Self-Healing and Reprocessable Oleic Acid-Based Elastomer with Dynamic S-S Bonds as Solvent-Free Reusable Adhesive on Copper Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
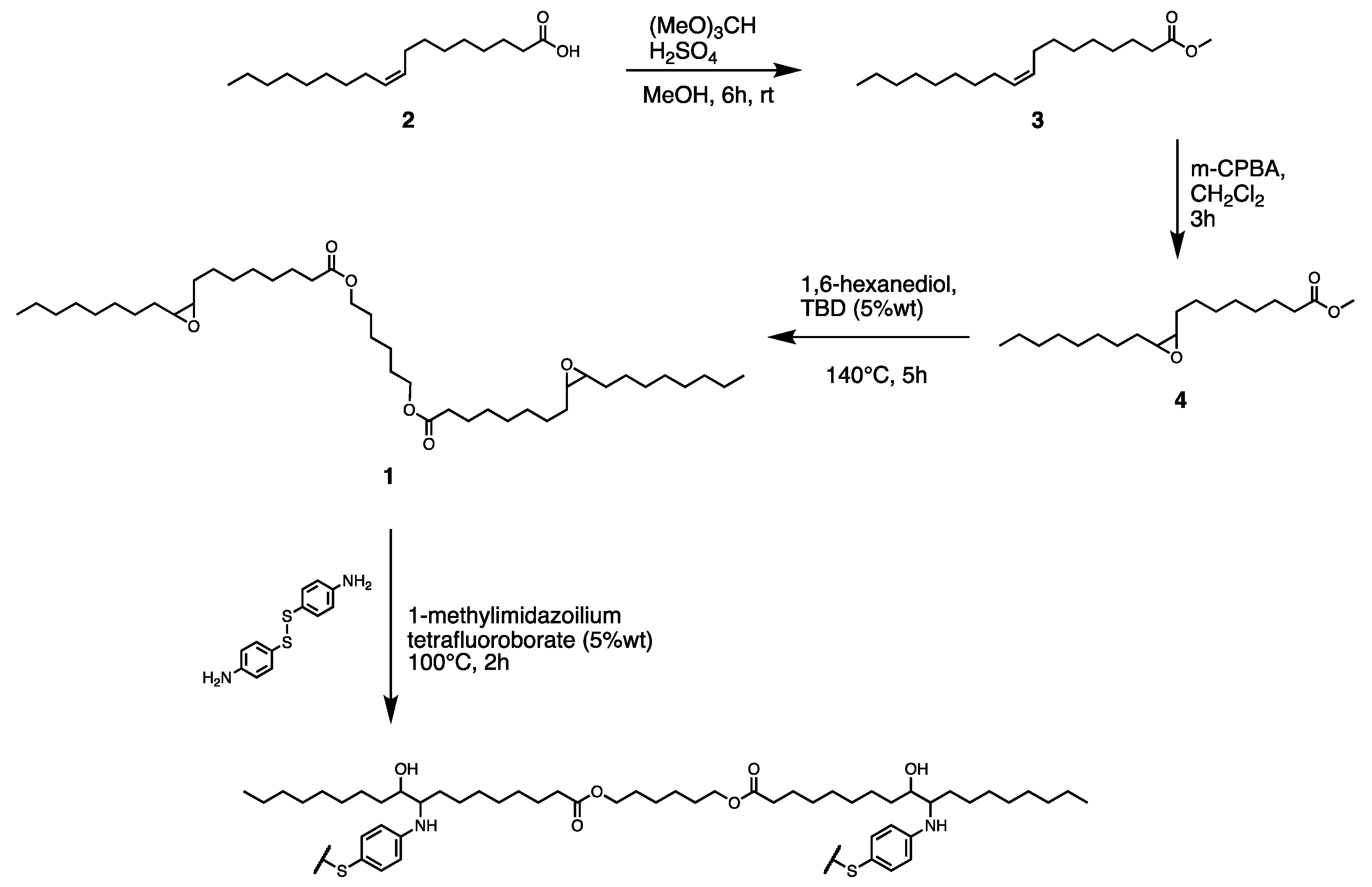
2.2. Preparations
2.2.1. Methyl Oleate (3) Preparation [32]
2.2.2. Methyl Oleate Epoxidized (4) Preparation
2.2.3. 1,6-Hexanediyl Dioleate Epoxidized (1) Synthesis
2.2.4. 1-Methylimidazolium Tetrafluoroborate Preparation [33]
2.2.5. Polymerization Procedure
2.3. Methods
2.3.1. Solubility in Organic Solvent
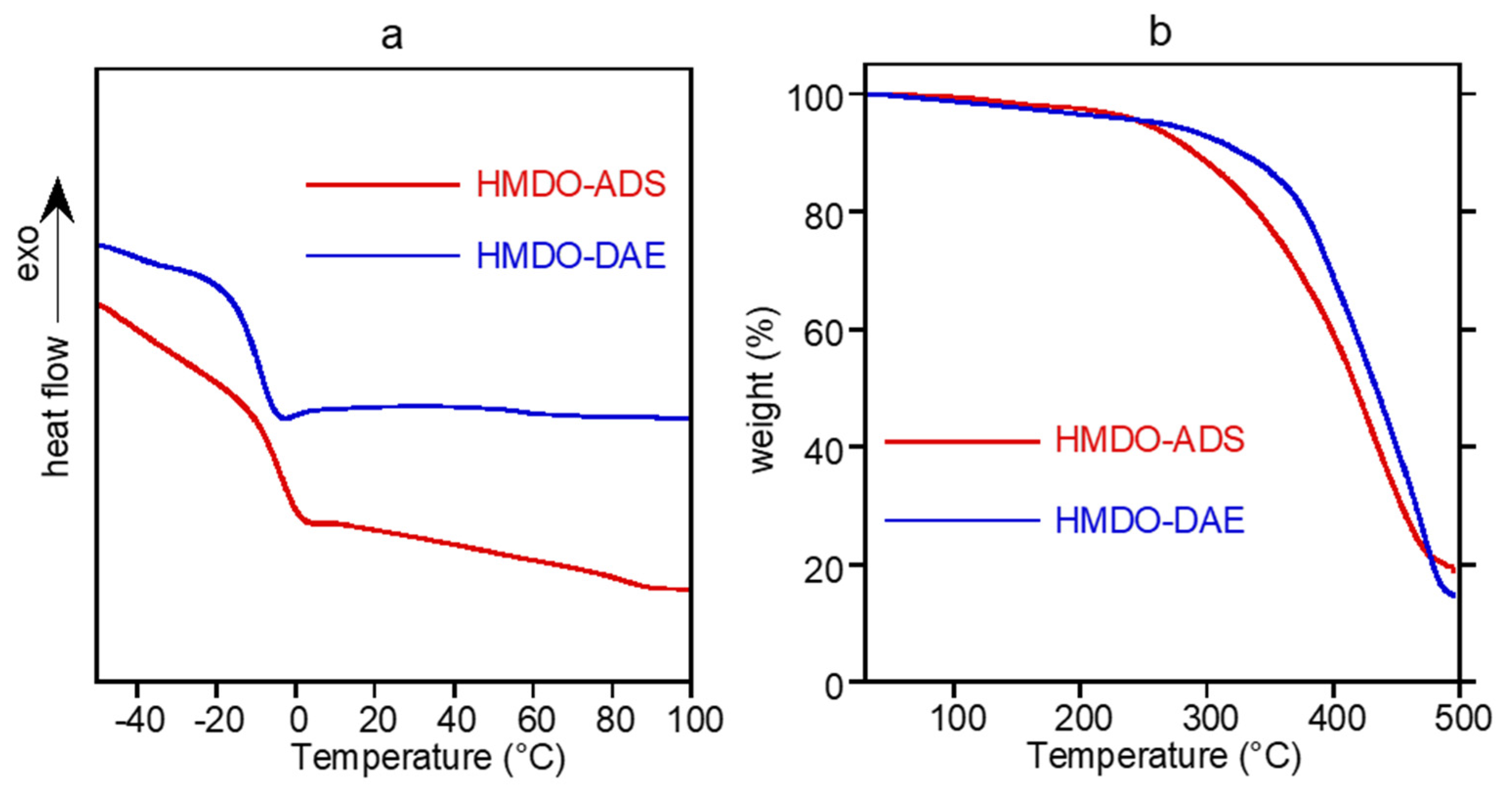
2.3.2. Thermal Analysis
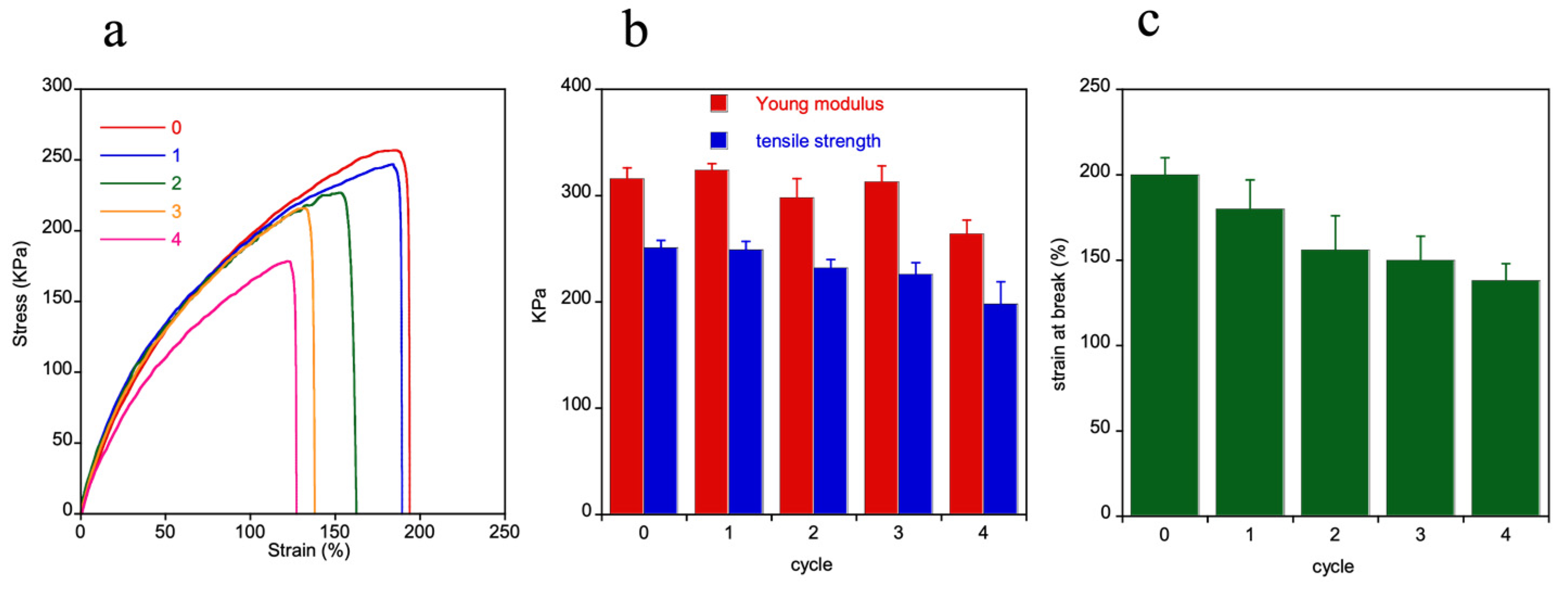
2.3.3. Material Reprocessing
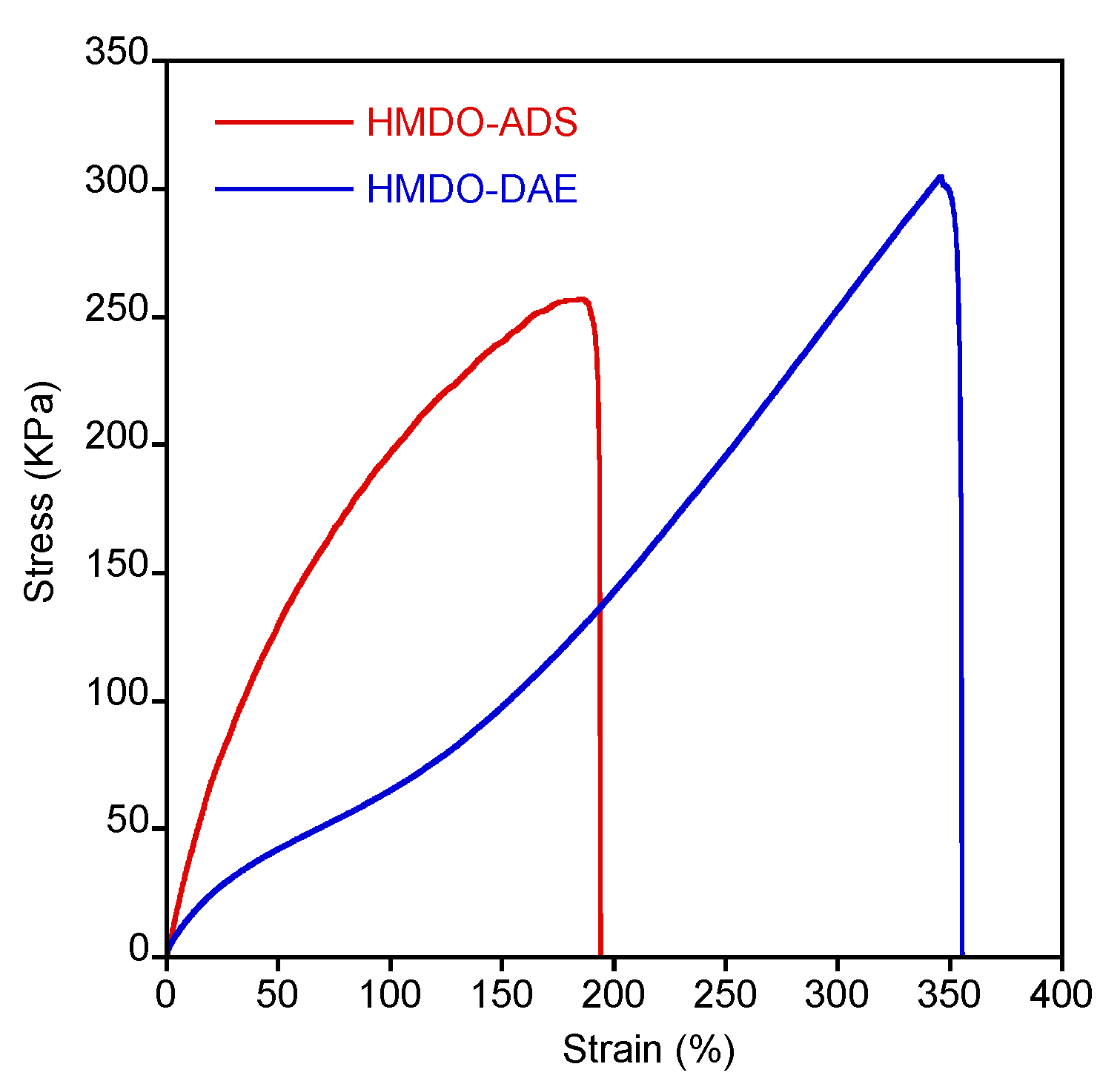
2.3.4. Tensile Test
2.3.5. Adhesion Test and Contact Angle
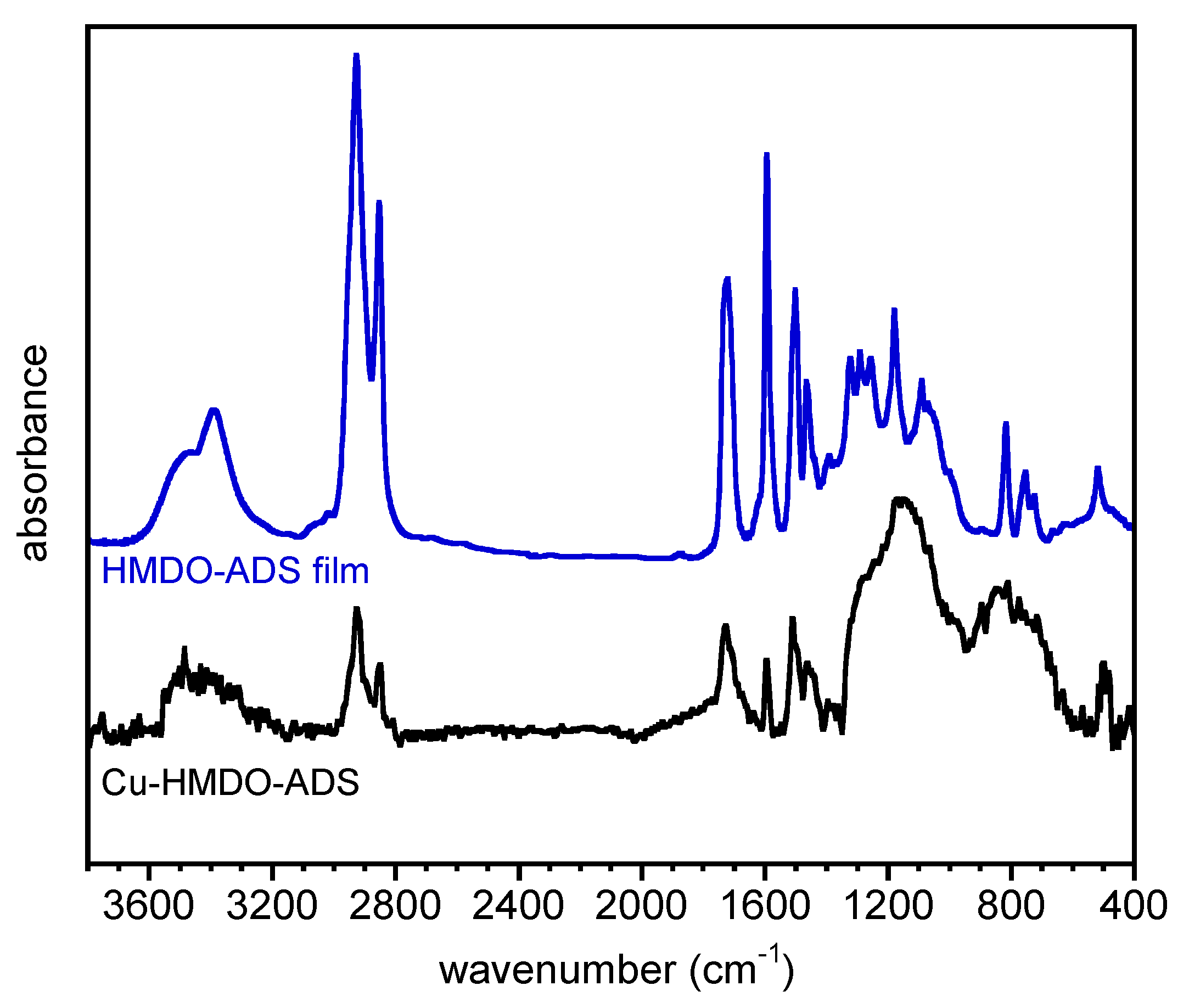
2.3.6. FTIR Spectroscopy
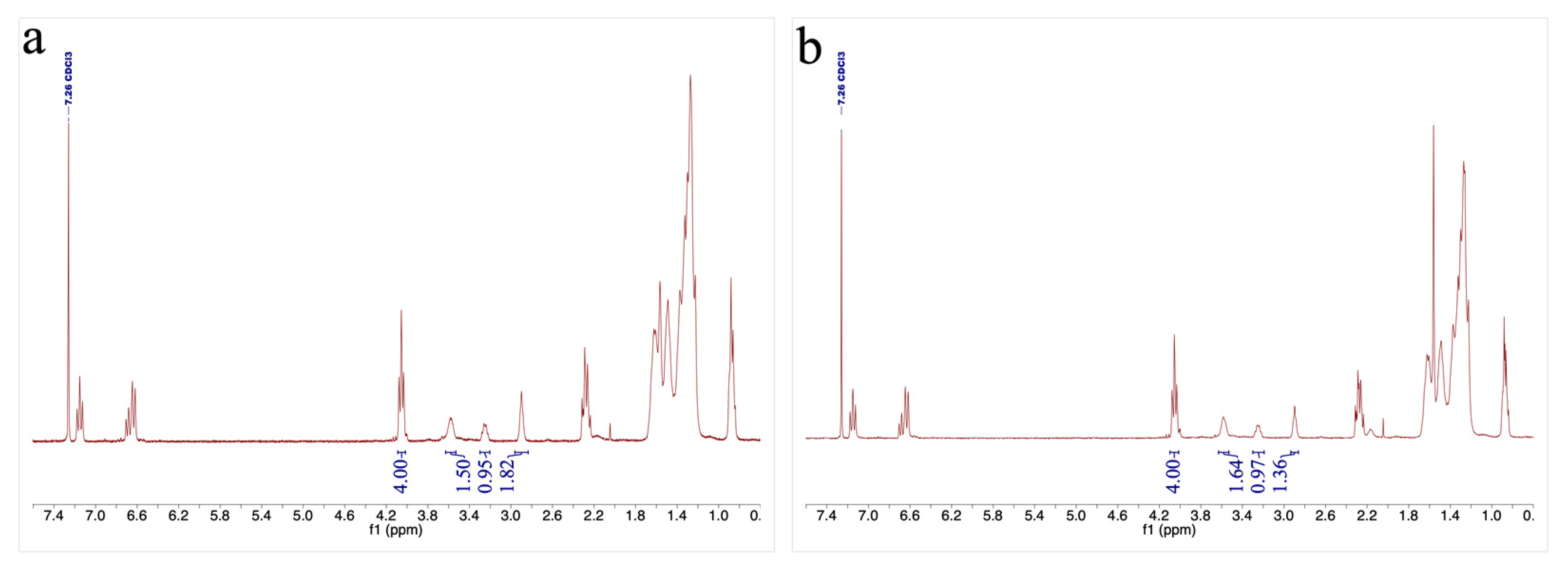
2.3.7. NMR Spectroscopy
2.3.8. Thin-Layer Chromatography
3. Results and Discussion
3.1. HMDO-ADS Synthesis and Swelling Tests
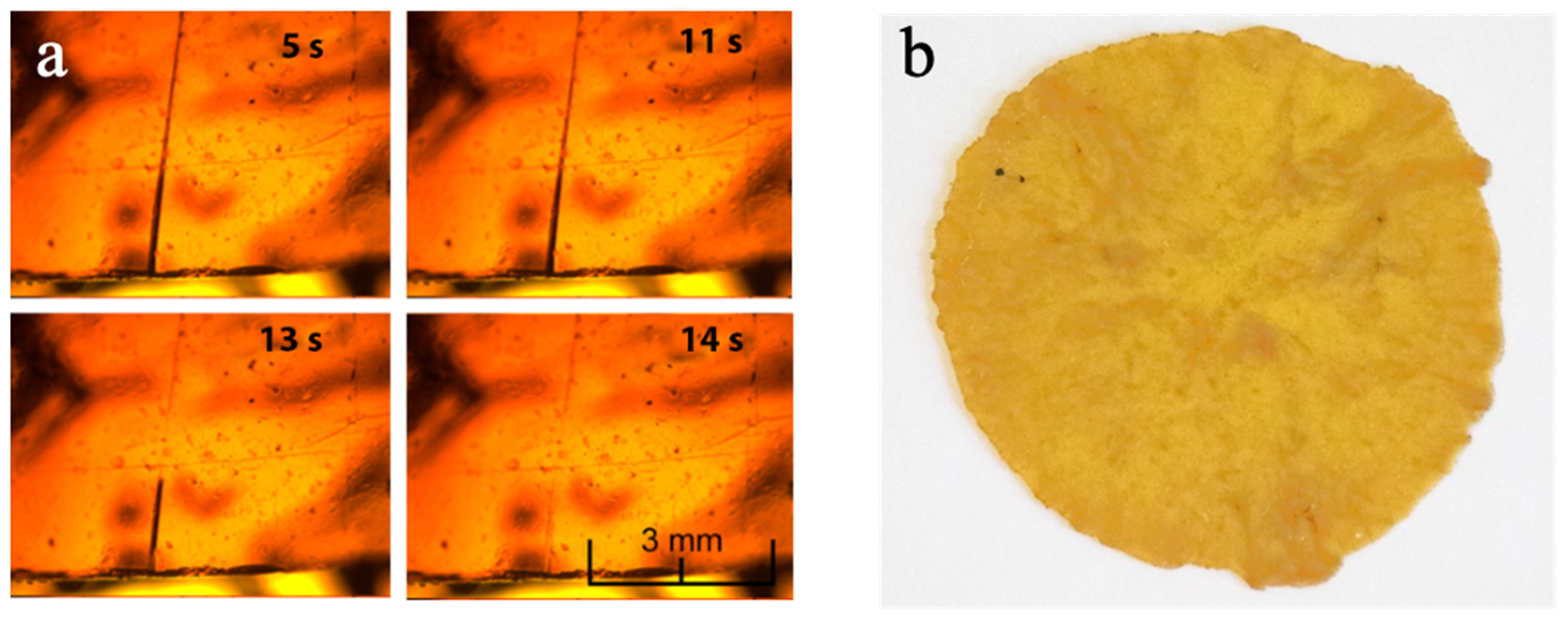
3.2. Self-Healing and Reprocessability
3.3. Contact Angle Results
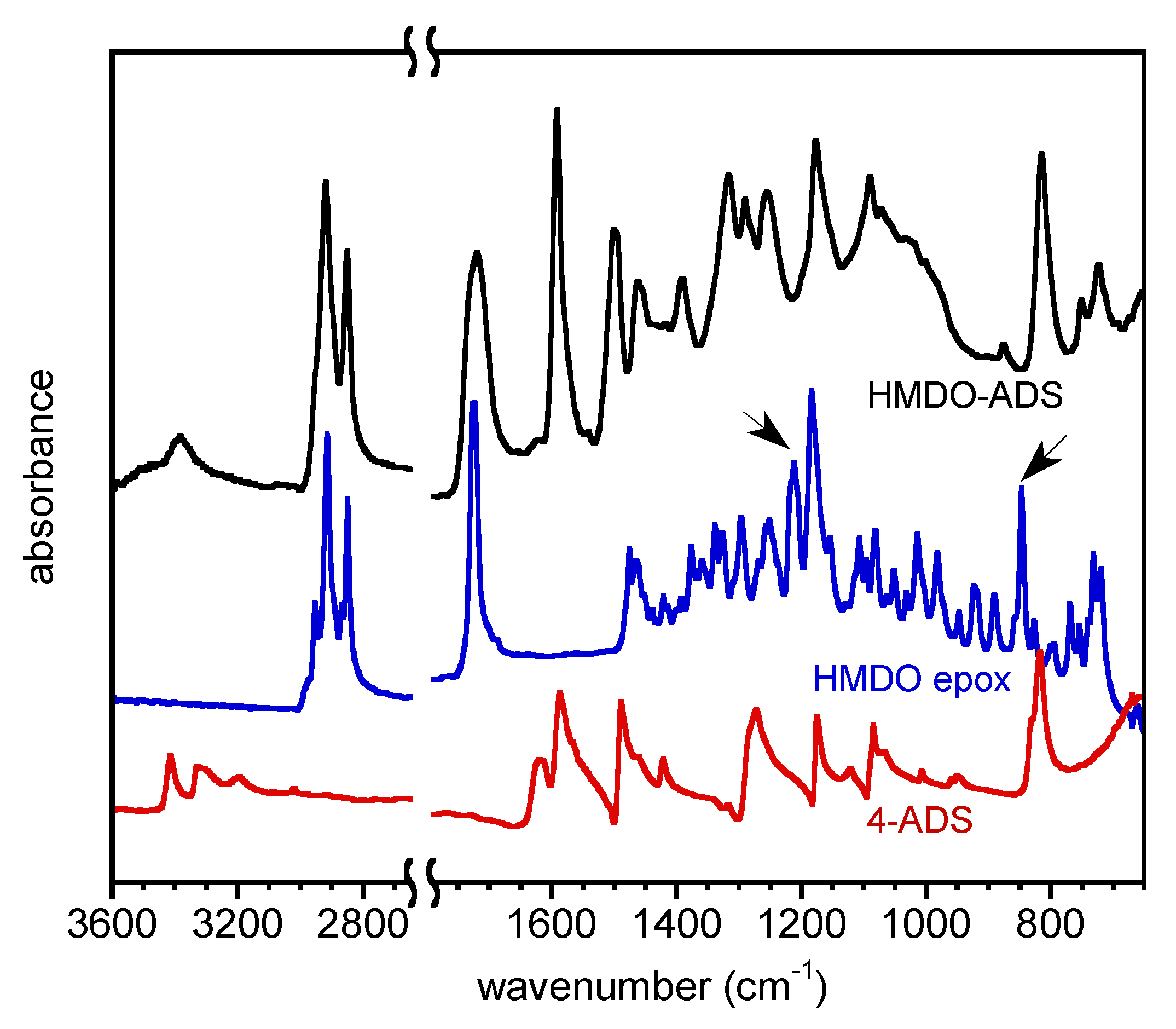
3.4. FTIR Spectroscopy
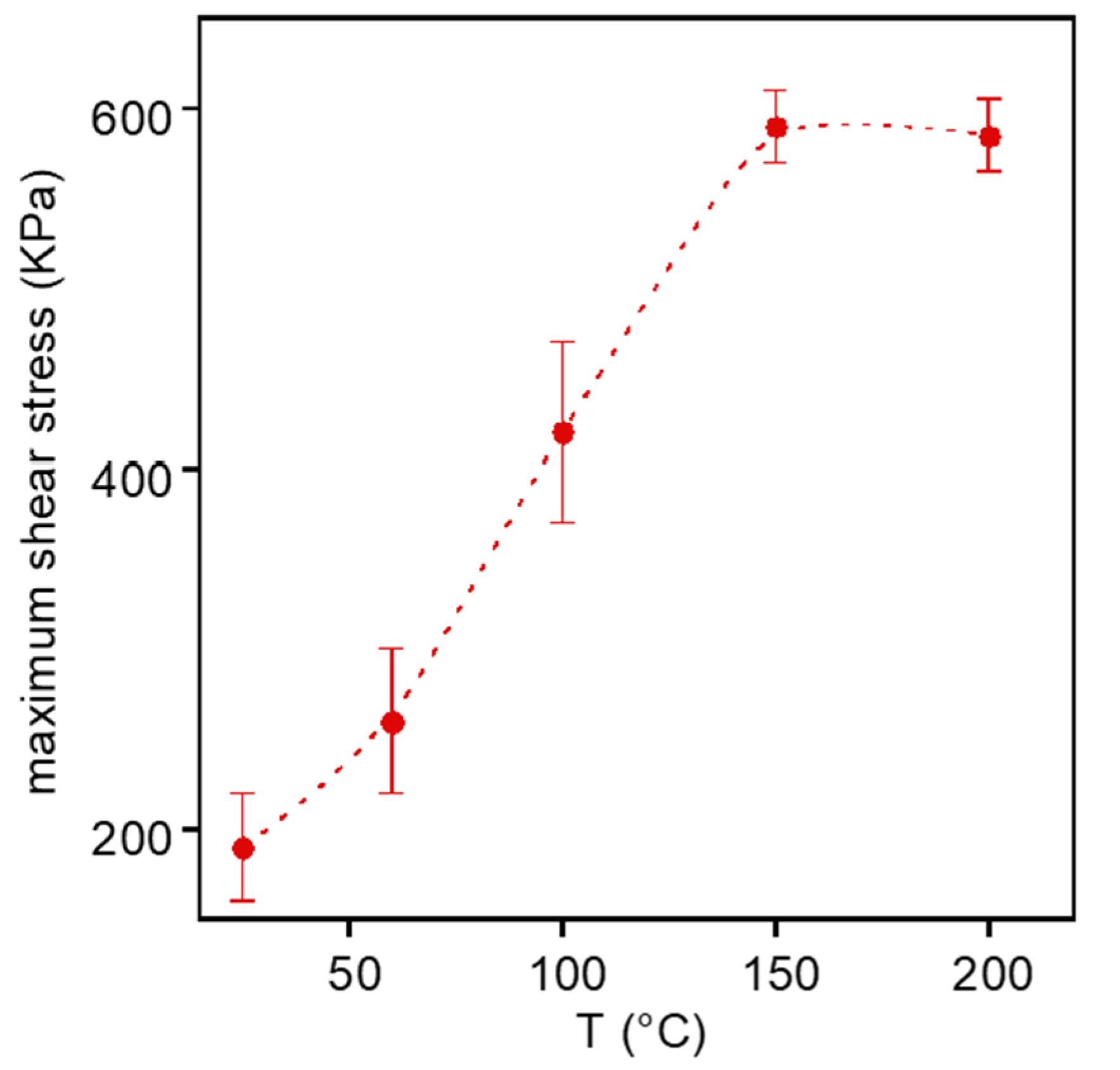
3.5. Mechanical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryberg, M.W.; Hauschild, M.Z.; Wang, F.; Averous-Monnery, S.; Laurent, A. Global Environmental Losses of Plastics Across their Value Chains. Resour. Conserv. Recycl. 2019, 151, 104459. [Google Scholar] [CrossRef]
- Monteiro, R.C.P.; Ivar do Sul, J.A.; Costa, M.F. Plastic Pollution in Islands of the Atlantic Ocean. Environ. Pollut. 2018, 238, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; An, Y.J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ma, S.; Wang, S.; Liu, Y.; Abu Taher, M.; Wang, B.; Huang, K.; Xu, X.; Han, Y.; Zhu, J. Green and Facile Preparation of Readily Dual-Recyclable Thermosetting Polymers with Superior Stability Based on Asymmetric Acetal. Macromolecules 2020, 53, 1474–1485. [Google Scholar] [CrossRef]
- Li, S.; Xu, C.; Yang, W.; Tang, Q. Thermoplastic polyurethanes stemming from castor oil: Green synthesis and their application in wood bonding. Coatings 2017, 7, 159. [Google Scholar] [CrossRef]
- Memon, H.; Wei, Y.; Zhu, C. Recyclable and Reformable Epoxy Resins Based on Dynamic Covalent Bonds – Present, Past, and Future. Polym. Test. 2022, 105, 107420. [Google Scholar] [CrossRef]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide vitrimers. ACS Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef]
- Pettazzoni, L.; Leonelli, F.; Martinelli, A.; Migneco, M.L.; Alfano, S.; Di Luca, D.; Celio, L.; Di Lisio, V. Transamidation-Based Vitrimers from Renewable Sources. J. Appl. Polym. Sci. 2022, 139, e52408. [Google Scholar] [CrossRef]
- Chapelle, C.; Quienne, B.; Bonneaud, C.; David, G.; Caillol, S. Diels-Alder Chitosan Based Dissociative Covalent Adaptable Networks. Carbohydr. Polym. 2021, 253, 117222. [Google Scholar] [CrossRef]
- Imato, K.; Takahara, A.; Otsuka, H. Self-healing of a Cross-Linked Polymer with Dynamic Covalent Linkages at Mild Temperature and Evaluation at Macroscopic and Molecular Level. Macromolecules 2015, 48, 5632–5639. [Google Scholar] [CrossRef]
- Luo, C.; Yang, W.; Qi, W.; Chen, Z.; Lin, J.; Bian, X.; He, S. Cost-Efficient and Recyclable Epoxy Vitrimer Composite with Low Initial Viscosity Based on Exchangeable Disulfide Crosslinks. Polym. Test. 2022, 113, 107670. [Google Scholar] [CrossRef]
- Zheng, N.; Hou, J.; Xu, Y.; Fang, Z.; Zou, W.; Zhao, Q.; Xie, T. Catalyst-Free Thermoset Polyurethane with Permanent Shape Reconfigurability and Highly Tunable Triple-Shape Memory Performance. ACS Macro Lett. 2017, 6, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Jiang, T. Preparation and Properties of a Self-Healing Waterborne Polyurethane Based on Dynamic Disulfide Bond. Polymers 2021, 13, 2936. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Liu, G.L.; Li, Y.D.; Weng, Y.; Zeng, J.B. Biobased High-Performance Epoxy Vitrimer with UV Shielding for Recyclable Carbon Fiber Reinforced Composites. ACS Sustain. Chem. Eng. 2021, 9, 4638. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, D.; Russell, T.P. Biobased Dynamic Polymer Networks with Rapid Stress Relaxation. ACS Sustain. Chem. Eng. 2021, 9, 11091. [Google Scholar] [CrossRef]
- Chu, Y.H.; Kung, Y.L. A Study on Vegetable Oil Blends. Food Chem. 1998, 62, 191–195. [Google Scholar] [CrossRef]
- Moore, K.M.; Knauft, D.A. The Inheritance of High Oleic Acid in Peanut. J. Hered. 1989, 80, 252–253. [Google Scholar] [CrossRef]
- Gh, M.N.H.; Gimbun, J.; Nurdin, S. Transesterification of Waste Cooking Oil Using Chemically Treated Catalyst. J. Appl. Sci. 2014, 14, 1425–1429. [Google Scholar]
- Nguyenthi, T.X.; Bazile, J.P.; Bessières, D. Density Measurements of Waste Cooking Oil Biodiesel and Diesel Blends Over Extended Pressure and Temperature Ranges. Energies 2018, 11, 1212. [Google Scholar] [CrossRef]
- Elkacmi, R.; Kamil, N.; Bennajah, M.; Kitane, S. Extraction of Oleic Acid from Moroccan Olive Mill Wastewater. Biomed. Res. Int. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Loizides, M.I.; Loizidou, X.I.; Orthodoxou, D.L.; Petsa, D. Circular Bioeconomy in Action: Collection and Recycling of Domestic Used Cooking Oil Through a Social, Reverse Logistics System. Recycling 2019, 4, 16. [Google Scholar] [CrossRef]
- Kumar, S.; Negi, S. Transformation of Waste Cooking Oil into C-18 Fatty Acids Using a Novel Lipase Produced by Penicillium Chrysogenum Through Solid State Fermentation. 3 Biotech 2015, 5, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Capiel, G.; Hernández, E.; Marcovich, N.E.; Mosiewicki, M.A. Stress Relaxation Behavior of Weldable Crosslinked Polymers Based on Methacrylated Oleic and Lauric Acids. Eur. Polym. J. 2020, 132, 109740. [Google Scholar] [CrossRef]
- Hood, C.; Ghazani, S.M.; Marangoni, A.G.; Pensini, E. Flexible Polymeric Biomaterials from Epoxidized Soybean Oil, Epoxidized Oleic Acid, and Citric Acid as Both a Hardener and Acid Catalyst. J. App. Polym. Sci. 2022, 139. [Google Scholar] [CrossRef]
- Martin, R.; Rekondo, A.; De Luzuriaga, A.R.; Casuso, P.; Dupin, D.; Cabañero, G.; Grande, H.J.; Odriozola, I. Dynamic Sulfur Chemistry as a Key Tool in the Design of Self-Healing Polymers. Smart Mater. Struct. 2016, 25, 084017. [Google Scholar] [CrossRef]
- Caprioli, F.; Martinelli, A.; Gazzoli, D.; Di Castro, V.; Decker, F. Enhanced Protective Properties and Structural Order of Self-Assembled Monolayers of Aromatic Thiols on Copper in Contact with Acidic Aqueous Solution. J. Phys. Chem. C. 2012, 116, 4628–4636. [Google Scholar] [CrossRef]
- Caprioli, F.; Martinelli, A.; Di Castro, V.; Decker, F. Effect of Various Terminal Groups on Long-Term Protective Properties of Aromatic SAMs on Copper in Acidic Environment. J. Electroanal. Chem. 2013, 693, 86–94. [Google Scholar] [CrossRef]
- Wong, C.K.Y.; Yuen, M.M.F.; Xi, B. Thiol-Based Self-Assembled Nanostructures in Promoting Interfacial Adhesion for Copper-Epoxy Joint. Appl. Phys. Lett. 2009, 94, 263102. [Google Scholar] [CrossRef]
- Muller, R.; Heckmann, M.; Paul, T.; Stratmann, M. New Adhesion Promoters for Copper Lead Frames and Epoxy Resin. J. Adhes. 2000, 72, 65–83. [Google Scholar] [CrossRef]
- Denayer, J.; Delhalle, J.; Mekhalif, Z. Aminealkylthiol and Dithiol Self-Assembly as Adhesion Promoter Between Copper Substrate and Epoxy Resin. Appl. Surf. Sci. 2011, 257, 10686–10691. [Google Scholar] [CrossRef]
- Xue, G.; Wang, Y.; Chen, Y.; Tsai, S.; Jiang, L. Adhesion Promotion at High Temperature for Epoxy Resin or Polyimide onto Metal by a Two-Component Coupling System of Polybenzimidazole and 4-Aminophenil Disulfide. J. Appl. Polym. Sci. 1995, 58, 2221–2227. [Google Scholar] [CrossRef]
- de Espinosa, L.M.; Gevers, A.; Woldt, B.; Graß, M.; Meier, M.A. Sulfur-Containing Fatty Acid-Based Plasticizers via Thiol-ene Addition and Oxidation: Synthesis and Evaluation in PVC Formulations. Green Chem. 2014, 16, 1883–1896. [Google Scholar] [CrossRef]
- Biswas, A.; Sharma, B.K.; Doll, K.M.; Erhan, S.Z.; Willett, J.L.; Cheng, H.N. Synthesis of an Amine-Oleate Derivative Using an Ionic Liquid Catalyst. J. Agric. Food Chem. 2009, 57, 8136–8141. [Google Scholar] [CrossRef]
- Matxain, J.M.; Asua, J.M.; Ruiperez, F. Design of New Disulfide-Based Organic Compounds for the Improvement of Self-Healing Materials. Phys. Chem. Chem. Phys. 2016, 18, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Jablonsky, M.J.; Mays, J.W. NMR and FT-IR Studies of Sulfonated Styrene-Based Homopolymers and Copolymers. Polymer 2002, 43, 5125–5132. [Google Scholar] [CrossRef]
- Rajkumar, G.; Sagunthala, R.; Sethuraman, M.G. Investigation of Inhibiting Properties of Self-Assembled Films of 4-Aminothiophenol on Copper in 3.5% NaCl. J. Adhes. Sci. Technol. 2015, 29, 1107–1117. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Li, F.; Xue, G. Enhancing Effect of Chemically Reduced Gold on Surface Raman Scattering for Organic Sulfides Chemisorbed on Iron. J. Raman Spectrosc. 2001, 32, 881–884. [Google Scholar] [CrossRef]
- Cohen, E.; Binshtok, O.; Dotan, A.; Dodiuk, H. Prospective Materials for Biodegradable and/or Biobased Pressure-Sensitive Adhesives: A Review. J. Adhes Sci. Technol. 2013, 27, 1998–2013. [Google Scholar] [CrossRef]
- Ke, X.; Tang, S.; Dong, Z.; Ren, K.; Yu, P.; Xu, X.; Yang, J.; Luo, J.; Li, J. An Instant, Repeatable and Universal Supramolecular Adhesive Based on Natural Small Molecules for Dry/Wet Environments. J. Chem. Eng. 2022, 442, 136206. [Google Scholar] [CrossRef]
- He, M.; Huang, Y.; Sun, J.; Dan, Y.; Zhao, W.; Jiang, L. Dynamic Crosslinked Poly(Acrylic Acid-Co-Acrylonitrile)/Polyethylene Glycol Networks as Reworkable Adhesives. React. Funct. Polym. 2022, 176, 105283. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, L.; Jie, S.; Li, B.-G. Upgrade SBS into Vitrimers with Excellent Mechanical and Physical Properties. Eur. Polym. J. 2022, 180, 111600. [Google Scholar] [CrossRef]
- Sohn, S. Various Ways to Control the Bulk Properties of Pressure Sensitive Adhesives. J. Adhes. Sci. Technol. 2003, 17, 703–723. [Google Scholar] [CrossRef]
- Dhilipkumar, T.; Rajesh, M. Enhancing Shear Strength and Structural Stiffness of Composite Joint with Carbon Nanotube Reinforced Adhesive through Co-Bonding Technique. J. Adhes. Sci. Technol. 2021, 36, 2143–2157. [Google Scholar] [CrossRef]
- Di Mauro, C.; Malburet, S.; Graillot, A.; Mija, A. Recyclable, Repairable, and Reshapable (3R) Thermoset Materials with Shape Memory Properties from Bio-Based Epoxidized Vegetable Oils. ACS Appl. Bio Mater. 2020, 3, 8094–8104. [Google Scholar] [CrossRef] [PubMed]

| Polymer | Liquid | SR | GF (%) |
|---|---|---|---|
| HMDO-ADS | THF | 9.95 | 91 |
| MeOH | 0.28 | 98 | |
| AcOEt | 2.73 | 80 | |
| HMDO-DAE | AcOEt | 10.8 | 75 |
| Sample | Advancing Contact Angle (°) |
|---|---|
| Bare copper | 78 ± 5 |
| HMDO-ADS film | 93 ± 2 |
| HMDO-DAE film | 97 ± 5 |
| Cu-ADS | 92 ± 3 |
| Cu-HMDO-ADS | 109 ± 2 |
| Cu-HMDO-DAE | 77 ± 5 |
| Sample | Maximum Shear Strength (KPa) | Failure | Maximum Shear Strength (KPa) | Failure |
|---|---|---|---|---|
| substrate | Cu | Al | ||
| HMDO-DAE | 460 ± 50 | adhesive | 300 ± 50 | adhesive |
| HMDO-ADS | 590 ± 20 | cohesive | 350 ± 10 | adhesive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pettazzoni, L.; Leonelli, F.; Marrani, A.G.; Migneco, L.M.; Vetica, F.; Celio, L.; Napoleone, V.; Alfano, S.; Colecchia, A.; Amato, F.; et al. Self-Healing and Reprocessable Oleic Acid-Based Elastomer with Dynamic S-S Bonds as Solvent-Free Reusable Adhesive on Copper Surface. Polymers 2022, 14, 4919. https://doi.org/10.3390/polym14224919
Pettazzoni L, Leonelli F, Marrani AG, Migneco LM, Vetica F, Celio L, Napoleone V, Alfano S, Colecchia A, Amato F, et al. Self-Healing and Reprocessable Oleic Acid-Based Elastomer with Dynamic S-S Bonds as Solvent-Free Reusable Adhesive on Copper Surface. Polymers. 2022; 14(22):4919. https://doi.org/10.3390/polym14224919
Chicago/Turabian StylePettazzoni, Luca, Francesca Leonelli, Andrea Giacomo Marrani, Luisa Maria Migneco, Fabrizio Vetica, Lorenzo Celio, Valerio Napoleone, Sara Alfano, Andrea Colecchia, Francesco Amato, and et al. 2022. "Self-Healing and Reprocessable Oleic Acid-Based Elastomer with Dynamic S-S Bonds as Solvent-Free Reusable Adhesive on Copper Surface" Polymers 14, no. 22: 4919. https://doi.org/10.3390/polym14224919
APA StylePettazzoni, L., Leonelli, F., Marrani, A. G., Migneco, L. M., Vetica, F., Celio, L., Napoleone, V., Alfano, S., Colecchia, A., Amato, F., Di Lisio, V., & Martinelli, A. (2022). Self-Healing and Reprocessable Oleic Acid-Based Elastomer with Dynamic S-S Bonds as Solvent-Free Reusable Adhesive on Copper Surface. Polymers, 14(22), 4919. https://doi.org/10.3390/polym14224919






