Flexible Polyurethane Foams Reinforced by Functionalized Polyhedral Oligomeric Silsesquioxanes: Structural Characteristics and Evaluation of Thermal/Flammability Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polyurethane Foams
2.3. Methods
3. Results
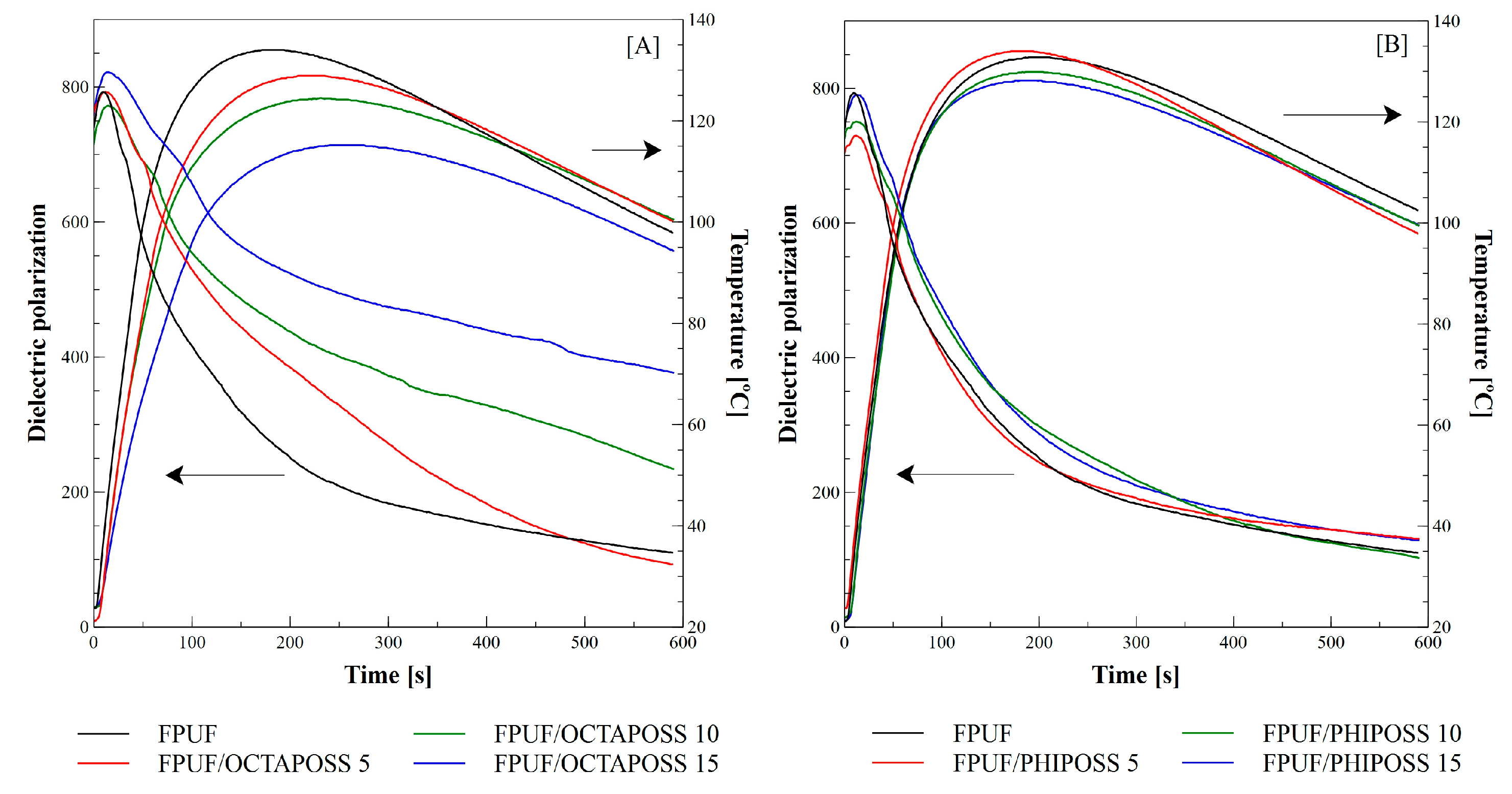
3.1. Foaming Process
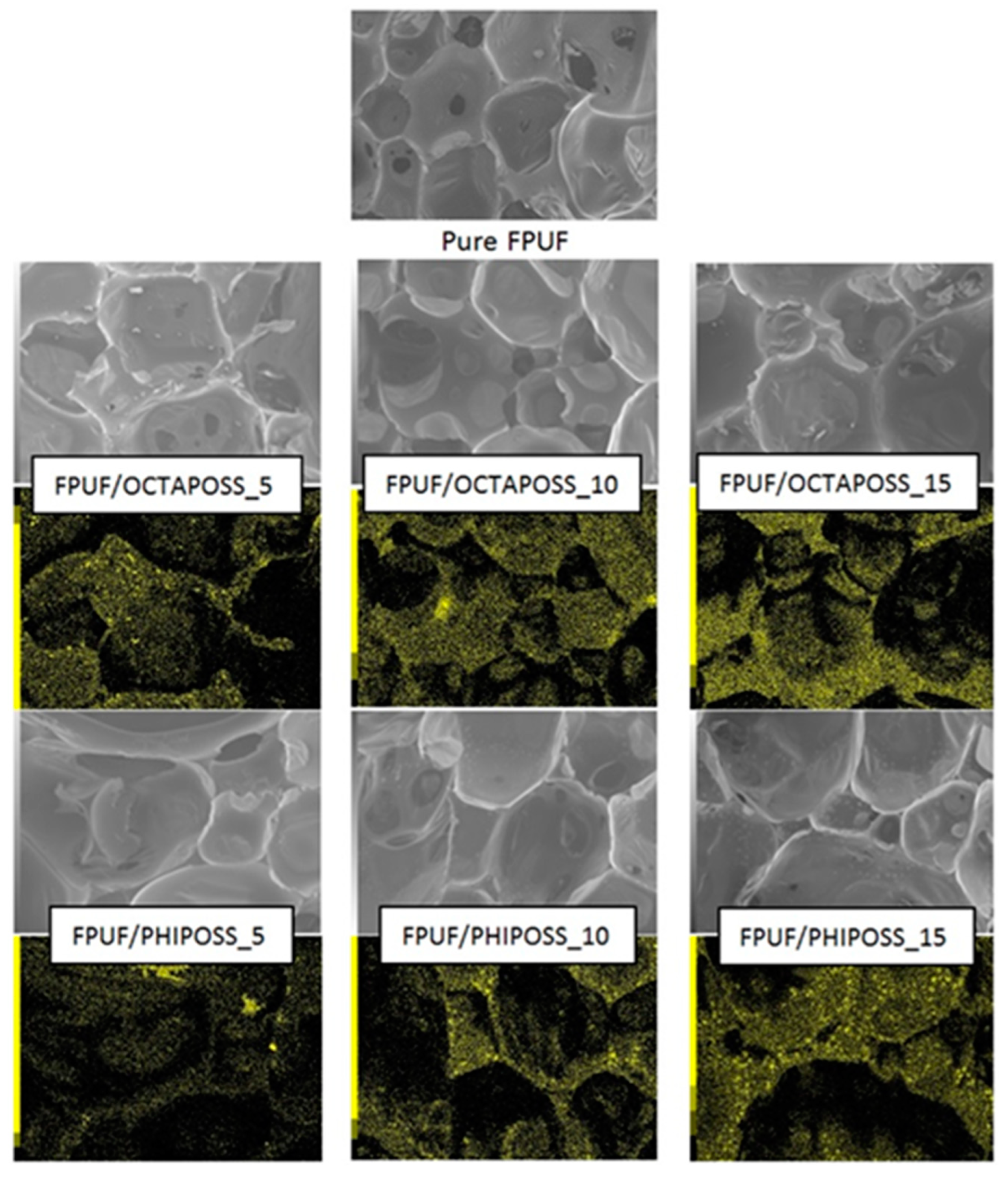
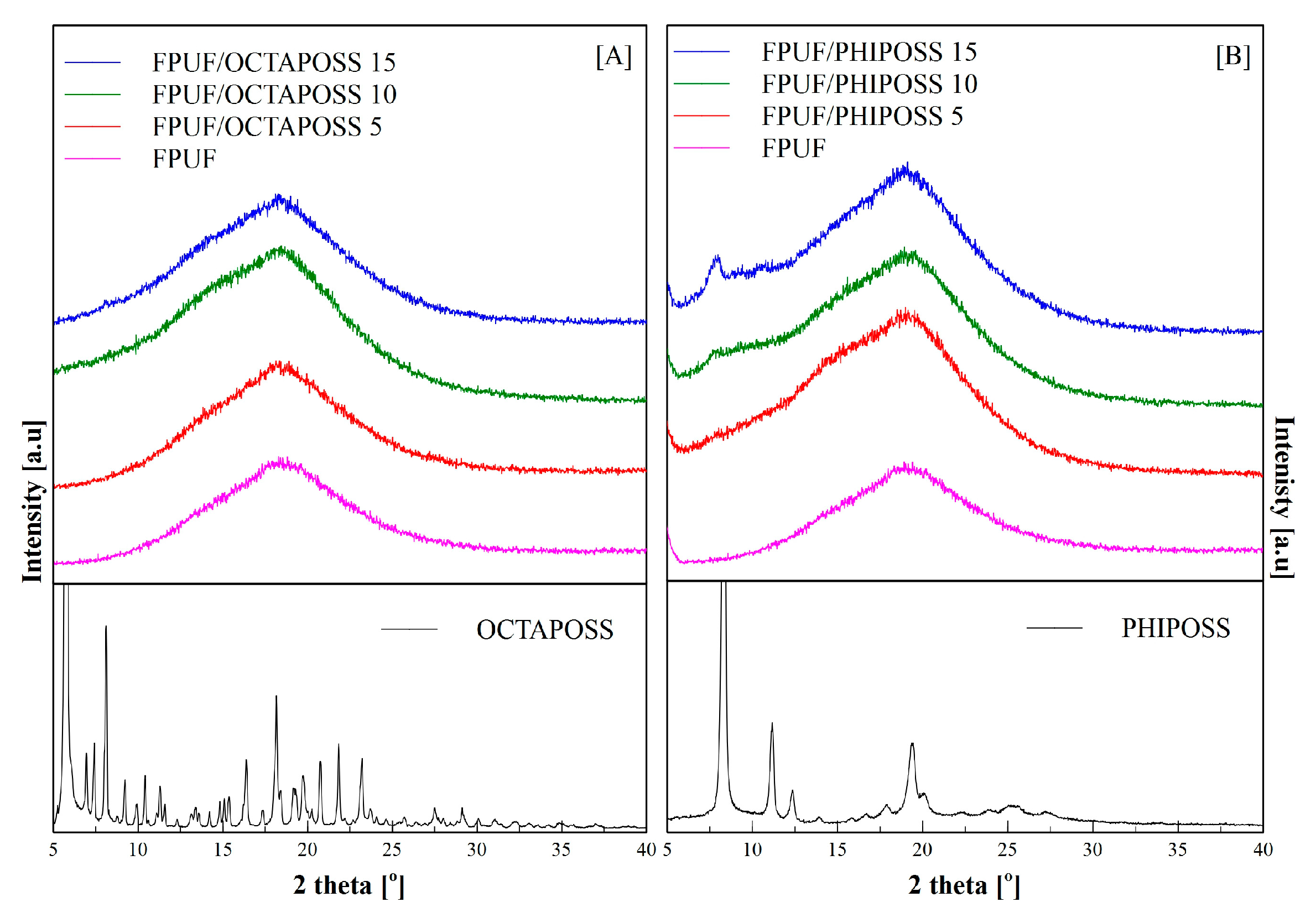
3.2. Structure and Morphology
3.3. Physicochemical and Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Javni, I.; Song, K.; Lin, J.; Petrovic, Z.S. Structure and properties of flexible polyurethane foams with nano- and micro-fillers. J. Cell Plast. 2011, 47, 357–372. [Google Scholar] [CrossRef]
- Liu, P.S.; Chen, G.F. Porous Materials: Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Artavia, L.D.; Macosko, C.W. Polyurethane flexible foam formation. In Low-Density Cellular Plastics; Hilyard, N.C., Cunningham, A., Eds.; Chapmann Hall: London, UK, 1994; pp. 22–55. [Google Scholar]
- Lee, S.T.; Ramesh, N.S. Polymeric Foams. Mechanisms and Materials; CRC Press LLC: Boca Raton, FL, USA, 2004. [Google Scholar]
- Randall, D.; Lee, S. The Polyurethane Book; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Ashida, K. Polyurethane and Related Foams Chemistry and Technology; Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Whittaker, R.E. The Mechanical Behavior of Microporous Polyurethane Foams. J. Appl. Poly. Sci. 1971, 15, 1205. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge Press: Cambridge, UK, 1999. [Google Scholar]
- Moreland, J.C.; Wilkes, G.L.; Turner, R.B. Viscoelastic behavior of flexible slabstock polyurethane foams: Dependence on temperature and relative humidity. I. Tensile and compression stress (load) relaxation. J. Appl. Poly. Sci. 1994, 52, 549–568. [Google Scholar] [CrossRef]
- Moreland, J.C.; Wilkes, G.L.; Turner, R.B. Viscoelastic behavior of flexible slabstock polyurethane foam as a function of temperature and relative humidity. II. Compressive creep behawior. J. Appl. Poly. Sci. 1994, 52, 569–576. [Google Scholar] [CrossRef]
- Aou, K.; Ge, S.; Mowery, D.M.; Zeigler, R.C.; Gamboa, R.R. Two-domain morphology in viscoelastic polyurethane foams. Polymer 2015, 56, 37–45. [Google Scholar] [CrossRef]
- Kaushiva, B.D. Structure-Property Telationships of Flexible Polyurethane Foams. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 1999. [Google Scholar]
- Kickelbick, G. Introduction to Hybrid Materials; WILEY-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Zheng, L.; Kasi, R.M.; Farris, R.J.; Coughlin, E.B. Synthesis and thermal properties of hybrid copolymers of syndiotactic polystyrene and polyhedral oligomeric silsesquioxane. J. Polym. Sci. A Polym. Chem. 2002, 39, 2920. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Yen, Y.-C.; Chang, F.-C. Self-supporting polymer from a POSS derivative. Macromol. Rapid Commun. 2011, 32, 927–932. [Google Scholar] [CrossRef]
- Wu, J.; Haddad, T.H.; Mather, P.T. Vertex group effects in entangled polystyrene-polyhedral oligosilsesquioxane (POSS) copolymers. Macromolecules 2009, 42, 1142. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Guan, D. Preparation and properties of epoxy resin/polyhedral oligomeric silsesquioxane hybrid materials. Polym. Bull. 2016, 73, 113–123. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, S.; Nie, K. Morphology and Thermomechanical Properties of Organic−Inorganic Hybrid Composites Involving Epoxy Resin and an Incompletely Condensed Polyhedral Oligomeric Silsesquioxane. Macromolecules 2005, 38, 5088–5097. [Google Scholar] [CrossRef]
- Xu, H.; Kuo, S.W.; Lee, J.S.; Chang, F.C. Preparations, Thermal Properties, and Tg Increase Mechanism of Inorganic/Organic Hybrid Polymers Based on Polyhedral Oligomeric Silsesquioxanes. Macromolecules 2002, 35, 8788–8793. [Google Scholar] [CrossRef]
- Pagacza, J.; Hebda, E.; Janowski, B.; Sternik, D.; Jancia, M.; Pielichowski, K. Thermal decomposition studies on polyurethane elastomers reinforced with polyhedral silsesquioxanes by evolved gas analysis. Polym. Degrad. Stab. 2018, 149, 129–142. [Google Scholar] [CrossRef]
- Kalia, S.; Pielichowski, K. Polymer/POSS Nanocomposites and Hybrid Materials: Preparation, Properties, Applications; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Montero, B.; Bellas, R.; Ramirez, C.; Rico, M.; Bouza, R. Flame retardancy and thermal stability of organic–inorganic hybrid resins based on polyhedral oligomeric silsesquioxanes and montmorillonite clay. Compos. Part B 2014, 63, 67. [Google Scholar] [CrossRef]
- Chen, S.; Gao, J.; Han, H.; Wang, C. Mechanical and thermal properties of epoxy-POSS reinforced-(biphenyl diol formaldehyde/epoxy hybrid resin) composites. Iran. Polym. J. 2014, 23, 609. [Google Scholar] [CrossRef]
- Gnanasekaran, D.; Walter, P.A.; Reddy, B.S.R. Influence of moieties on morphology, thermal, and dielectric properties in polyamide-polyhedral oligomeric silsequioxanes nanocomposites. Polym. Eng. Sci. 2013, 53, 1637. [Google Scholar] [CrossRef]
- Hebda, E.; Bukowczan, A.; Michałowski, S.; Wroński, S.; Urbaniak, P.; Kaczmarek, M.; Hutnik, E.; Romaniuk, A.; Wolun-Cholewa, M.; Pielichowski, K. Examining the influence of functionalized POSS on the structure and bioactivity of flexible polyurethane foams. Mater. Sci. Eng. C 2020, 108, 110370. [Google Scholar] [CrossRef] [PubMed]
- Michałowski, S.; Hebda, E.; Pielichowski, K. Thermal stability and flammability of polyurethane foams chemically reinforced with POSS. J. Therm. Anal. Calorim. 2017, 130, 155–163. [Google Scholar] [CrossRef]
- Michałowski, S.; Pielichowski, K. 1,2-Propanediolizobutyl POSS as a co-flame retardant for rigid polyurethane foams. J. Therm. Anal. Calorim. 2018, 134, 1351–1358. [Google Scholar] [CrossRef]
- Pagacz, J.; Hebda, E.; Michałowski, S.; Ozimek, J.; Sternik, D.; Pielichowski, K. Polyurethane foams chemically reinforced with POSS—Thermal degradation studies. Thermochim. Acta 2016, 642, 95–104. [Google Scholar] [CrossRef]
- Hebda, E.; Ozimek, J.; Raftopoulos, K.N.; Michałowski, S.; Pielichowski, J.; Jancia, M.; Pielichowski, K. Synthesis and morphology of rigid polyurethane foams with POSS as pendant groups or chemical crosslinks. Polym. Adv. Technol. 2015, 26, 932–940. [Google Scholar] [CrossRef]
- Mantz, R.A.; Jones, P.F.; Chaffee, K.P.; Lichtenhan, J.D.; Gilman, J.W.; Ismail, I.M.K.; Burmeister, M.J. Thermolysis of polyhedral oligomeric silsesquioxane (POSS) macromers and POSS-siloxane copolymers. Chem. Mater. 1996, 8, 1250–1259. [Google Scholar] [CrossRef]
- Bourbigot, S.; Turf, T.; Bellayer, S.; Duquesne, S. Polyhedral oligomeric silsesquioxane as flame retardant for thermoplastic polyurethane. Polym. Degrad. Stab. 2009, 94, 1230–1237. [Google Scholar] [CrossRef]
- Belot, V.; Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. Redistribution reactions in silsesquioxane gels. J. Mater. Sci. Lett. 1990, 9, 1052–1054. [Google Scholar] [CrossRef]
- Liu, X.; Salmeia, K.A.; Rentsch, D.; Hao, J.; Gaan, S. Thermal decomposition and flammability of rigid PU foams containing some DOPO derivatives and other phosphorus compounds. J. Anal. Appl. Pyrolysis 2017, 124, 219–229. [Google Scholar] [CrossRef]
- Vahabi, H.; Ferry, L.; Longuet, C.; Otazaghine, B.; Negrell-Guirao, C.; David, G.; Lopez-Cuesta, J.-M. Combination effect of polyhedral oligomeric silsesquioxane (POSS) and a phosphorus modified PMMA, flammability and thermal stability properties. Mater. Chem. Phys. 2012, 136, 762–770. [Google Scholar] [CrossRef]
- Sun, Y.; Tong, D.; Li, Z. Ceramization of Carborane-POSS and Coating on Carbon Fiber via Precursor Infiltration-Pyrolysis (PIP). Method. Fibers Polym. 2019, 20, 1564–1576. [Google Scholar] [CrossRef]
- Le Bras, M.; Bourbigot, S.; Duquesne, S.; Jama, C.; Wilkie, C.H. Fire Retardancy of Polymers: New Applications of Mineral Fillers; Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar]
- Hiltz, J.A. Analytical pyrolysis gas chromatography/mass spectrometry (py-GC/MS) of poly(ether urethane)s, poly(ether urea)s and poly(ether urethane-urea). J. Anal. Appl. Pyrolysis 2015, 113, 248–258. [Google Scholar] [CrossRef]
- Garrido, M.A.; Gerecke, A.C.; Heeb, N.; Font, R.; Cones, J.A. Isocyanate emissions from pyrolysis of mattresses containing polyurethane foam. Chemosphere 2017, 168, 667. [Google Scholar] [CrossRef]

| Reagents | FPUF | PHIPOSS | OCTAPOSS | ||||
|---|---|---|---|---|---|---|---|
| Polyol [%] | 100 | ||||||
| PHI-POSS [%wt.] | - | 5 | 10 | 15 | - | - | - |
| OCTA-POSS [%wt.] | - | - | - | - | 5 | 10 | 15 |
| Water [%] | 4 | ||||||
| Surfactant [%] | 1.5 | ||||||
| Catalyst [%] | 1.0 | ||||||
| TDI, NCO index | 1.0 | ||||||
| Measurement | FPUF [%] | FPUF/PHIPOSS [%] | FPUF/OCTAPOSS [%] | ||||
|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 5 | 10 | 15 | ||
| Apparent Density [kg/m3] | 28.8 ± 0.1 | 32.6 ± 1.4 | 33.7 ± 1.6 | 36.3 ± 1.5 | 30.7 ± 1.4 | 31.4 ± 1.6 | 32.3 ± 1.4 |
| Compression Strength [kPa] | 12.2 ± 2.3 | 14.4 ± 3.1 | 14.4 ± 0.8 | 20.3 ± 3.9 | 20.3 ± 2.2 | 24.0 ± 0.9 | 24.4 ± 1.9 |
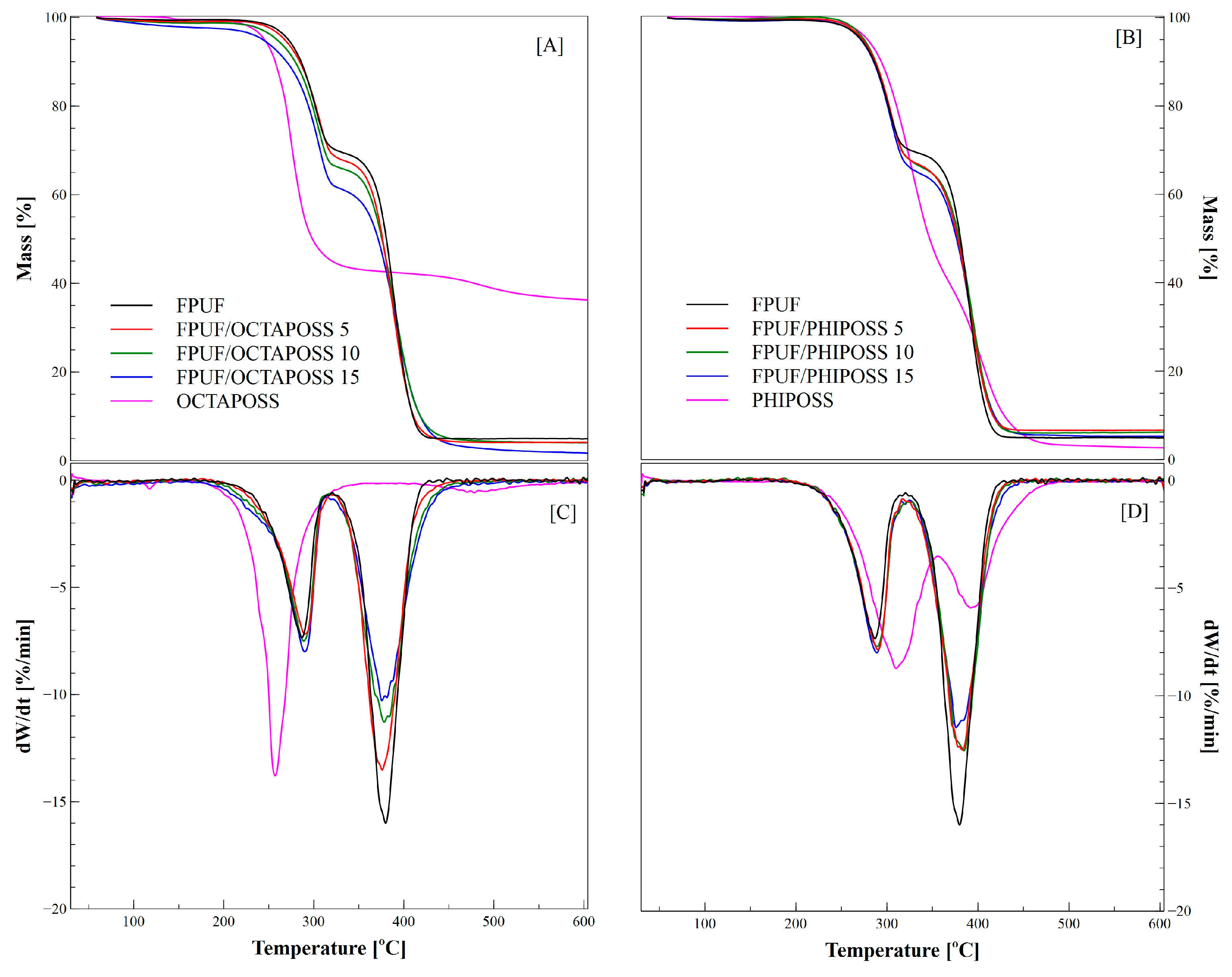
| Samples | Tonset [°C] | ΔTonset [°C] | TDTGmax1 [°C] | TDTGmax2 [°C] | DTGmax1 [%/min] | DTGmax2 [%/min] | m1 [%] | Residual Mass [%] |
|---|---|---|---|---|---|---|---|---|
| FPUF | 259.5 | - | 286.8 | 379.8 | −7.3 | −16.0 | 30.6 | 4.9 |
| FPUF/OCTAPOSS 5 | 260.7 | 1.2 | 290.1 | 376.0 | −7.2 | −13.5 | 31.3 | 4.1 |
| FPUF/OCTAPOSS 10 | 259.5 | 0.0 | 288.8 | 378.1 | −7.5 | −11.3 | 32.8 | 4.1 |
| FPUF/OCTAPOSS 15 | 259.4 | 0.1 | 290.0 | 375.6 | −3.1 | −10.3 | 36.2 | 1.7 |
| FPUF/PHIPOSS 5 | 261.8 | 2.3 | 290.2 | 382.8 | −7.9 | −12.5 | 32.7 | 6.6 |
| FPUF/PHIPOSS 10 | 260.9 | 1.4 | 289.4 | 384.6 | −7.7 | −12.5 | 33.8 | 6.2 |
| FPUF/PHIPOSS 15 | 261.1 | 0.6 | 288.9 | 376.0 | −7.7 | −12.0 | 35.5 | 5.2 |
| OCTAPOSS | 237.8 | −21.3 | 257.3 | 478.5 | −13.8 | −0.6 | 57.3 | 36.3 |
| PHIPOSS | 272.4 | 12.9 | 312.3 | 393.7 | −8.6 | −5.9 | 60.1 | 2.7 |
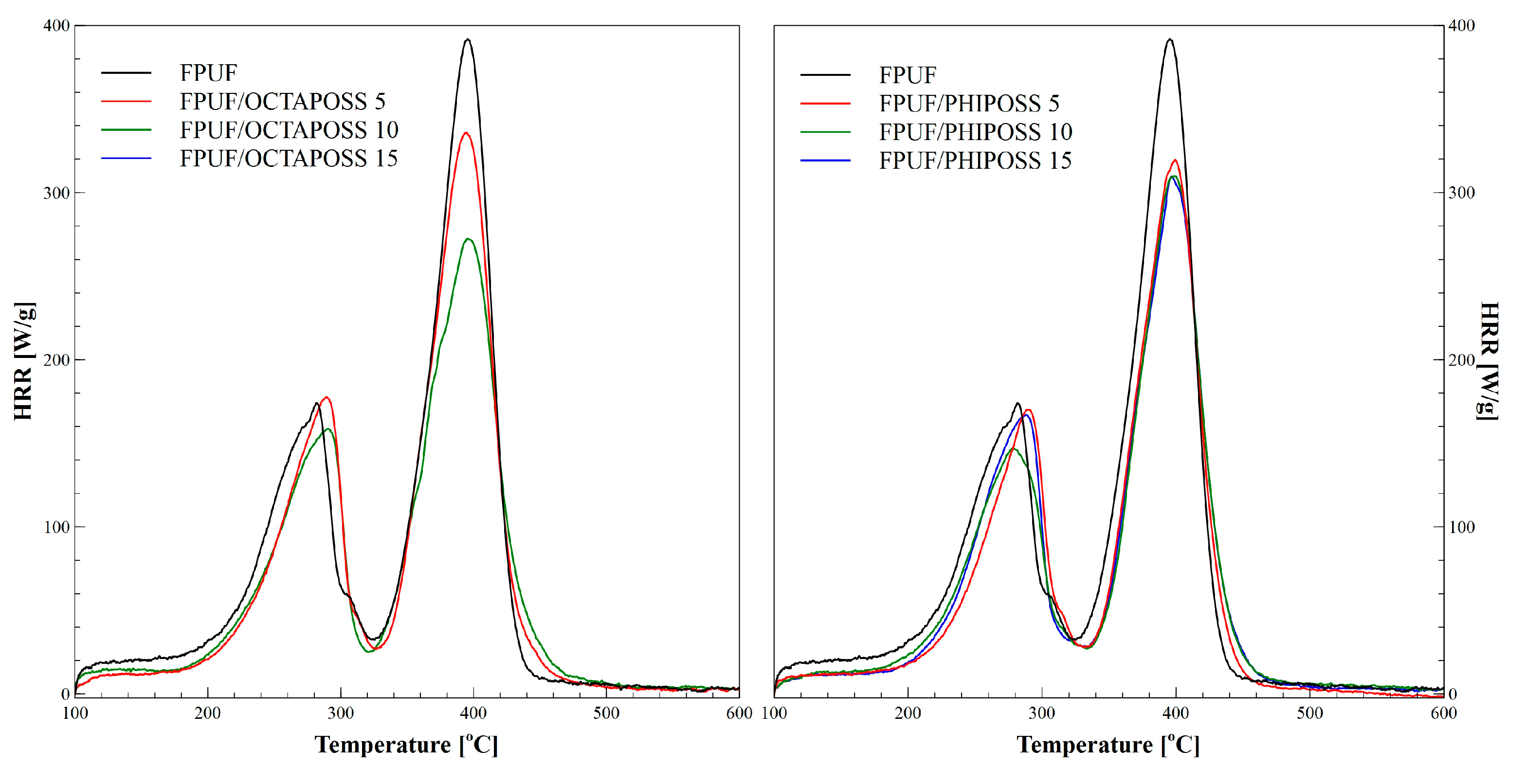
| Sample | pHRR2 [W/g] | THR [kJ/g] | HRC [J/gK] | T2 [°C] |
|---|---|---|---|---|
| EPUF | 387.0 ± 29.0 | 35.2 ± 2.8 | 419 ± 28 | 395 ± 4 |
| EPUF/OCTAPOSS 5 | 323.1 ± 11.0 | 31.7 ± 2.7 | 351 ± 12 | 395 ± 3 |
| EPUF/OCTAPOSS 10 | 261.4 ± 15.1 | 29.9 ± 1.2 | 277 ± 17 | 388 ± 9 |
| EPUF/OCTAPOSS 15 | 228.1 ± 9.2 | 30.2 ± 0.8 | 248 ± 12 | 387 ± 3 |
| EPUF/PHIPOSS 5 | 314.7 ± 16.4 | 31.1 ± 2.9 | 342 ± 21 | 399 ± 1 |
| EPUF/PHIPOSS 10 | 310.0 ± 14.6 | 29.9 ± 0.9 | 337 ± 18 | 396 ± 1 |
| EPUF/PHIPOSS 15 | 307.9 ± 21.4 | 31.3 ± 2.1 | 333 ± 24 | 396 ± 2 |
| Compounds | Retention Time [min] | Identified | ||||
|---|---|---|---|---|---|---|
| OCTAPOSS | PHIPOSS | FPUF | FPUF/ OCTA POSS 15 | FPUF/ PHI POSS 15 | ||
| Carbon dioxide | 1.2 | x | x | x | x | |
| 2-methyl-1-Propene | 1.7 | x | ||||
| Acetone | 1.8 | x | ||||
| Propene | 1.9 | x | x | x | ||
| 1-methoxy-2-propanone | 2.0 | x | x | x | ||
| Tetrahydrofuran | 2.3 | x | ||||
| Tetramethyl-oxirane | 2.8 | x | ||||
| [(1,1-dimethyl-2-propenyl)oxy]dimethyl-silane | 3.7 | x | x | x | ||
| 1,1′-[ethylidenebis(oxy)]bis-propane | 3.8 | x | x | x | ||
| 1-(1-methylethoxy)- 2-propanone | 4.0 | x | x | x | ||
| Allyldimethyl-silanol | 5.4 | x | x | x | ||
| 4-methyl-3-heptanol | 5.6 | x | x | x | ||
| 3,3′-oxybis-1-propanol | 5.8 | x | x | x | ||
| 3-Buten-1-ol TBDS derivative (isomers) | 6.6 | x | ||||
| tri(propylene glycol) propyl ether | 7.3 | x | x | x | ||
| 5-methyl-3-heptanol | 7.4 | x | x | x | ||
| 1,3-diisocyanato-2-methyl-benzene | 9.1 | x | x | x | ||
| 7-amino-1,3-dihydro-indol-2-one | 9.5 | x | x | x | ||
| 4-(methyleneamino)phenyldimethyl amine | 9.6 | x | x | x | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hebda, E.; Bukowczan, A.; Michałowski, S.; Pielichowski, K. Flexible Polyurethane Foams Reinforced by Functionalized Polyhedral Oligomeric Silsesquioxanes: Structural Characteristics and Evaluation of Thermal/Flammability Properties. Polymers 2022, 14, 4743. https://doi.org/10.3390/polym14214743
Hebda E, Bukowczan A, Michałowski S, Pielichowski K. Flexible Polyurethane Foams Reinforced by Functionalized Polyhedral Oligomeric Silsesquioxanes: Structural Characteristics and Evaluation of Thermal/Flammability Properties. Polymers. 2022; 14(21):4743. https://doi.org/10.3390/polym14214743
Chicago/Turabian StyleHebda, Edyta, Artur Bukowczan, Sławomir Michałowski, and Krzysztof Pielichowski. 2022. "Flexible Polyurethane Foams Reinforced by Functionalized Polyhedral Oligomeric Silsesquioxanes: Structural Characteristics and Evaluation of Thermal/Flammability Properties" Polymers 14, no. 21: 4743. https://doi.org/10.3390/polym14214743
APA StyleHebda, E., Bukowczan, A., Michałowski, S., & Pielichowski, K. (2022). Flexible Polyurethane Foams Reinforced by Functionalized Polyhedral Oligomeric Silsesquioxanes: Structural Characteristics and Evaluation of Thermal/Flammability Properties. Polymers, 14(21), 4743. https://doi.org/10.3390/polym14214743