Sponges from Plasma Treated Cellulose Nanofibers Grafted with Poly(ethylene glycol)methyl Ether Methacrylate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
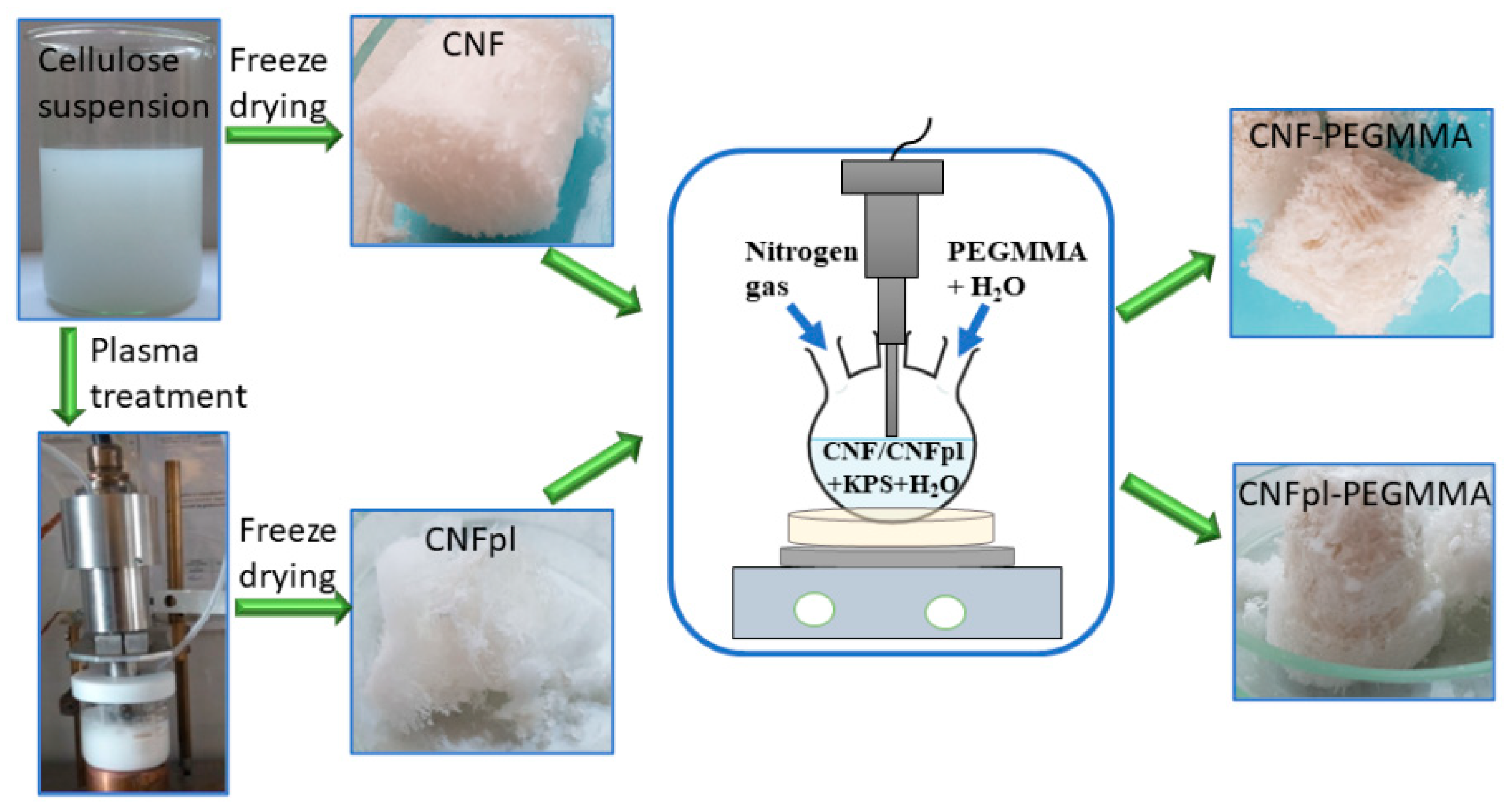
2.2.1. Plasma Treatment of Cellulose Suspension
2.2.2. Grafting Reaction
2.2.3. Characterization
3. Results and Discussion
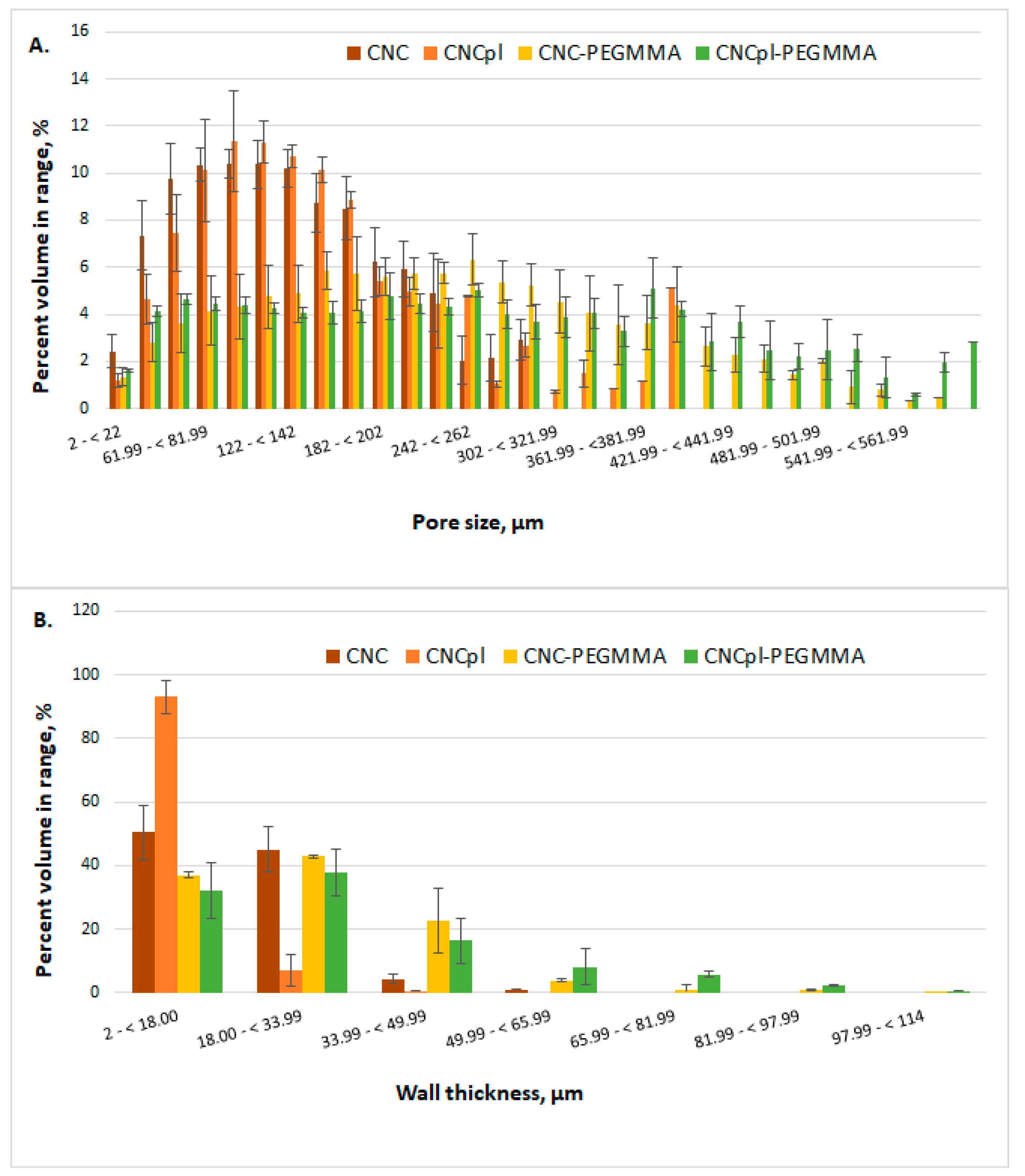
3.1. Morphological Investigation
3.2. Structural Investigation
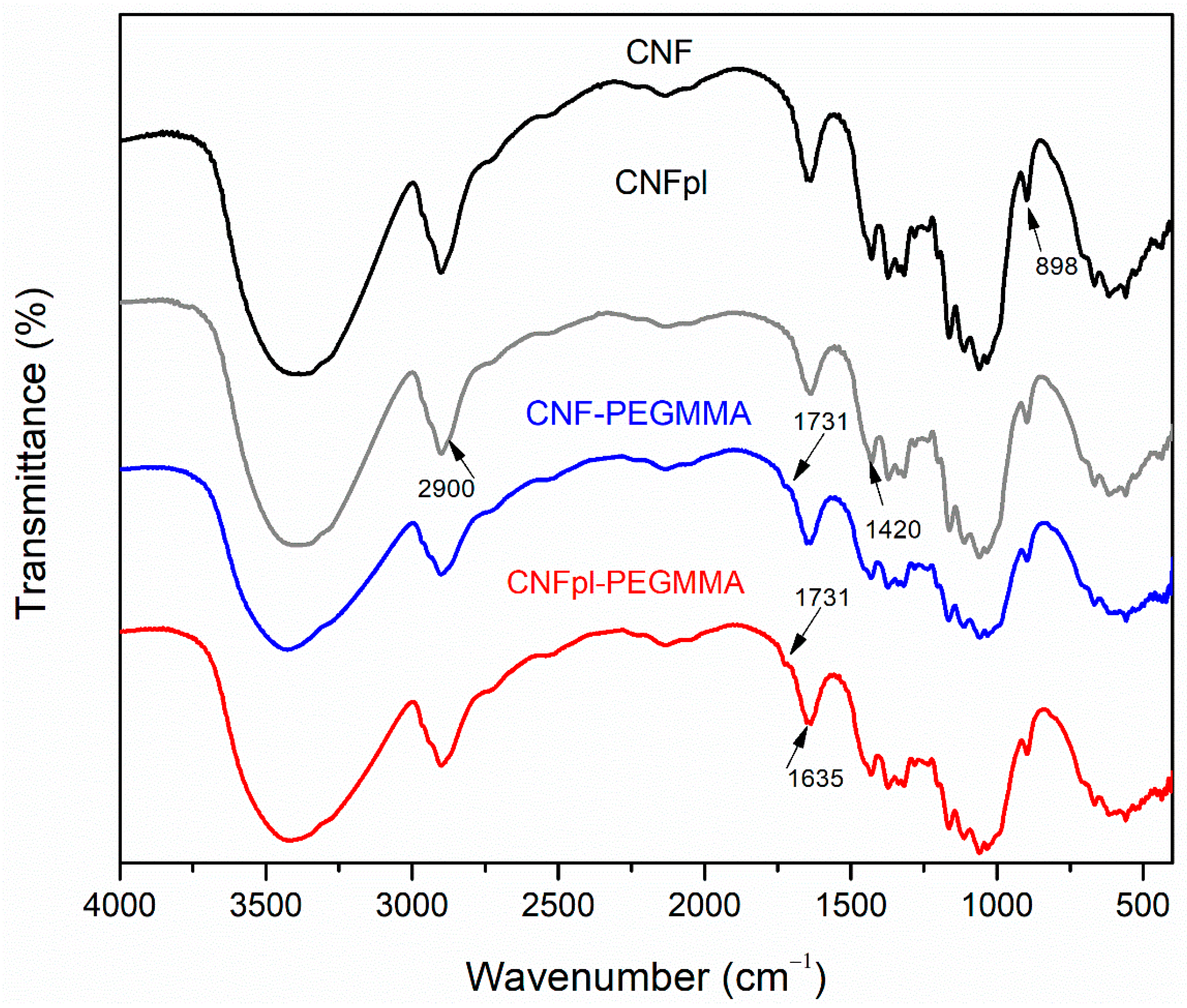
3.2.1. FTIR Spectroscopy
3.2.2. Contact Angle Analysis
3.2.3. Investigation of the Crystalline Structure by XRD
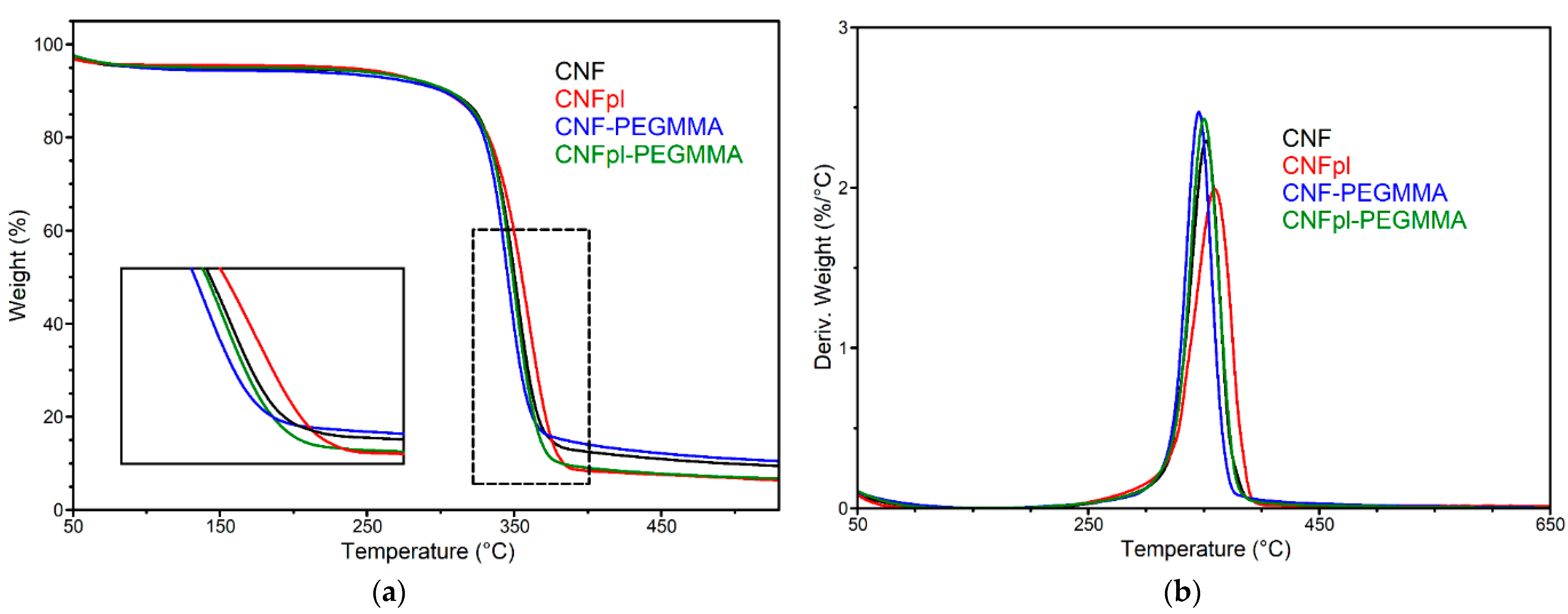
3.3. Thermal Stability
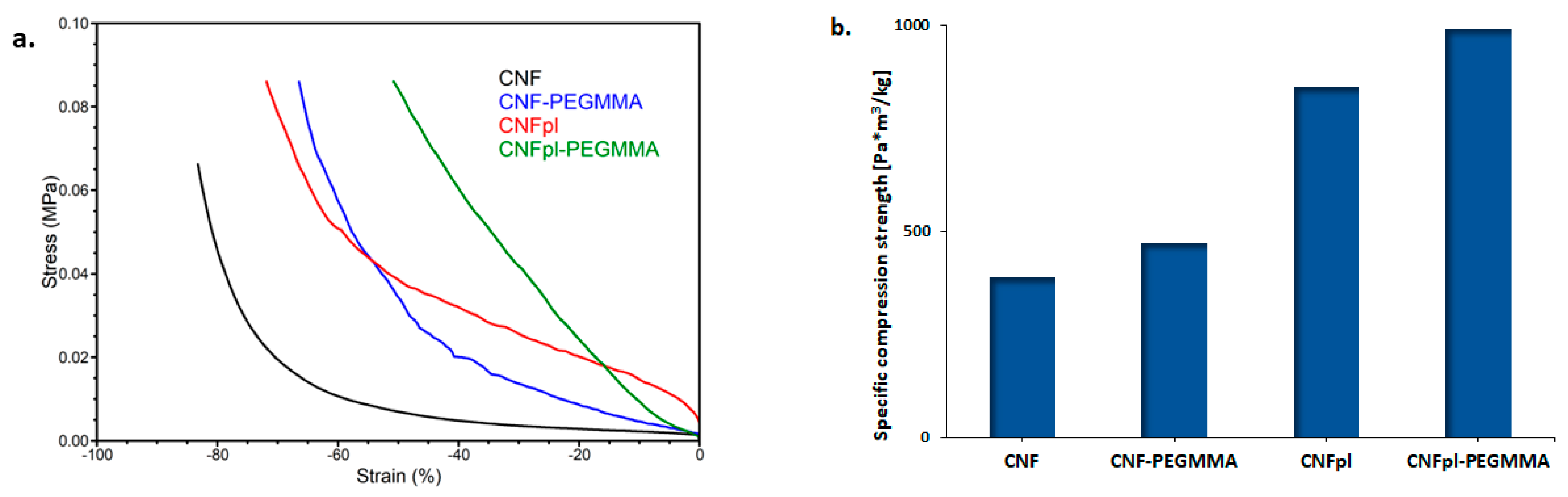
3.4. Mechanical Properties
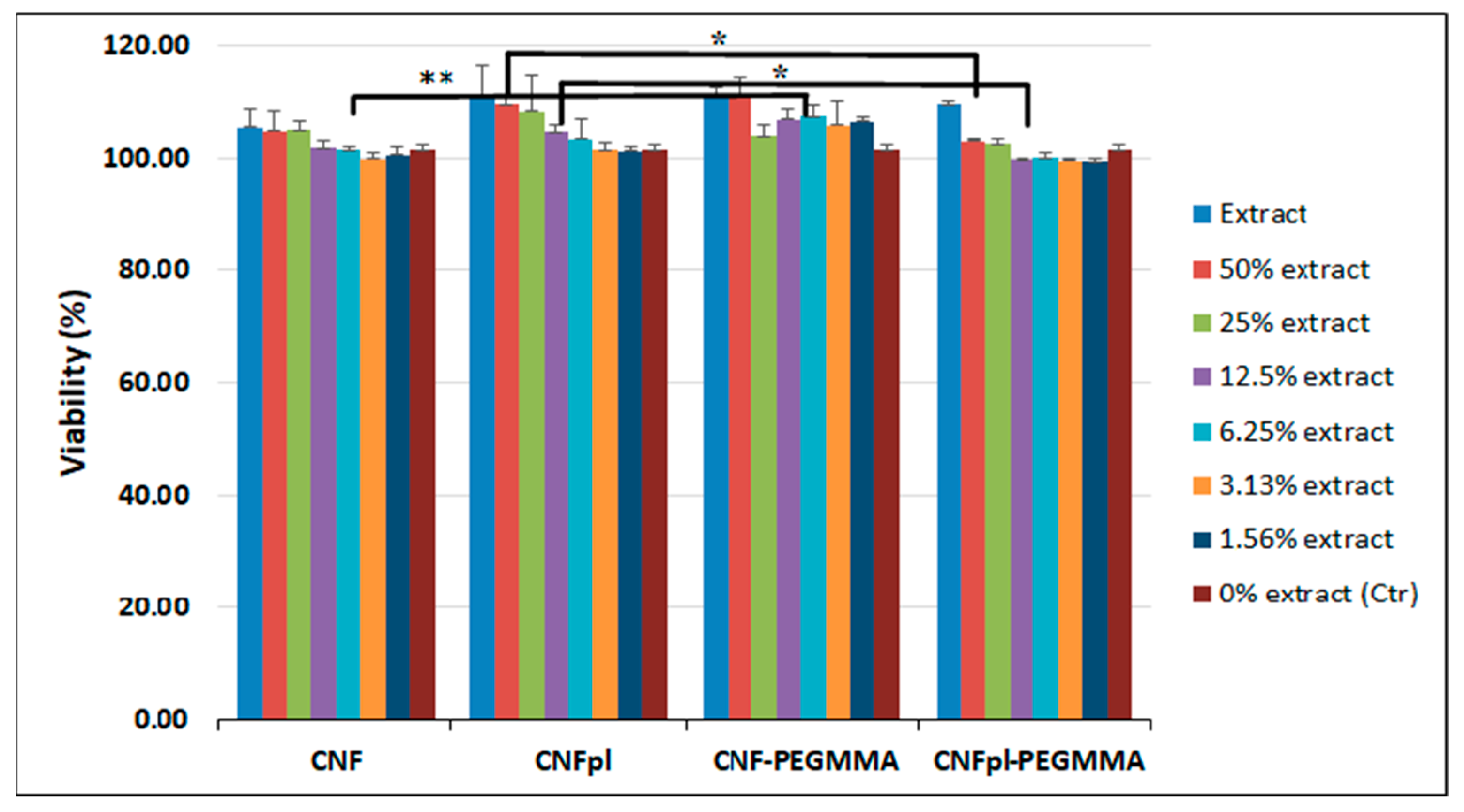
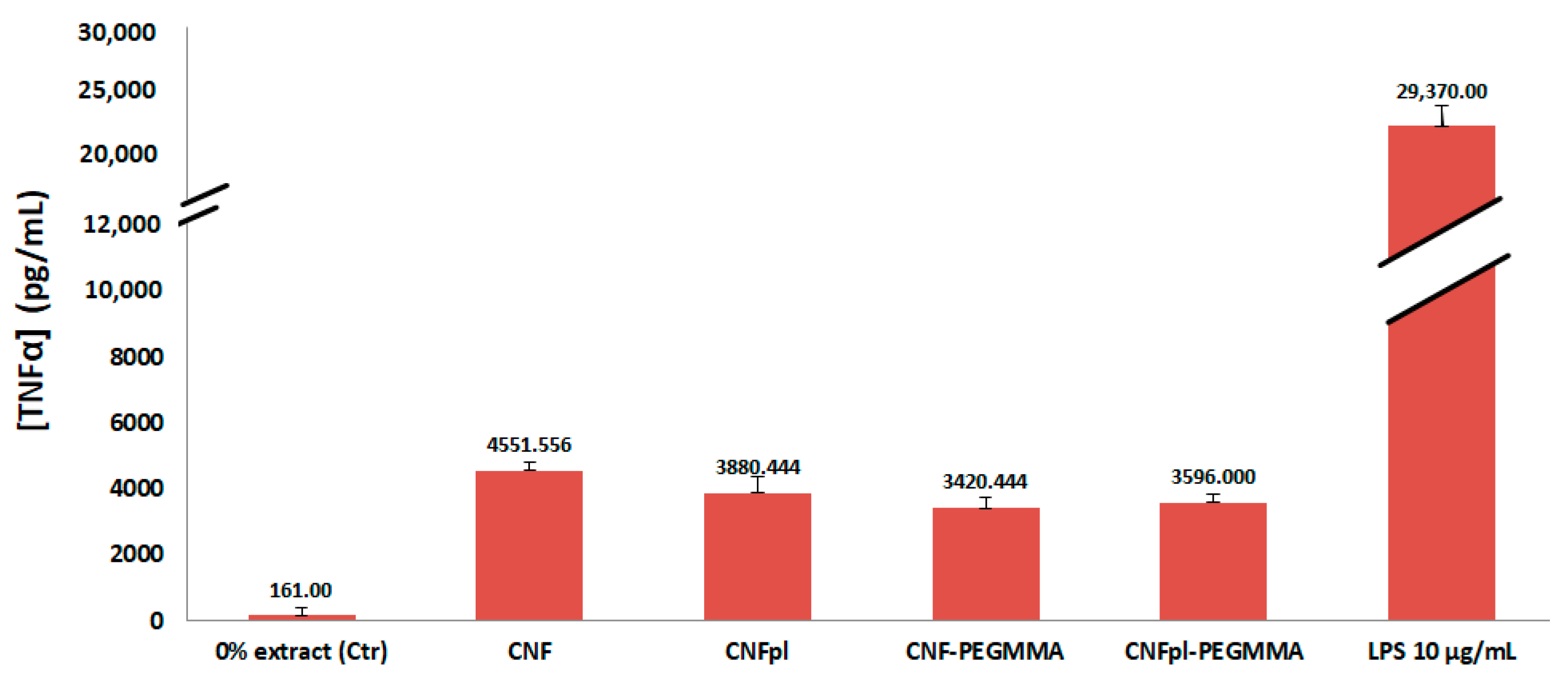
3.5. Biocompatibility Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Randhawa, A.; Dutta, S.D.; Ganguly, K.; Patil, T.V.; Patel, D.K.; Lim, K.T. A Review of Properties of Nanocellulose, Its Synthesis, and Potential in Biomedical Applications. Appl. Sci. 2022, 12, 7090. [Google Scholar] [CrossRef]
- Bretel, G.; Rull-Barrull, J.; Nongbe, M.C.; Terrier, J.-P.; Le Grognec, E.; Felpin, F.-X. Hydrophobic Covalent Patterns on Cellulose Paper through Photothiol-X Ligations. ACS Omega 2018, 3, 9155–9159. [Google Scholar] [CrossRef] [PubMed]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, Z.; Wu, Q.; Kuang, Y. Acetylated cellulose nanocrystals with high-crystallinity obtained by one-step reaction from the traditional acetylation of cellulose. Carbohydr. Polym. 2019, 229, 115553. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sanchez-Salvador, J.L.; Balea, A.; Blanco, A.; Negro, C. Optimization of reagent consumption in TEMPO-mediated oxidation of Eucalyptus cellulose to obtain cellulose nanofibers. Cellulose 2022, 29, 6611–6627. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Laitinen, O.; Ämmälä, A.; Liimatainen, H. Amino-modified cellulose nanocrystals with adjustable hydrophobicity from combined regioselective oxidation and reductive amination. Carbohydr. Polym. 2016, 136, 581–587. [Google Scholar] [CrossRef]
- Wang, Y.; Lwal, A.L.J.; Wang, Q.; Zhou, J.; Dufresne, A.; Lin, N. Regulating surface sulfonation on cellulose nanocrystals and self-assembly behaviors. Chem. Commun. 2020, 56, 10958–10961. [Google Scholar] [CrossRef]
- De Nino, A.; Tallarida, M.A.; Algieri, V.; Olivito, F.; Costanzo, P.; De Filpo, G.; Maiuolo, L. Sulfonated Cellulose-Based Magnetic Composite as Useful Media for Water Remediation from Amine Pollutants. Appl. Sci. 2020, 10, 8155. [Google Scholar] [CrossRef]
- Wohlhauser, S.; Delepierre, G.; Labet, M.; Morandi, G.; Thielemans, W.; Weder, C.; Zoppe, J.O. Grafting Polymers from Cellulose Nanocrystals: Synthesis, Properties, and Applications. Macromolecules 2018, 51, 6157–6189. [Google Scholar] [CrossRef] [Green Version]
- Gök, Ö.; Alkan, C. Poly(ethylene glycol)s grafted celluloses as solid–solid phase change materials for different thermal energy storage application temperatures and through isophorone linkage. J. Therm. Anal. 2020, 146, 1511–1523. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Wang, A.; Li, Y.; Yu, F.; Chen, Y. Polyethylene glycol-grafted cellulose-based gel polymer electrolyte for long-life Li-ion batteries. Appl. Surf. Sci. 2022, 593, 153411. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Dong, L.; Ren, S.; Wu, Q.; Lei, T. Thermoresponsive poly(poly(ethylene glycol) methylacrylate)s grafted cellulose nanocrystals through SI-ATRP polymerization. Cellulose 2017, 24, 4189–4203. [Google Scholar] [CrossRef]
- Macke, N.; Hemmingsen, C.M.; Rowan, S.J. The effect of polymer grafting on the mechanical properties of PEG -grafted cellulose nanocrystals in poly(lactic acid). J. Appl. Polym. Sci. 2022. [Google Scholar] [CrossRef]
- Singam, A.; Killi, N.; Patel, P.R.; Gundloori, R.V.N. PEGylated ethyl cellulose micelles as a nanocarrier for drug delivery. RSC Adv. 2021, 11, 30532–30543. [Google Scholar] [CrossRef] [PubMed]
- Chiulan, I.; Panaitescu, D.M.; Radu, E.-R.; Vizireanu, S.; Sătulu, V.; Biţă, B.; Gabor, R.A.; Nicolae, C.A.; Raduly, M.; Rădiţoiu, V. Influence of TEMPO oxidation on the properties of ethylene glycol methyl ether acrylate grafted cellulose sponges. Carbohydr. Polym. 2021, 272, 118458. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, D.M.; Vizireanu, S.; Nicolae, C.A.; Frone, A.N.; Casarica, A.; Carpen, L.G.; Dinescu, G. Treatment of Nanocellulose by Submerged Liquid Plasma for Surface Functionalization. Nanomaterials 2018, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Couturaud, B.; Baldo, A.; Mas, A.; Robin, J.J. Improvement of the interfacial compatibility between cellulose and poly(l-lactide) films by plasma-induced grafting of l-lactide: The evaluation of the adhesive properties using a peel test. J. Colloid Interface Sci. 2015, 448, 427–436. [Google Scholar] [CrossRef]
- Pertile, R.A.; Andrade, F.K.; Alves, C., Jr.; Gama, M. Surface modification of bacterial cellulose by nitrogen-containing plasma for improved interaction with cells. Carbohydr. Polym. 2010, 82, 692–698. [Google Scholar] [CrossRef]
- Lusi, A.; Hu, H.; Bai, X. Producing high yield of levoglucosan by pyrolyzing nonthermal plasma-pretreated cellulose. Green Chem. 2020, 22, 2036–2048. [Google Scholar]
- Czaderna-Lekka, A.; Kozanecki, M.; Matusiak, M.; Kadlubowski, S. Phase transitions of poly(oligo(ethylene glycol) methyl ether methacrylate)-water systems. Polymer 2021, 212, 123247. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Park, B.-D. Optimum oxidation for direct and efficient extraction of carboxylated cellulose nanocrystals from recycled MDF fibers by ammonium persulfate. Carbohydr. Polym. 2020, 251, 117029. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I.; Casarica, A.; Nicolae, C.A.; Ghiurea, M.; Trusca, R.; Damian, C.M. Structural and morphological characterization of bacterial cellulose nano-reinforcements prepared by mechanical route. Mater. Des. 2016, 110, 790–801. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zeni, M.; Zattera, A.J. Crystalline properties and decomposition kinetics of cellulose fibers in wood pulp obtained by two pulping processes. Polym. Degrad. Stab. 2011, 96, 679–685. [Google Scholar] [CrossRef]
- Vizireanu, S.; Panaitescu, D.M.; Nicolae, C.A.; Frone, A.N.; Chiulan, I.; Ionita, M.D.; Satulu, V.; Carpen, L.G.; Petrescu, S.; Birjega, R.; et al. Cellulose defibrillation and functionalization by plasma in liquid treatment. Sci. Rep. 2018, 8, 15473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radakisnin, R.; Majid, M.S.A.; Jamir, M.R.M.; Tahir, M.F.M.; Meng, C.E.; Al Alshahrani, H. Physical, thermal, and mechanical properties of highly porous polylactic acid/cellulose nanofibre scaffolds prepared by salt leaching technique. Nanotechnol. Rev. 2021, 10, 1469–1483. [Google Scholar] [CrossRef]
- Yang, Q.; Su, W.; Hu, J.; Xu, Y.; Liu, Z.; Hui, L. Synthesis of Superhydrophobic Cellulose Stearoyl Ester for Oil/Water Separation. Nanomaterials 2022, 12, 1964. [Google Scholar] [CrossRef]
- Li, Q.; Renneckar, S. Supramolecular structure characterization of molecularly thin cellulose I nanoparticles. Biomacromolecules 2011, 12, 650–659. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar]
- Wang, Y.-X.; Xin, Y.; Yin, J.-Y.; Huang, X.-J.; Wang, J.-Q.; Hu, J.-L.; Geng, F.; Nie, S.-P. Revealing the architecture and solution properties of polysaccharide fractions from Macrolepiota albuminosa (Berk.) Pegler. Food Chem. 2022, 368, 130772. [Google Scholar] [CrossRef] [PubMed]
- Sinha, E.; Panigrahi, S. Effect of Plasma Treatment on Structure, Wettability of Jute Fiber and Flexural Strength of its Composite. J. Compos. Mater. 2009, 43, 1791–1802. [Google Scholar] [CrossRef]
- Hernández-Varela, J.D.; Chanona-Pérez, J.J.; Benavides, H.A.C.; Sodi, F.C.; Vicente-Flores, M. Effect of ball milling on cellulose nanoparticles structure obtained from garlic and agave waste. Carbohydr. Polym. 2020, 255, 117347. [Google Scholar] [CrossRef]
- Abouzayed, F.I.; El-Nassr, N.T.A.; Abouel-Enein, S.A. Synthesis, characterization of functionalized grafted cellulose and its environmental application in uptake of copper (II), manganese (II) and iron (III) ions. J. Mol. Struct. 2022, 1270, 133907. [Google Scholar] [CrossRef]
- Neto, J.S.S.; de Queiroz, H.F.M.; Aguiar, R.A.A.; Banea, M.D. A Review on the Thermal Characterisation of Natural and Hybrid Fiber Composites. Polymers 2021, 13, 4425. [Google Scholar] [CrossRef]
- da Silva, L.P.; Cerqueira, M.T.; Sousa, R.A.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Engineering cell-adhesive gellan gum spongy-like hydrogels for regenerative medicine purposes. Acta Biomater. 2014, 10, 4787–4797. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, M.; Shi, T.; Chen, H.; Wang, H. Preparation of Nanocellulose Aerogel from the Poplar (Populus tomentosa) Catkin Fiber. Forests 2019, 10, 749. [Google Scholar] [CrossRef] [Green Version]
- Osorio, D.A.; Lee, B.E.J.; Kwiecien, J.M.; Wang, X.; Shahid, I.; Hurley, A.L.; Cranston, E.D.; Grandfield, K. Cross-linked cellulose nanocrystal aerogels as viable bone tissue scaffolds. Acta Biomater. 2019, 87, 152–165. [Google Scholar] [CrossRef]
- Surendran, G.; Sherje, A.P. Cellulose nanofibers and composites: An insight into basics and biomedical applications. J. Drug Deliv. Sci. Technol. 2022, 75, 103601. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Yang, L.; Wei, Y.; Wang, X.; Wang, Z.; Tao, L. Cytotoxicity study of polyethylene glycol derivatives. RSC Adv. 2017, 7, 18252–18259. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Chian, K.S.; Chan-Park, M.B.E.; Lee, S.T. Effect of argon-plasma treatment on proliferation of human-skin–derived fibroblast on chitosan membrane in vitro. J. Biomed. Mater. Res. Part A 2005, 73, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Techaikool, P.; Daranarong, D.; Kongsuk, J.; Boonyawan, D.; Haron, N.; Harley, W.S.; Thomson, K.A.; Foster, L.J.R.; Punyodom, W. Effects of plasma treatment on biocompatibility of poly[(L-lactide)-co-(ϵ-caprolactone)] and poly[(L-lactide)-co-glycolide] electrospun nanofibrous membranes. Polym. Int. 2017, 66, 1640–1650. [Google Scholar] [CrossRef]
- Park, G.H.; Ko, T.-J.; Min, H.S.; Kim, M.S.; Kim, J.-A.; Jeon, B.; Kim, Y.; Huang, Y.; Jin, X.; Wufuer, M.; et al. The cytotoxicity and skin irritation of nanostructured cellulose surface fabricated by a plasma-induced method. Cellulose 2019, 26, 9737–9749. [Google Scholar] [CrossRef]
- Fahmi, M.Z.; Haris, A.; Permana, A.J.; Wibowo, D.L.N.; Purwanto, B.; Nikmah, Y.L.; Idris, A. Bamboo leaf-based carbon dots for efficient tumor imaging and therapy. RSC Adv. 2018, 8, 38376–38383. [Google Scholar] [CrossRef] [PubMed]




| Sample | d110, nm | d200, nm | D110, nm | D200, nm | C, % |
|---|---|---|---|---|---|
| CNF | 0.548 | 0.392 | 4.122 | 4.718 | 79.5 |
| CNFpl | 0.541 | 0.394 | 4.122 | 4.162 | 73.8 |
| CNF-PEGMMA | 0.543 | 0.394 | 4.198 | 4.717 | 75.0 |
| CNFpl-PEGMMA | 0.544 | 0.394 | 4.105 | 4.288 | 75.9 |
| Sample | T5% (°C) | Tonset (°C) | Tmax (°C) | R700 °C |
|---|---|---|---|---|
| CNF | 103.2 | 331.5 | 351.9 | 8.2 |
| CNFpl | 232.8 | 331.0 | 359.4 | 3.9 |
| CNF-PEGMMA | 95.7 | 328.6 | 345.7 | 9.2 |
| CNFpl-PEGMMA | 184.4 | 331.3 | 350.2 | 5.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiulan, I.; Panaitescu, D.M.; Serafim, A.; Radu, E.R.; Ioniţă, G.; Rădiţoiu, V.; Gabor, A.R.; Nicolae, C.-A.; Ghiurea, M.; Baciu, D.D. Sponges from Plasma Treated Cellulose Nanofibers Grafted with Poly(ethylene glycol)methyl Ether Methacrylate. Polymers 2022, 14, 4720. https://doi.org/10.3390/polym14214720
Chiulan I, Panaitescu DM, Serafim A, Radu ER, Ioniţă G, Rădiţoiu V, Gabor AR, Nicolae C-A, Ghiurea M, Baciu DD. Sponges from Plasma Treated Cellulose Nanofibers Grafted with Poly(ethylene glycol)methyl Ether Methacrylate. Polymers. 2022; 14(21):4720. https://doi.org/10.3390/polym14214720
Chicago/Turabian StyleChiulan, Ioana, Denis Mihaela Panaitescu, Andrada Serafim, Elena Ruxandra Radu, Gabriela Ioniţă, Valentin Rădiţoiu, Augusta Raluca Gabor, Cristian-Andi Nicolae, Marius Ghiurea, and Dora Domnica Baciu. 2022. "Sponges from Plasma Treated Cellulose Nanofibers Grafted with Poly(ethylene glycol)methyl Ether Methacrylate" Polymers 14, no. 21: 4720. https://doi.org/10.3390/polym14214720
APA StyleChiulan, I., Panaitescu, D. M., Serafim, A., Radu, E. R., Ioniţă, G., Rădiţoiu, V., Gabor, A. R., Nicolae, C.-A., Ghiurea, M., & Baciu, D. D. (2022). Sponges from Plasma Treated Cellulose Nanofibers Grafted with Poly(ethylene glycol)methyl Ether Methacrylate. Polymers, 14(21), 4720. https://doi.org/10.3390/polym14214720










