Thermal Degradation of Organophosphorus Flame Retardants
Abstract
1. Introduction
2. Experimental
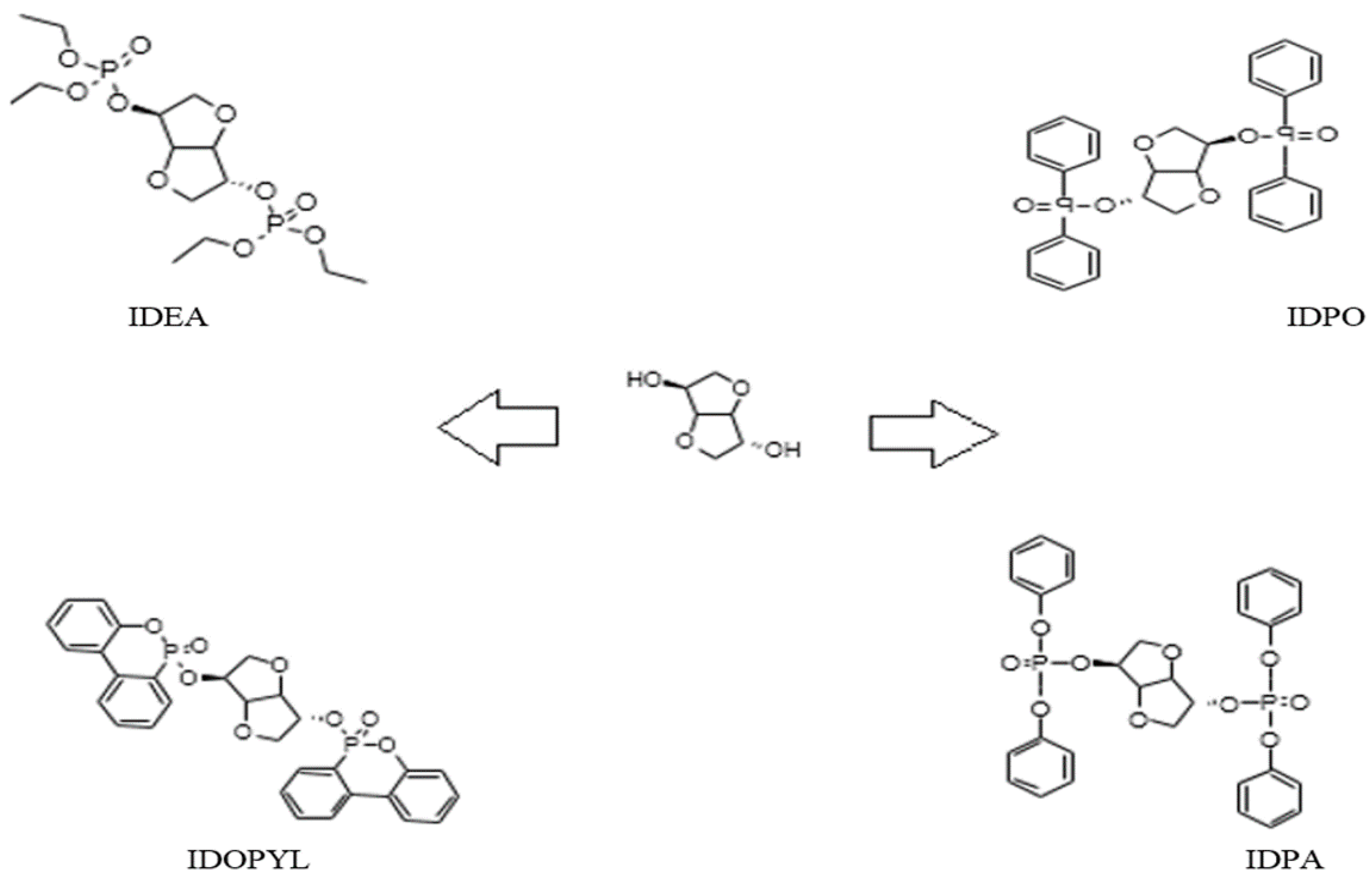
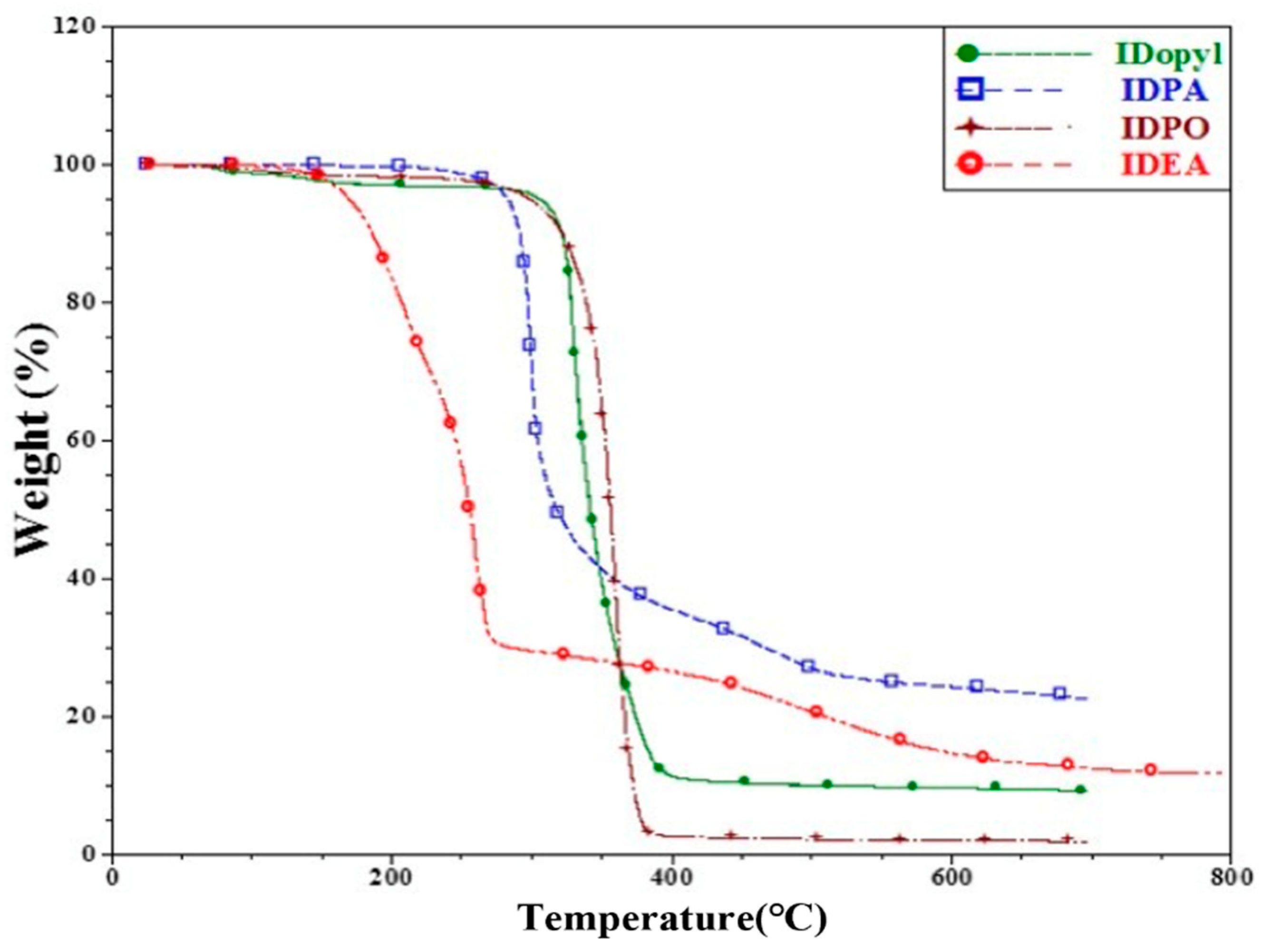
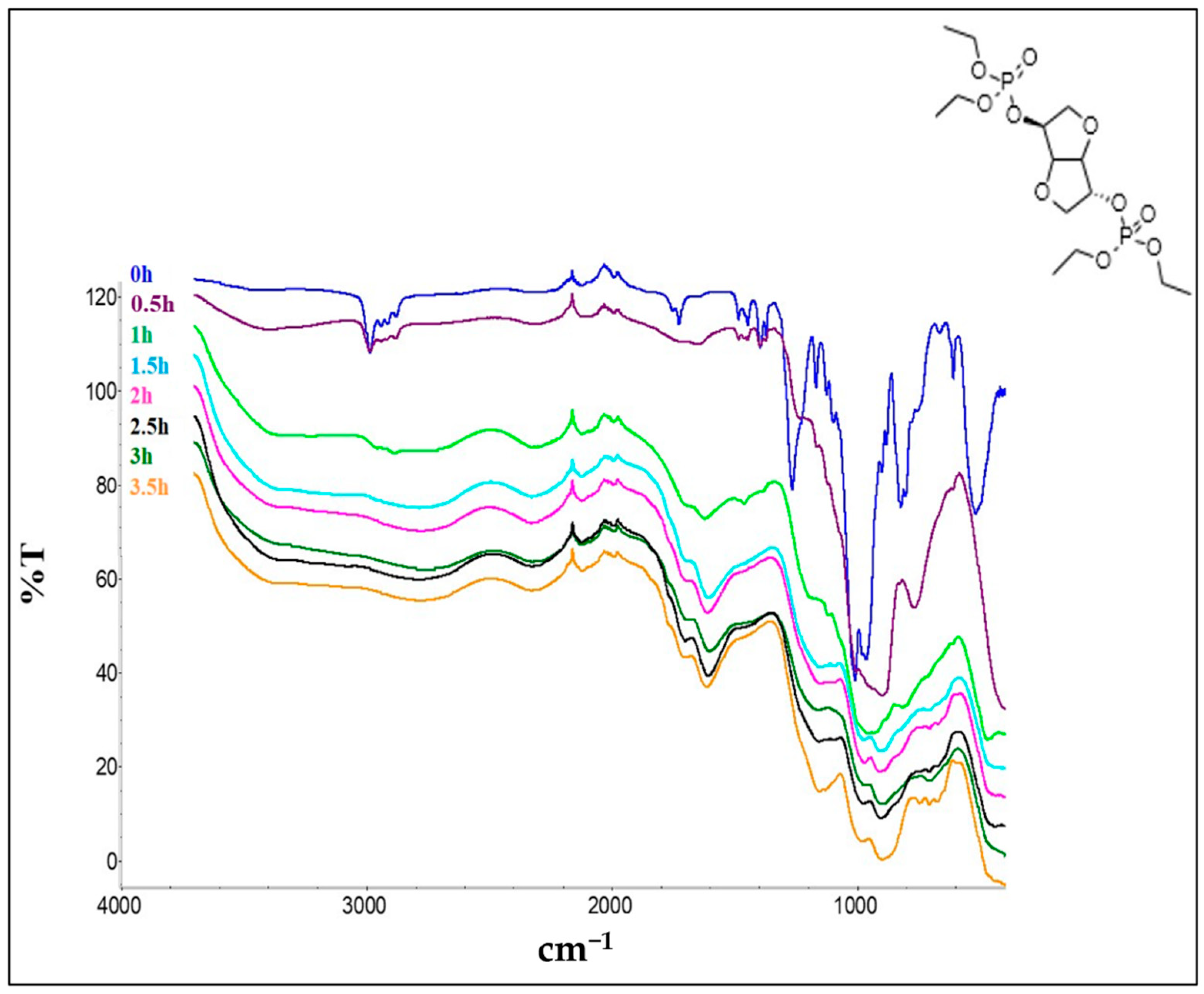
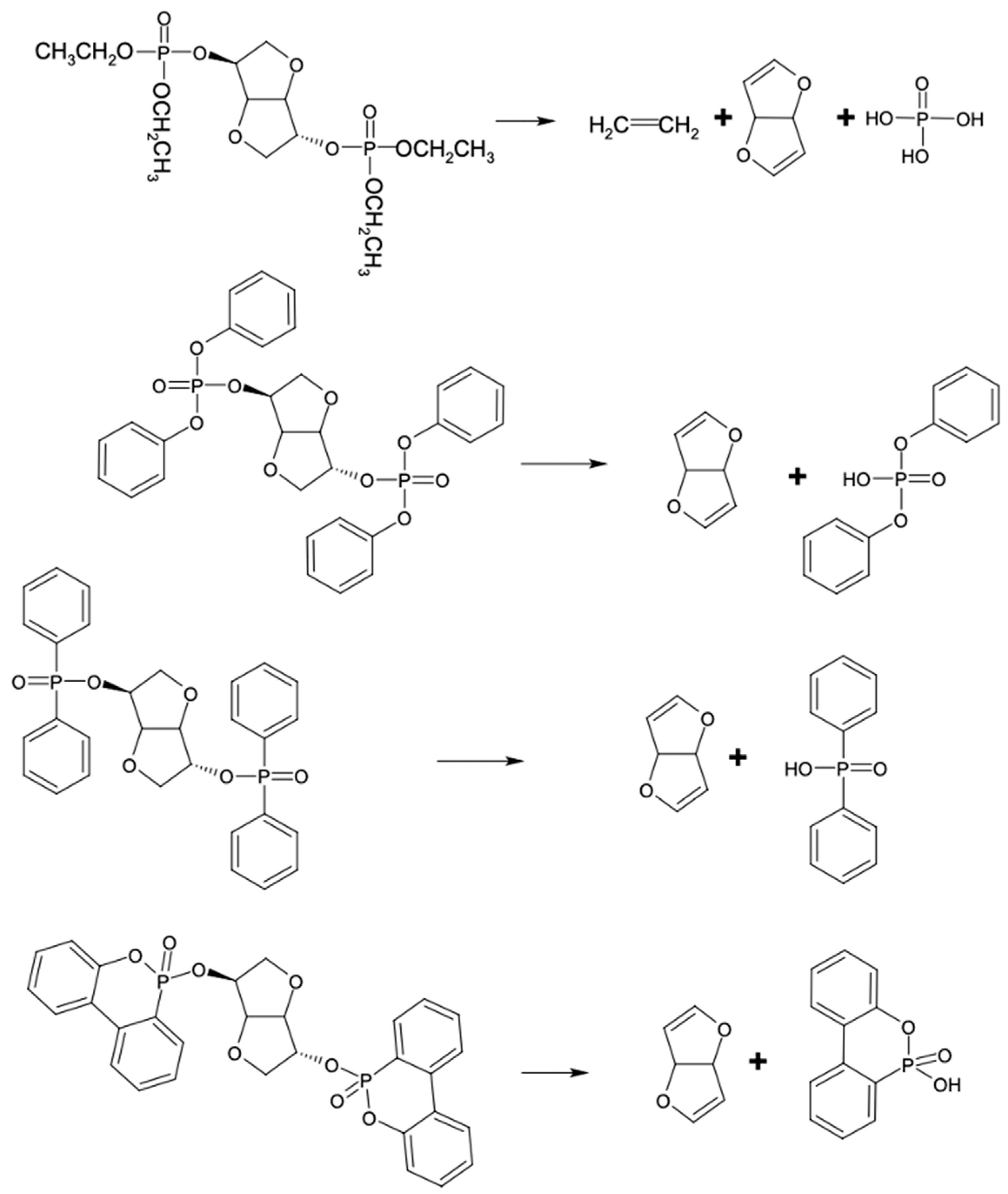
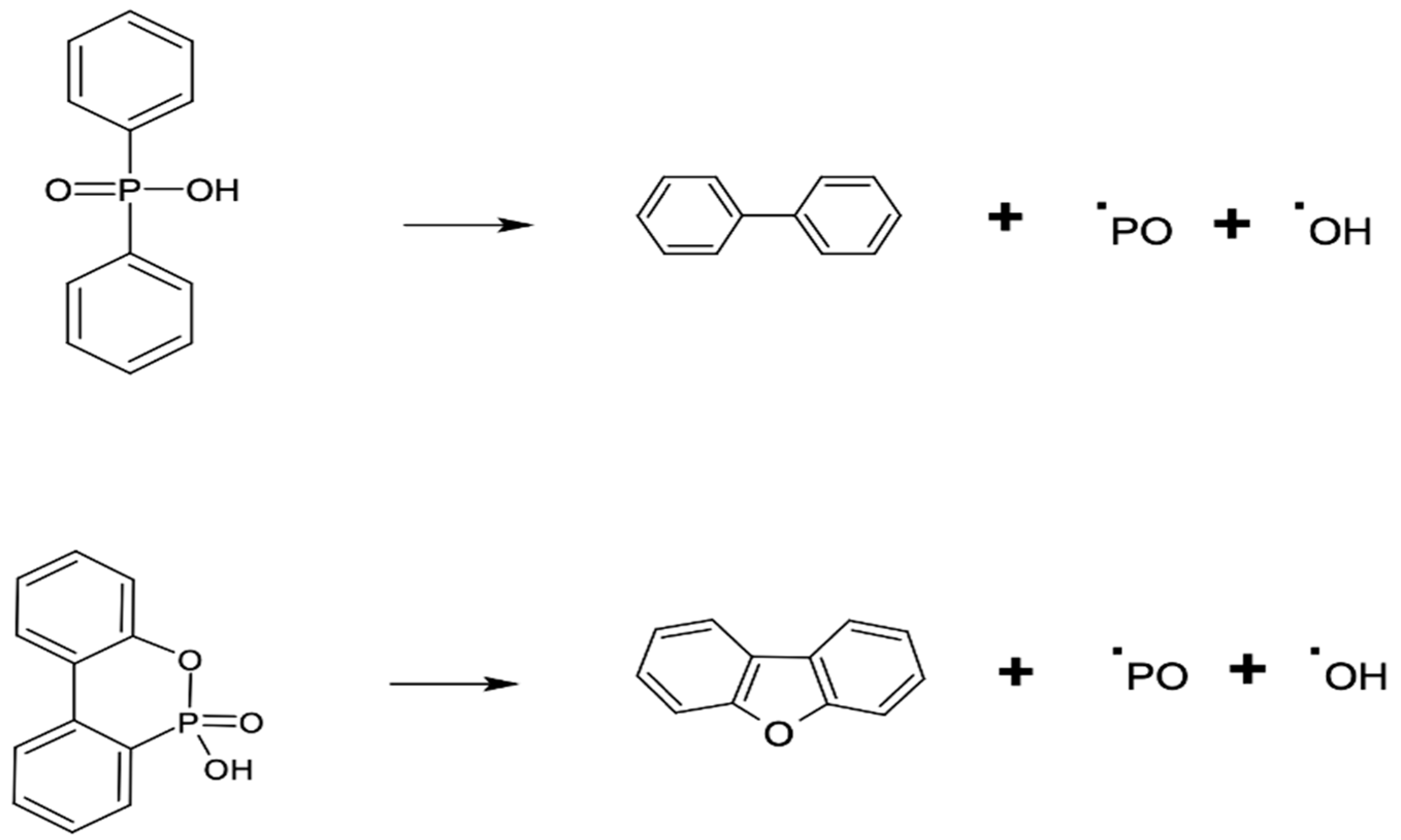
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staudinger, H. Uber Polymerization. Chem. Ber. 1920, 53, 1073–1085. [Google Scholar] [CrossRef]
- Carothers, W.A. An Introduction to the General Theory of Condensation Polymers. J. Am. Chem. Soc. 1929, 51, 2548–2559. [Google Scholar] [CrossRef]
- Furukawa, Y. Inventing Polymer Science; University of Pennsylvania Press: Philadelphia, PA, USA, 1998. [Google Scholar]
- Hermes, M.E. Enough for One Lifetime: Wallace Carothers, Inventor of Nylon; Chemical Heritage Foundation: Philadelphia, PA, USA, 1996. [Google Scholar]
- Hounshell, D.A.; Smith, J.K., Jr. Science and Corporate Strategy: Dupont R&D, 1920–1980; Cambridge University Press: New York, NY, USA, 1988. [Google Scholar]
- Lyons, J.W. The Chemistry and Uses of Fire Retardants; Wiley Interscience, Inc.: New York, NY, USA, 1970. [Google Scholar]
- Alaee, M.; Arias, P.; Sjodin, A.; Bergman, A. An Overview of Commercially Used Brominated Flame Retardants, their Application, their Use Patterns in Different Countries/Regions and Possible Modes of Release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact During their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Cristale, J.; Bele, T.G.A.; Lacorte, S.; de Marchi, M.R.R. Occurence of Flame Retardants in Landfills: A Case Study in Brazil. Environ. Res. 2019, 168, 420–427. [Google Scholar] [CrossRef]
- Osako, M.; Kim, Y.-J.; Sakai, S.-I. Leaching of Brominated Flame Retardants in Leachate from Landfills in Japan. Chemosphere 2004, 57, 1571–1579. [Google Scholar] [CrossRef]
- Zhang, M.; Buekens, A.; Li, X. Brominated Flame Retardants and the Formation of Dioxins and Furans in Fires. J. Hazard. Mater. 2016, 304, 26–39. [Google Scholar] [CrossRef]
- Behnisch, P.A.; Hosoe, K.; Sakai, S. Brominated Dioxin-like Compounds: In Vitro Assessment in Comparison to Classical Dioxin-like Compounds and Other Polyaromatic Compounds. Environ. Int. 2003, 29, 861–877. [Google Scholar] [CrossRef]
- Darnerud, P.O. Brominated Flame Retardants as Possible Endocrine Disruptors. Int. J. Androl. 2008, 31, 152. [Google Scholar] [CrossRef]
- Hamers, T.; Kamstra, J.H.; Sonnevelt, E.; Murk, A.J.; Kester, M.H.A.; Anderson, P.L.; Legler, J.; Brouwer, A. In Vitro Profiling of the Endocrine-disrupting Potency of Brominated Flame Retardants. Toxicol. Sci. 2006, 92, 157–173. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Merkwart, J.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef] [PubMed]
- Howell, B.A.; Daniel, Y.G. Isosorbide as a Platform for the Generation of New Biobased Organophosphorus Flame Retardants. Insi. Chem. Biochem. 2020, 1. [Google Scholar] [CrossRef]
- Factor, A. The Chemistry of Polymer Burning and Flame Retardance. J. Chem. Enduc. 1974, 51, 453–456. [Google Scholar] [CrossRef]
- Granzow, A. Flame Retardation by Phosphorus Compounds. Accounts Chem. Res. 1978, 11, 177–183. [Google Scholar] [CrossRef]
- Braun, U.; Balbanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Doering, M.; Perez, R.; Sandler, J.K.W.; Alstadt, V.; et al. Influence of the Oxidation State of PHosphorus on the Decomposition and Fire Behaviorof Flame-retarded Epoxy Resin Composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Modesti, M.; Besco, S.; Hrelja, D.; Donadi, S. Influence of Phosphorus Valency on the Thermal Behavior of Flame Retarded Polyurethane Foam. Polym. Degrad. Stab. 2011, 96, 1455–1461. [Google Scholar] [CrossRef]
- Sonnier, R.; Negrell-Guirao, C.; Vahabi, H.; Otazaghine, B.; David, G.; Lopez-Cuesta, J.M. Relationship Between the Molecular Structure and Flammability of Polymers: Study of Phosphonate Functions Using a Microscale Combustion Calorimeter. Polymer 2012, 53, 1258–1266. [Google Scholar] [CrossRef]
- Schartel, B.; Perret, B.; Dittrich, B.; Ciesielski, M.; Kramer, J.; Mueller, P.; Alstadt, V.; Zhang, L.; Doering, M. Flame Retardancy of Polymers: The Role of Specific Reactions in the Condensed Phase. Macromol. Mater. Eng. 2016, 301, 9–35. [Google Scholar]
- Schafer, A.; Seibold, S.; Lohstroh, W.; Walter, O.; Doering, M. Synthesis and Properties of Flame-retardant Epoxy Resins Based on DOPO and One of Its Analogs DPPO. J. Appl. Polym. Sci. 2007, 105, 685–696. [Google Scholar] [CrossRef]
- Liang, S.; Hemberger, P.; Neisins, N.M.; Bodi, A.; Grutzmacher, H.; Gaan, S. Elucidating the Thermal Decomposition of Dimethyl Methylphosphonate by Vacuum Ultraviolet (VUV) Photoionization: Pathways to the PO Radical, a Key Species in Flame-retardant Mechanisms. Chem. Eur. J. 2015, 21, 1073–1080. [Google Scholar] [CrossRef]
- Howell, B.A.; Lienhart, G.W. Phosphorus Esters of 1-Dopyl-1,2-(4-hydroxyphenyl)ethene as Effective Flame Retardants for Polymeric Materials. Fire Mater. 2020, 44, 1127–1134. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Synthesis and Characterization of Isosorbide bis-Phosphorus Esters. Heteroat. Chem. 2017, 28, e21369. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Flame Retardant Properties of Isosorbide bis-Phosphorus Esters. Polym. Degrad. Stab. 2017, 140, 25–31. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Thermal Degradation of bis-Phosphorus Esters of Isosorbide. J. Therm. Anal. Calorim. 2018, 131, 363–369. [Google Scholar] [CrossRef]
- Howell, B.A.; Daniel, Y.G. Incorporation of Comonomer exo-5-(Diphenylphosphato)isorsorbide-2-endo-acrylate to Generate Flame Retardant Poly(styrene). Polymers 2019, 11, 2033. [Google Scholar] [CrossRef]
- Howell, B.A.; Ostrander, E.A. Thermal Degradation of Flame-retardant Compounds Derived from Castor Oil. J. Therm. Anal. Calorim. 2019, 138, 3961–3975. [Google Scholar] [CrossRef]
- Howell, B.A.; Zhang, T.; Smith, P.B. Biobased Hyperbranched Poly(ester)s of Precise Structure for the Release of Therapeutics. Med. Res. Arch. 2021, 9. [Google Scholar] [CrossRef]
- Zhang, T.; Howell, B.A.; Smith, P.B. Rational Synthesis of Hyperbranched Poly(ester)s. Ind. Eng. Chem. Res. 2017, 56, 1661–1670. [Google Scholar] [CrossRef]
- Zhang, T.; Howell, B.A.; Dumitrascu, A.; Martin, S.J.; Smith, P.B. Synthesis and Characterization of Glycerol-Adipic Acid Hyperbranched Poly(ester)s. Polymer 2014, 55, 5065–5072. [Google Scholar] [CrossRef]
- Howell, B.A.; Lazar, S.T. Biobased Plasticizers from Glycerol/Adipic Acid Hyperbranched Poly(ester)s. Ind. Eng. Chem. Res. 2019, 58, 17227–17234. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howell, B.A. Thermal Degradation of Organophosphorus Flame Retardants. Polymers 2022, 14, 4929. https://doi.org/10.3390/polym14224929
Howell BA. Thermal Degradation of Organophosphorus Flame Retardants. Polymers. 2022; 14(22):4929. https://doi.org/10.3390/polym14224929
Chicago/Turabian StyleHowell, Bob A. 2022. "Thermal Degradation of Organophosphorus Flame Retardants" Polymers 14, no. 22: 4929. https://doi.org/10.3390/polym14224929
APA StyleHowell, B. A. (2022). Thermal Degradation of Organophosphorus Flame Retardants. Polymers, 14(22), 4929. https://doi.org/10.3390/polym14224929





