In Situ Ring-Opening Polymerization of L-lactide on the Surface of Pristine and Aminated Silica: Synthesis and Metal Ions Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
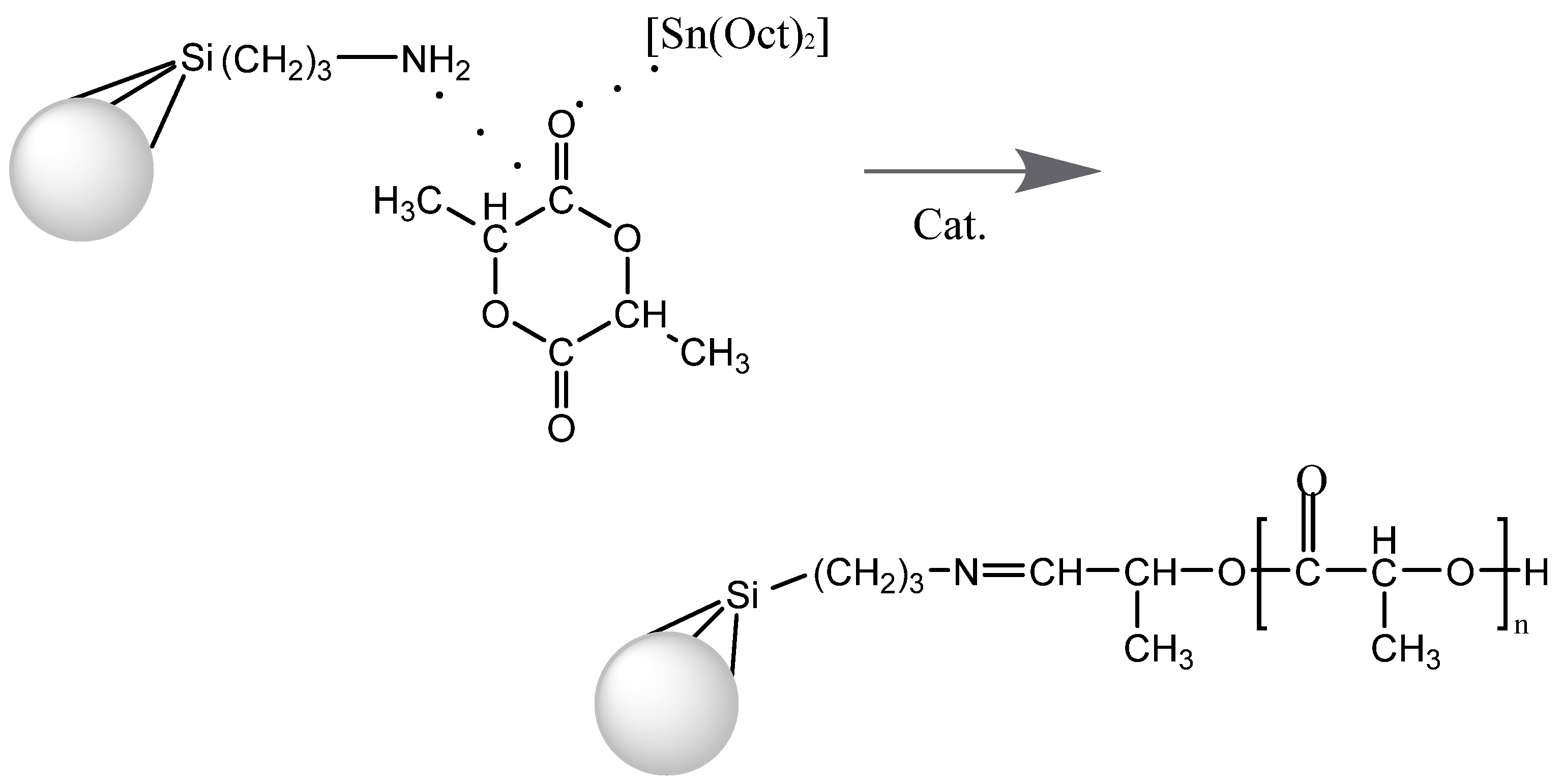
2.2. Amine-Functionalized Porous Silica Samples (Silochrom-NH2)
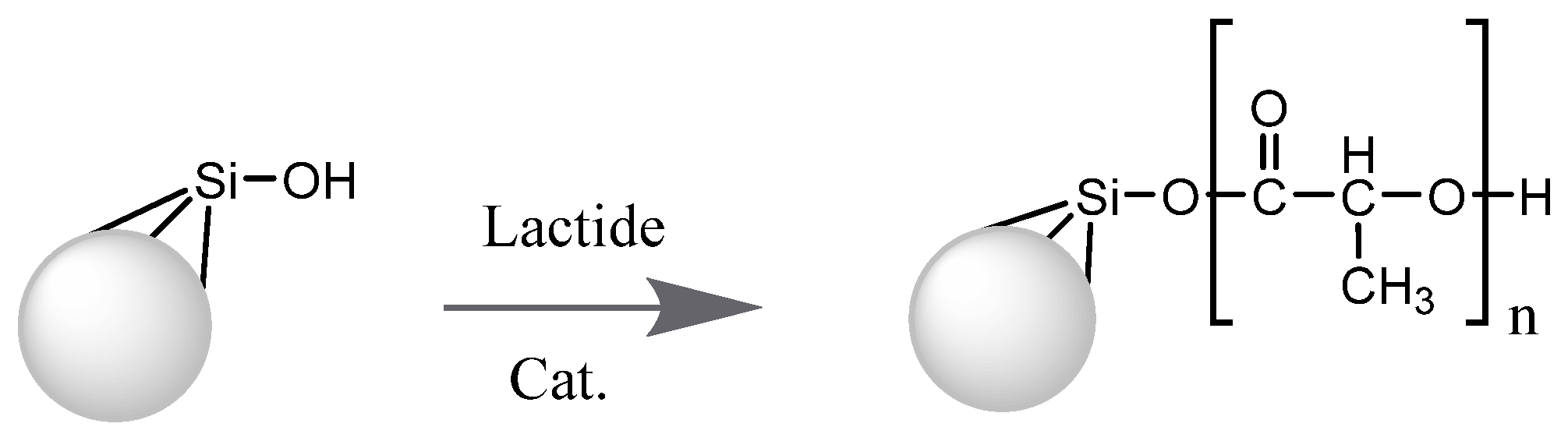
2.3. Synthesis of Silochrom/PLA and Silochrom-NH2/PLA Composites
2.4. Methods
3. Results and Discussion
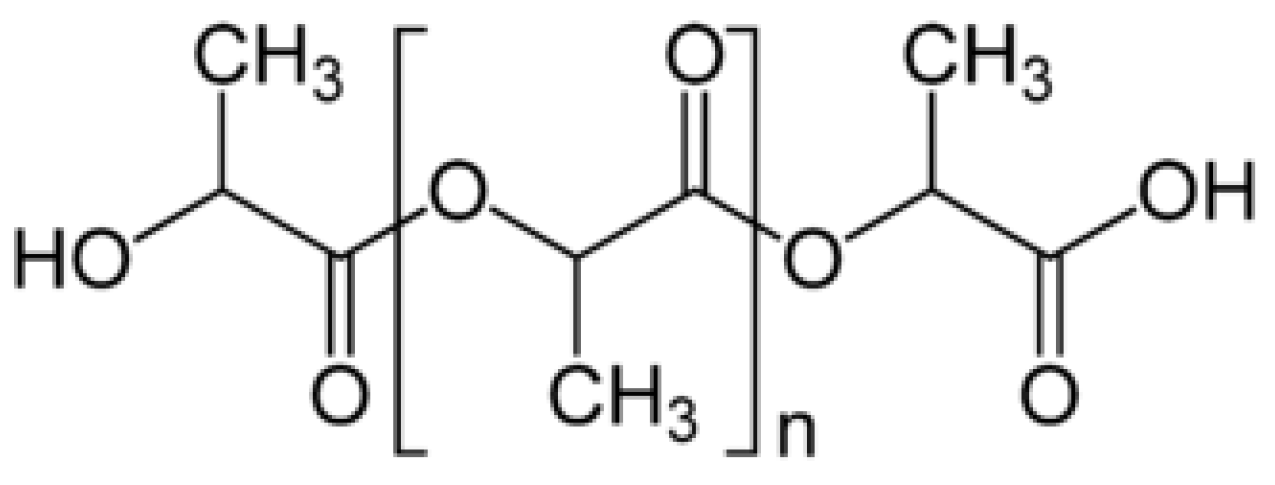
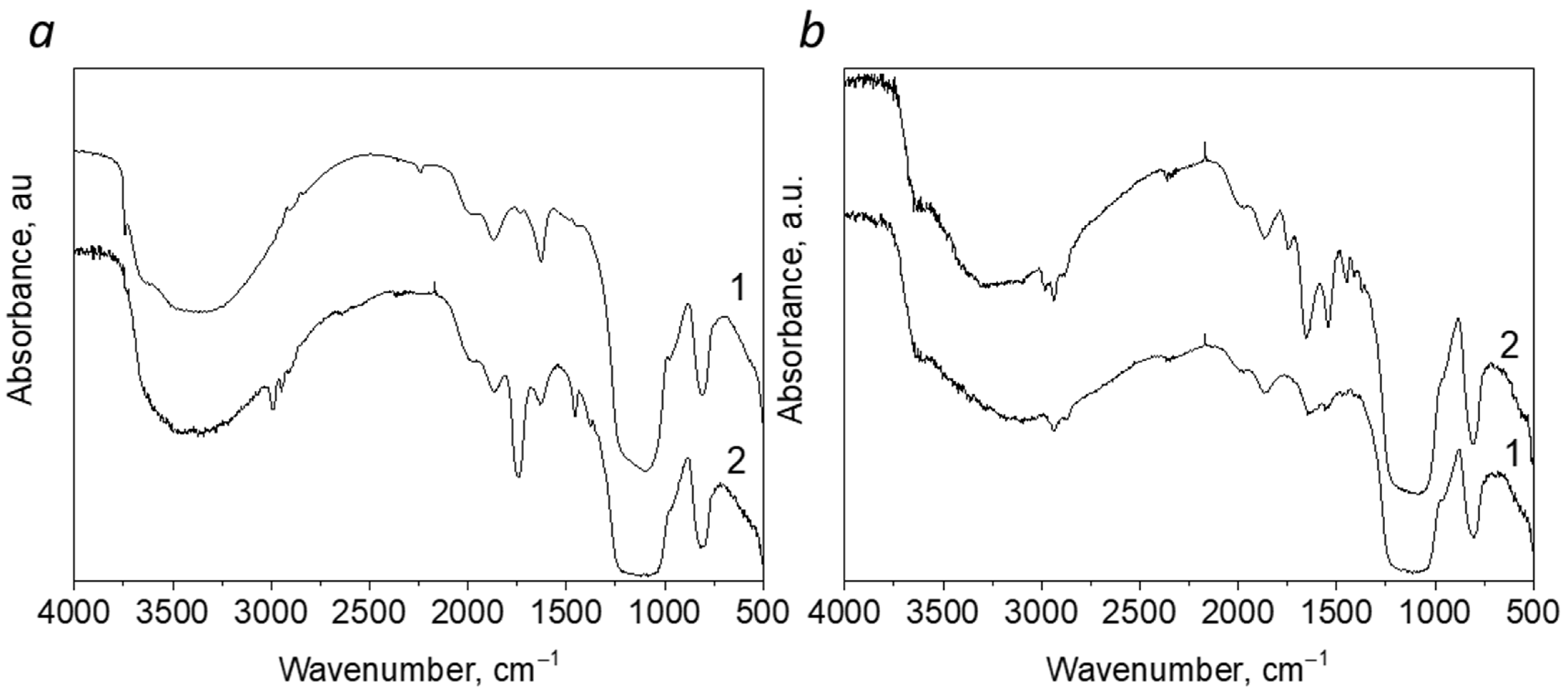
3.1. Preparation of Polylactic Acid/Silica Composites
3.2. Adsorption Studies
3.3. Effect of pH
3.4. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Olivera, S.; Hu, C.; Nagananda, G.S.; Reddy, N.; Venkatesh, K.; Muralidhara, H.B. Multipurpose Composite for Heavy Metal Sorption, Antimicrobial, and Antioxidant Applications. Int. J. Environ. Sci. Technol. 2019, 16, 2017–2030. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Witt, K. Sorption of Cu(II), Zn(II) and Pb(II) Ions in an Aqueous Solution on the PVC-Acetylacetone Composites. Polymers 2019, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.-R.; Kim, S.W.; Lee, C.-H.; Choi, E.-K.; Oh, M.H.; Seo, S.N.; Park, H.-J.; Hamm, S.-Y. Performance of Composite Mineral Adsorbents for Removing Cu, Cd, and Pb Ions from Polluted Water. Sci. Rep. 2019, 9, 13598. [Google Scholar] [CrossRef]
- Ren, C.; Ding, X.; Li, W.; Wu, H.; Yang, H. Highly Efficient Adsorption of Heavy Metals onto Novel Magnetic Porous Composites Modified with Amino Groups. J. Chem. Eng. Data 2017, 62, 1865–1875. [Google Scholar] [CrossRef]
- Goliszek, M.; Kołodyńska, D.; Pylypchuk, I.V.; Sevastyanova, O.; Podkościelna, B. Synthesis of Lignin-Containing Polymer Hydrogels with Tunable Properties and Their Application in Sorption of Nickel(II) Ions. Ind. Crops Prod. 2021, 164, 113354. [Google Scholar] [CrossRef]
- Esakkimuthu, E.S.; DeVallance, D.; Pylypchuk, I.; Moreno, A.; Sipponen, M.H. Multifunctional Lignin-Poly (Lactic Acid) Biocomposites for Packaging Applications. Front. Bioeng. Biotechnol. 2022, 10, 1025076. [Google Scholar] [CrossRef]
- Guillaume, S.M. Recent Advances in Ring-Opening Polymerization Strategies toward α,ω-Hydroxy Telechelic Polyesters and Resulting Copolymers. Eur. Polym. J. 2013, 49, 768–779. [Google Scholar] [CrossRef]
- Shrivastava, A. 2—Polymerization. In Plastics Design Library; Shrivastava, A.B.T.-I.P.E., Ed.; William Andrew Publishing: Burlington, MA, USA, 2018; pp. 17–48. ISBN 978-0-323-39500-7. [Google Scholar]
- Seisenbaeva, G.A.; Ali, L.M.A.; Vardanyan, A.; Gary-Bobo, M.; Budnyak, T.M.; Kessler, V.G.; Durand, J.O. Mesoporous Silica Adsorbents Modified with Amino Polycarboxylate Ligands—Functional Characteristics, Health and Environmental Effects. J. Hazard Mater. 2021, 406, 124698. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Piątek, J.; Pylypchuk, I.V.; Klimpel, M.; Sevastyanova, O.; Lindströ, M.E.; Gun’ko, V.M.; Slabon, A. Membrane-Filtered Kraft Lignin−Silica Hybrids as Bio-Based Sorbents for Cobalt(II) Ion Recycling. ACS Omega 2020, 5, 10847–10856. [Google Scholar] [CrossRef]
- Piątek, J.; de Bruin-Dickason, C.N.; Jaworski, A.; Chen, J.; Budnyak, T.; Slabon, A. Glycine-Functionalized Silica as Sorbent for Cobalt(II) and Nickel(II) Recovery. Appl. Surf. Sci. 2020, 530, 147299. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Vlasova, N.N.; Golovkova, L.P.; Slabon, A.; Tertykh, V.A. Bile Acids Adsorption by Chitoan-Fumed Silica Enterosorbent. Colloids Interface Sci. Commun. 2019, 32, 100194. [Google Scholar] [CrossRef]
- Mishra, A.K. (Ed.) Sol-Gel Based Nanoceramic Materials: Preparation, Properties and Applications; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Pylypchuk, I.V.; Lindströ, M.E.; Sevastyanova, O. Electrostatic Deposition of the Oxidized Kraft Lignin onto the Surface of Aminosilicas: Thermal and Structural Characteristics of Hybrid Materials. ACS Omega 2019, 4, 22530–22539. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Qiu, X.; Sun, J.; Deng, M.; Chen, X.; Jing, X. Grafting Polymerization of L-Lactide on the Surface of Hydroxyapatite Nano-Crystals. Polymer 2004, 45, 6699–6706. [Google Scholar] [CrossRef]
- Lalanne-Tisné, M.; Mees, M.A.; Eyley, S.; Zinck, P.; Thielemans, W. Organocatalyzed Ring Opening Polymerization of Lactide from the Surface of Cellulose Nanofibrils. Carbohydr. Polym. 2020, 250, 116974. [Google Scholar] [CrossRef] [PubMed]
- Alba, A.; du Boullay, O.T.; Martin-Vaca, B.; Bourissou, D. Direct Ring-Opening of Lactide with Amines: Application to the Organo-Catalyzed Preparation of Amide End-Capped PLA and to the Removal of Residual Lactide from PLA Samples. Polym. Chem. 2015, 6, 989–997. [Google Scholar] [CrossRef]
- Vouyiouka, S.N.; Papaspyrides, C.D. 4.34—Mechanistic Aspects of Solid-State Polycondensation; Matyjaszewski, K., Möller, M.B.T.-P.S.A.C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 857–874. ISBN 978-0-08-087862-1. [Google Scholar]
- Piątek, J.; Afyon, S.; Budnyak, T.M.; Budnyk, S.; Sipponen, M.H.; Slabon, A. Sustainable Li-Ion Batteries: Chemistry and Recycling. Adv. Energy Mater. 2020, 11, 2003456. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Modersitzki, S.; Pylypchuk, I.V.; Piątek, J.; Jaworski, A.; Sevastyanova, O.; Lindström, M.E.; Slabon, A. Tailored Hydrophobic/Hydrophilic Lignin Coatings on Mesoporous Silica for Sustainable Cobalt(II) Recycling. ACS Sustain Chem. Eng. 2020, 8, 16262–16273. [Google Scholar] [CrossRef]
- Ehsani, M.; Khodabakhshi, K.; Asgari, M. Lactide Synthesis Optimization: Investigation of the Temperature, Catalyst and Pressure Effects. e-Polymers 2014, 14, 353–361. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef]
- Pilipenko, A.T.; Pyatniskyi, I.V. Analytical Chemistry; Mir: Moscow, Russia, 1990. [Google Scholar]
- Gun’ko, V.M.; Turov, V.V. (Eds.) Nuclear Magnetic Resonance Studies of Interfacial Phenomena, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Scholten, J.J.F. Characterization of Porous Solids; Proceedings of the Applied Catalysis; Rodríguez-Reinoso, F., McEnaney, B., Rouquerol, J., Unger, K., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 45, pp. 165–167. [Google Scholar]
- Ahmed, J.; Varshney, S.K. Polylactides—Chemistry, Properties and Green Packaging Technology: A Review. Int. J. Food Prop. 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characterstic Group Frequencies. Chichester; New York, Brisbane, Toronto, 1980; Elsevier: Amsterdam, The Netherlands, 1981; p. 174. Available online: https://www.sciencedirect.com/science/article/pii/0022286081852805?via%3Dihub (accessed on 3 October 2022).
- Nakanisi, K. IR-Spectra and Structure of Organic Compounds; Mir: Moscow, Russia, 1965. [Google Scholar]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0470011130. [Google Scholar]
- Stloukal, P.; Novák, I.; Mičušík, M.; Procházka, M.; Kucharczyk, P.; Chodák, I.; Lehocký, M.; Sedlařík, V. Effect of plasma treatment on the release kinetics of a chemotherapy drug from biodegradable polyester films and polyester urethane films. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 161–173. [Google Scholar] [CrossRef]
- Abdul Hamid, Z.A.; Tham, C.Y.; Ahmad, Z. Preparation and Optimization of Surface-Engineered Poly(Lactic Acid) Microspheres as a Drug Delivery Device. J. Mater. Sci. 2018, 53, 4745–4758. [Google Scholar] [CrossRef]
- Bennettand, H.; Wiley, G.J.O.; Benninghoven, A.; Janssen, K.T.F.; Tumpner, J.; Wer, H.W. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database (Beamson, G.; Briggs, D.). J. Chem. Educ. 1993, 70, A25. [Google Scholar] [CrossRef]
- Abomosallam, M.; Elalfy, M.; Zheng, Z.; Nagata, K.; Suzuki, M. Adsorption Kinetics and Thermodynamics of Toxic Metal Ions onto Chitosan Nanoparticles Extracted from Shrimp Shells. Nanotechnol. Environ. Eng. 2022, 7, 35–47. [Google Scholar] [CrossRef]
- Singh, S.; Kapoor, D.; Khasnabis, S.; Singh, J.; Ramamurthy, P.C. Mechanism and Kinetics of Adsorption and Removal of Heavy Metals from Wastewater Using Nanomaterials. Environ. Chem. Lett. 2021, 19, 2351–2381. [Google Scholar] [CrossRef]
- Hameed, B.H.; Din, A.T.M.; Ahmad, A.L. Adsorption of Methylene Blue onto Bamboo-Based Activated Carbon: Kinetics and Equilibrium Studies. J. Hazard Mater. 2007, 141, 819–825. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Bartczak, P.; Jesionowski, T. Enhanced Removal of Hazardous Dye Form Aqueous Solutions and Real Textile Wastewater Using Bifunctional Chitin/Lignin Biosorbent. Int. J. Biol. Macromol. 2017, 99, 754–764. [Google Scholar] [CrossRef]
- Ripan, R.; Chetyanu, I. Inorganic Chemistry; MIR: Moscow, Russia, 1972. [Google Scholar]

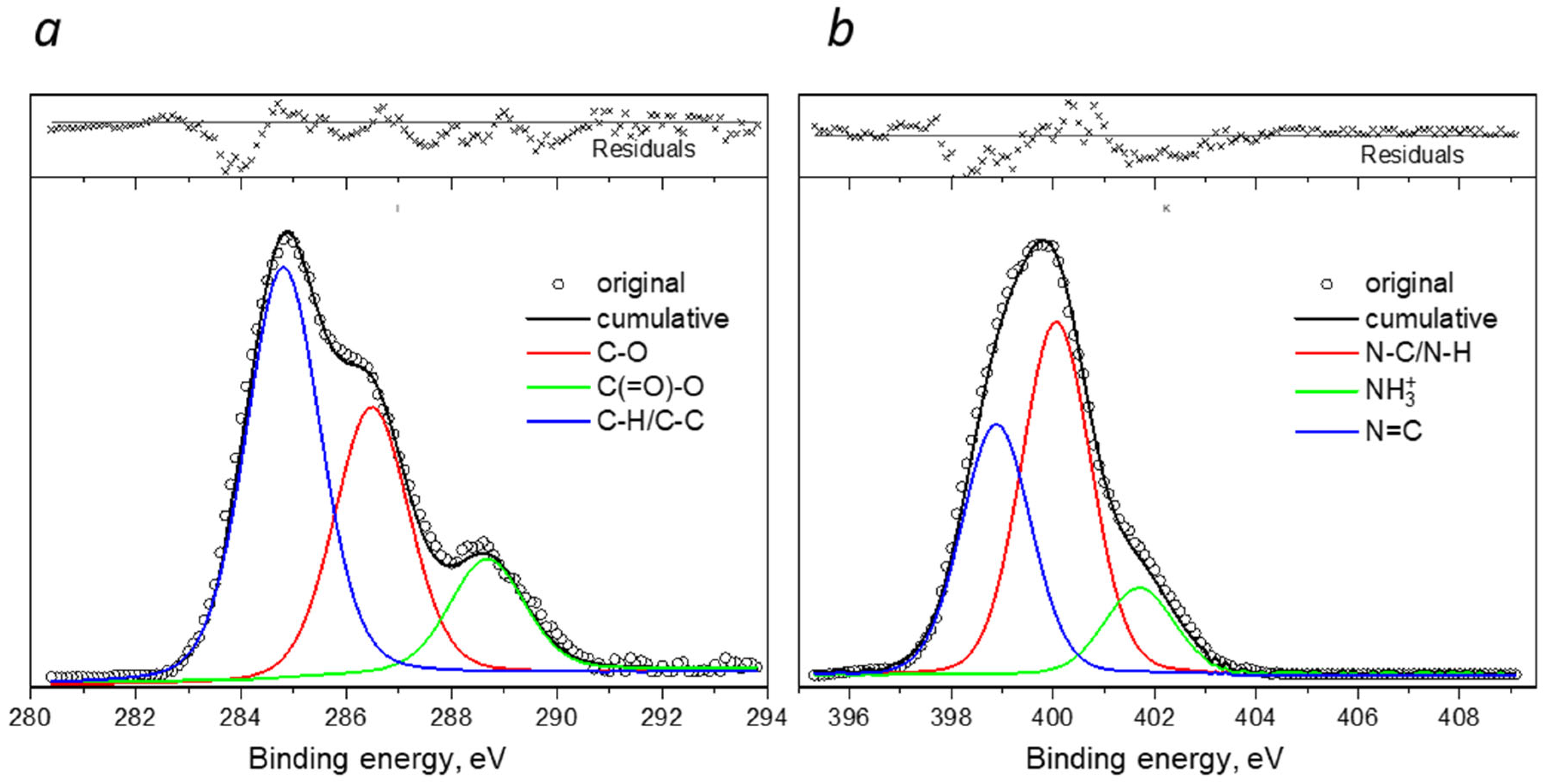
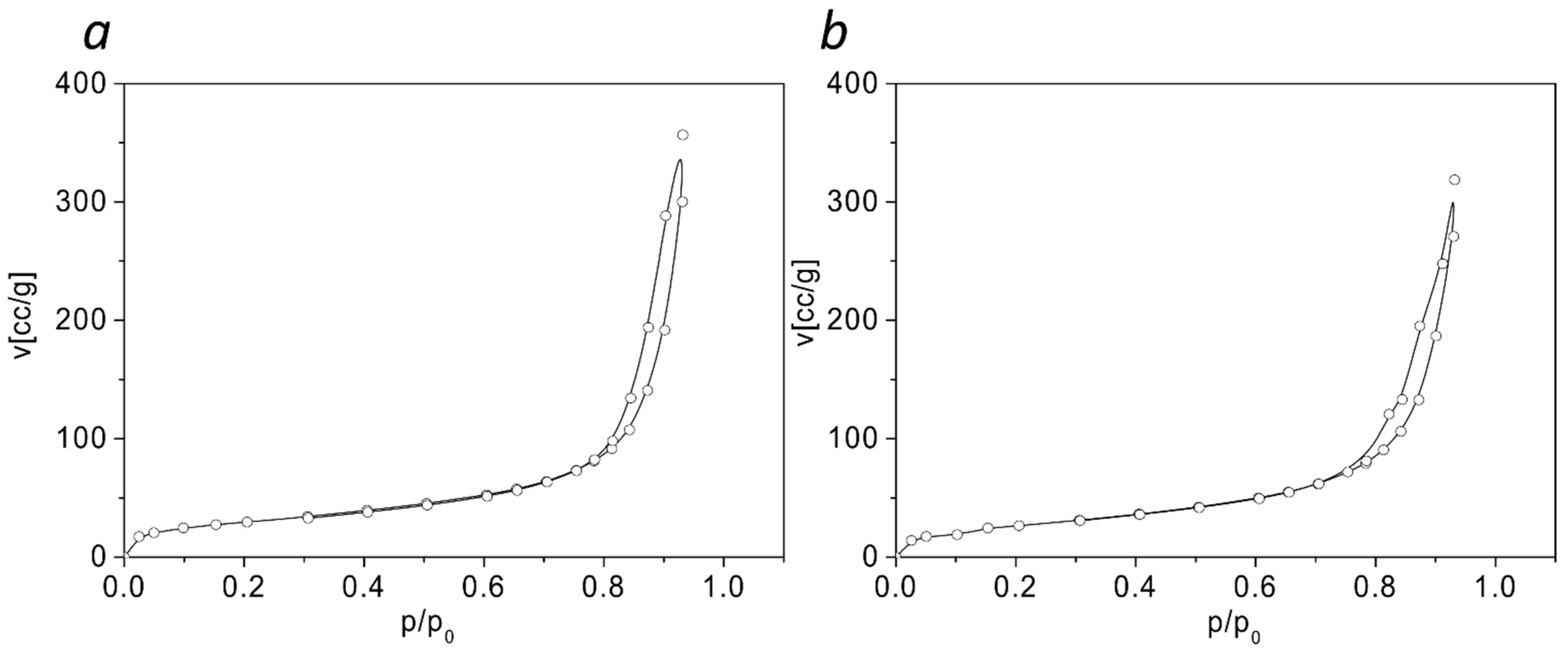
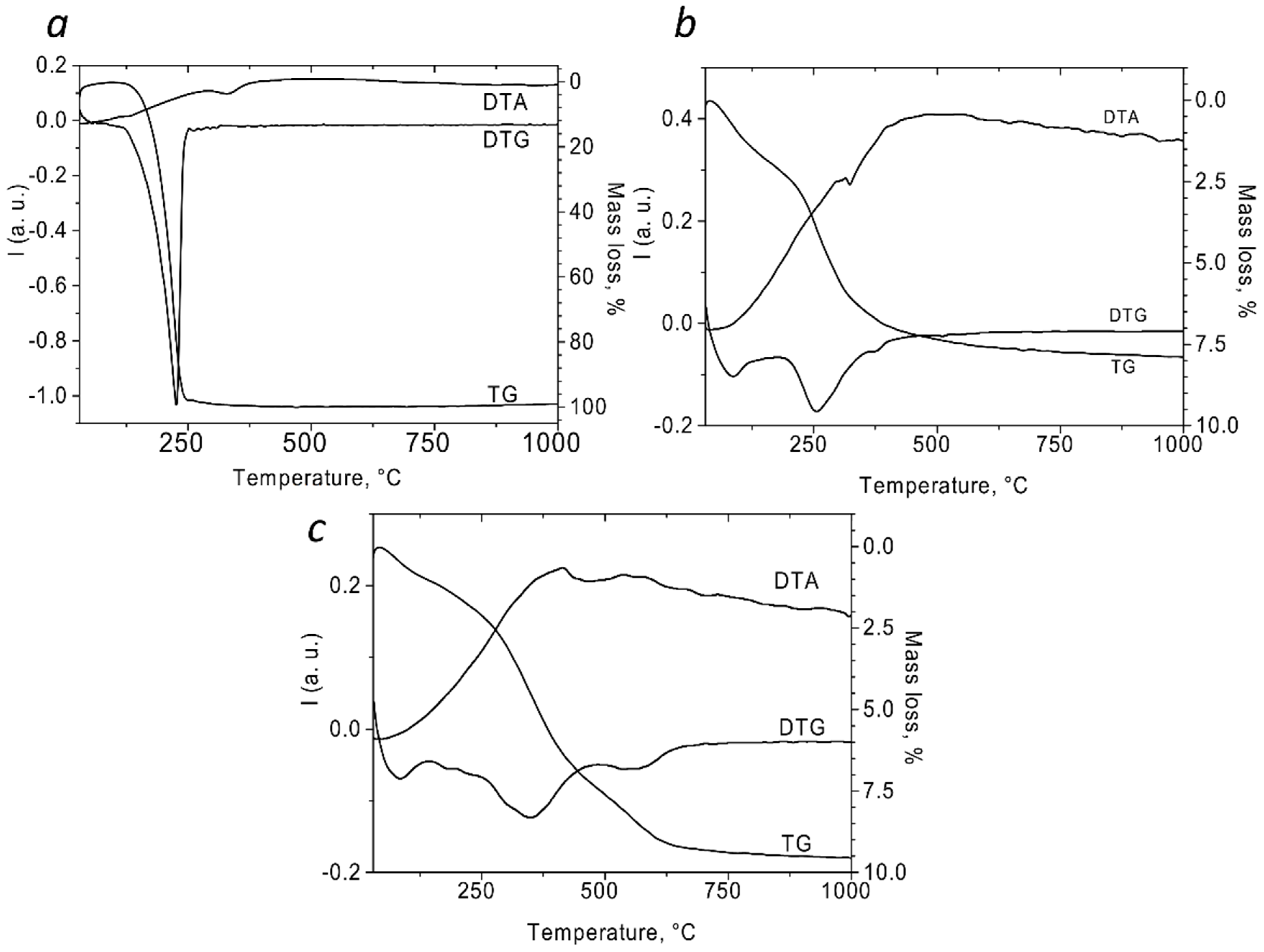
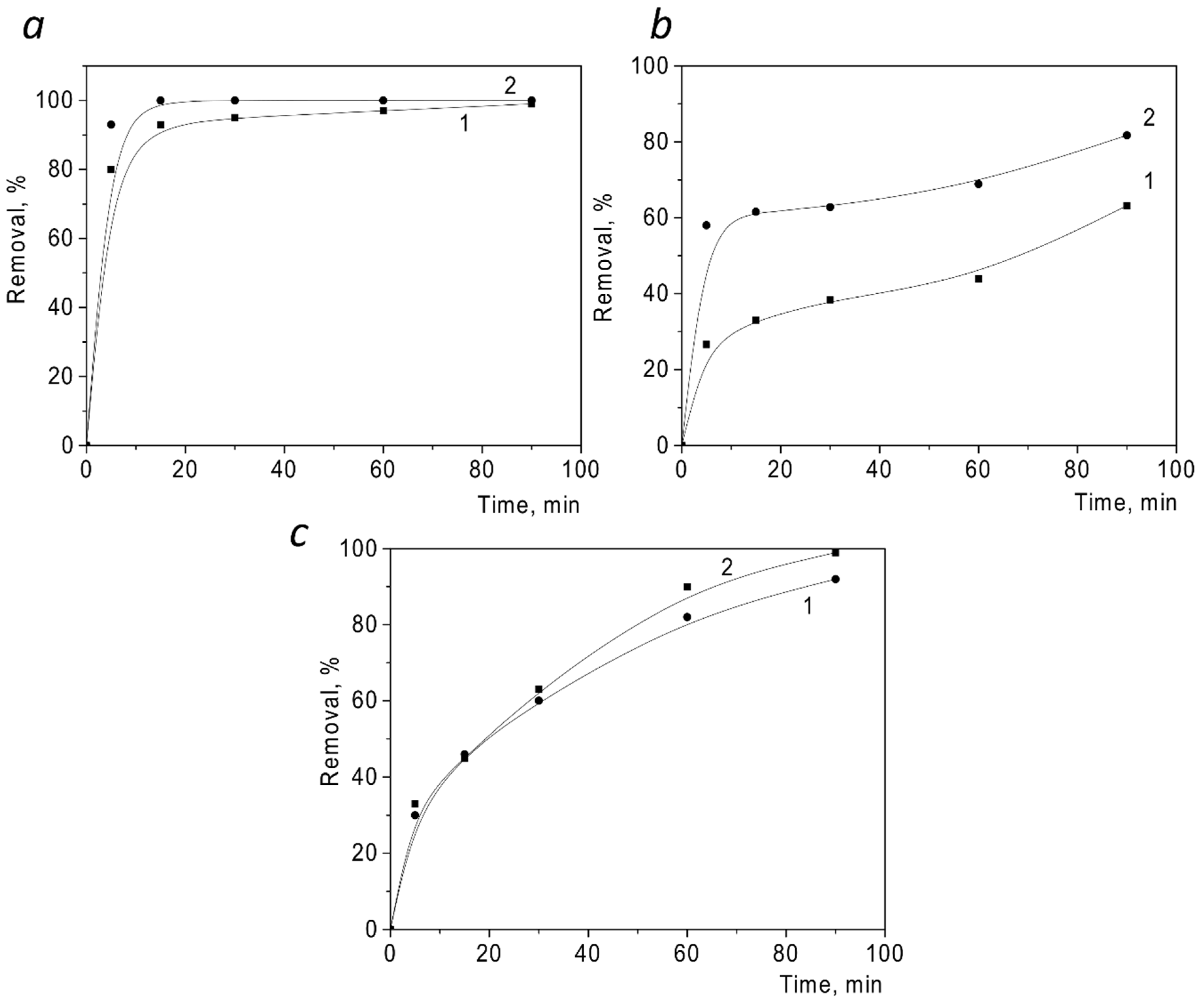

| Samples | Specific Surface Area, m2/g | Total Pore Volume, cm3/g | Mean Pore Diameter, nm |
|---|---|---|---|
| Silochrom | 143 | 0.95 | 17 |
| Silochrom/PLA | 107 | 0.55 | 12–15 |
| Silochrom-NH2/PLA | 101 | 0.49 | 12–15 |
| Removal Efficiency, % | |||||
|---|---|---|---|---|---|
| pH = 1.68 | pH = 4.01 | Distilled Water | pH = 6.86 | ||
| Co(II) | Silochrom-NH2/PLA | 0 | 6.3 | 81.7 | 73.9 |
| Silochrom/PLA | 0 | 2.1 | 63.0 | 27.8 | |
| Pb(II) | Silochrom-NH2/PLA | 3.4 | 0.7 | 99.3 | 99.7 |
| Silochrom/PLA | 0 | 25.0 | 99.5 | 89.9 | |
| Cu(II) | Silochrom-NH2/PLA | 0 | 64.0 | 86.4 | 99.0 |
| Silochrom/PLA | 0 | 60.0 | 70.2 | 92.7 | |
| Isotherm Model/ Parameter, Unit | Silochrom/PLA | Silochrom-NH2/PLA | ||||
|---|---|---|---|---|---|---|
| Cu2+ | Co2+ | Pb2+ | Cu2+ | Co2+ | Pb2+ | |
| Langmuir | ||||||
| qm, mmol/g | 0.12 | 0.04 | 0.66 | 0.31 | 0.06 | 1.44 |
| KL, L/mol | 7.949 | 70.909 | 17.976 | 5.604 | 8.531 | 49.163 |
| R2 | 0.9356 | 0.9951 | 0.80430 | 5.604 | 0.9855 | 0.9775 |
| Freundlich | ||||||
| KF, mmol/g | 1.59 | 2.59 | 1.12 | 1.96 | 13.89 | 2.55 |
| 1/n | 1.310 | 1.941 | 0.751 | 0.952 | 0.591 | 0.484 |
| R2 | 0.8757 | 0.8178 | 0.9709 | 0.9056 | 0.9665 | 0.9123 |
| Temkin | ||||||
| bT, kJ/mol | 73.652 | 415.700 | 28.719 | 38.202 | 533.180 | 15.158 |
| KT | 47.24 | 629.38 | 1669.66 | 47.20 | 712.91 | 5118.5 |
| R2 | 0.9028 | 0.8591 | 0.8304 | 0.7011 | 0.3294 | 0.9527 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polishchuk, L.M.; Kozakevych, R.B.; Kusyak, A.P.; Tertykh, V.A.; Tkachenko, O.; Strømme, M.; Budnyak, T.M. In Situ Ring-Opening Polymerization of L-lactide on the Surface of Pristine and Aminated Silica: Synthesis and Metal Ions Extraction. Polymers 2022, 14, 4995. https://doi.org/10.3390/polym14224995
Polishchuk LM, Kozakevych RB, Kusyak AP, Tertykh VA, Tkachenko O, Strømme M, Budnyak TM. In Situ Ring-Opening Polymerization of L-lactide on the Surface of Pristine and Aminated Silica: Synthesis and Metal Ions Extraction. Polymers. 2022; 14(22):4995. https://doi.org/10.3390/polym14224995
Chicago/Turabian StylePolishchuk, Liliia M., Roman B. Kozakevych, Andrii P. Kusyak, Valentin A. Tertykh, Oleg Tkachenko, Maria Strømme, and Tetyana M. Budnyak. 2022. "In Situ Ring-Opening Polymerization of L-lactide on the Surface of Pristine and Aminated Silica: Synthesis and Metal Ions Extraction" Polymers 14, no. 22: 4995. https://doi.org/10.3390/polym14224995
APA StylePolishchuk, L. M., Kozakevych, R. B., Kusyak, A. P., Tertykh, V. A., Tkachenko, O., Strømme, M., & Budnyak, T. M. (2022). In Situ Ring-Opening Polymerization of L-lactide on the Surface of Pristine and Aminated Silica: Synthesis and Metal Ions Extraction. Polymers, 14(22), 4995. https://doi.org/10.3390/polym14224995









