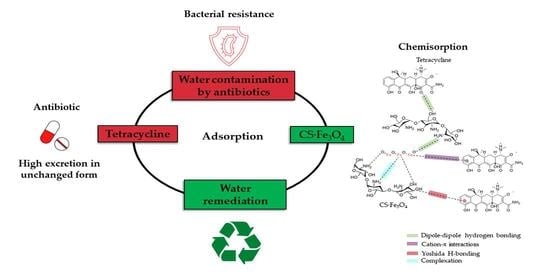
Highly Efficient Adsorption of Tetracycline Using Chitosan-Based Magnetic Adsorbent
Abstract
1. Introduction
2. Materials and Methods
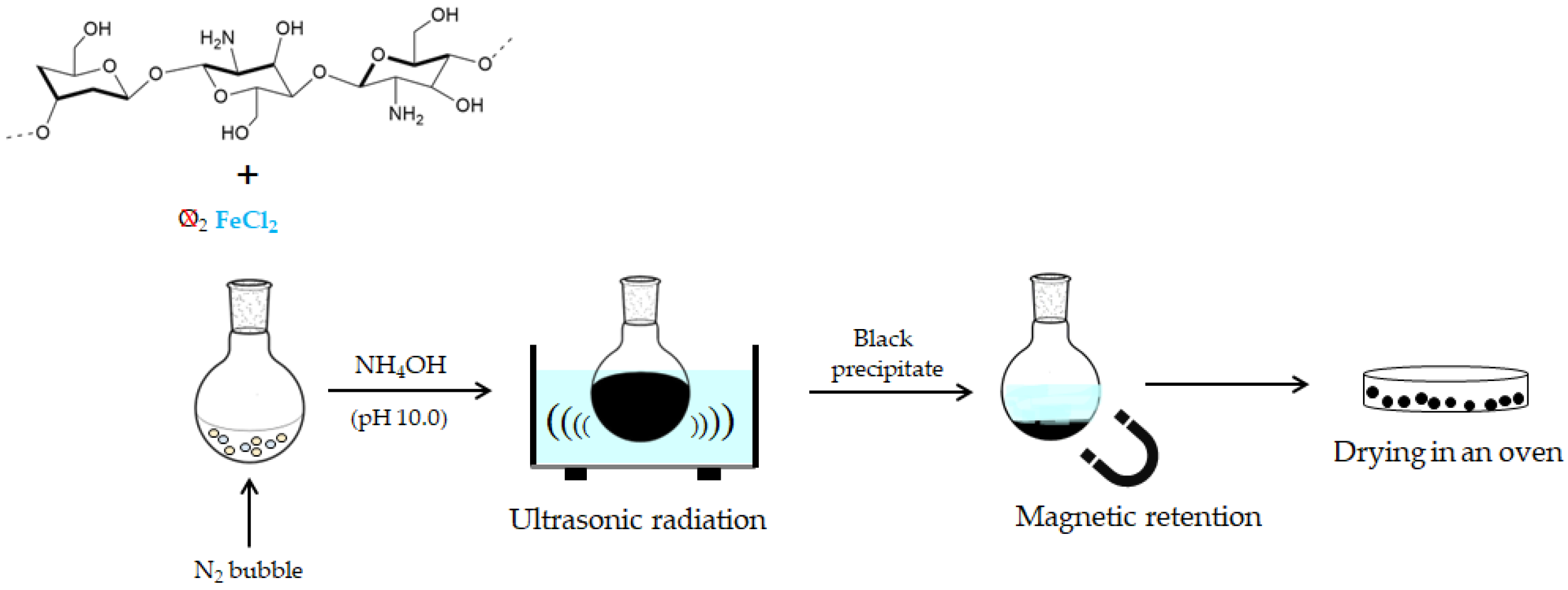
2.1. Synthesis of Magnetic Chitosan
2.2. Adsorbent Characterization
2.3. Adsorption Procedure and Mathematical Modeling
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
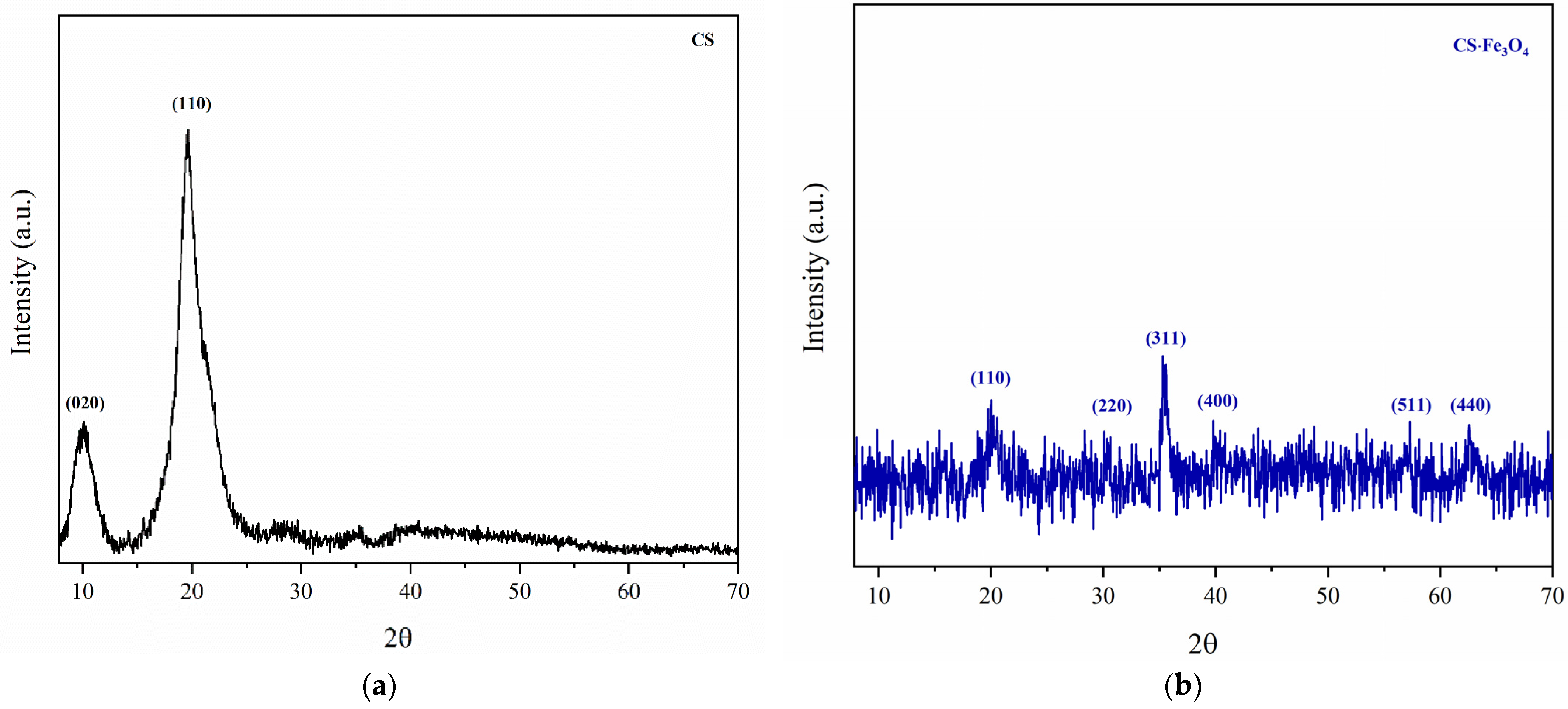
3.2. X-ray Diffraction (XRD)
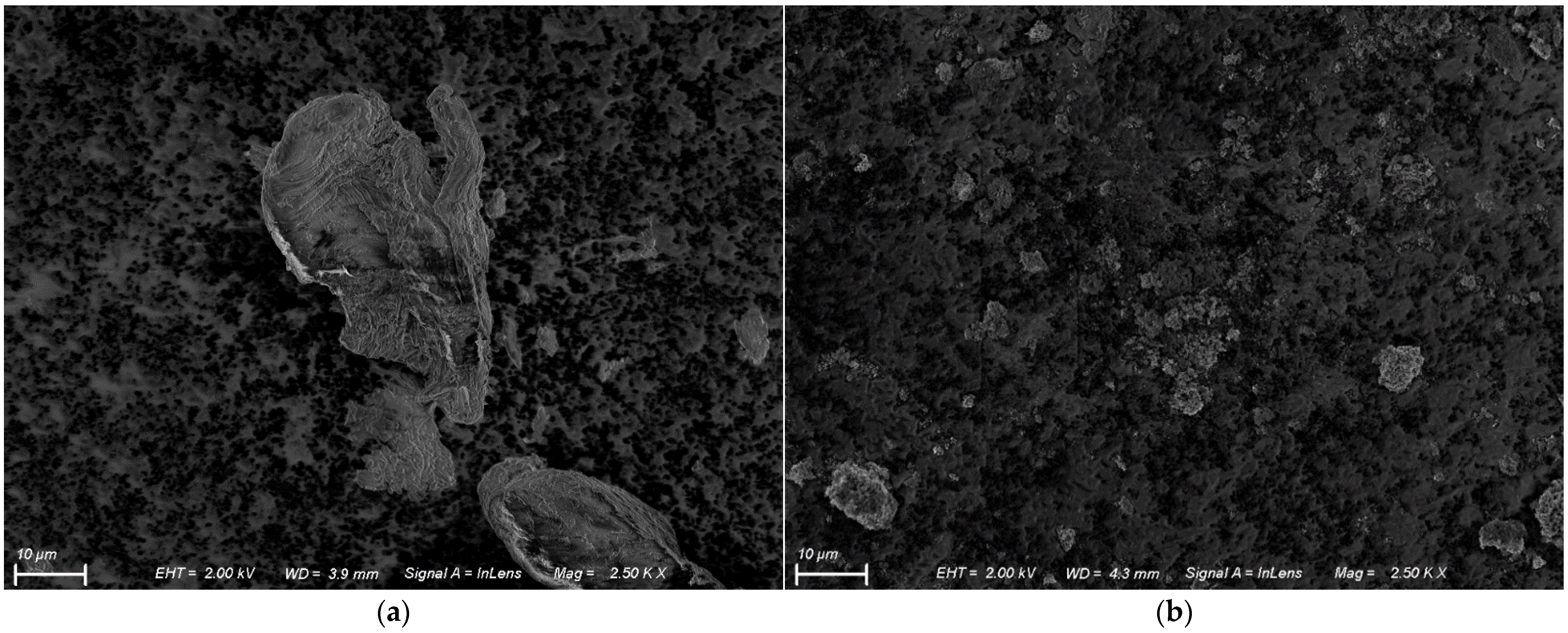
3.3. Scanning Electron Microscopy (SEM)
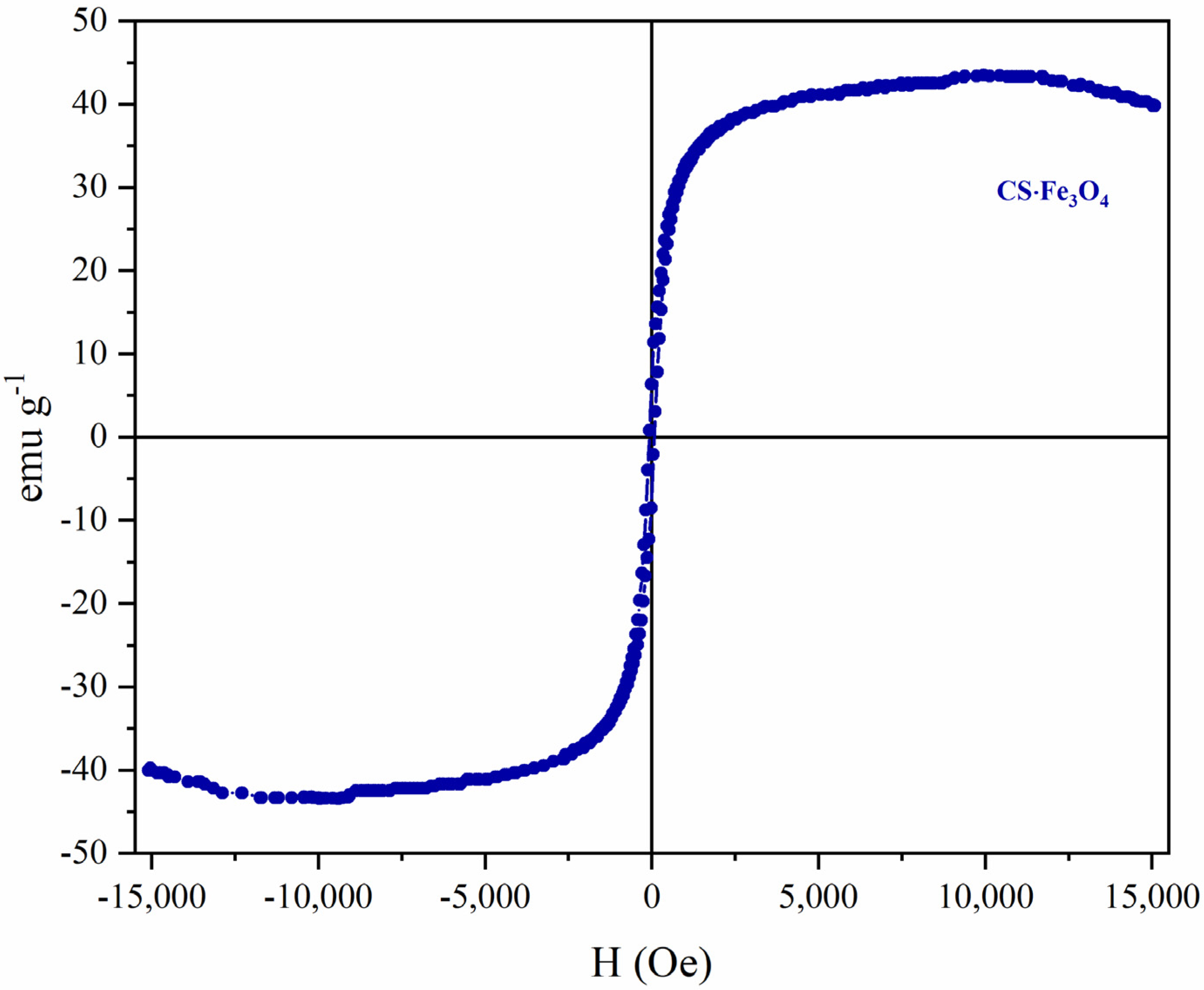
3.4. Vibrating Sample Magnetometer (VSM)
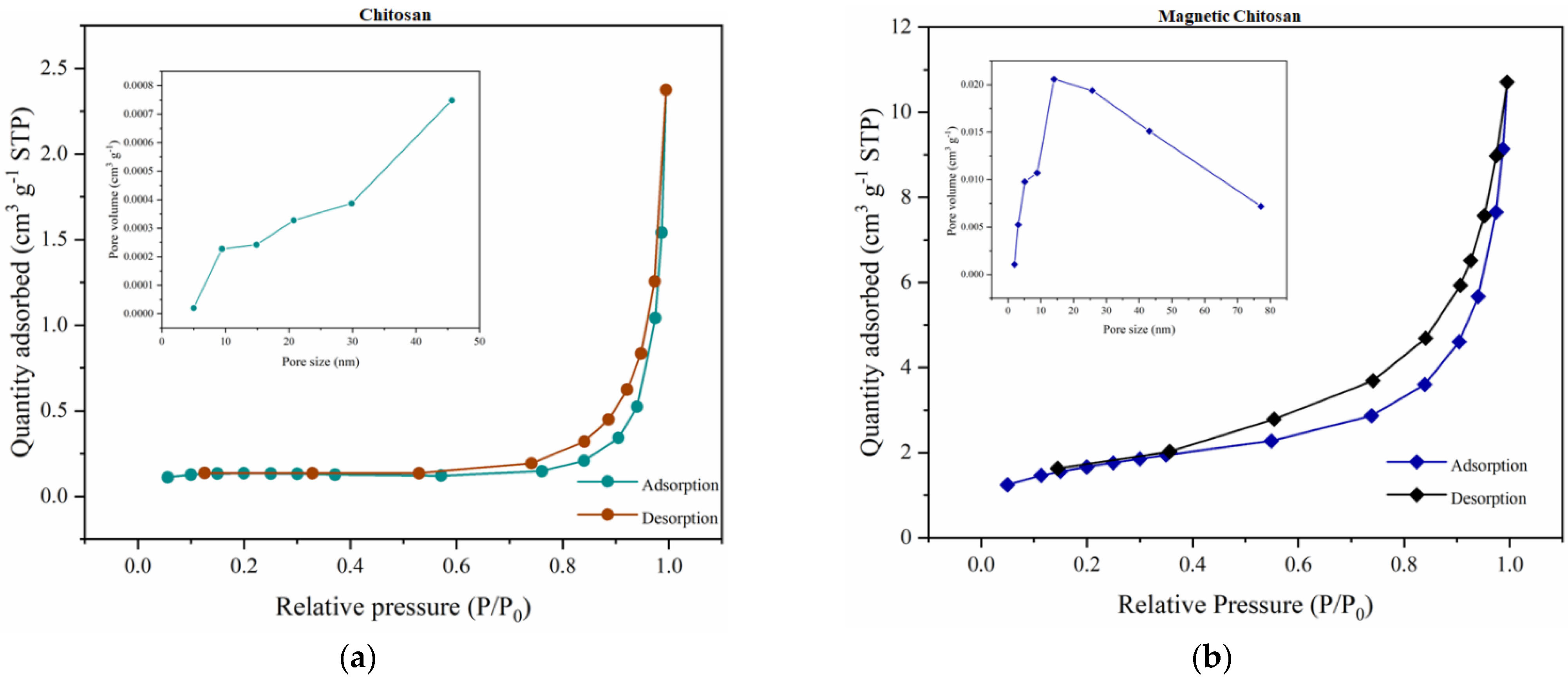
3.5. Nitrogen Porosimetry
3.6. Tetracycline Adsorption
3.6.1. Effect of Magnetite Incorporation onto Chitosan Surface
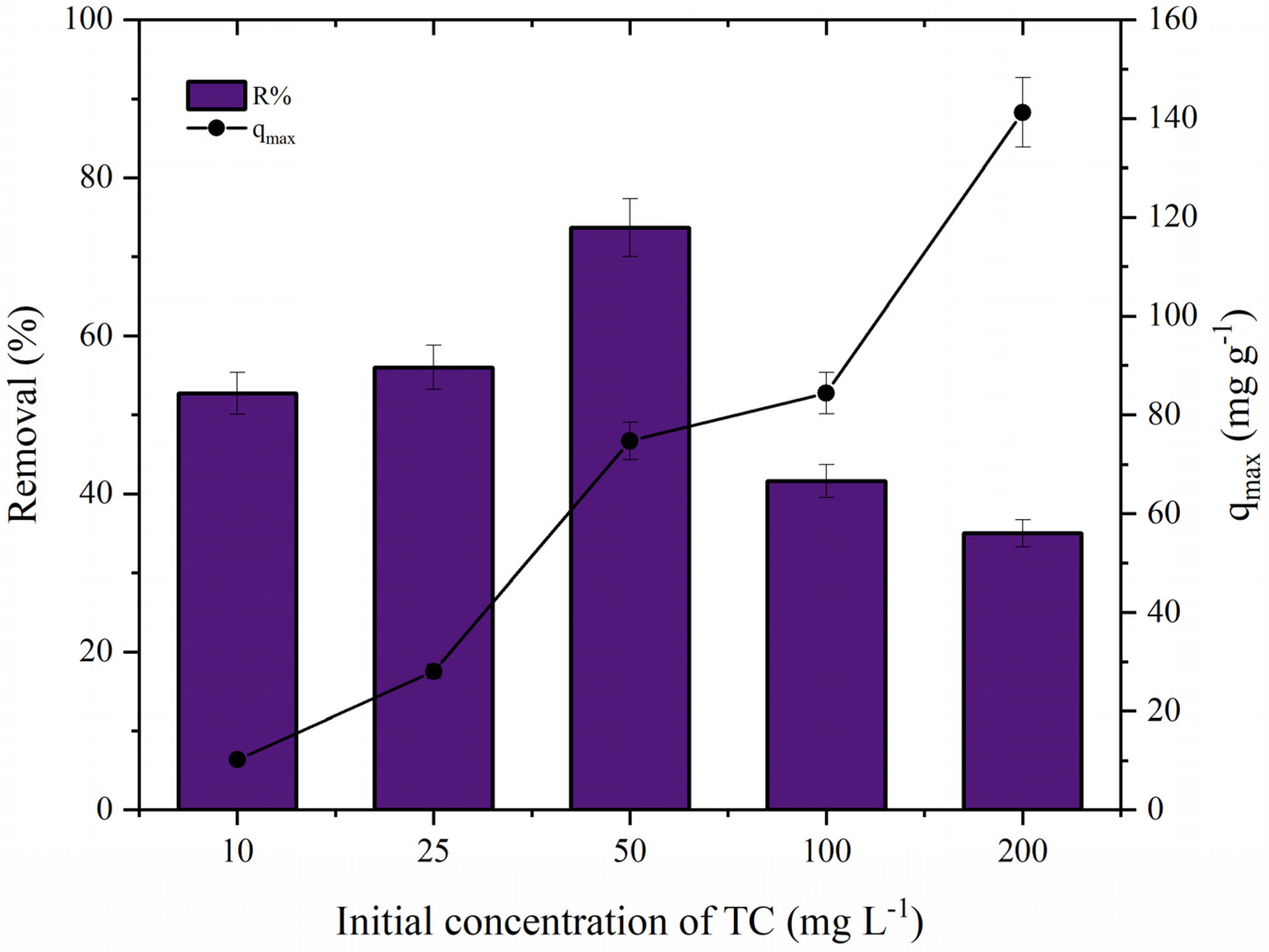
3.6.2. Effect of Initial Concentration of TC and Adsorbent Dosage
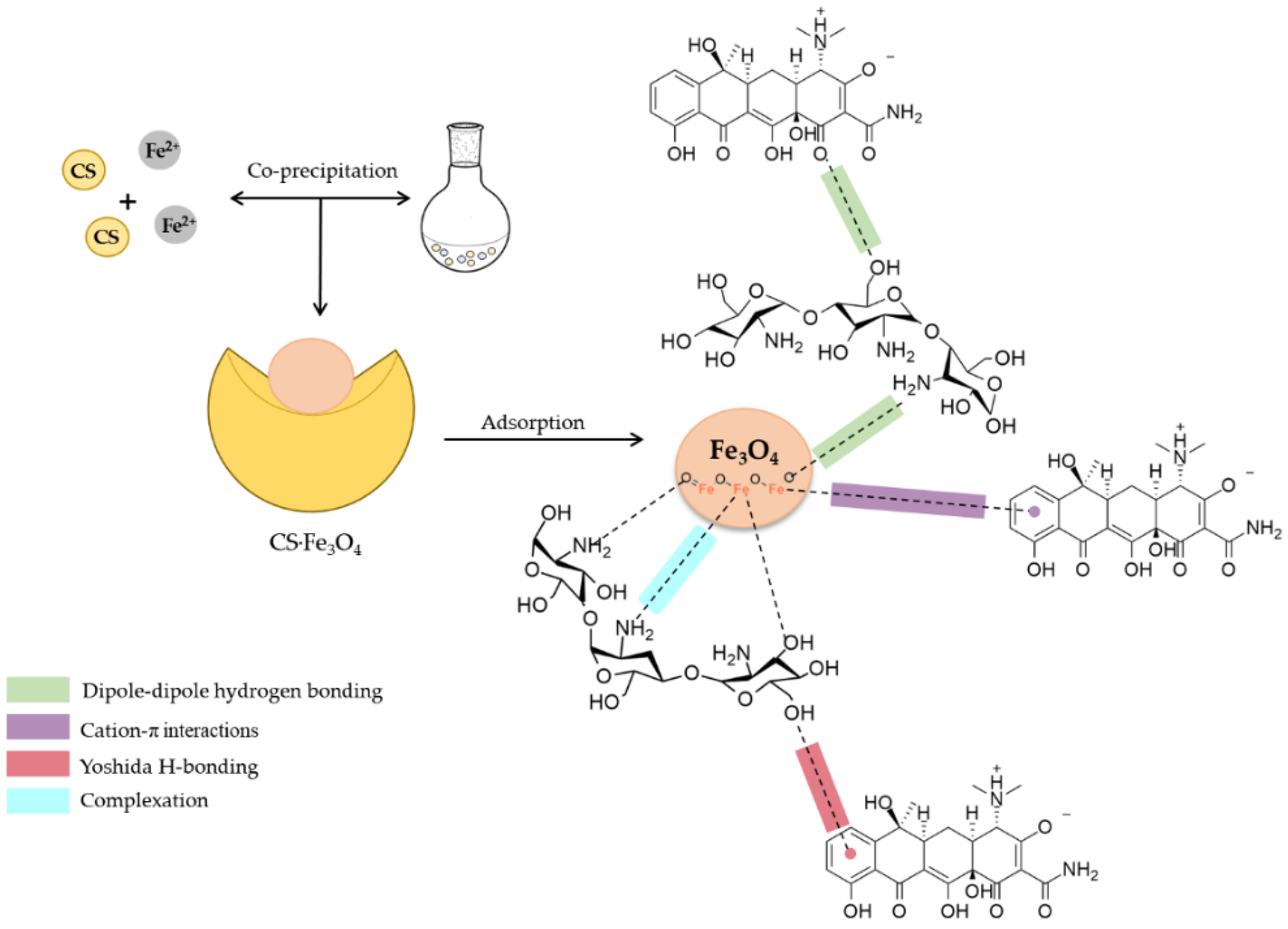
3.6.3. Effect of pH and Adsorption Mechanisms
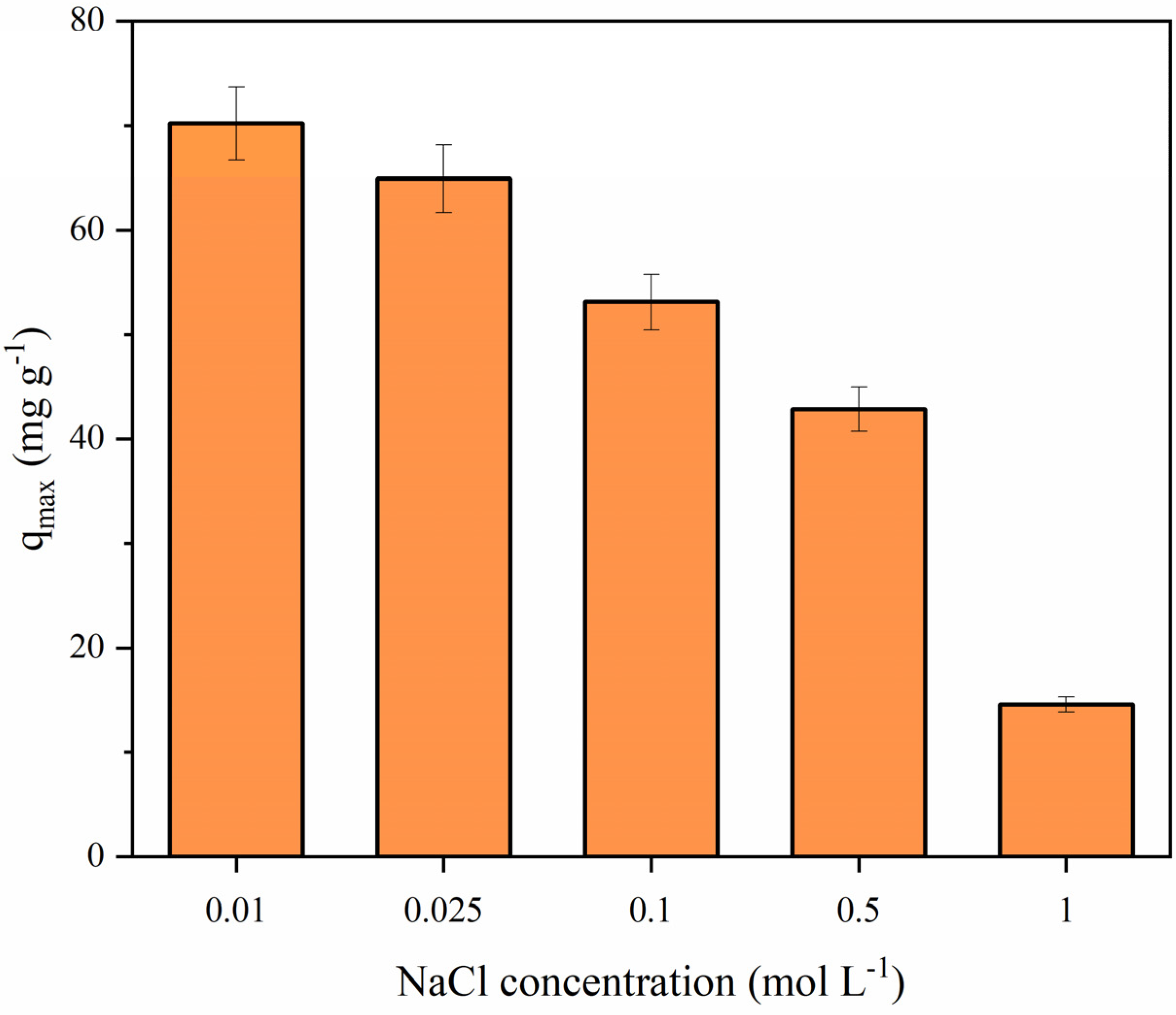
3.6.4. Effect of Ionic Strength
3.6.5. Kinetic Modeling
3.6.6. Adsorption Equilibrium Isotherms and Thermodynamic Study
3.6.7. Comparative Study on the Adsorption Capacity Using Different Adsorbent Materials
| Adsorbents | T (K) | pH | qmax (mg g−1) | Reference |
|---|---|---|---|---|
| Carbon nanotube with 5.9% oxygen content | 298 | 4.0 | 210.43 | [34] |
| Nanocrystalline cellulose | 288 | 5.0 | 6.47 | [55] |
| Fe3O4 nanoparticles | 302 | 7.0 | 19.6 | [16] |
| Reduced graphene oxide | 298 | 7.0 | 44.23 | [51] |
| α-Fe2O3/reduced graphene oxide | 298 | 4.0 | 18.47 | [51] |
| Halloysite/chitosan nanocomposite | 298 | 8.5 | 15.6 | [54] |
| Tricaprylmethylammonium chloride-conjugated chitosan hydrogel | 318 | 7.0 | 22.42 | [52] |
| Mesoporous cage metal-organic framework | 323 | 3.32 | 442.5 | [53] |
| Copper/cobalt ferrite@chitosan | 298 | 3.5 | 4.48 | [1] |
| Graphene oxide-functionalized magnetic particles | 298 | - | 39.1 | [56] |
| Rice husk ash | 313 | 5.0 | 8.37 | [57] |
| Magnetic chitosan | 293 | 7.0 | 211.21 | This work |
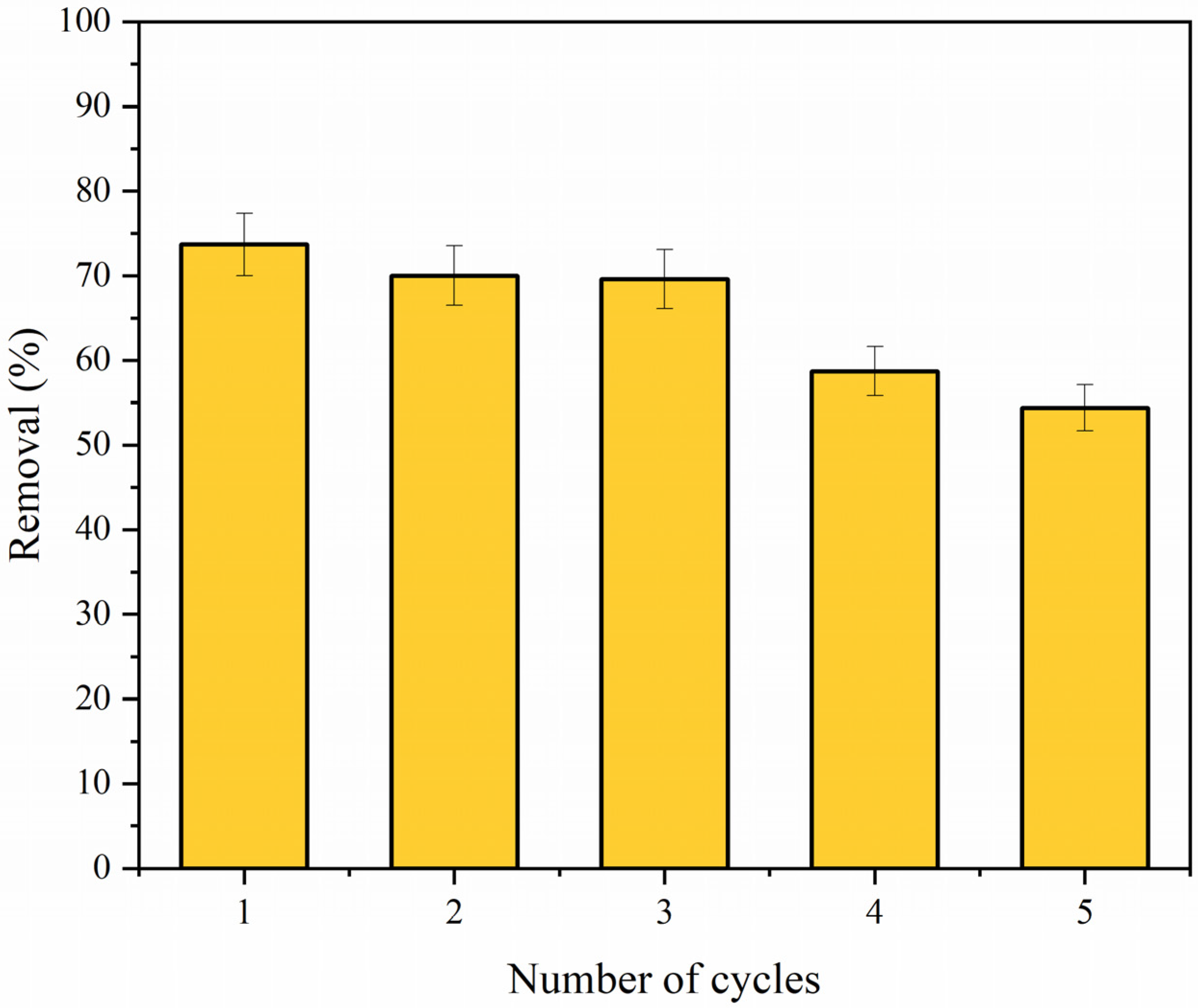
3.6.8. Regeneration and Reuse
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasiri, A.; Rajabi, S.; Amiri, A.; Fattahizade, M.; Hasani, O.; Lalehzari, A.; Hashemi, M. Adsorption of tetracycline using CuCoFe2O4@ Chitosan as a new and green magnetic nanohybrid adsorbent from aqueous solutions: Isotherm, kinetic and thermodynamic study. Arab. J. Chem. 2022, 15, 104014. [Google Scholar] [CrossRef]
- Da Silva Bruckmann, F.; Ledur, C.M.; da Silva, I.Z.; Dotto, G.L.; Rhoden, C.R.B. A DFT theoretical and experimental study about tetracycline adsorption onto magnetic graphene oxide. J. Mol. Liq. 2022, 1188, 35337. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, M.; Li, J.; Yang, S.; Sun, Y.; Sun, Q.; Wang, S.; Lu, L.; Zhang, K.; Xu, J.; et al. A review on pollution situation and treatment methods of tetracycline in groundwater. Sep. Sci. Technol. 2020, 55, 1005–1021. [Google Scholar] [CrossRef]
- Pollock, A.J.; Seibert, T.; Allen, D.B. Severe and persistent thyroid dysfunction associated with tetracycline-antibiotic treatment in youth. J. Pediatr. 2016, 173, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.; Salama, R.S.; El-Hakam, S.A.; Khder, A.S.; Ahmed, A.I. Synthesis of 12-tungestophosphoric acid supported on Zr/MCM-41 composite with excellent heterogeneous catalyst and promising adsorbent of methylene blue. Colloid Surf. A Physicochem. Eng. Asp. 2021, 631, 127753. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Alswat, A.A.; Salama, R.S. Gold-selenide quantum dots supported onto cesium ferrite nanocomposites for the efficient degradation of rhodamine B. Heliyon 2022, 8, e09652. [Google Scholar] [CrossRef]
- Alayli, A.; Nadaroglu, H.; Turgut, E. Nanobiocatalyst beds with Fenton process for removal of methylene blue. Appl. Water Sci. 2021, 11, 32. [Google Scholar] [CrossRef]
- Sani, O.N.; Yazdani, M.; Taghavi, M. Catalytic ozonation of ciprofloxacin using γ-Al2O3 nanoparticles in synthetic and real wastewaters. J. Water Process Eng. 2019, 32, 100894. [Google Scholar] [CrossRef]
- Bakry, A.M.; Alamier, W.M.; Salama, R.S.; El-Shall, M.S.; Awad, F.S. Remediation of water containing phosphate using ceria nanoparticles decorated partially reduced graphene oxide (CeO2-PRGO) composite. Surf. Interfaces 2022, 31, 102006. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Karamesouti, M.; Mitropoulos, A.C.; Kyzas, G.Z. A review for coffee adsorbents. J. Mol. Liq. 2017, 229, 555–565. [Google Scholar] [CrossRef]
- Abegunde, S.M.; Idowu, K.S.; Adejuwon, O.M.; Adeyemi-Adejolu, T. A review on the influence of chemical modification on the performance of adsorbents. Environ. Dev. Sustain. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Periyasamy, S.; Viswanathan, N. Hydrothermal synthesis of hydrocalumite assisted biopolymeric hybrid composites for efficient Cr (VI) removal from water. New J. Chem. 2018, 42, 3371–3382. [Google Scholar] [CrossRef]
- Da Rosa Salles, T.; De Bitencourt Rodrigues, H.; Da Silva Bruckmann, F.; Alves LC, S.; Mortari, S.R.; Rhoden CR, B. Graphene oxide optimization synthesis for application on laboratory of Universidade Franciscana. Discip. Sci. Sér. Ciên. Nat. Tecnol. 2020, 21, 15–26. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, T.; Liu, J.; Li, D. Adsorption of tetracycline antibiotics from an aqueous solution onto graphene oxide/calcium alginate composite fibers. RSC Adv. 2018, 8, 2616–2621. [Google Scholar] [CrossRef]
- Da Silva Bruckmann, F.; Zuchetto, T.; Ledur, C.M.; dos Santos, C.L.; da Silva, W.L.; Fagan, S.B.; da Silva, I.Z.; Rhoden CR, B. Methylphenidate adsorption onto graphene derivatives: Theory and experiment. New J. Chem. 2022, 4283, 46–4291. [Google Scholar] [CrossRef]
- Zhai, W.; He, J.; Han, P.; Zeng, M.; Gao, X.; He, Q. Adsorption mechanism for tetracycline onto magnetic Fe3O4 nanoparticles: Adsorption isotherm and dynamic behavior, location of adsorption sites and interaction bonds. Vacuum 2022, 195, 110634. [Google Scholar] [CrossRef]
- Shahraki, S.; Delarami, H.S.; Khosravi, F.; Nejat, R. Improving the adsorption potential of chitosan for heavy metal ions using aromatic ring-rich derivatives. J. Colloid Interface Sci. 2020, 576, 79–89. [Google Scholar] [CrossRef]
- Da Rosa Salles, T.; Da Silva Bruckamann, F.; Viana, A.R.; Krause, L.M.F.; Mortari, S.R.; Rhoden, C.R.B. Magnetic nanocrystalline cellulose: Azithromycin adsorption and in vitro biological activity against melanoma cells. J. Polym. Environ. 2022, 30, 2695–2713. [Google Scholar] [CrossRef]
- Li, Z.; Sellaoui, L.; Gueddida, S.; Dotto, G.L.; Lamine, A.B.; Bonilla-Petriciolet, A.; Badawi, M. Adsorption of methylene blue on silica nanoparticles: Modelling analysis of the adsorption mechanism via a double layer model. J. Mol. Liq. 2020, 319, 114348. [Google Scholar] [CrossRef]
- Giraldo, J.D.; Rivas, B.L. Direct ionization and solubility of chitosan in aqueous solutions with acetic acid. Polym. Bull. 2021, 78, 1465–1488. [Google Scholar] [CrossRef]
- Nunes, F.B.; Da Silva Bruckmann, F.; Da Rosa Salles, T.; Rhoden, C.B.R. Study of phenobarbital removal from the aqueous solutions employing magnetite-functionalized chitosan. Environ. Sci. Pollut. Res. 2022, 29, 1–14. [Google Scholar] [CrossRef]
- Bruckmann, F.S.; Rossato Viana, A.; Tonel, M.Z.; Fagan, S.B.; Garcia, W.J.D.S.; Oliveira, A.H.D.; Dorneles, L.S.; Mortari, S.R.; Da Silva, W.L.; Da Silva, I.Z.; et al. Influence of magnetite incorporation into chitosan on the adsorption of the methotrexate and in vitro cytotoxicity. Environ. Sci. Pollut. Res. 2022, 29, 70413–70434. [Google Scholar] [CrossRef]
- Liu, E.; Zheng, X.; Xu, X.; Zhang, F.; Liu, E.; Wang, Y.; Li, C.; Yan, Y. Preparation of diethylenetriamine-modified magnetic chitosan nanoparticles for adsorption of rare-earth metal ions. New J. Chem. 2017, 41, 7739–7750. [Google Scholar] [CrossRef]
- Rhoden, C.R.B.; Bruckmann, F.S.; Salles, T.R.; Kaufmann Jr, C.G.; Mortari, S.R. Study from the influence of magnetite onto removal of hydrochlorothiazide from aqueous solutions applying magnetic graphene oxide. J. Water Process. Eng. 2021, 43, 102262. [Google Scholar] [CrossRef]
- Wang, X.; Tang, R.; Zhang, Y.; Yu, Z.; Qi, C. Preparation of a novel chitosan based biopolymer dye and application in wood dyeing. Polymers 2016, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Zhu, X.J.; Sun, H.W.; Chi, G.R.; Xu, J.X.; Sun, Y.L. Control synthesis of magnetic Fe3O4–chitosan nanoparticles under UV irradiation in aqueous system. Curr. Appl. Phys. 2010, 10, 828–833. [Google Scholar] [CrossRef]
- da Silva Bruckmann, F.; Pimentel, A.C.; Viana, A.R.; da Rosa Salles, T.; Krause, L.M.F.; Mortari, S.R.; Silva, I.Z.; Rhoden, C.R.B. Synthesis, characterization and cytotoxicity evaluation of magnetic nanosilica in L929 cell line. Discip. Sci. Sér. Ciên. Nat. Tecnol. 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Peng, J.; Wang, X.; Lou, T. Preparation of chitosan/gelatin composite foam with ternary solvents of dioxane/acetic acid/water and its water absorption capacity. Polym. Bull. 2020, 77, 5227–5244. [Google Scholar] [CrossRef]
- Hao, G.; Hu, Y.; Shi, L.; Chen, J.; Cui, A.; Weng, W.; Osako, K. Physicochemical characteristics of chitosan from swimming crab (Portunus trituberculatus) shells prepared by subcritical water pretreatment. Sci. Rep. 2021, 11, 1646. [Google Scholar] [CrossRef]
- Prabha, G.; Raj, V. Preparation and characterization of chitosan—Polyethylene glycol-polyvinylpyrrolidone-coated superparamagnetic iron oxide nanoparticles as carrier system: Drug loading and in vitro drug release study. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 808–816. [Google Scholar] [CrossRef]
- Ates, B.; Ulu, A.; Köytepe, S.; Noma, S.A.A.; Kolat, V.S.; Izgi, T. Magnetic-propelled Fe3O4–chitosan carriers enhance L-asparaginase catalytic activity: A promising strategy for enzyme immobilization. RSC Adv. 2018, 8, 36063–36075. [Google Scholar] [CrossRef] [PubMed]
- Pylypchuk, I.V.; Kołodyńska, D.; Kozioł, M.; Gorbyk, P.P. Gd-DTPA adsorption on chitosan/magnetite nanocomposites. Nanoscale Res. Lett. 2016, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Freire, T.M.; Fechine, L.M.; Queiro, D.C.; Freire, R.M.; Denardin, J.C.; Ricardo, N.M.; Rodrigues, T.N.B.; Gondim, D.R.; Junior, I.J.S.; Fechine, P. Magnetic Porous Controlled Fe3O4–Chitosan Nanostructure: An Ecofriendly Adsorbent for Efficient Removal of Azo Dyes. Nanomaterials 2020, 10, 1194. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Ma, J.; Han, S. Adsorption of tetracycline from aqueous solutions onto multi-walled carbon nanotubes with different oxygen contents. Sci. Rep. 2014, 4, 5326. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, C.; Roata, I.C.; Pascu, A.; Stanciu, E.M. Diffusion and controlled release in physically crosslinked poly (vinyl alcohol)/iota-carrageenan hydrogel blends. Polymers 2020, 12, 1544. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhao, Y.; Wang, H.; Tan, X.; Yang, Y.; Liu, Y. Efficient removal of tetracycline from aqueous media with a Fe3O4 nanoparticles@ graphene oxide nanosheets assembly. Int. J. Environ. Res. Public Health 2017, 14, 1495. [Google Scholar] [CrossRef]
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Strong adsorption properties and mechanism of action with regard to tetracycline adsorption of double-network polyvinyl alcohol-copper alginate gel beads. J. Hazard. Mater. 2022, 422, 126863. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Bi, J.; Zheng, S.; Xu, Z.; Zhu, D.; Alvarez, P.J. Adsorption of tetracycline on single-walled and multi-walled carbon nanotubes as affected by aqueous solution chemistry. Environ. Toxicol. Chem. 2010, 29, 2713–2719. [Google Scholar] [CrossRef]
- Liang, J.; Fang, Y.; Luo, Y.; Zeng, G.; Deng, J.; Tan, X.; Tang, N.; Li, X.; He, X.; Feng, C.; et al. Magnetic nanoferromanganese oxides modified biochar derived from pine sawdust for adsorption of tetracycline hydrochloride. Environ. Sci. Pollut. Res. 2019, 26, 5892–5903. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, X.; Zhao, Y.; Cui, L.; Huang, Z.; Long, J.; Xu, J.; Deng, J.; Wu, C.; Liao, W. Decontamination of tetracycline by thiourea-dioxide–reduced magnetic graphene oxide: Effects of pH, ionic strength, and humic acid concentration. J. Colloid Interface Sci. 2017, 495, 68–77. [Google Scholar] [CrossRef]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.; Bandoch, G.F.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Agarry, S.E.; Aworanti, O.A. Kinetics, Isothermal and Thermodynamic Modelling Studies of Hexavalent Chromium Ions Adsorption from Simulated Wastewater onto Parkia biglobosa-Sawdust Derived Acid-Steam. Act. Carbon. Appl. J. Envir. Eng. Sci. 2017, 3, 58–76. [Google Scholar] [CrossRef]
- De Souza, F.M.; Dos Santos, O.A.A.; Vieira, M.G.A. Adsorption of herbicide 2,4-D from aqueous solution using organo-modified bentonite clay. Environ. Sci. Pollut. Res. 2019, 26, 18329–18342. [Google Scholar] [CrossRef]
- Gago, D.; Chagas, R.; Ferreira, L.M.; Velizarov, S.; Coelhoso, I. A novel cellulose-based polymer for efficient removal of methylene blue. Membranes 2020, 10, 13. [Google Scholar] [CrossRef]
- Cazalbou, S.; Bertrand, G.; Drouet, C. Tetracycline-loaded biomimetic apatite: An adsorption study. J. Phys. Chem. B 2015, 119, 3014–3024. [Google Scholar] [CrossRef] [PubMed]
- Acosta, R.; Fierro, V.; De Yuso, A.M.; Nabarlatz, D.; Celzard, A. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 2016, 149, 168–176. [Google Scholar] [CrossRef]
- Chaabane, L.; Beyou, E.; Baouab, M.H.V. Preparation of a novel zwitterionic graphene oxide-based adsorbent to remove of heavy metal ions from water: Modeling and comparative studies. Adv. Powder Technol. 2021, 32, 2502–2516. [Google Scholar] [CrossRef]
- Wojtacha-Rychter, K.; Howaniec, N.; Smoliński, A. Effect of porous structure of coal on propylene adsorption from gas mixtures. Sci. Rep. 2020, 10, 11277. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Wang, S.; Bai, L.; Deng, Y.; Tao, J. Effect of pore size distribution and amination on adsorption capacities of polymeric adsorbents. Molecules 2021, 26, 5267. [Google Scholar] [CrossRef]
- Huízar-Félix, A.M.; Aguilar-Flores, C.; Martínez-de-la Cruz, A.; Barandiarán, J.M.; Sepúlveda-Guzmán, S.; Cruz-Silva, R. Removal of tetracycline pollutants by adsorption and magnetic separation using reduced graphene oxide decorated with α-Fe2O3 nanoparticles. Nanomaterials 2019, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Ranjbari, S.; Tanhaei, B.; Ayati, A.; Khadempir, S.; Sillanpää, M. Efficient tetracycline adsorptive removal using tricaprylmethylammonium chloride conjugated chitosan hydrogel beads: Mechanism, kinetic, isotherms and thermodynamic study. Int. J. Biol. Macromol. 2020, 155, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ding, C.; Li, Y.; Ke, H.; Cheng, G. Efficient removal of tetracycline hydrochloride from aqueous solution by mesoporous cage MOF-818. SN Appl. Sci. 2020, 2, 669. [Google Scholar] [CrossRef]
- Erdem, S.; Öztekin, M.; Açıkel, Y.S. Investigation of tetracycline removal from aqueous solutions using halloysite/chitosan nanocomposites and halloysite nanotubes/alginate hydrogel beads. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100576. [Google Scholar] [CrossRef]
- Rathod, M.; Haldar, S.; Basha, S. Nanocrystalline cellulose for removal of tetracycline hydrochloride from water via biosorption: Equilibrium, kinetic and thermodynamic studies. Ecol. Eng. 2015, 84, 240–249. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, S.; Li, J. Fast and highly efficient tetracyclines removal from environmental waters by graphene oxide functionalized magnetic particles. Chem. Eng. J. 2013, 225, 679–685. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Duan, L.; Yang, H.; Gao, J. Tetracycline adsorption onto rice husk ash, an agricultural waste: Its kinetic and thermodynamic studies. J. Mol. Liq. 2016, 222, 487–494. [Google Scholar] [CrossRef]




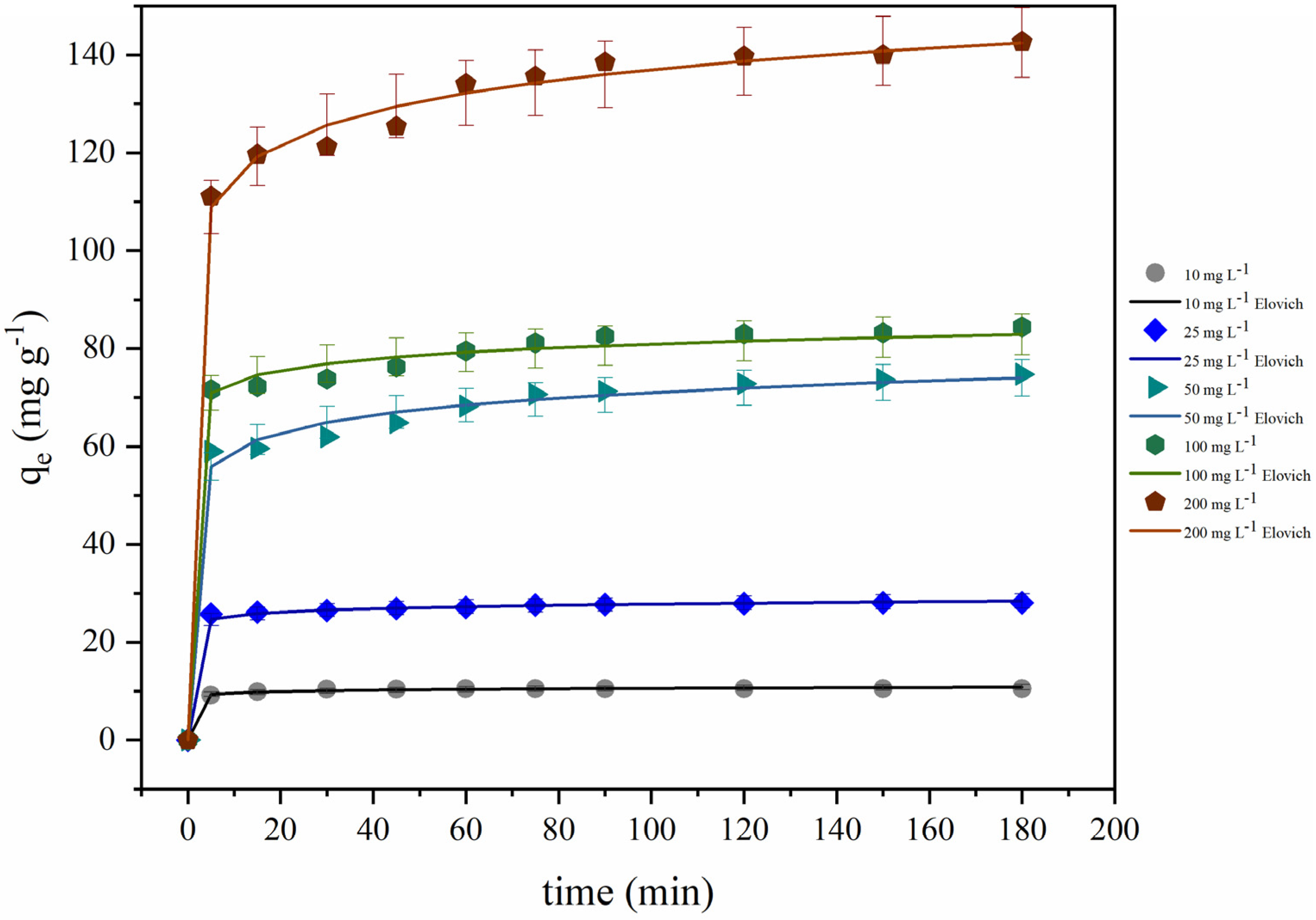
| Concentration (mg L−1) | 10 | 25 | 50 | 100 | 200 |
|---|---|---|---|---|---|
| Pseudo-first-order model (PFO) | |||||
| q1 (mg g−1) | 10.43 | 27.31 | 68.73 | 79.71 | 133.17 |
| k1 (min−1) | 0.409 | 0.586 | 0.378 | 0.452 | 0.348 |
| R2 | 0.996 | 0.994 | 0.974 | 0.973 | 0.964 |
| R2adj | 0.995 | 0.992 | 0.967 | 0.966 | 0.955 |
| ARE (%) | 0.93 | 1.79 | 5.47 | 3.75 | 4.58 |
| SSE | 0.02 | 0.36 | 6.41 | 4.24 | 9.51 |
| Pseudo-second-order model (PSO) | |||||
| q2 (mg g−1) | 10.61 | 27.61 | 71.08 | 81.39 | 137.54 |
| k2 (g mg−1 min−1) | 0.112 | 0.080 | 0.009 | 0.013 | 0.004 |
| R2 | 0.999 | 0.996 | 0.987 | 0.984 | 0.982 |
| R2adj | 0.999 | 0.995 | 0.983 | 0.980 | 0.977 |
| ARE (%) | 0.39 | 1.14 | 4.51 | 3.22 | 3.13 |
| SSE | 0.11 | 0.20 | 5.97 | 8.54 | 6.65 |
| Elovich model | |||||
| α (mg g−1 min−1) | 1.27 | 3.91 | 6.33 | 11.81 | 21.23 |
| β (g mg−1) | 2.51 | 0.958 | 0.300 | 0.197 | 0.106 |
| R2 | 0.997 | 0.998 | 0.993 | 0.996 | 0.996 |
| R2adj | 0.996 | 0.997 | 0.991 | 0.995 | 0.995 |
| ARE (%) | 0.40 | 0.85 | 2.34 | 1.81 | 1.35 |
| SSE | 0.04 | 0.13 | 2.72 | 2.77 | 5.05 |
| Temperature | 20 °C | 30 °C | 40 °C |
|---|---|---|---|
| Langmuir | |||
| qmax (mg g−1) | 86.81 | 85.37 | 92.12 |
| KL (L mg−1) | 0.201 | 0.069 | 0.045 |
| R2 | 0.977 | 0.965 | 0.906 |
| R2adj | 0.971 | 0.956 | 0.882 |
| ARE (%) | 3.94 | 4.56 | 8.84 |
| SSE | 9.04 | 9.55 | 24.97 |
| Freundlich | |||
| KF ((mg g−1) (L−1)−1/n | 6.59 | 3.28 | 2.51 |
| n | 43.48 | 20.22 | 13.56 |
| R2 | 0.967 | 0.956 | 0.892 |
| R2adj | 0.958 | 0.945 | 0.865 |
| ARE (%) | 4.87 | 5.21 | 9.55 |
| SSE | 13.46 | 11.89 | 28.85 |
| Sips | |||
| qs (mg g−1) | 74.71 | 65.01 | 57.03 |
| Ks (L mg−1) | 0.069 | 0.064 | 0.049 |
| ns | 5.20 | 3.81 | 2.74 |
| R2 | 0.998 | 0.992 | 0.987 |
| R2adj | 0.997 | 0.990 | 0.983 |
| ARE (%) | 0.81 | 3.29 | 3.02 |
| SSE | 0.42 | 4.66 | 3.84 |
| T (K) | ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 (kJ mol−1 K−1) |
|---|---|---|---|
| 293.15 | −3.25 | −27.29 | −0.082 |
| 313.15 | −1.65 | ||
| 333.15 | −1.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Bruckmann, F.; Schnorr, C.E.; da Rosa Salles, T.; Nunes, F.B.; Baumann, L.; Müller, E.I.; Silva, L.F.O.; Dotto, G.L.; Bohn Rhoden, C.R. Highly Efficient Adsorption of Tetracycline Using Chitosan-Based Magnetic Adsorbent. Polymers 2022, 14, 4854. https://doi.org/10.3390/polym14224854
da Silva Bruckmann F, Schnorr CE, da Rosa Salles T, Nunes FB, Baumann L, Müller EI, Silva LFO, Dotto GL, Bohn Rhoden CR. Highly Efficient Adsorption of Tetracycline Using Chitosan-Based Magnetic Adsorbent. Polymers. 2022; 14(22):4854. https://doi.org/10.3390/polym14224854
Chicago/Turabian Styleda Silva Bruckmann, Franciele, Carlos Eduardo Schnorr, Theodoro da Rosa Salles, Franciane Batista Nunes, Luiza Baumann, Edson Irineu Müller, Luis F. O. Silva, Guilherme L. Dotto, and Cristiano Rodrigo Bohn Rhoden. 2022. "Highly Efficient Adsorption of Tetracycline Using Chitosan-Based Magnetic Adsorbent" Polymers 14, no. 22: 4854. https://doi.org/10.3390/polym14224854
APA Styleda Silva Bruckmann, F., Schnorr, C. E., da Rosa Salles, T., Nunes, F. B., Baumann, L., Müller, E. I., Silva, L. F. O., Dotto, G. L., & Bohn Rhoden, C. R. (2022). Highly Efficient Adsorption of Tetracycline Using Chitosan-Based Magnetic Adsorbent. Polymers, 14(22), 4854. https://doi.org/10.3390/polym14224854