Preparation of 3D Printed Polylactic Acid/Bacterial Cellulose Composite Scaffold for Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
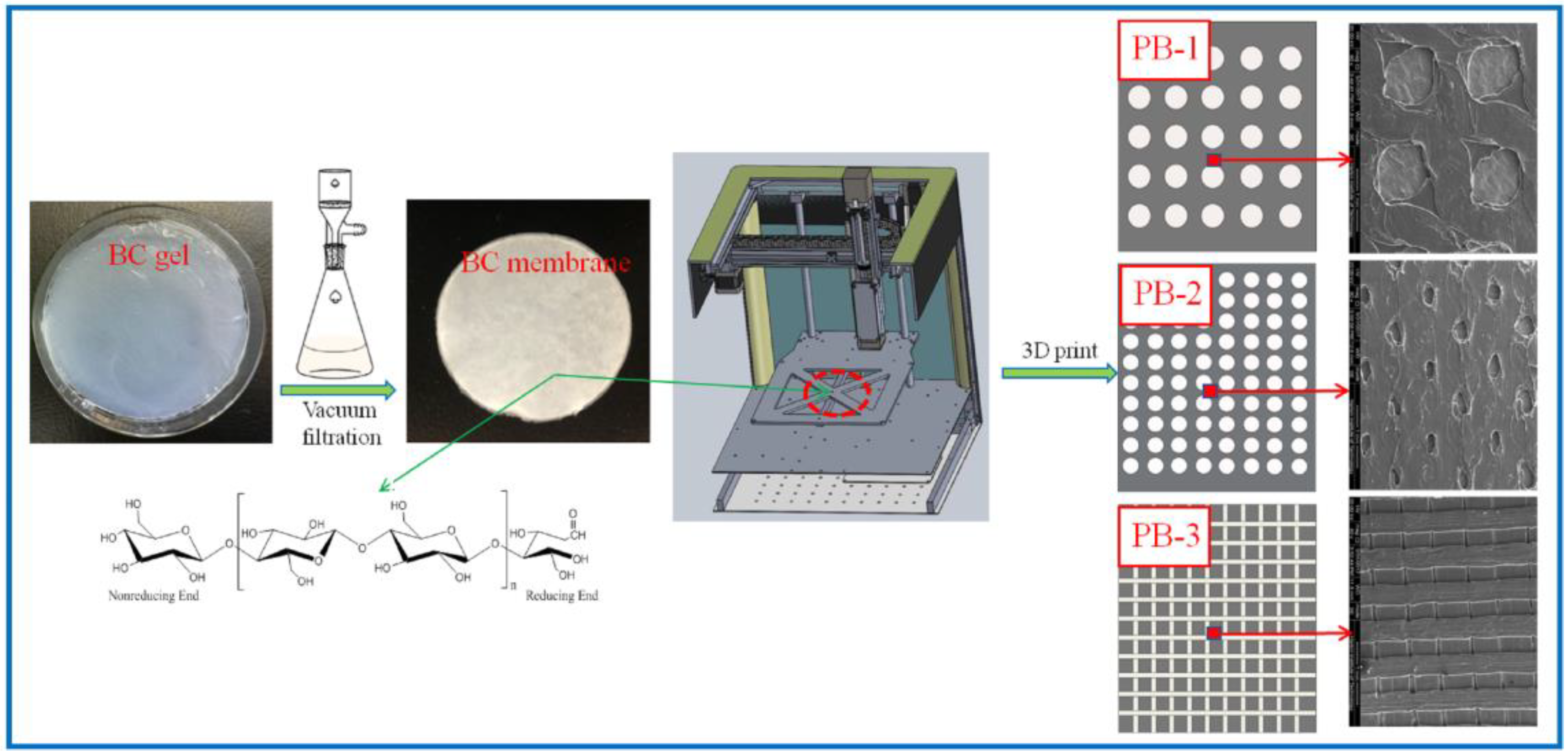
2.2. Preparation of BC Membrane
2.3. Preparation of 3D-Printed PLA/BC Composite Scaffolds
- (1)
- Modeling: established the 3D model of the required porous membrane material support in the computer, and decomposed the model into 5 layer 20 μM sheets;
- (2)
- Feeding: added PLA powder for 3D printing into the barrel of the 3D printer;
- (3)
- Printing: PLA was printed onto the BC membrane material placed on the three-dimensional fluctuation platform by layer printing. Among them, the mass of the PLA used in PLA/BC-1, PLA/BC-2, and PLA/BC-3 were 80 mg, 120 mg, 160 mg, respectively.
2.4. Characterization and Performances Study of PLA/BC Composite Scaffolds
2.5. Biological Properties Evaluation of PLA/BC Composite Scaffolds
3. Results and Discussion
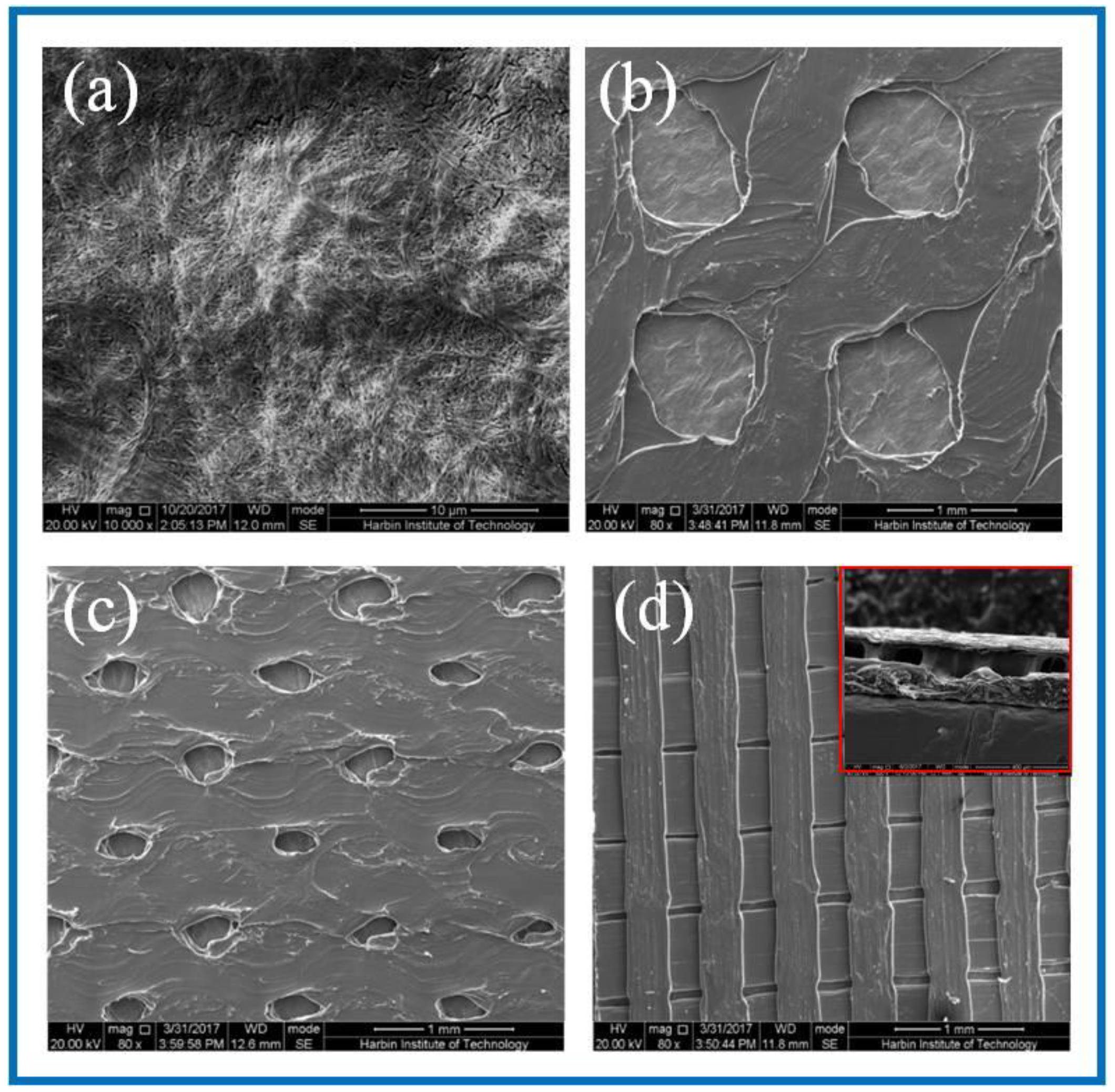
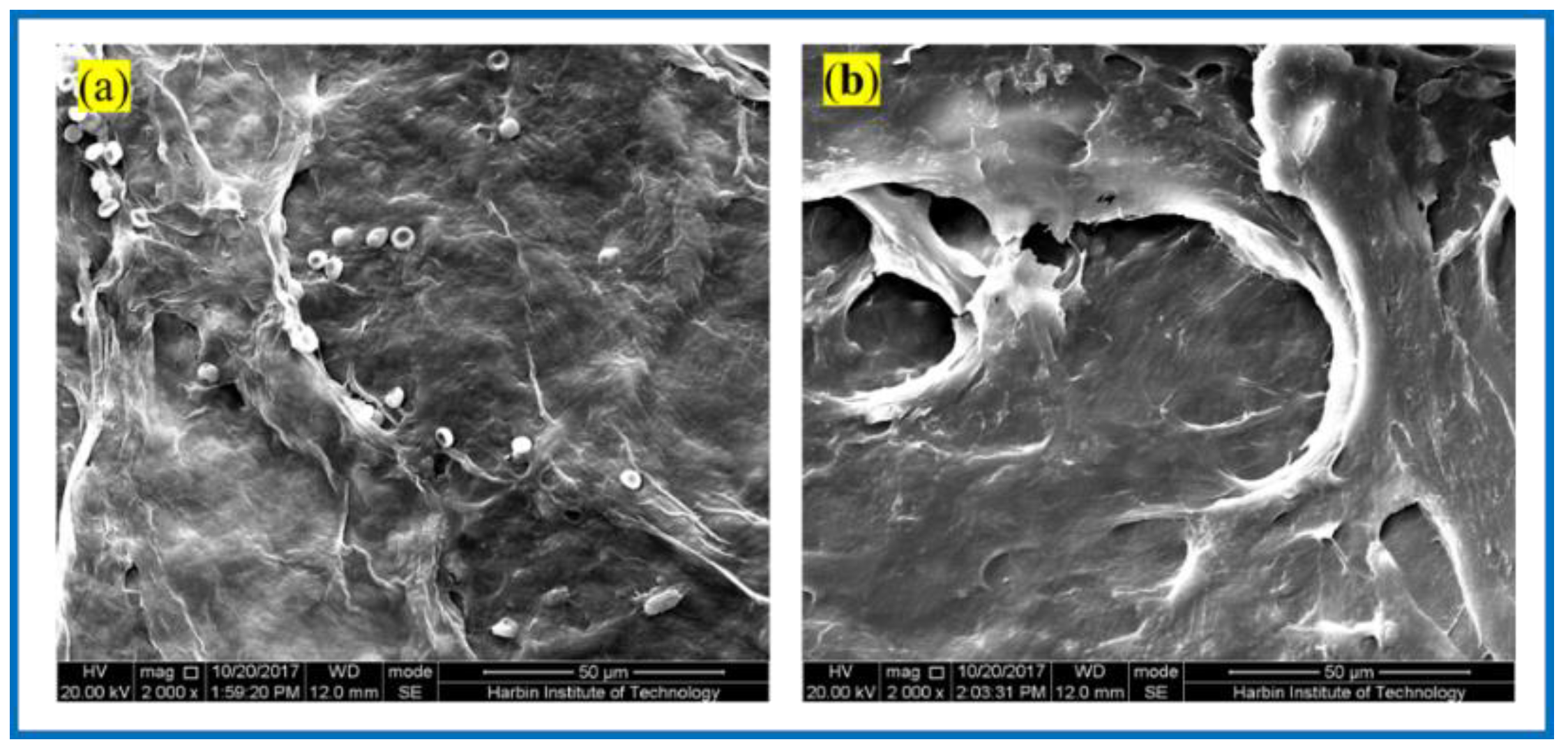
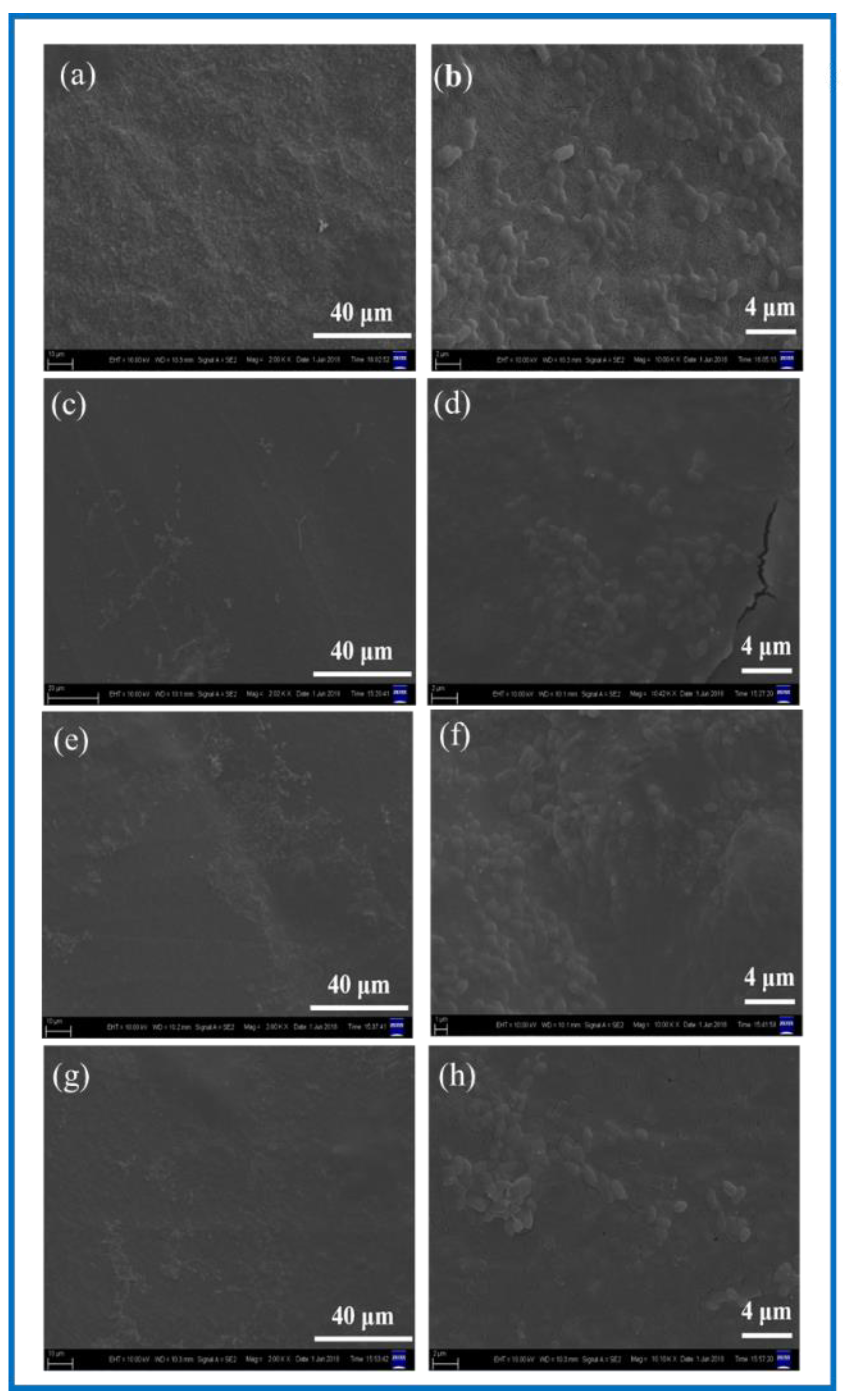
3.1. Structural Morphology Observation
3.2. Characterization and Performance Study of PLA/BC Scaffolds
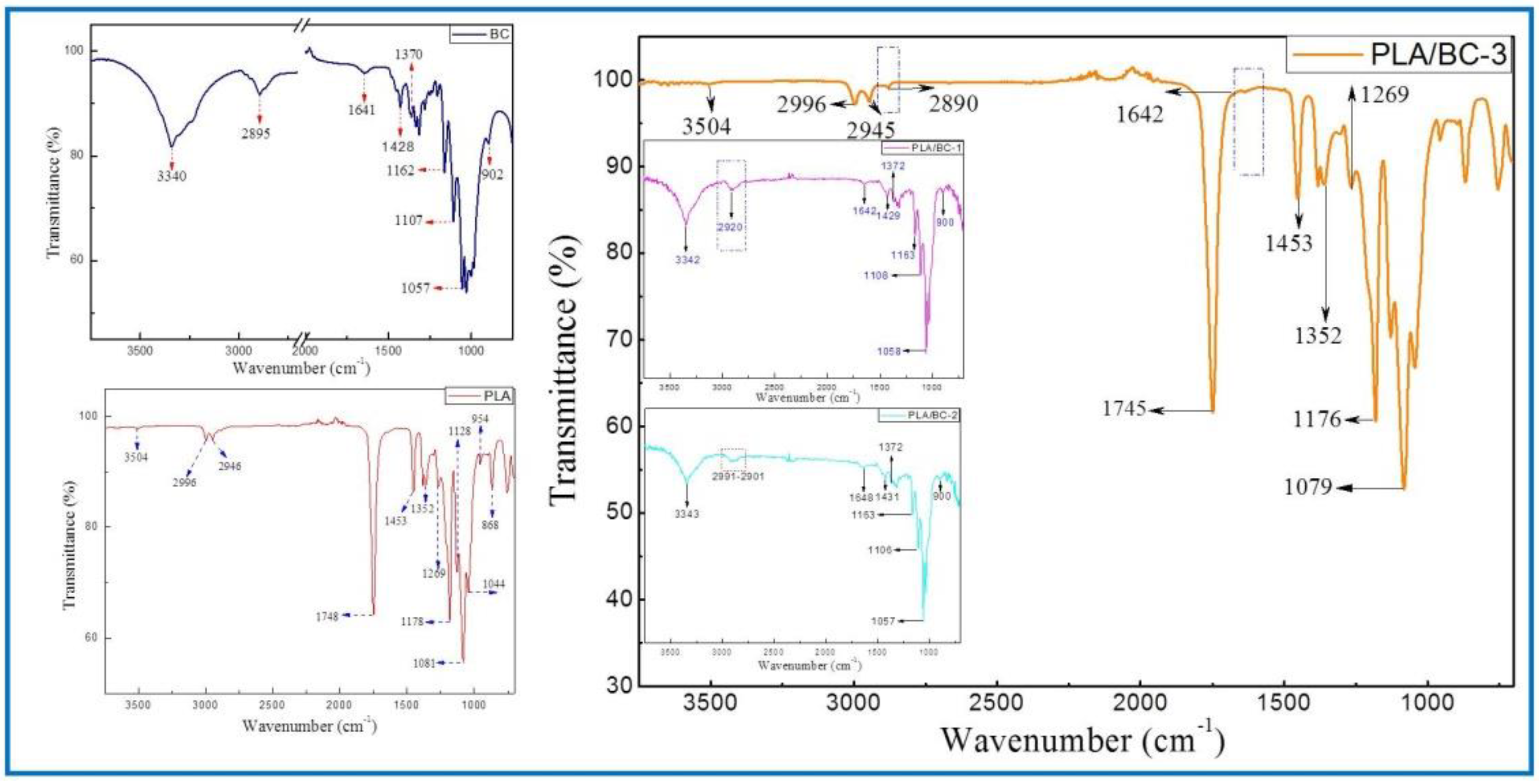
3.2.1. IR Studies of PLA/BC Composite Scaffolds
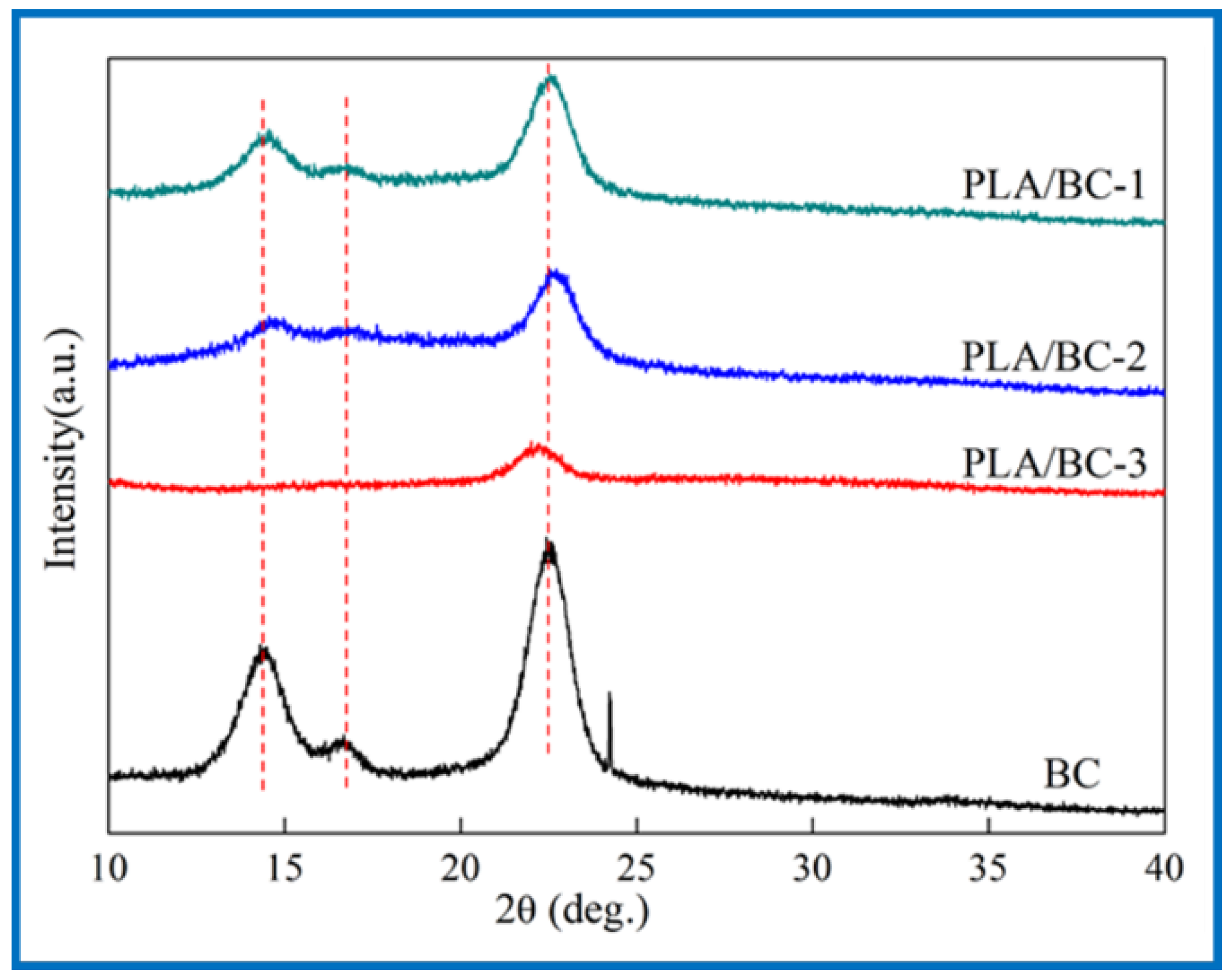
3.2.2. XRD Analysis of PLA/BC Composite Scaffolds
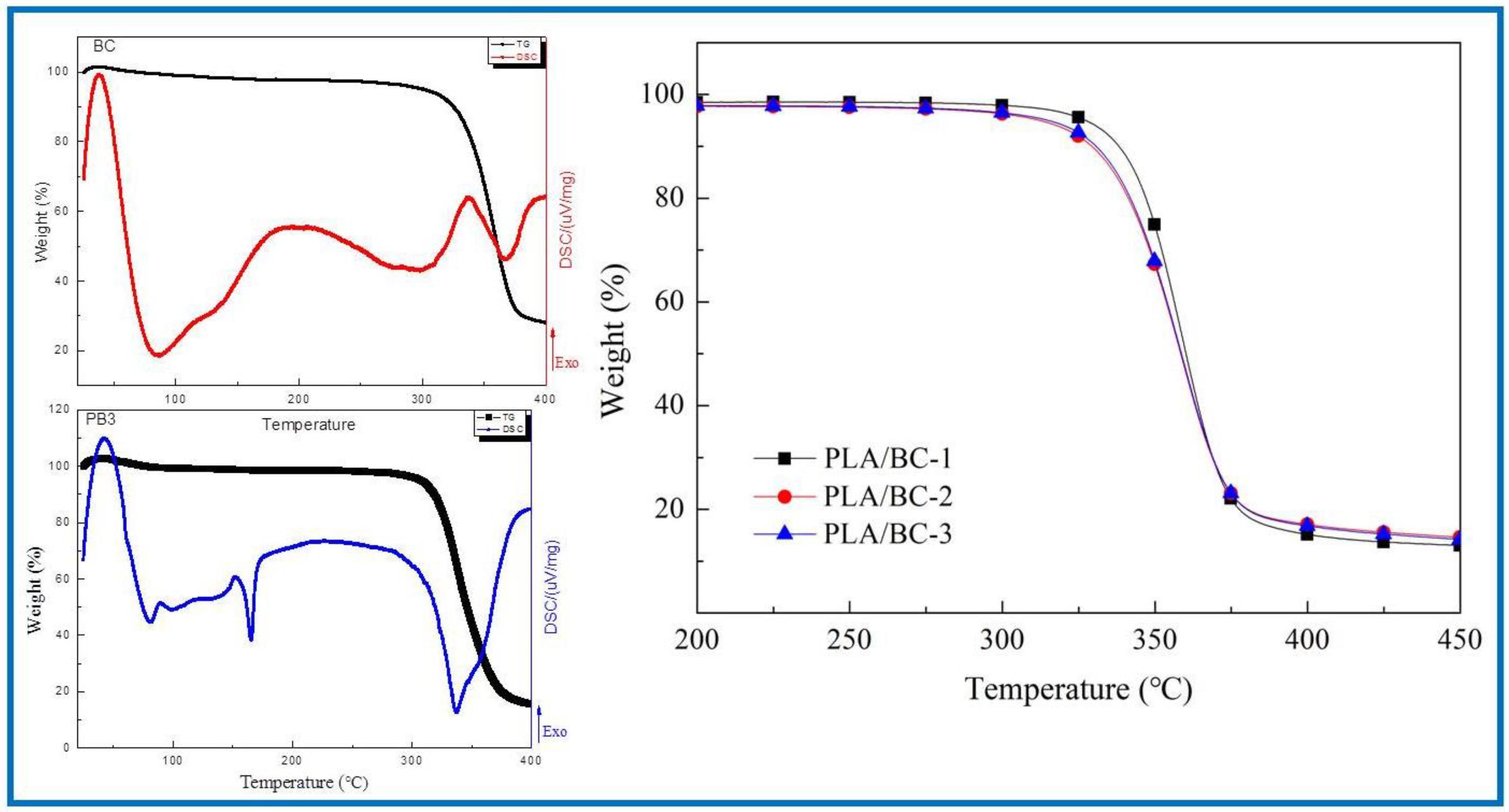
3.2.3. Thermal Properties of PLA/BC Composite Scaffolds
3.2.4. Porosity Test of PLA/BC Composite Scaffolds
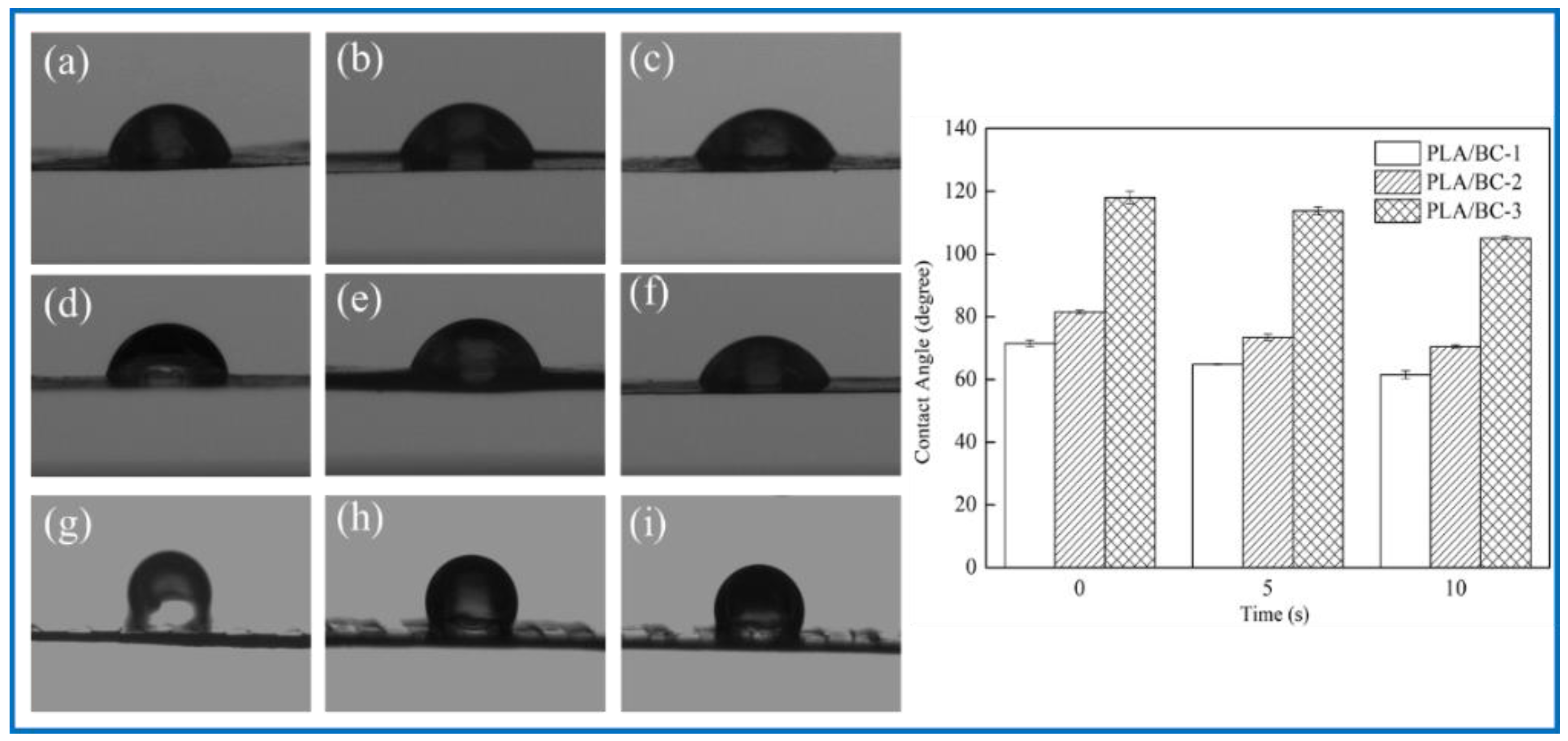
3.2.5. Effect of PLA Loading on Hydrophilicity of the Composite Scaffolds
3.2.6. Mechanical Properties of PLA/BC Composite Scaffolds
3.3. Biological Properties Evaluation of PLA/BC Composite Scaffolds
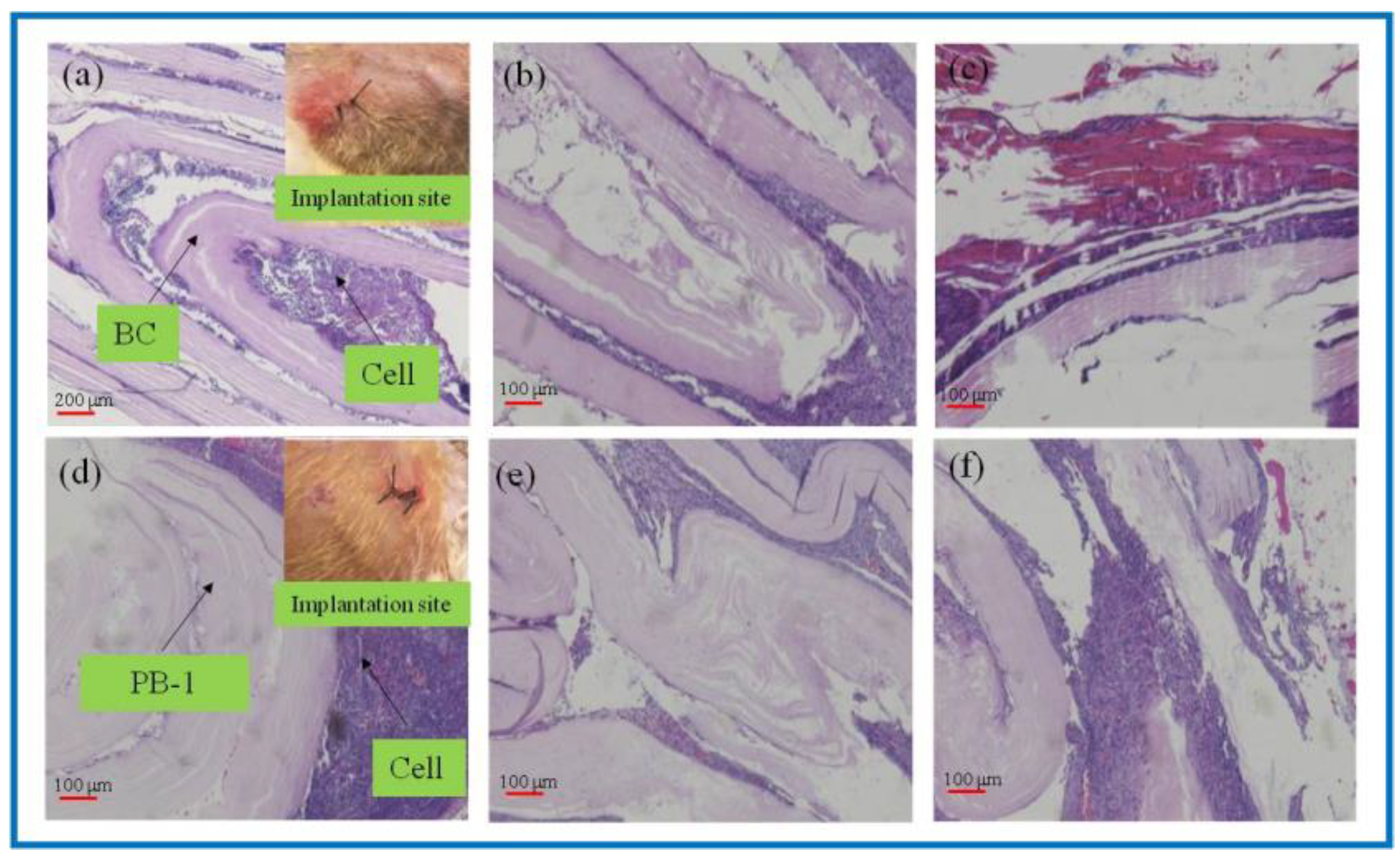
3.3.1. Degradation Properties In Vivo
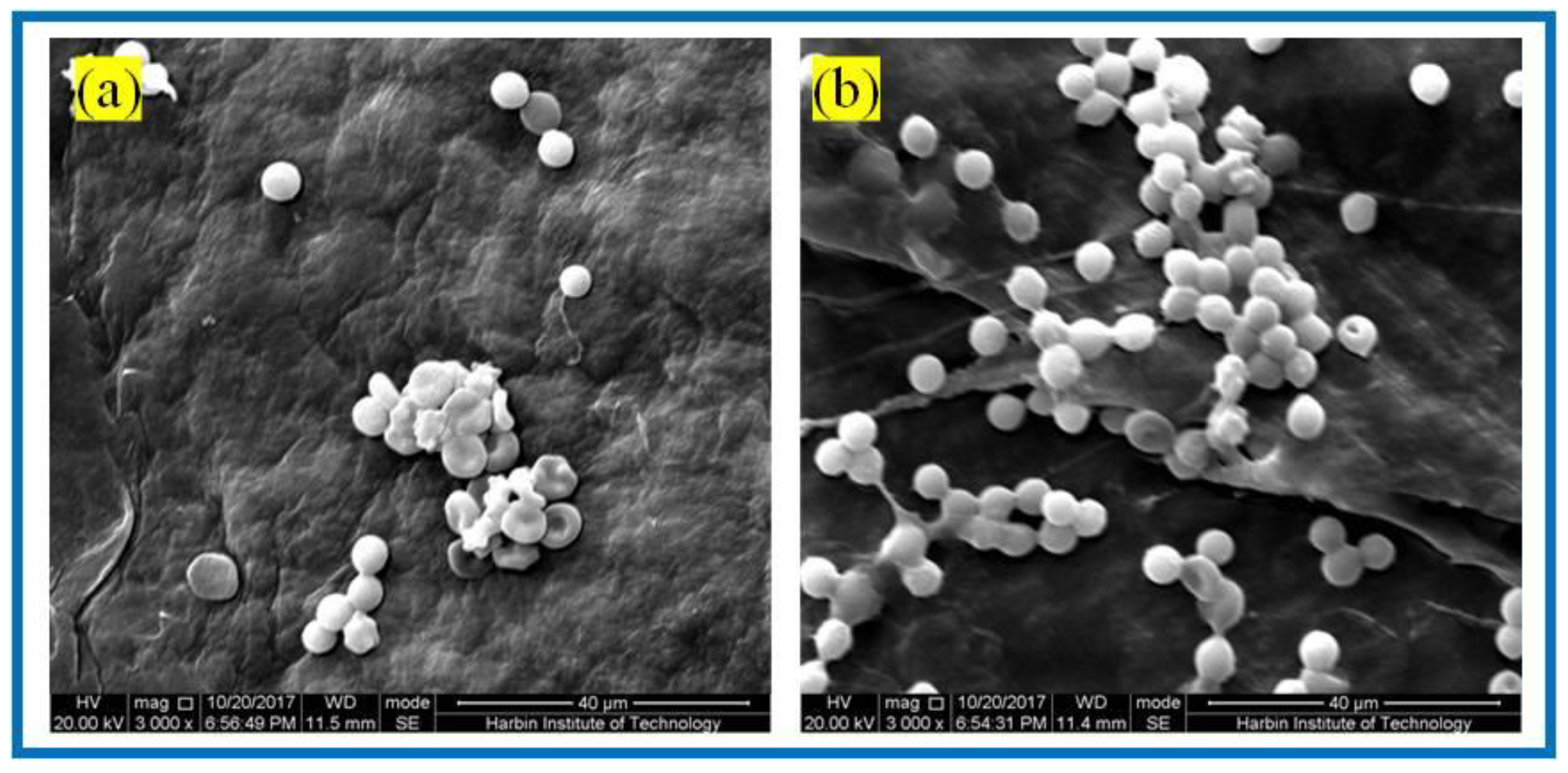
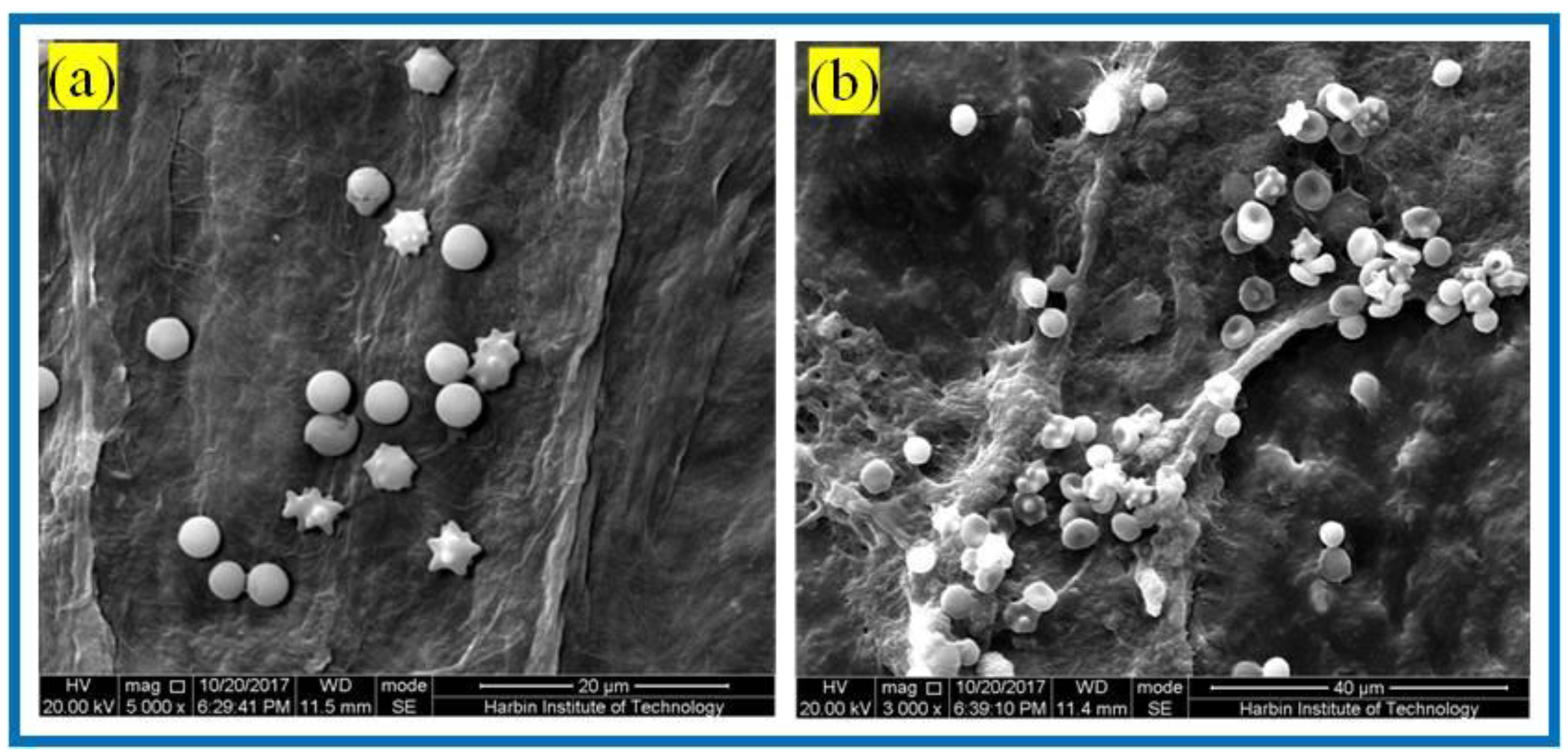
3.3.2. Erythrocyte Fixation Experiments
3.3.3. Platelet Adhesion Experiments
3.3.4. Cytotoxicity Experiment
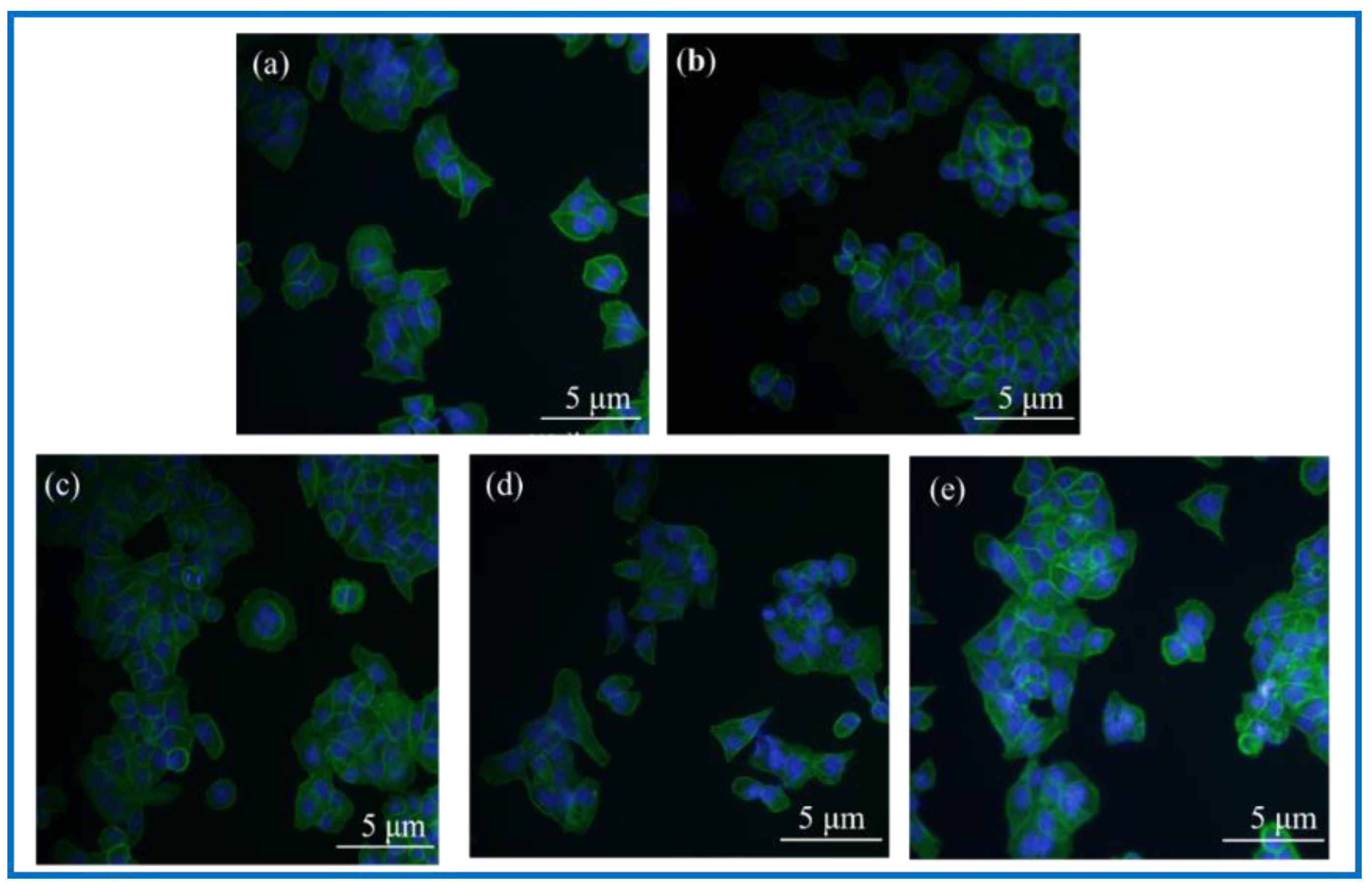
3.3.5. SCs Adhesion Experiment
3.3.6. Analysis of Immunological Staining Results
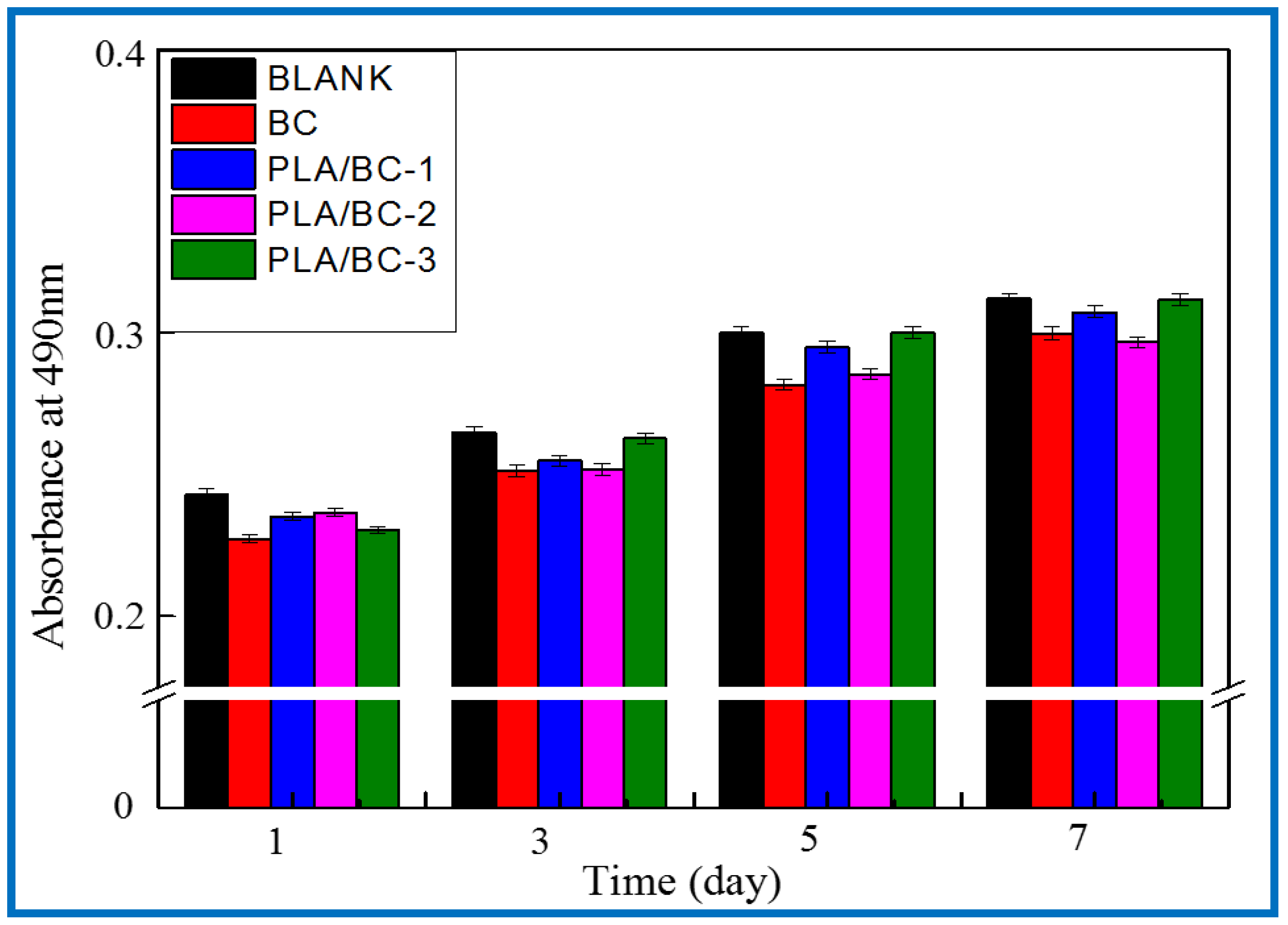
3.3.7. Cell Proliferation Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Lohmann, C.H.; Romero, J.; Schwartz, Z. Bone and Cartilage Tissue Engineering. Clin. Plast. Surg. 1999, 26, 629–645. [Google Scholar] [CrossRef]
- Rai, V.; Dilisio, M.F.; Dietz, N.E.; Agrawal, D.K. Recent Strategies in Cartilage Repair: A Systemic Review of the Scaffold Development and Tissue Engineering. J. Biomed. Mater. Res. Part A 2017, 105, 2343–2354. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Z.; Xiao, W.; Yu, F.; Zhang, W. Primary Observation on PLGA/TCP Scaffolds Incorporating Icariin in Repairing the Defect of Knee Cartilage Accordance with the Biological Characteristics of the Rabbits. China Mod. Med. 2016, 23, 7–10. [Google Scholar]
- Semina, E.V.; Rubina, K.A.; Sysoeva, V.Y.; Stepanova, V.V.; Tkachuk, V.A. Three-dimensional Model of Biomatrix as A Method of Studying Blood Vessels and Nerve Growth in Tissue Engineering Structures. Mosc. Univ. Chem. Bull. 2016, 71, 172–177. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Biol. 2022, 13, 100186. [Google Scholar] [CrossRef]
- McCarthy, R.R.; Ullah, M.W.; Booth, P.; Pei, E.; Yang, G. The use of bacterial polysaccharides in bioprinting. Biotechnol. Adv. 2019, 37, 107448. [Google Scholar] [CrossRef]
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and its Applications in Tissue Engineering and Regenerative Medicine. Int. J. Biol. Macromol. 2018, 107, 261–275. [Google Scholar] [CrossRef]
- Aljohani, W.; Ullah, M.W.; Li, W.; Shi, L.; Zhang, X.; Yang, G. Three-dimensional printing of alginate-gelatin-agar scaffolds using free-form motor assisted microsyringe extrusion system. J. Polym. Res. 2018, 25, 62–71. [Google Scholar] [CrossRef]
- Shi, L.; Hu, Y.; Ullah, M.W.; Ullah, I.; Ou, H.; Zhang, W.; Xiong, L.; Zhang, X. Cryogenic free-form extrusion bioprinting of decellularized small intestinal submucosa for potential applications in skin tissue engineering. Biofabrication 2019, 11, 035023. [Google Scholar] [CrossRef]
- Zhang, W.; Ullah, I.; Shi, L.; Zhang, Y.; Ou, H.; Zhou, J.; Ullah, M.W.; Zhang, X.; Li, W. Fabrication and characterization of porous polycaprolactone scaffold via extrusion-based cryogenic 3D printing for tissue engineering. Mater. Des. 2019, 180, 107946. [Google Scholar] [CrossRef]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Varsavas, S.D.; Kaynak, C. Effects of glass fiber reinforcement and thermoplastic elastomer blending on the mechanical performance of polylactide. Compos. Commun. 2018, 8, 24–30. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on polylactic acid (PLA) based sustainable materials for durable applications: Focus on toughness and heat resistance ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Liebscher, M.; Tzounis, L. Three-Dimensional Printed Antimicrobial Objects of Polylactic Acid (PLA)-Silver Nanoparticle Nanocomposite Filaments Produced by an In-Situ Reduction Reactive Melt Mixing Process. Biomimetics 2020, 5, 42. [Google Scholar] [CrossRef]
- Divakara Shetty, S.; Shetty, N. Investigation of mechanical properties and applications of polylactic acids—A review. Mater. Res. Express 2019, 6, 112002. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Inkinen, S.; Hakkarainen, M.; Albertsson, A.-C.; Södergård, A. From Lactic Acid to Poly(lactic acid) (PLA): Characterization and Analysis of PLA and Its Precursors. Biomacromolecules 2011, 12, 523–532. [Google Scholar] [CrossRef]
- Bhaskar, B.; Owen, R.; Bahmaee, H.; Wally, Z.; Sreenivasa Rao, P.; Reilly, G.C. Composite Porous Scaffold of Polyethylene Glycol (PEG)/Polylactic Acid (PLA) Support Improved Bone Matrix eposition in Vitro Compared to PLA-only Scaffolds. J. Biomed. Mater. Res. Part A 2018, 106, 1334–1340. [Google Scholar] [CrossRef]
- Lin, Q.; Jiang, P.; Ren, S.; Liu, S.; Ji, Y.; Huang, Y.; Yu, W.; Fontaine, G.; Bourbigot, S. Advanced functional materials based on bamboo cellulose fibers with different crystal structures. Compos. Part A Appl. Sci. Manuf. 2022, 154, 106758. [Google Scholar] [CrossRef]
- Graninger, G.; Kumar, S.; Garrett, G.; Falzon, B.G. Effect of shear forces on dispersion-related properties of microcrystalline cellulose-reinforced EVOH composites for advanced applications. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106103. [Google Scholar] [CrossRef]
- Gebler, M.; Schoot Uiterkamp, A.J.M.; Visser, C. A global sustainability perspective on 3D printing technologies. Energy Policy 2014, 74, 158–167. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Tzounis, L.; Maniadi, A.; Velidakis, E.; Mountakis, N.; Kechagias, J.D. Sustainable additive manufacturing: Mechanical response of polyamide 12 over multiple recycling processes. Materials 2021, 14, 466. [Google Scholar] [CrossRef]
- Vidakis, N.; Maniadi, A.; Petousis, M.; Vamvakaki, M.; Kenanakis, G.; Koudoumas, E. Mechanical and Electrical Properties Investigation of 3D-Printed Acrylonitrile–Butadiene–Styrene Graphene and Carbon Nanocomposites. J. Mater. Eng. Perform. 2020, 29, 1909–1918. [Google Scholar] [CrossRef]
- Valino, A.D.; Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Messman, J.; Advincula, R.C. Advances in 3D printing of thermoplastic polymer composites and nanocomposites. Prog. Polym. Sci. 2019, 98, 101162. [Google Scholar] [CrossRef]
- Campbell, T.A.; Ivanova, O.S. 3D printing of multifunctional nanocomposites. Nano Today 2013, 8, 119–120. [Google Scholar] [CrossRef]
- Gnanasekaran, K.; Heijmans, T.; van Bennekom, S.; Woldhuis, H.; Wijnia, S.; de With, G.; Friedrich, H. 3D printing of CNT- and graphene-based conductive polymer nanocomposites by fused deposition modeling. Appl. Mater. Today 2017, 9, 21–28. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Spiridaki, M.; Kechagias, J.D. Mechanical Performance of Fused Filament Fabricated and 3D-Printed Polycarbonate Polymer and Polycarbonate/Cellulose Nanofiber Nanocomposites. Fibers 2021, 9, 74. [Google Scholar] [CrossRef]
- Zhou, L.; Ke, K.; Yang, M.B.; Yang, W. Recent progress on chemical modification of cellulose for high mechanical-performance Poly(lactic acid)/Cellulose composite: A review. Compos. Commun. 2021, 23, 100548. [Google Scholar] [CrossRef]
- Dong, J.; Mei, C.; Han, J.; Lee, S.; Wu, Q. 3D printed poly(lactic acid) composites with grafted cellulose nanofibers: Effect of nanofiber and post-fabrication annealing treatment on composite flexural properties. Addit. Manuf. 2019, 28, 621–628. [Google Scholar] [CrossRef]
- Ambone, T.; Torris, A.; Shanmuganathan, K. Enhancing the mechanical properties of 3D printed polylactic acid using nanocellulose. Polym. Eng. Sci. 2020, 60, 1842–1855. [Google Scholar] [CrossRef]
- Kim, Y.; Ullah, M.W.; Ul-lslam, M.; Khan, S.; Jang, J.H.; Park, J.K. Self-assembly of bio-cellulose nanofibrils through intermediate phase in a cell-free enzyme system. Biochem. Eng. J. 2019, 142, 135–144. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul-lslam, M.; Khan, S.; Kim, Y.; Park, J.K. Innovative production of bio-cellulose using a cell-free system derived from a single cell line. Carbohydr. Polym. 2015, 132, 286–294. [Google Scholar] [CrossRef]
- Lotfi, M.; Bagherzadeh, R.; Naderi-Meshkin, H.; Mahdipour, E.; Mafinezhad, A.; Sadeghnia, H.R.; Esmaily, H.; Maleki, M.; Hasssanzadeh, H.; Ghayaour-Mobarhan, M. Hybird Chitosan-β-glycerol Phosphate-gelatin Nano/Micro Fibrous Scaffolds with Suitable Mechanical and Biological Properties for Tissue Engineering. Biopolymers 2016, 105, 163–175. [Google Scholar] [CrossRef]
- Nelson, C.; Khan, Y.; Laurencin, C.T. Nanofiber–microsphere (nano-micro) Matrices for Bone Regenerative Engineering: A Convergence Approach Toward Matrix Design. Regen. Biomater. 2014, 1, 3–9. [Google Scholar] [CrossRef]
- Yin, N.; Stilwell, M.D.; Santos, T.M.A.; Wang, H.; Weibel, D.B. Agarose Particle-Templated Porous Bacterial Cellulose and Its Application in Cartilage Growth in Vitro. Acta Biomater. 2015, 12, 129–138. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Yang, F.; Shao, Y.; Zhang, X.; Dai, K. Modification and Evaluation of Micro-nano Structured Porous Bacterial Cellulose Scaffold for Bone Tissue Engineering. Mater. Sci. Eng. C 2017, 75, 1034–1041. [Google Scholar] [CrossRef]
- Zhu, C.; Li, F.; Zhou, X.; Lin, L.; Zhang, T. Kombucha-Synthesized Bacterial Cellulose: Preparation, Characterization, and Biocompatibility Evaluation. J. Biomed. Mater. Res. Part A 2014, 102, 1548–1557. [Google Scholar] [CrossRef]
- Gutiérrez-Hernández, J.M.; Escobar-García, D.M.; Escalante, A.; Flores, H.; González, F.J.; Gatenholm, P.; Toriz, G. In Vitro Evaluation of Osteoblastic Cells on Bacterial Cellulose Modified with Multi-walled Carbon Nanotubes as Scaffold for Bone Regeneration. Mater. Sci. Eng. C 2017, 75, 445–453. [Google Scholar] [CrossRef]
- Fujisawa, S.; Okita, Y.; Fukuzumi, H.; Saito, T.; Isogai, A. Preparation and Characterization of TEMPO-oxidized Cellulose Nanofibril Films with Free Carboxyl Groups. Carbohydr. Polym. 2011, 84, 579–583. [Google Scholar] [CrossRef]
- Urbina, L.; Algar, I.; García-Astrain, C.; Gabilondo, N.; González, A.; Corcuera, M.; Eceiza, A.; Retegi, A. Biodegradable Composites with Improved Barrier Properties and Transparency from the Impregnation of PLA to Bacterial Cellulose Membranes. J. Appl. Polym. Sci. 2016, 133, 258–261. [Google Scholar] [CrossRef]
- Bodin, A.; Bäckdahl, H.; Fink, H.; Gustafsson, L.; Risberg, B.; Gatenholm, P. Influence of Cultivation Conditions on Mechanical and Morphological Properties of Bacterial Cellulose Tubes. Biotechnol. Bioeng. 2010, 97, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, Y.; Chanzy, H. The Hydrogen Bond Network in Iβ, Cellulose as Observed by Infrared Spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Atalla, R.H.; Vanderhart, D.L. Native Cellulose: A Composite of Two Distinct Crystalline Forms. Science 1984, 223, 283–285. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Chen, S.; Feng, C.; Chen, S.; Yin, N.; Yang, J.; Wang, H.; Xu, Y. Facilely Green Synthesis of Silver Nanoparticles into Bacterial Cellulose. Cellulose 2015, 22, 373–383. [Google Scholar] [CrossRef]
- Tokoh, C.; Takabe, K.; Fujita, M.; Saiki, H. Cellulose Synthesized by Acetobacter Xylinum in the Presence of Acetyl Glucomannan. Cellulose 1998, 5, 249–261. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for es-timating the degree of crystallinity of native cellulose using the X-raydiffractometer. Text. Res. J. 1959, 29, 785–794. [Google Scholar] [CrossRef]
| Group | Porosity (%) |
|---|---|
| BC | 93.75 (p < 0.03) |
| PLA/BC-1 | 87.85 (p < 0.02) |
| PLA/BC-2 | 85.58 (p < 0.01) |
| PLA/BC-3 | 80.95 (p < 0.02) |
| CV (%) Group | HUVEC |
|---|---|
| BC | 146.78 (p < 0.03) |
| PLA/BC-1 | 111.71 (p < 0.01) |
| PLA/BC-2 | 121.48 (p < 0.01) |
| PLA/BC-3 | 120.92 (p < 0.02) |
| CV (%) Days of Culture/d | BC | PLA/BC-1 | PLA/BC-2 | PLA/BC-3 |
|---|---|---|---|---|
| 1 | 93.52 | 96.85 | 97.41 | 94.81 |
| 3 | 94.85 | 96.20 | 95.07 | 99.17 |
| 5 | 93.88 | 98.31 | 95.08 | 99.99 |
| 7 | 96.06 | 98.47 | 95.05 | 99.86 |
| RGR (%) | Grade of Toxicity |
|---|---|
| 80–100 | 0 |
| 60–79 | 1 |
| 40–59 | 2 |
| 20–39 | 3 |
| 0–19 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, Y.; Wang, F.; Huang, Y.; He, J. Preparation of 3D Printed Polylactic Acid/Bacterial Cellulose Composite Scaffold for Tissue Engineering Applications. Polymers 2022, 14, 4756. https://doi.org/10.3390/polym14214756
Wu Y, Wang Y, Wang F, Huang Y, He J. Preparation of 3D Printed Polylactic Acid/Bacterial Cellulose Composite Scaffold for Tissue Engineering Applications. Polymers. 2022; 14(21):4756. https://doi.org/10.3390/polym14214756
Chicago/Turabian StyleWu, Yadong, Yunfeng Wang, Fang Wang, Yudong Huang, and Jinmei He. 2022. "Preparation of 3D Printed Polylactic Acid/Bacterial Cellulose Composite Scaffold for Tissue Engineering Applications" Polymers 14, no. 21: 4756. https://doi.org/10.3390/polym14214756
APA StyleWu, Y., Wang, Y., Wang, F., Huang, Y., & He, J. (2022). Preparation of 3D Printed Polylactic Acid/Bacterial Cellulose Composite Scaffold for Tissue Engineering Applications. Polymers, 14(21), 4756. https://doi.org/10.3390/polym14214756








