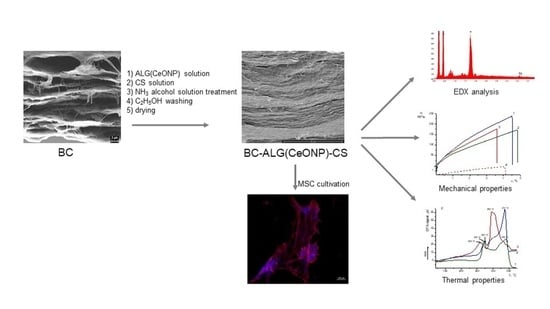
Bacterial Cellulose Composites with Polysaccharides Filled with Nanosized Cerium Oxide: Characterization and Cytocompatibility Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. The Biosynthesis of BC
2.2. The Citrate-Stabilized Cerium Dioxide Nanoparticles
2.3. Preparation of Composite Films
2.4. Characterization of the Control and Composite Films
2.5. Cultivation of Multipotent Mesenchymal Stem Cells (MMSCs)
3. Results and Discussion
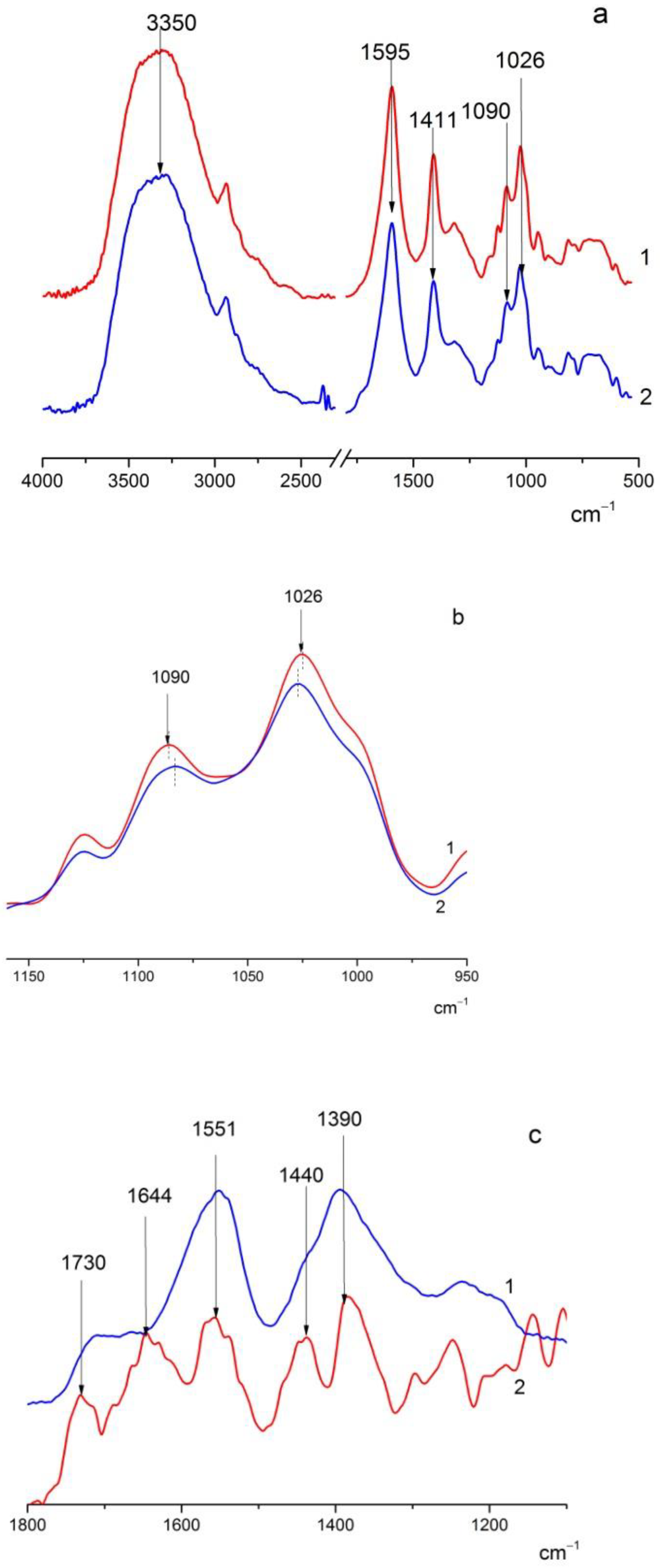
3.1. FTIR Spectra of the Studied Films
3.2. Morphology of the Composite Films
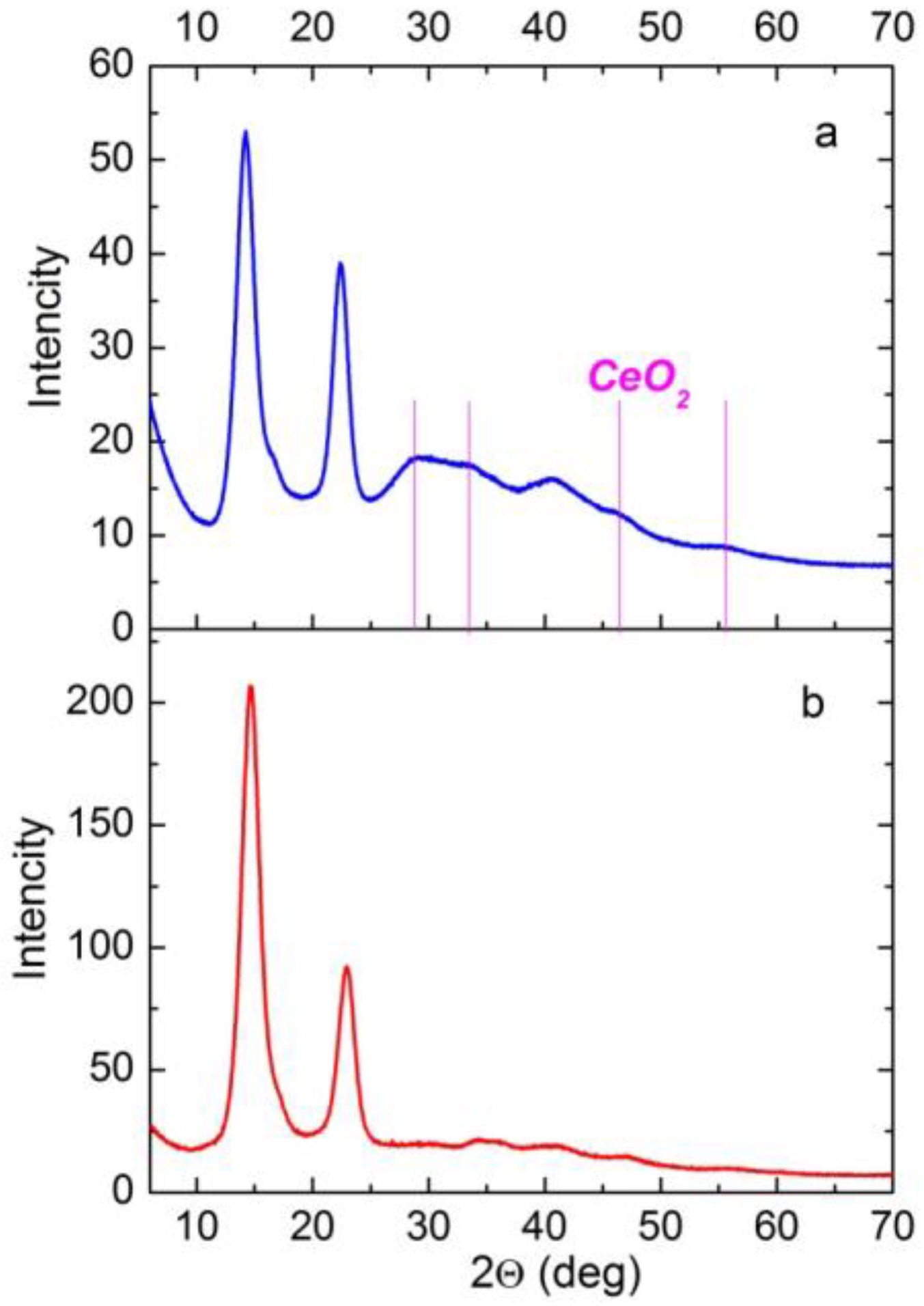
3.3. X-ray Diffraction (XRD) of the Composite Films
3.4. Swelling of Composite Films in Water
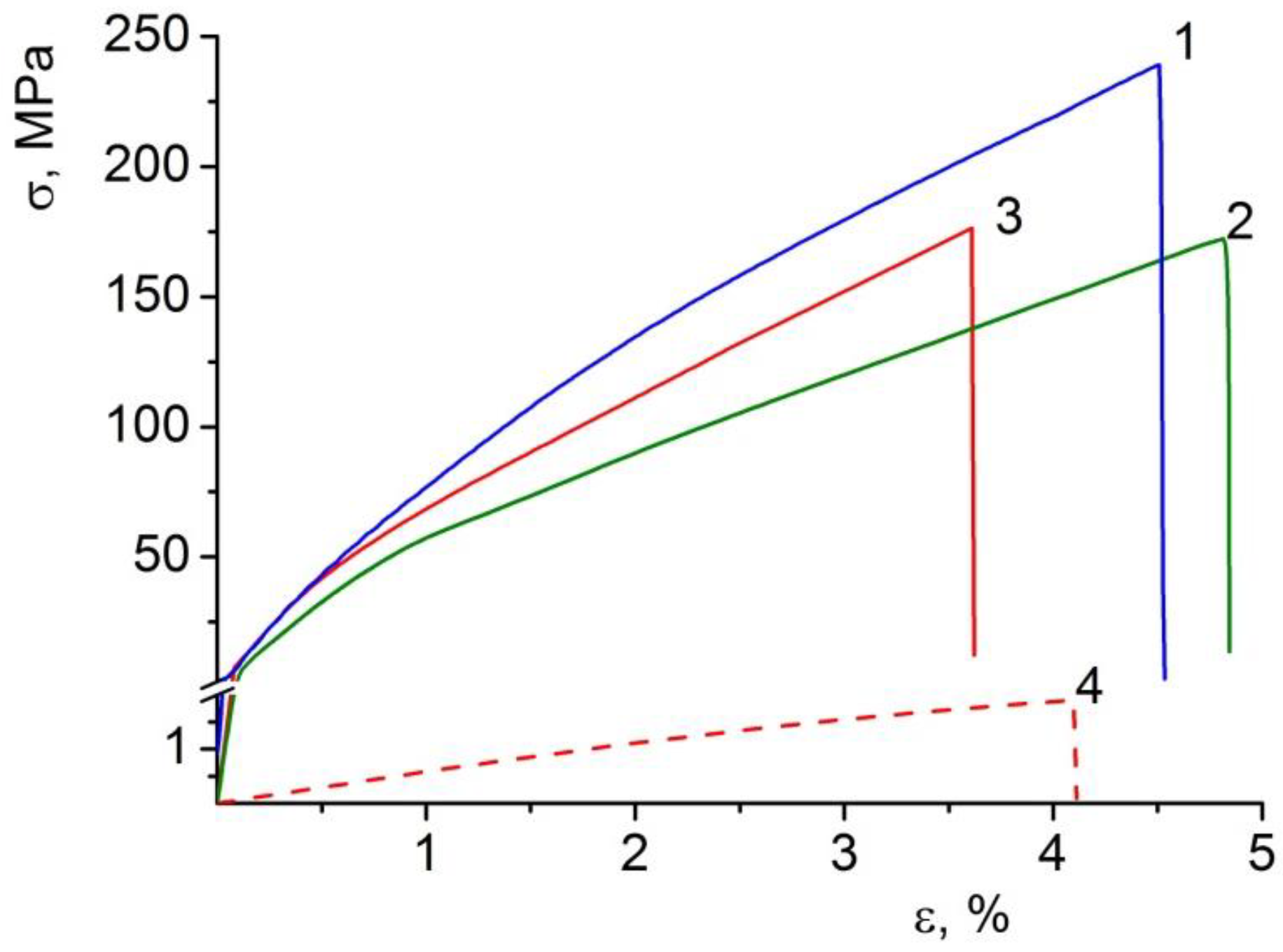
3.5. Mechanical Properties of the Composite Films
3.6. Thermal Analysis of the Composite Films
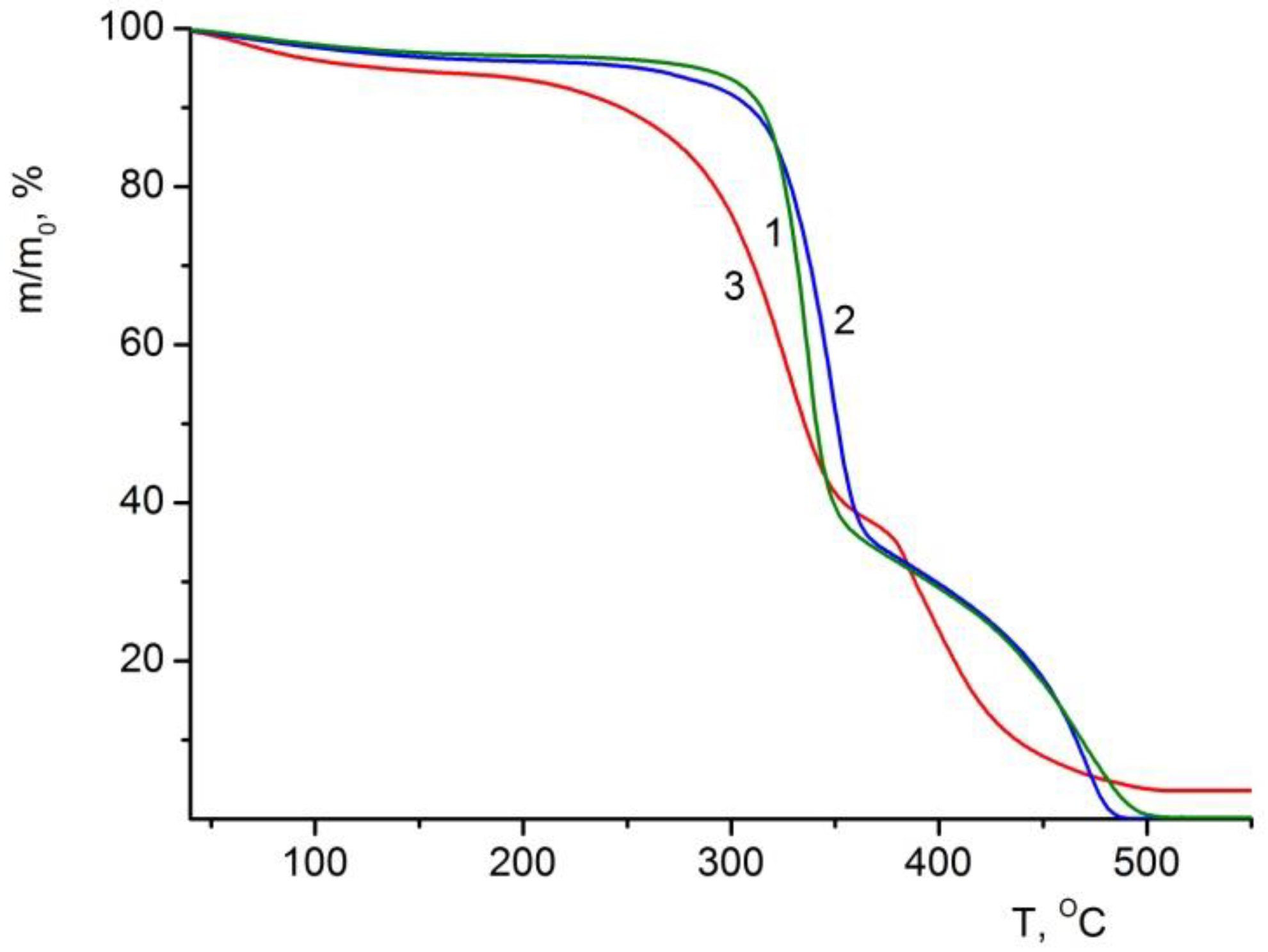
3.6.1. TGA of the Composite Films
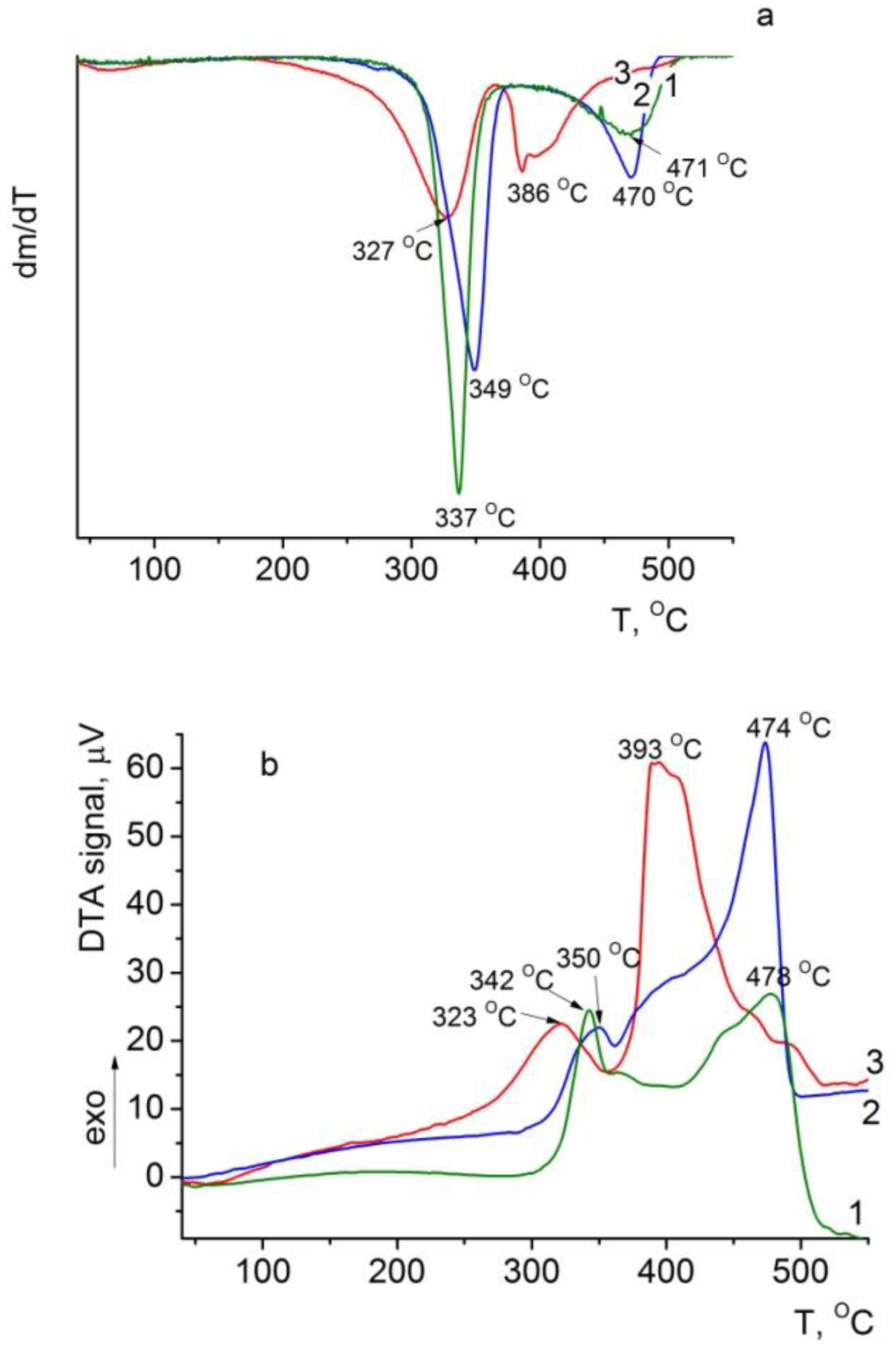
3.6.2. DTG and DTA of the Composite Films
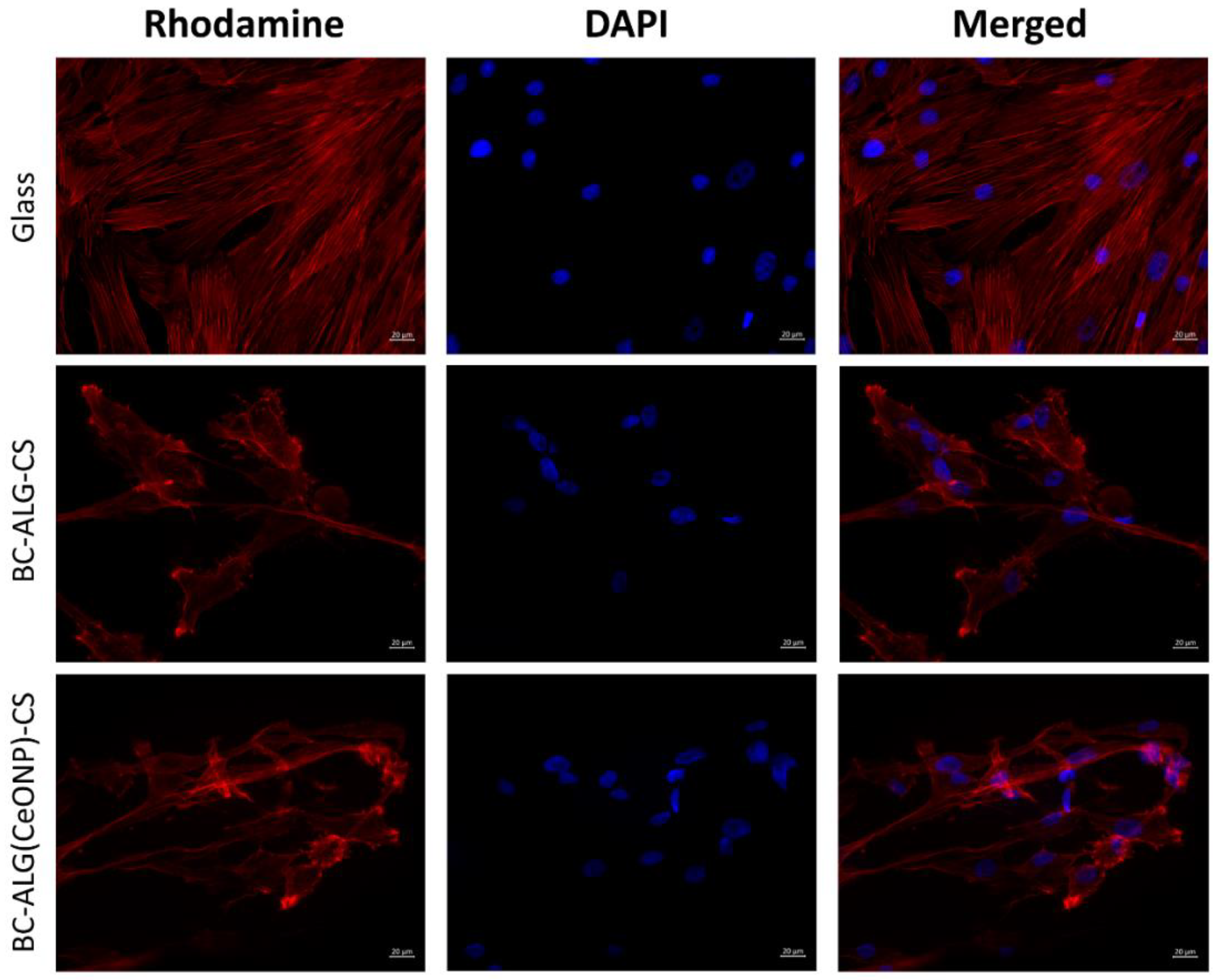
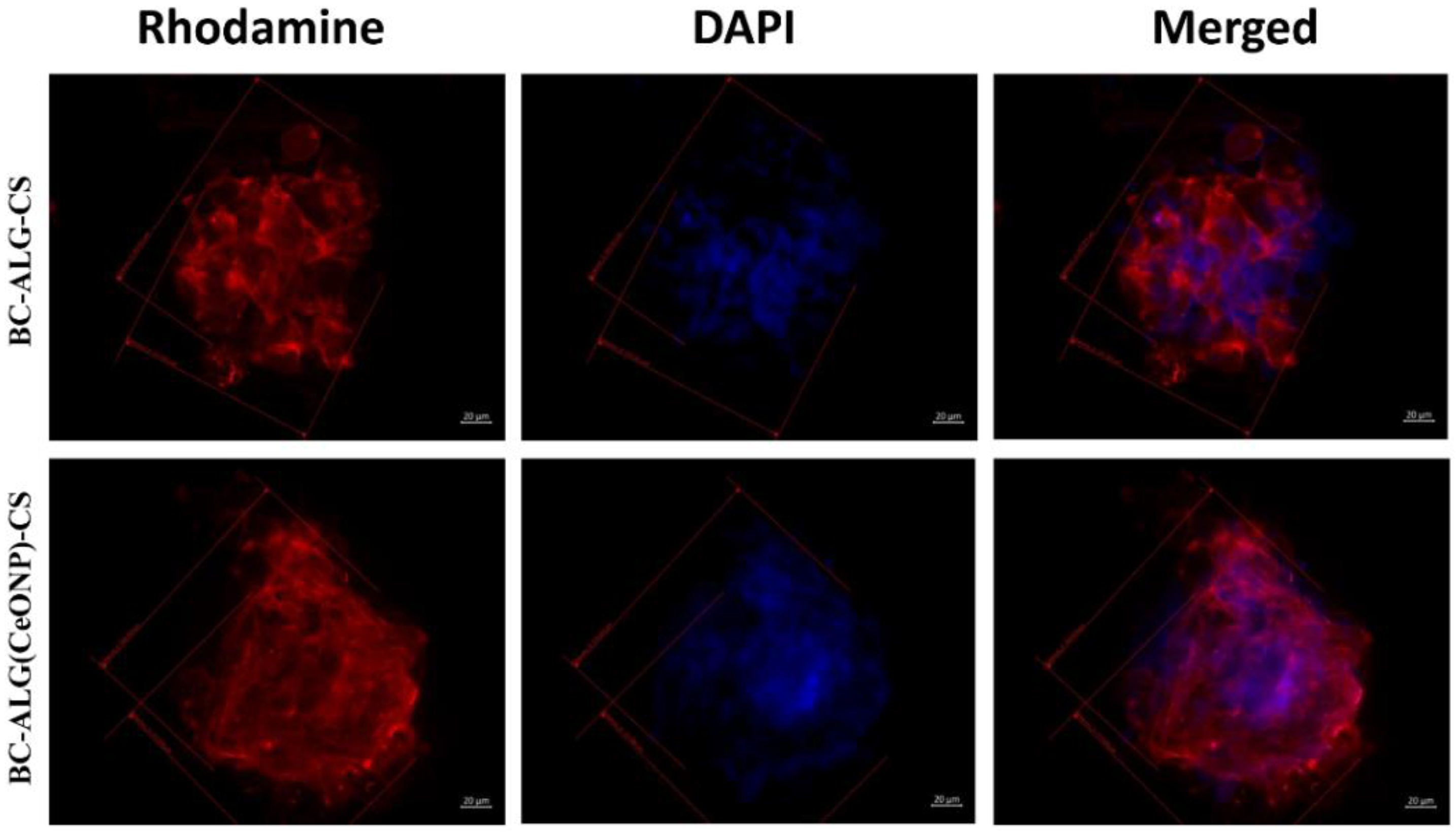
3.7. Cultivation of Multipotent Mesenchymal Stem Cells (MMSCs)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Pal Singh Chauhan, N.; Jadoun, S.; Dehghani Soltani, M.; Zarrintaj, P. Polyacrylic acid nanoplatforms: Antimicrobial, tissue engineering, and cancer theranostic applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef] [PubMed]
- Kalaycıoğlu, Z.; Kahya, N.; Adımcılar, V.; Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Erim, F.B. Antibacterial nano cerium oxide/chitosan/cellulose acetate composite films as potential wound dressing. Eur. Polym. J. 2020, 133, 109777. [Google Scholar] [CrossRef]
- Iijima, K.; Tsuji, Y.; Kuriki, I.; Kakimoto, A.; Nikaido, Y.; Ninomiya, R.; Iyoda, T.; Fukai, F.; Hashizume, M. Control of cell adhesion and proliferation utilizing polysaccharide composite film scaffolds. Colloids Surf. B Biointerfaces 2017, 160, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Montes, E. Polysaccharides: Sources, Characteristics, Properties, and Their Application in Biodegradable Films. Polysaccharides 2022, 3, 480–501. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Majumder, S.; Gupta, K.C.; Kumar, A. Dextran based amphiphilic nano-hybrid hydrogel system incorporated with curcumin and cerium oxide nanoparticles for wound healing. Colloids Surf. B Biointerfaces 2020, 195, 111263. [Google Scholar] [CrossRef]
- Fan, C.; Liao, L.; Zhang, C.; Liu, L. A tough double network hydrogel for cartilage tissue engineering. J. Mater. Chem. B 2013, 1, 4251–4258. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Mostafavi, E.; Saleh, B.; Soltantabar, P.; Webster, T.J. Chitosan/pva hydrogels incorporated with green synthesized cerium oxide nanoparticles for wound healing applications. Eur. Polym. J. 2020, 134, 109853. [Google Scholar] [CrossRef]
- Raja, I.S.; Fathima, N.N. Gelatin-cerium oxide nanocomposite for enhanced excisional wound healing. ACS Appl. Bio Mater. 2018, 1, 487–495. [Google Scholar] [CrossRef]
- Weaver, J.D.; Stabler, C.L. Antioxidant cerium oxide nanoparticle hydrogels for cellular encapsulation. Acta Biomater. 2015, 16, 136–144. [Google Scholar] [CrossRef]
- Lai, W.F.; Tang, R.; Wong, W.T. Ionically crosslinked complex gels loaded with oleic acid-containing vesicles for transdermal drug delivery. Pharmaceutics 2020, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Bahu, J.O.; de Andrade, L.R.M.; de Melo Barbosa, R.; Crivellin, S.; da Silva, A.P.; Souza, S.D.A.; Cárdenas Concha, V.O.; Severino, P.; Souto, E.B. Plant Polysaccharides in Engineered Pharmaceutical Gels. Bioengineering 2022, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.E.G.; Bossard, F.; Rinaudo, M. Electrospun biomaterials from chitosan blends applied as scaffold for tissue regeneration. Polymers 2021, 13, 1037. [Google Scholar] [CrossRef] [PubMed]
- Naseri-Nosar, M.; Farzamfar, S.; Sahrapeyma, H.; Ghorbani, S.; Bastami, F.; Vaez, A.; Salehi, M. Cerium oxide nanoparticle-containing poly (ε-caprolactone)/gelatin electrospun film as a potential wound dressing material: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2017, 81, 366–372. [Google Scholar] [CrossRef]
- Petrova, V.A.; Chernyakov, D.D.; Poshina, D.N.; Gofman, I.V.; Romanov, D.P.; Mishanin, A.I.; Golovkin, A.S.; Skorik, Y.A. Electrospun bilayer chitosan/hyaluronan material and its compatibility with mesenchymal stem cells. Materials 2019, 12, 2016. [Google Scholar] [CrossRef]
- Radwan-Praglowska, J.; Janus, L.; Piatkowski, M.; Bogdal, D.; Matysek, D. Hybrid bilayer pla/chitosan nanofibrous scaffolds doped with zno, fe3o4, and au nanoparticles with bioactive properties for skin tissue engineering. Polymers 2020, 12, 159. [Google Scholar] [CrossRef]
- Bhattarai, N.; Li, Z.; Edmondson, D.; Zhang, M. Alginate-based nanofibrous scaffolds: Structural, mechanical, and biological properties. Adv. Mater. 2006, 18, 1463–1467. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Progress in the field of electrospinning for tissue engineering applications. Adv. Mater. 2009, 21, 3343–3351. [Google Scholar] [CrossRef]
- Moniri, M.; Boroumand Moghaddam, A.; Azizi, S.; Abdul Rahim, R.; Bin Ariff, A.; Zuhainis Saad, W.; Navaderi, M.; Mohamad, R. Production and status of bacterial cellulose in biomedical engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef]
- Petrova, V.A.; Khripunov, A.K.; Golovkin, A.S.; Mishanin, A.I.; Gofman, I.V.; Romanov, D.P.; Migunova, A.V.; Arkharova, N.A.; Klechkovskaya, V.V.; Skorik, Y.A. Bacterial cellulose (komagataeibacter rhaeticus) biocomposites and their cytocompatibility. Materials 2020, 13, 4558. [Google Scholar] [CrossRef]
- Ahmed, J.; Gultekinoglu, M.; Edirisinghe, M. Bacterial cellulose micro-nano fibres for wound healing applications. Biotechnol. Adv. 2020, 41, 107549. [Google Scholar] [CrossRef] [PubMed]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, S.; Ullah, M.W.; Park, J.K. Bacterial cellulose composites: Synthetic strategies and multiple applications in bio-medical and electro-conductive fields. Biotechnol. J. 2015, 10, 1847–1861. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Ullah, M.W.; Israr, M.; Jang, J.H.; Park, J.K. Nano-gold assisted highly conducting and biocompatible bacterial cellulose-pedot:Pss films for biology-device interface applications. Int. J. Biol. Macromol. 2018, 107, 865–873. [Google Scholar] [CrossRef]
- Jasim, A.; Ullah, M.W.; Shi, Z.; Lin, X.; Yang, G. Fabrication of bacterial cellulose/polyaniline/single-walled carbon nanotubes membrane for potential application as biosensor. Carbohydr. Polym. 2017, 163, 62–69. [Google Scholar] [CrossRef]
- Ciechańska, D. Multifunctional bacterial cellulose/chitosan composite materials for medical applications. Fibres Text. East. Eur. 2004, 12, 69–72. [Google Scholar]
- Kim, J.; Cai, Z.; Lee, H.S.; Choi, G.S.; Lee, D.H.; Jo, C. Preparation and characterization of a bacterial cellulose/chitosan composite for potential biomedical application. J. Polym. Res. 2011, 18, 739–744. [Google Scholar] [CrossRef]
- Krontiras, P.; Gatenholm, P.; Hagg, D.A. Adipogenic differentiation of stem cells in three-dimensional porous bacterial nanocellulose scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 195–203. [Google Scholar] [CrossRef]
- Nakayama, A.; Kakugo, A.; Gong, J.P.; Osada, Y.; Takai, M.; Erata, T.; Kawano, S. High mechanical strength double-network hydrogel with bacterial cellulose. Adv. Funct. Mater. 2004, 14, 1124–1128. [Google Scholar] [CrossRef]
- Seifert, M.; Hesse, S.; Kabrelian, V.; Klemm, D. Controlling the water content of never dried and reswollen bacterial cellulose by the addition of water-soluble polymers to the culture medium. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 463–470. [Google Scholar] [CrossRef]
- Yang, J.; Lv, X.; Chen, S.; Li, Z.; Feng, C.; Wang, H.; Xu, Y. In situ fabrication of a microporous bacterial cellulose/potato starch composite scaffold with enhanced cell compatibility. Cellulose 2014, 21, 1823–1835. [Google Scholar] [CrossRef]
- Woehl, M.A.; Ono, L.; Riegel Vidotti, I.C.; Wypych, F.; Schreiner, W.H.; Sierakowski, M.R. Bioactive nanocomposites of bacterial cellulose and natural hydrocolloids. J. Mater. Chem. B 2014, 2, 7034–7044. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Mozafari, M. Cerium oxide nanoparticles: Recent advances in tissue engineering. Materials 2020, 13, 3072. [Google Scholar] [CrossRef]
- Krpetic, Z.; Anguissola, S.; Garry, D.; Kelly, P.M.; Dawson, K.A. Nanomaterials: Impact on cells and cell organelles. Adv. Exp. Med. Biol. 2014, 811, 135–156. [Google Scholar]
- Shcherbakov, A.B.; Zholobak, N.M.; Ivanov, V.K. 8-biological, biomedical and pharmaceutical applications of cerium oxide. In Cerium Oxide (ceo₂): Synthesis, Properties and Applications; Scirè, S., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–358. [Google Scholar]
- Bhattacharya, D.; Tiwari, R.; Bhatia, T.; Purohit, M.P.; Pal, A.; Jagdale, P.; Mudiam, M.K.R.; Chaudhari, B.P.; Shukla, Y.; Ansari, K.M.; et al. Accelerated and scarless wound repair by a multicomponent hydrogel through simultaneous activation of multiple pathways. Drug Deliv. Transl. Res. 2019, 9, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakov, A.B.; Reukov, V.V.; Yakimansky, A.V.; Krasnopeeva, E.L.; Ivanova, O.S.; Popov, A.L.; Ivanov, V.K. Ceo2 nanoparticle-containing polymers for biomedical applications: A review. Polymers 2021, 13, 924. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Naik, P. Synthesis and biomedical applications of cerium oxide nanoparticles—A review. Biotechnol. Rep. 2018, 17, 1–5. [Google Scholar] [CrossRef]
- Siposova, K.; Huntosova, V.; Shlapa, Y.; Lenkavska, L.; Macajova, M.; Belous, A.; Musatov, A. Advances in the study of cerium oxide nanoparticles: New insights into antiamyloidogenic activity. ACS Appl. Bio. Mater. 2019, 2, 1884–1896. [Google Scholar] [CrossRef]
- Rozhin, P.; Melchionna, M.; Fornasiero, P.; Marchesan, S. Nanostructured ceria: Biomolecular templates and (bio)applications. Nanomaterials 2021, 11, 2259. [Google Scholar] [CrossRef] [PubMed]
- Gofman, I.V.; Nikolaeva, A.L.; Khripunov, A.K.; Ivankova, E.M.; Shabunin, A.S.; Yakimansky, A.V.; Romanov, D.P.; Popov, A.L.; Ermakov, A.M.; Solomevich, S.O.; et al. Bacterial cellulose-based nanocomposites containing ceria and their use in the process of stem cell proliferation. Polymers 2021, 13, 1999. [Google Scholar] [CrossRef] [PubMed]
- Arkharova, N.A.; Severin, A.V.; Khripunov, A.K.; Krasheninnikov, S.V.; Tkachenko, A.A.; Orekhov, A.S.; Davydova, G.A.; Rakova, E.V.; Klechkovskaya, V.V. Composite films based on bacterial cellulose and nanocrystals of hydroxyapatite: Morphology, structure, and properties. Polym. Sci.-Ser. A 2019, 61, 650–658. [Google Scholar] [CrossRef]
- Ivanova, O.S.; Shekunova, T.O.; Ivanov, V.K.; Shcherbakov, A.B.; Popov, A.L.; Davydova, G.A.; Selezneva, I.I.; Kopitsa, G.P.; Tret’yakov, Y.D. One-stage synthesis of ceria colloid solutions for biomedical use. Dokl. Chem. 2011, 437, 103–106. [Google Scholar] [CrossRef]
- Petrova, V.A.; Elokhovskiy, V.Y.; Raik, S.V.; Poshina, D.N.; Romanov, D.P.; Skorik, Y.A. Alginate gel reinforcement with chitin nanowhiskers modulates rheological properties and drug release profile. Biomolecules 2019, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Kononova, S.V.; Kruchinina, E.V.; Petrova, V.A.; Baklagina, Y.G.; Klechkovskaya, V.V.; Orekhov, A.S.; Vlasova, E.N.; Popova, E.N.; Gubanova, G.N.; Skorik, Y.A. Pervaporation membranes of a simplex type with polyelectrolyte layers of chitosan and sodium hyaluronate. Carbohydr. Polym. 2019, 209, 10–19. [Google Scholar] [CrossRef]
- Barteau, M.A. Organic reactions at well-defined oxide surfaces. Chem. Rev. 1996, 96, 1413–1430. [Google Scholar] [CrossRef]
- Mullins, D.R. The surface chemistry of cerium oxide. Surf. Sci. Rep. 2015, 70, 42–85. [Google Scholar] [CrossRef]
- Lee, C.M.; Gu, J.; Kafle, K.; Catchmark, J.; Kim, S.H. Cellulose produced by gluconacetobacter xylinus strains atcc 53524 and atcc 23768: Pellicle formation, post-synthesis aggregation and fiber density. Carbohydr. Polym. 2015, 133, 270–276. [Google Scholar] [CrossRef]
- Passi, M.; Kumar, V.; Packirisamy, G. Theranostic nanozyme: Silk fibroin based multifunctional nanocomposites to combat oxidative stress. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110255. [Google Scholar] [CrossRef]
- Atisme, T.B.; Yu, C.Y.; Tseng, E.N.; Chen, Y.C.; Hsu, P.K.; Chen, S.Y. Interface interactions in conjugated polymer composite with metal oxide nanoparticles. Nanomaterials 2019, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.G.W.; Sakai, Y.; Shafizadeh, F. A kinetic model for pyrolysis of cellulose. J. Appl. Polym. Sci. 1979, 23, 3271–3280. [Google Scholar] [CrossRef]
- de Britto, D.; Campana-Filho, S.P. Kinetics of the thermal degradation of chitosan. Thermochim. Acta 2007, 465, 73–82. [Google Scholar] [CrossRef]
- López, F.A.; Mercê, A.L.R.; Alguacil, F.J.; López-Delgado, A. A kinetic study on the thermal behaviour of chitosan. J. Therm. Anal. Calorim. 2008, 91, 633–639. [Google Scholar] [CrossRef]
- Werner, K.; Pommer, L.; Broström, M. Thermal decomposition of hemicelluloses. J. Anal. Appl. Pyrolysis 2014, 110, 130–137. [Google Scholar] [CrossRef]
- Gubanova, G.N.; Petrova, V.A.; Kononova, S.V.; Popova, E.N.; Smirnova, V.E.; Bugrov, A.N.; Klechkovskaya, V.V.; Skorik, Y.A. Thermal properties and structural features of multilayer films based on chitosan and anionic polysaccharides. Biomolecules 2021, 11, 762. [Google Scholar] [CrossRef]
- Vesel, A.; Junkar, I.; Cvelbar, U.; Kovac, J.; Mozetic, M. Surface modification of polyester by oxygen- and nitrogen-plasma treatment. Surf. Interface Anal. 2008, 40, 1444–1453. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef]
- Tsai, A.C.; Liu, Y.; Yuan, X.; Ma, T. Compaction, fusion, and functional activation of three-dimensional human mesenchymal stem cell aggregate. Tissue Eng. Part A 2015, 21, 1705–1719. [Google Scholar] [CrossRef]
- Marx, V. Cell culture: A better brew. Nature 2013, 496, 253–258. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]


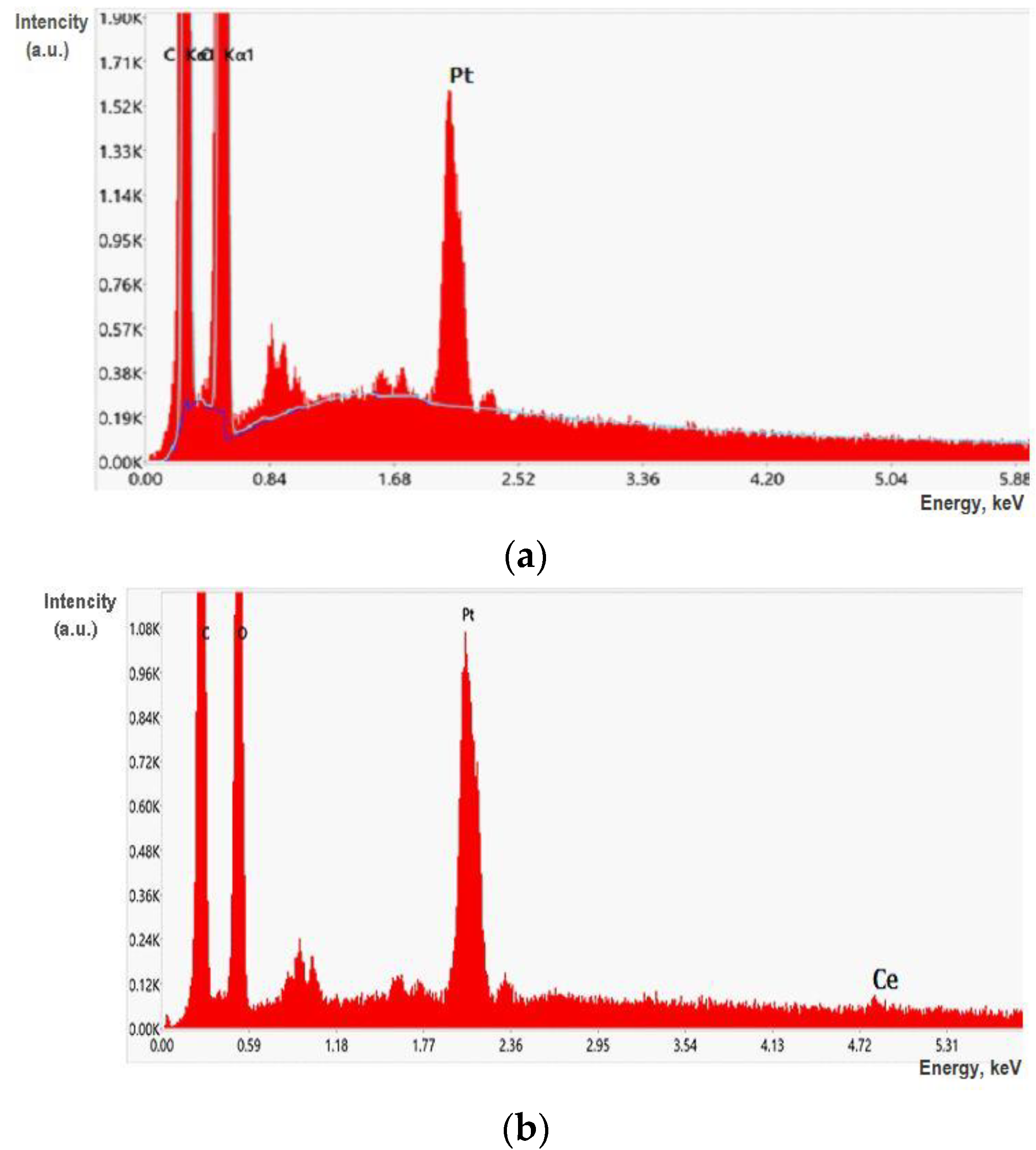

| Sample | Swelling in Water (g/g) |
|---|---|
| Bacterial cellulose (BC) | 1.9 |
| BC–sodium alginate (ALG)–chitosan (CS) | 24.0 |
| BC–ALG-cerium oxide nanoparticles (CeONP)–CS | 42.0 |
| Sample | E | σb (MPa) | εb (%) |
|---|---|---|---|
| BC dry | 6.07 ± 0.18 GPa | 236 ± 11 | 4.7 ± 0.6 |
| BC–ALG–CS dry | 7.13 ± 0.08 GPa | 172 ± 9 | 4.9 ± 0.2 |
| BC–ALG(CeONP)–CS dry | 8.21 ± 0.53 GPa | 176 ± 7 | 3.6 ± 0.4 |
| BC–ALG(CeONP)–CS swollen in water | 39 ± 2 MPa | 1.9 ± 0.2 | 4.1 ± 0.3 |
| Sample | Volatile Impurities, % | Thermal Stability Indices (°C) | |
|---|---|---|---|
| τ5 | τ10 | ||
| BC | 3.5 | 310 | 319 |
| BC–ALG–CS | 4.1 | 304 | 320 |
| BC–ALG(CeONP)–CS | 5.4 | 251 | 276 |
| Sample | Nonadhered Cells | Type of Colonies | Maximal Longitudinal Site of Spheroids (µm) | Cell Migration from Spheroid |
|---|---|---|---|---|
| Glass | – | monolayer | ||
| BC–ALG–CS | multiple | monolayer+ single spheroids | 154 ± 2 | + |
| BC–ALG(CeONP)–CS | single | monolayer+ spheroids | 163 ± 5 | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, V.A.; Gofman, I.V.; Golovkin, A.S.; Mishanin, A.I.; Dubashynskaya, N.V.; Khripunov, A.K.; Ivan’kova, E.M.; Vlasova, E.N.; Nikolaeva, A.L.; Baranchikov, A.E.; et al. Bacterial Cellulose Composites with Polysaccharides Filled with Nanosized Cerium Oxide: Characterization and Cytocompatibility Assessment. Polymers 2022, 14, 5001. https://doi.org/10.3390/polym14225001
Petrova VA, Gofman IV, Golovkin AS, Mishanin AI, Dubashynskaya NV, Khripunov AK, Ivan’kova EM, Vlasova EN, Nikolaeva AL, Baranchikov AE, et al. Bacterial Cellulose Composites with Polysaccharides Filled with Nanosized Cerium Oxide: Characterization and Cytocompatibility Assessment. Polymers. 2022; 14(22):5001. https://doi.org/10.3390/polym14225001
Chicago/Turabian StylePetrova, Valentina A., Iosif V. Gofman, Alexey S. Golovkin, Alexander I. Mishanin, Natallia V. Dubashynskaya, Albert K. Khripunov, Elena M. Ivan’kova, Elena N. Vlasova, Alexandra L. Nikolaeva, Alexander E. Baranchikov, and et al. 2022. "Bacterial Cellulose Composites with Polysaccharides Filled with Nanosized Cerium Oxide: Characterization and Cytocompatibility Assessment" Polymers 14, no. 22: 5001. https://doi.org/10.3390/polym14225001
APA StylePetrova, V. A., Gofman, I. V., Golovkin, A. S., Mishanin, A. I., Dubashynskaya, N. V., Khripunov, A. K., Ivan’kova, E. M., Vlasova, E. N., Nikolaeva, A. L., Baranchikov, A. E., Skorik, Y. A., Yakimansky, A. V., & Ivanov, V. K. (2022). Bacterial Cellulose Composites with Polysaccharides Filled with Nanosized Cerium Oxide: Characterization and Cytocompatibility Assessment. Polymers, 14(22), 5001. https://doi.org/10.3390/polym14225001