in Mortars: Analysis of Microstructure and Strength. Polymers 2022, 14, 1753. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and Incorporation of Functional Ingredients in Edible Films and Coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of Starch Type on the Physico-Chemical Properties of Edible Films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- Development and Application of a Principal Component Analysis Model to Quantify the Green Ethylene Content in Virgin Impact Copolymer Resins During Their Synthesis on an Industrial Scale|SpringerLink. Available online: https://link.springer.com/article/10.1007/s10924-022-02557-4 (accessed on 24 October 2022).
- Olabarrieta, I.; Cho, S.-W.; Gällstedt, M.; Sarasua, J.-R.; Johansson, E.; Hedenqvist, M.S. Aging Properties of Films of Plasticized Vital Wheat Gluten Cast from Acidic and Basic Solutions. Biomacromolecules 2006, 7, 1657–1664. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Jiménez, A.; Talens, P.; Chiralt, A. Properties of Starch–Hydroxypropyl Methylcellulose Based Films Obtained by Compression Molding. Carbohydr. Polym. 2014, 109, 155–165. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Vicente, A.A.; Reis, M.A.M.; Torres-Giner, S.; Lagaron, J.M. Antimicrobial and Antioxidant Performance of Various Essential Oils and Natural Extracts and Their Incorporation into Biowaste Derived Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Layers Made from Electrospun Ultrathin Fibers. Nanomaterials 2019, 9, 144. [Google Scholar] [CrossRef]
- Wang, L.; Auty, M.A.E.; Kerry, J.P. Physical Assessment of Composite Biodegradable Films Manufactured Using Whey Protein Isolate, Gelatin and Sodium Alginate. J. Food Eng. 2010, 96, 199–207. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, X.; Chen, J.; He, J. Effects of Cinnamon Essential Oil on the Physical, Mechanical, Structural and Thermal Properties of Cassava Starch-Based Edible Films. Int. J. Biol. Macromol. 2021, 184, 574–583. [Google Scholar] [CrossRef]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.M.H.; Imani, A. Development of Bioactive Composite Films from Chitosan and Carboxymethyl Cellulose Using Glutaraldehyde, Cinnamon Essential Oil and Oleic Acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef]
- Dong, M.; Tian, L.; Li, J.; Jia, J.; Dong, Y.; Tu, Y.; Liu, X.; Tan, C.; Duan, X. Improving Physicochemical Properties of Edible Wheat Gluten Protein Films with Proteins, Polysaccharides and Organic Acid. LWT 2022, 154, 112868. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Guerra, Y.; Puello-Polo, E.; Marquez, E. Effects of Different Concentrations of Arsine on the Synthesis and Final Properties of Polypropylene. Polymers 2022, 14, 3123. [Google Scholar] [CrossRef]
- Owens, D.K. Some thermodynamic aspects of polymer adhesion. J. Appl. Polym. Sci. 1970, 14, 1725–1730. [Google Scholar] [CrossRef]
- Arezoo, E.; Mohammadreza, E.; Maryam, M.; Abdorreza, M.N. The Synergistic Effects of Cinnamon Essential Oil and Nano TiO2 on Antimicrobial and Functional Properties of Sago Starch Films. Int. J. Biol. Macromol. 2020, 157, 743–751. [Google Scholar] [CrossRef] [PubMed]
- do Evangelho, J.A.; da Silva Dannenberg, G.; Biduski, B.; el Halal, S.L.M.; Kringel, D.H.; Gularte, M.A.; Fiorentini, A.M.; da Rosa Zavareze, E. Antibacterial Activity, Optical, Mechanical, and Barrier Properties of Corn Starch Films Containing Orange Essential Oil. Carbohydr. Polym. 2019, 222, 114981. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-Based Composite Edible Films Containing Origanum vulgare L. Essential Oil. Ind. Crops Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- Kavoosi, G.; Rahmatollahi, A.; Mohammad Mahdi Dadfar, S.; Mohammadi Purfard, A. Effects of Essential Oil on the Water Binding Capacity, Physico-Mechanical Properties, Antioxidant and Antibacterial Activity of Gelatin Films. LWT—Food Sci. Technol. 2014, 57, 556–561. [Google Scholar] [CrossRef]
- Sun, S.; Song, Y.; Zheng, Q. Morphologies and Properties of Thermo-Molded Biodegradable Plastics Based on Glycerol-Plasticized Wheat Gluten. Food Hydrocoll. 2007, 21, 1005–1013. [Google Scholar] [CrossRef]
- Hernández Fernández, J.; Cano, H.; Guerra, Y.; Puello Polo, E.; Ríos-Rojas, J.F.; Vivas-Reyes, R.; Oviedo, J. Identification and Quantification of Microplastics in Effluents of Wastewater Treatment Plant by Differential Scanning Calorimetry (DSC). Sustainability 2022, 14, 4920. [Google Scholar] [CrossRef]
- Fernández, J.H.; Guerra, Y.; Cano, H. Detection of Bisphenol A and Four Analogues in Atmospheric Emissions in Petrochemical Complexes Producing Polypropylene in South America. Molecules 2022, 27, 4832. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Mendieta, J.R.; Ortega-Toro, R. In-Depth Study from Gluten/PCL-Based Food Packaging Films Obtained under Reactive Extrusion Conditions Using Chrome Octanoate as a Potential Food Grade Catalyst. Food Hydrocoll. 2021, 111, 106255. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Al-Maqtari, Q.A.; Mohammed, J.K.; Al-Ansi, W.; Cui, H.; Lin, L. Enhancement of Antioxidant Activity, Antifungal Activity, and Oxidation Stability of Citrus Reticulata Essential Oil Nanocapsules by Clove and Cinnamon Essential Oils. Food Biosci. 2021, 43, 101226. [Google Scholar] [CrossRef]
- Surendhiran, D.; Roy, V.C.; Park, J.-S.; Chun, B.-S. Fabrication of Chitosan-Based Food Packaging Film Impregnated with Turmeric Essential Oil (TEO)-Loaded Magnetic-Silica Nanocomposites for Surimi Preservation. Int. J. Biol. Macromol. 2022, 203, 650–660. [Google Scholar] [CrossRef]
- Keshari, D.; Tripathi, A.D.; Agarwal, A.; Rai, S.; Srivastava, S.K.; Kumar, P. Effect of α-Dl Tocopherol Acetate (Antioxidant) Enriched Edible Coating on the Physicochemical, Functional Properties and Shelf Life of Minimally Processed Carrots (Daucus carota subsp. Sativus). Future Foods 2022, 5, 100116. [Google Scholar] [CrossRef]
- Ben-Fadhel, Y.; Cingolani, M.C.; Li, L.; Chazot, G.; Salmieri, S.; Horak, C.; Lacroix, M. Effect of γ-Irradiation and the Use of Combined Treatments with Edible Bioactive Coating on Carrot Preservation. Food Packag. Shelf Life 2021, 28, 100635. [Google Scholar] [CrossRef]

| Films | Starch | Wheat Gluten | Glycerol | Cinnamon Essential Oil (EOCN) | Turmeric Essential Oil (EOTU) |
|---|---|---|---|---|---|
| F1 | 0.800 | 0.000 | 0.200 | 0.000 | 0.000 |
| F2 | 0.000 | 0.800 | 0.200 | 0.000 | 0.000 |
| F3 | 0.782 | 0.000 | 0.195 | 0.023 | 0.000 |
| F4 | 0.000 | 0.782 | 0.195 | 0.023 | 0.000 |
| F5 | 0.782 | 0.000 | 0.195 | 0.000 | 0.023 |
| F6 | 0.000 | 0.782 | 0.195 | 0.000 | 0.023 |
| Film | Thickness (µm) | L * | a * | b * | h * | C * | ΔE |
|---|---|---|---|---|---|---|---|
| F1 | 102 ± 11 b | 91.3 ± 1.2 c | −1.16 ± 0.03 d | 5.62 ± 0.12 c | 101.7 ± 0.5 c | 5.7 ± 0.2 c | --- |
| F2 | 91 ± 13 a | 38.1 ± 0.3 b | −0.67 ± 0.02 c | 6.79 ± 0.08 e | 95.6 ± 0.6 b | 6.8 ± 0.2 d | --- |
| F3 | 98 ± 9 ab | 36.1 ± 0.2 b | −0.48 ± 0.03 b | −0.05 ± 0.02 a | 264 ± 2 d | 0.48 ± 0.05 a | 55.49 ± 0.5 c* |
| F4 | 100 ± 16 b | 37.8 ± 0.3 b | −0.47 ± 0.04 b | 6.16 ± 0.03 d | 94.4 ± 0.5 b | 6.2 ± 0.9 cd | 0.70 ± 0.02 a** |
| F5 | 82 ± 14 a | 31.6 ± 0.3 a | −0.27 ± 0.02 a | 0.61 ± 0.03 b | 114.2 ± 0.6 d | 0.66 ± 0.02 b | 59.928 ± 0.3 d* |
| F6 | 111 ± 12 b | 37.2 ± 0.2 b | −0.21 ± 0.02 a | 8.72 ± 0.04 f | 91.3 ± 0.4 a | 8.72 ± 0.05 e | 2.15 ± 0.05 b** |
| Film | MC (%) | WS (%) | WAC (%) |
|---|---|---|---|
| F1 | 11.58 ± 1.17 b | 45.7±12.8 b | 0.86 ± 0.03 a |
| F2 | 11.21 ± 0.22 b | 46.4 ± 9.4 b | 1.07 ± 0.54 b |
| F3 | 10.81 ± 0.51 ab | 38.1 ± 4.9 a | 1.24 ± 0.52 b |
| F4 | 11.32 ± 0.28 b | 52.5 ± 1.6 b | 1.36 ± 0.06 b |
| F5 | 10.04 ± 0.27 a | 32.7 ± 3.1 a | 1.22 ± 0.13 b |
| F6 | 10.74 ± 0.62 ab | 48.4 ± 4.3 b | 0.91 ± 0.14 a |
| Film | WVP (g·mm/kPa·h·m2) | CA-10s (°) | CA-30s (°) |
|---|---|---|---|
| F1 | 1.60 ± 0.10 b | 35.9 ± 0.7 a | 36.8 ± 0.7 a |
| F2 | 1.44 ± 0.09 a | 38.70 ± 1.01 b | 39.4 ± 0.8 b |
| F3 | 1.53 ± 0.09 ab | 36.8 ± 0.3 a | 37.6 ± 0.3 a |
| F4 | 1.57 ± 0.09 b | 39.1 ± 0.2 b | 39.9 ± 0.3 b |
| F5 | 1.29 ± 0.08 a | 36.9 ± 0.4 a | 37.8 ± 0.3 a |
| F6 | 1.75 ± 0.11 b | 38.9 ± 0.5 b | 39.9 ± 0.3 b |
| Sample | DPPH IC50 (EO mL/DPPH mg) | ABTS IC50 (EO mL/DPPH mg) |
|---|---|---|
| EOCN | 146.6 ± 1.5 a | 28.4 ± 0.5 a |
| EOTU | 14.2 ± 0.8 b | 2.62 ± 0.13 b |
| DPPH IC50 (film mg/DPPH mg) | ABTS IC50 (film mg/DPPH mg) | |
| F3 | 191.4 ± 1.3 c | 53.2 ± 0.8 c |
| F4 | 213 ± 1 d | 67.6 ± 0.9 d |
| F5 | 45.6 ± 0.9 a | 28.6 ± 0.5 a |
| F6 | 60.2 ± 0.8 b | 38.4 ± 0.5 b |
| Day | Films | Visual Aspect (%) | Softening (%) | Presence of Fungi |
|---|---|---|---|---|
| 0 | Control | 100 | 0 | 0 |
| F1 | 100 | 0 | 0 | |
| F2 | 100 | 0 | 0 | |
| F3 | 100 | 0 | 0 | |
| F4 | 100 | 0 | 0 | |
| F5 | 100 | 0 | 0 | |
| F6 | 100 | 0 | 0 | |
| 3 | Control | 60 | 40 | 0 |
| F1 | 60 | 40 | 0 | |
| F2 | 60 | 40 | 0 | |
| F3 | 80 | 20 | 0 | |
| F4 | 80 | 20 | 0 | |
| F5 | 100 | 0 | 0 | |
| F6 | 100 | 0 | 0 | |
| 6 | Control | 20 | 80 | 40 |
| F1 | 60 | 40 | 40 | |
| F2 | 60 | 40 | 20 | |
| F3 | 80 | 20 | 0 | |
| F4 | 80 | 20 | 0 | |
| F5 | 80 | 20 | 0 | |
| F6 | 100 | 0 | 0 | |
| 9 | Control | 0 | 100 | 80 |
| F1 | 40 | 60 | 60 | |
| F2 | 40 | 60 | 60 | |
| F3 | 80 | 20 | 20 | |
| F4 | 80 | 20 | 20 | |
| F5 | 80 | 20 | 20 | |
| F6 | 80 | 20 | 0 | |
| 12 | Control | 0 | 100 | 100 |
| F1 | 20 | 80 | 60 | |
| F2 | 20 | 80 | 80 | |
| F3 | 60 | 40 | 20 | |
| F4 | 60 | 40 | 40 | |
| F5 | 60 | 40 | 20 | |
| F6 | 60 | 40 | 20 | |
| 15 | Control | 0 | 100 | 100 |
| F1 | 0 | 100 | 80 | |
| F2 | 20 | 80 | 80 | |
| F3 | 40 | 60 | 40 | |
| F4 | 40 | 60 | 40 | |
| F5 | 60 | 40 | 40 | |
| F6 | 60 | 40 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera Leiva, A.F.; Hernández-Fernández, J.; Ortega Toro, R. Active Films Based on Starch and Wheat Gluten (Triticum vulgare) for Shelf-Life Extension of Carrots. Polymers 2022, 14, 5077. https://doi.org/10.3390/polym14235077
Rivera Leiva AF, Hernández-Fernández J, Ortega Toro R. Active Films Based on Starch and Wheat Gluten (Triticum vulgare) for Shelf-Life Extension of Carrots. Polymers. 2022; 14(23):5077. https://doi.org/10.3390/polym14235077
Chicago/Turabian StyleRivera Leiva, Andrés Felipe, Joaquín Hernández-Fernández, and Rodrigo Ortega Toro. 2022. "Active Films Based on Starch and Wheat Gluten (Triticum vulgare) for Shelf-Life Extension of Carrots" Polymers 14, no. 23: 5077. https://doi.org/10.3390/polym14235077
APA StyleRivera Leiva, A. F., Hernández-Fernández, J., & Ortega Toro, R. (2022). Active Films Based on Starch and Wheat Gluten (Triticum vulgare) for Shelf-Life Extension of Carrots. Polymers, 14(23), 5077. https://doi.org/10.3390/polym14235077