Composite Fibers Based on Polycaprolactone and Calcium Magnesium Silicate Powders for Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Powder Synthesis
2.2. Composites Preparation
2.3. Investigation Techniques
2.3.1. Physico-Chemical Characterization
2.3.2. Biological Evaluation
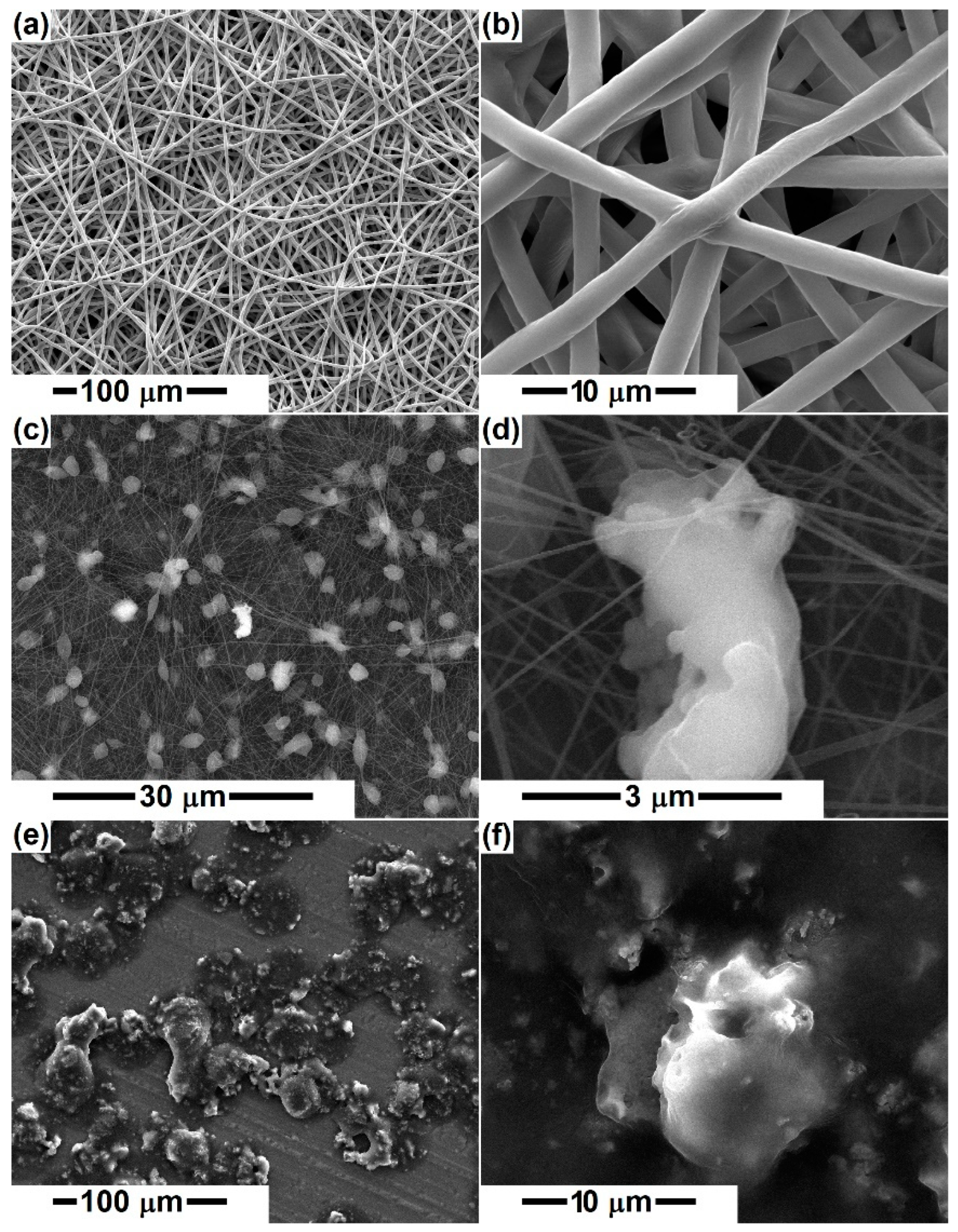
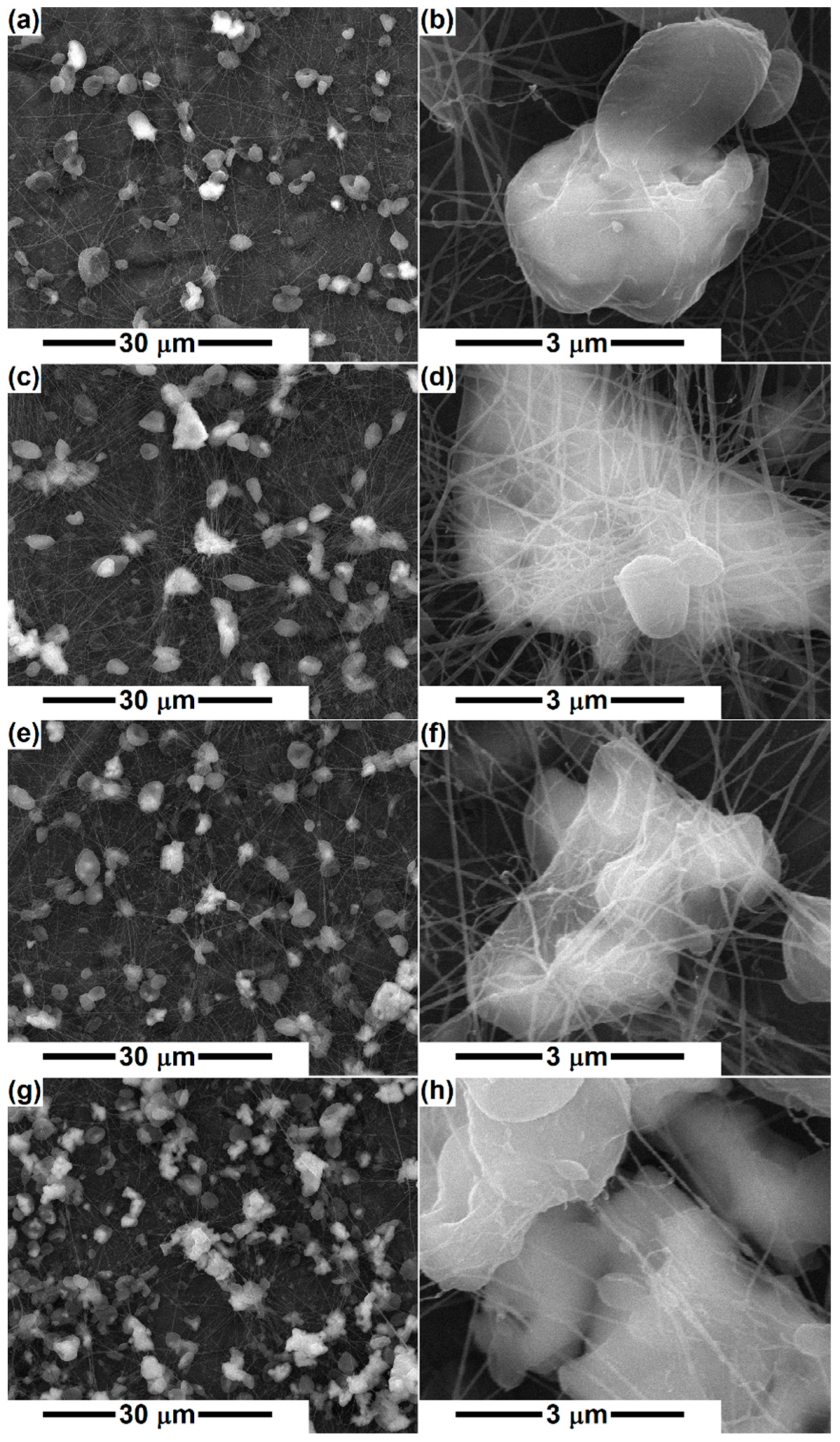
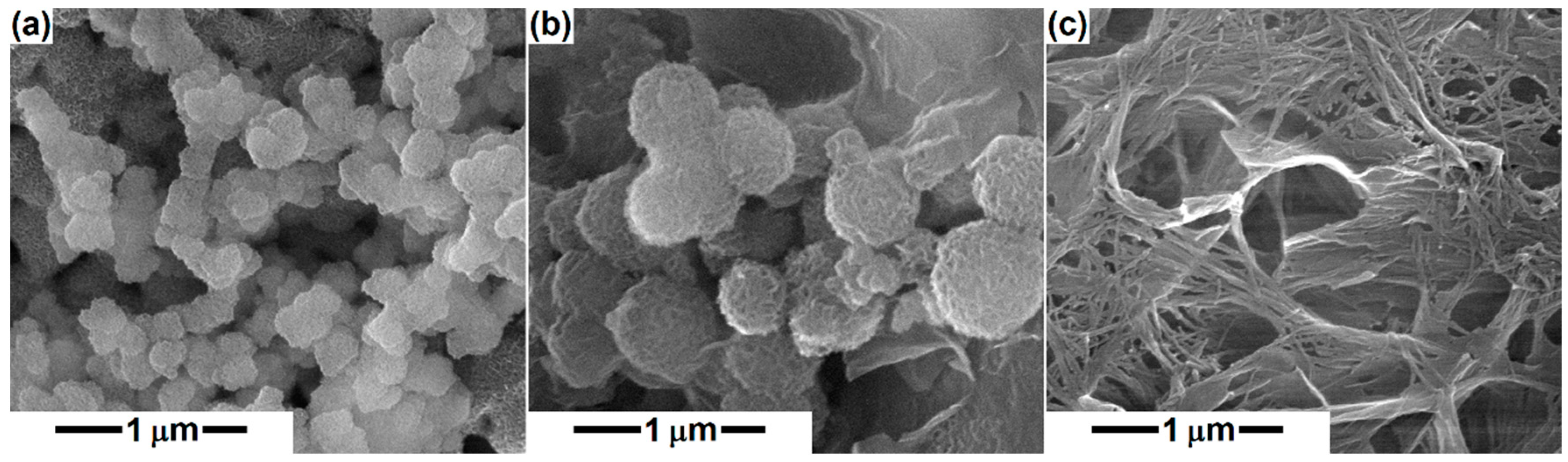
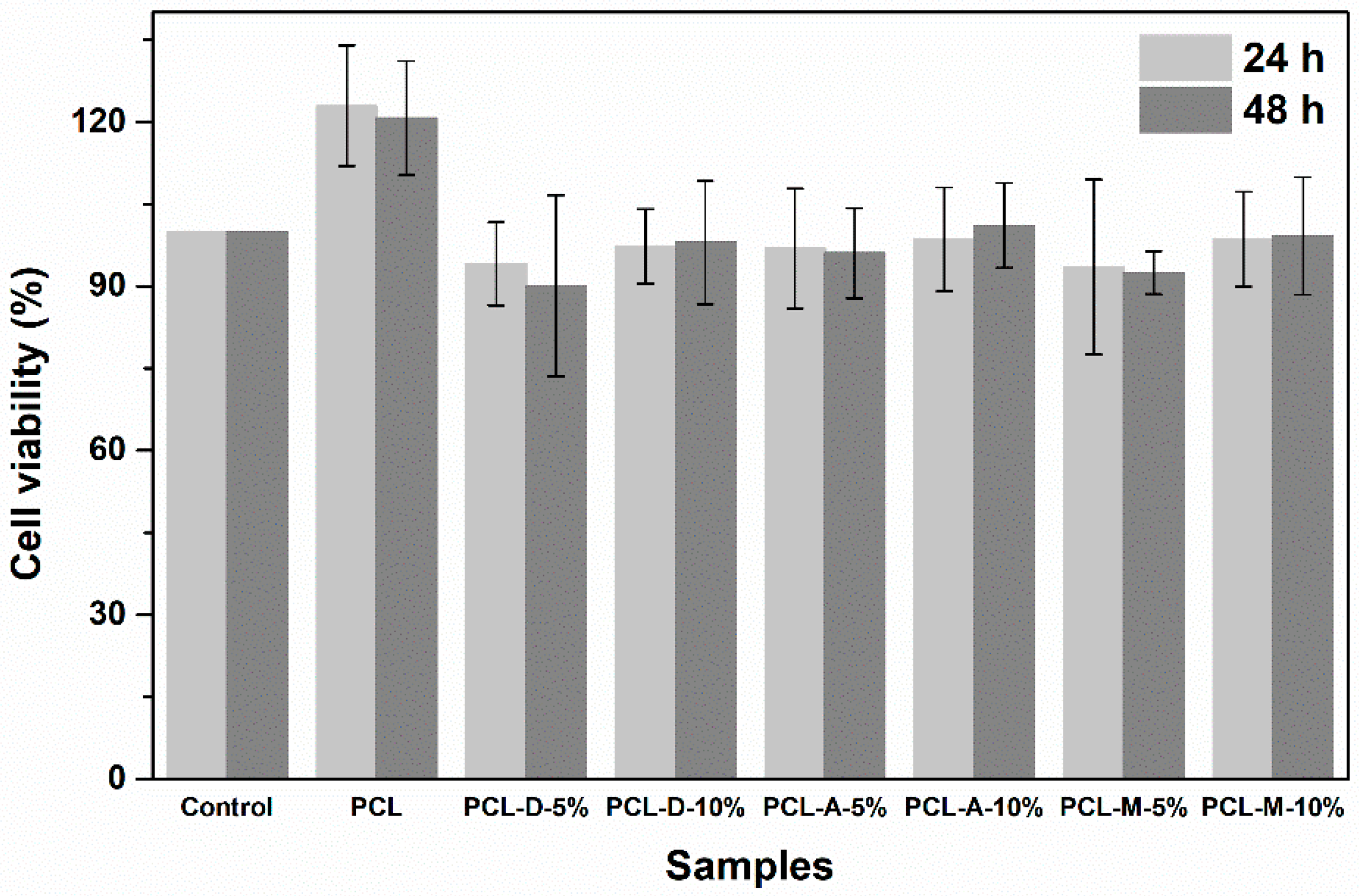
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinto, A.R.; Reis, R.L.; Neves, N.M. Scaffolds Based Bone Tissue Engineering: The Role of Chitosan. Tissue Eng. Part B Rev. 2011, 17, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Zamparini, F.; Degli Esposti, M.; Chiellini, F.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Highly Porous Polycaprolactone Scaffolds Doped with Calcium Silicate and Dicalcium Phosphate Dihydrate Designed for Bone Regeneration. Mater. Sci. Eng. C 2019, 102, 341–361. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Bahremandi-Toloue, E.; Karbasi, S. Recent Advances in Modification Strategies of Pre- and Post-Electrospinning of Nanofiber Scaffolds in Tissue Engineering. React. Funct. Polym. 2022, 172, 105202. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, V.; Baino, F.; Mauro, J.C.; Pickrell, G.; Evans, I.; Bretcanu, O. Mechanical Properties of Bioactive Glasses, Ceramics, Glass-Ceramics and Composites: State-of-the-Art Review and Future Challenges. Mater. Sci. Eng. C 2019, 104, 109895. [Google Scholar] [CrossRef]
- Arastouei, M.; Khodaei, M.; Atyabi, S.M.; Jafari Nodoushan, M. Poly Lactic Acid-Akermanite Composite Scaffolds Prepared by Fused Filament Fabrication for Bone Tissue Engineering. J. Mater. Res. Technol. 2020, 9, 14540–14548. [Google Scholar] [CrossRef]
- Salernitano, E.; Migliaresi, C. Composite Materials for Biomedical Applications: A Review. J. Appl. Biomater. Biomech. 2003, 1, 3–18. [Google Scholar]
- Sadeghzade, S.; Emadi, R.; Tavangarian, F.; Doostmohammadi, A. In Vitro Evaluation of Diopside/Baghdadite Bioceramic Scaffolds Modified by Polycaprolactone Fumarate Polymer Coating. Mater. Sci. Eng. C 2020, 106, 110176. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Sriramulu, M.; Shanmugam, S.; Drweesh, E.A.; Elnagar, M.M.; Mosa, E.S.; Sasikumar, S. Solution Combustion Synthesis of Functional Diopside, Akermanite, and Merwinite Bioceramics: Excellent Biomineralization, Mechanical Strength, and Antibacterial Ability. Mater. Today Commun. 2021, 27, 102365. [Google Scholar] [CrossRef]
- Duman, Ş. Effect of Akermanite Powders on Mechanical Properties and Bioactivity of Chitosan-Based Scaffolds Produced by 3D-Bioprinting. Ceram. Int. 2021, 10, 13912–13921. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Hashemi Beni, B.; Vashaee, D.; Tayebi, L. Nanostructured Merwinite Bioceramic Coating on Mg Alloy Deposited by Electrophoretic Deposition. Ceram. Int. 2014, 40, 9473–9484. [Google Scholar] [CrossRef]
- Tavangarian, F.; Zolko, C.A.; Davami, K. Synthesis, Characterization and Formation Mechanisms of Nanocrystalline Akermanite Powder. J. Mater. Res. Technol. 2021, 11, 792–800. [Google Scholar] [CrossRef]
- Tavangarian, F. Facile Synthesis and Structural Insight of Nanostructure Akermanite Powder. Ceram. Int. 2019, 7, 7871–7877. [Google Scholar] [CrossRef]
- Praharaj, S.; Venkatraman, S.K.; Vasantharaman, R.; Swamiappan, S. Sol-Gel Combustion Synthesis of Merwinite and Its Biomedical Applications. Mater. Lett. 2021, 300, 130108. [Google Scholar] [CrossRef]
- Sherikar, B.N.; Sahoo, B.; Umarji, A.M. One-Step Synthesis of Diopside (CaMgSi2O6) Ceramic Powder by Solution Combustion Method. Adv. Powder Technol. 2020, 31, 3492–3499. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R. Coating Biodegradable Magnesium Alloys with Electrospun Poly-L-Lactic Acid-Åkermanite-Doxycycline Nanofibers for Enhanced Biocompatibility, Antibacterial Activity, and Corrosion Resistance. Coat. Technol. 2019, 14, 124898. [Google Scholar] [CrossRef]
- Choudhary, R.; Venkatraman, S.K.; Chatterjee, A.; Vecstaudza, J.; Yáñez-Gascón, M.J.; Pérez-Sánchez, H.; Locs, J.; Abraham, J.; Swamiappan, S. Biomineralization, Antibacterial Activity and Mechanical Properties of Biowaste Derived Diopside Nanopowders. Adv. Powder Technol. 2019, 30, 1950–1964. [Google Scholar] [CrossRef]
- Abdollahi, S.; Paryab, A.; Khalilifard, R.; Anousheh, M.; Malek Khachatourian, A. The Fabrication and Characterization of Bioactive Akermanite/Octacalcium Phosphate Glass-Ceramic Scaffolds Produced via PDC Method. Ceram. Int. 2021, 47, 6653–6662. [Google Scholar] [CrossRef]
- Sayed, M.; Mahmoud, E.M.; Bondioli, F.; Naga, S.M. Developing Porous Diopside/Hydroxyapatite Bio-Composite Scaffolds via a Combination of Freeze-Drying and Coating Process. Ceram. Int. 2019, 45, 9025–9031. [Google Scholar] [CrossRef]
- Goudouri, O.M.; Theodosoglou, E.; Kontonasaki, E.; Will, J.; Chrissafis, K.; Koidis, P.; Paraskevopoulos, K.M.; Boccaccini, A.R. Development of Highly Porous Scaffolds Based on Bioactive Silicates for Dental Tissue Engineering. Mater. Res. Bull. 2014, 49, 399–404. [Google Scholar] [CrossRef]
- Myat-Htun, M. Enhanced Sinterability and in Vitro Bioactivity of Barium-Doped Akermanite Ceramic. Ceram. Int. 2020, 7, 19062–19068. [Google Scholar] [CrossRef]
- Hosseini, Y.; Emadi, R.; Kharaziha, M. Surface Modification of PCL-Diopside Fibrous Membrane via Gelatin Immobilization for Bone Tissue Engineering. Mater. Chem. Phys. 2017, 194, 356–366. [Google Scholar] [CrossRef]
- Shahrouzifar, M.R.; Salahinejad, E.; Sharifi, E. Co-Incorporation of Strontium and Fluorine into Diopside Scaffolds: Bioactivity, Biodegradation and Cytocompatibility Evaluations. Mater. Sci. Eng. C 2019, 103, 109752. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, E.A.; Ahmed, H.Y.; Farag, M.M. Combination of Na-Ca-Phosphate and Fluorapatite in Wollastonite-Diopside Glass-Ceramic: Degradation and Biocompatibility. J. Non-Cryst. Solids 2021, 566, 120888. [Google Scholar] [CrossRef]
- Yamagata, C.; Leme, D.R.; Rodrigues, V.G.; Eretides, G.T.; Dorion Rodas, A.C. Three Routes for the Synthesis of the Bioceramic Powder of the CaO-MgO-SiO2 System. Ceram. Int. 2022, 48, 9681–9691. [Google Scholar] [CrossRef]
- Feng, J.; Wu, D.; Long, M.; Lei, K.; Sun, Y.; Zhao, X. Diopside Glass-Ceramics Were Fabricated by Sintering the Powder Mixtures of Waste Glass and Kaolin. Ceram. Int. 2022, 48, 27088–27096. [Google Scholar] [CrossRef]
- Srinath, P.; Abdul Azeem, P.; Venugopal Reddy, K.; Chiranjeevi, P.; Bramanandam, M.; Prasada Rao, R. A Novel Cost-Effective Approach to Fabricate Diopside Bioceramics: A Promising Ceramics for Orthopedic Applications. Adv. Powder Technol. 2021, 32, 875–884. [Google Scholar] [CrossRef]
- Siqueira, I.A.W.B.; de Moura, N.K.; de Barros Machado, J.P.; Backes, E.H.; Roberto Passador, F.; de Sousa Trichês, E. Porous Membranes of the Polycaprolactone (PCL) Containing Calcium Silicate Fibers for Guided Bone Regeneration. Mater. Lett. 2017, 206, 210–213. [Google Scholar] [CrossRef]
- Torres, E.; Fombuena, V.; Vallés-Lluch, A.; Ellingham, T. Improvement of Mechanical and Biological Properties of Polycaprolactone Loaded with Hydroxyapatite and Halloysite Nanotubes. Mater. Sci. Eng. C 2017, 75, 418–424. [Google Scholar] [CrossRef]
- Chunyan, Z.; Lan, C.; Jiajia, L.; Dongwei, S.; Jun, Z.; Huinan, L. In Vitro Evaluation of Degradation, Cytocompatibility and Antibacterial Property of Polycaprolactone/Hydroxyapatite Composite Coating on Bioresorbable Magnesium Alloy. J. Magnes. Alloys 2021, 10, 2252–2265. [Google Scholar] [CrossRef]
- Mendes Soares, I.P.; Anselmi, C.; Kitagawa, F.A.; de Ribeiro, R.A.O.; Leite, M.L.; de Souza Costa, C.A.; Hebling, J. Nano-Hydroxyapatite-Incorporated Polycaprolactone Nanofibrous Scaffold as a Dentin Tissue Engineering-Based Strategy for Vital Pulp Therapy. Dent. Mater. 2022, 38, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A.; Shkarina, S.; Syromotina, D.S.; Melnik, E.V.; Shkarin, R.; Selezneva, I.I.; Ermakov, A.M.; Ivlev, S.I.; Cecilia, A.; Weinhardt, V.; et al. Characterization of Biomimetic Silicate- and Strontium-Containing Hydroxyapatite Microparticles Embedded in Biodegradable Electrospun Polycaprolactone Scaffolds for Bone Regeneration. Eur. Polym. J. 2019, 113, 67–77. [Google Scholar] [CrossRef]
- Sadeghi, A.; Razavi, S.M.A.; Shahrampour, D. Fabrication and Characterization of Biodegradable Active Films with Modified Morphology Based on Polycaprolactone-Polylactic Acid-Green Tea Extract. Int. J. Biol. Macromol. 2022, 205, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, J.; Fang, S.; Wang, H.; Bai, Y.; Zhao, Z.; Zhu, Q.; Wang, C.; Chen, G.; Jiang, H.; et al. Effect of Polycaprolactone Impregnation on the Properties of Calcium Silicate Scaffolds Fabricated by 3D Printing. Mater. Des. 2022, 220, 110856. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Gao, G.; Yin, X.; Pu, X.; Shi, B.; Liu, Y.; Huang, Z.; Wang, J.; Li, J.; et al. MBG/PGA-PCL Composite Scaffolds Provide Highly Tunable Degradation and Osteogenic Features. Bioact. Mater. 2022, 15, 53–67. [Google Scholar] [CrossRef]
- Ghaziof, S.; Shojaei, S.; Mehdikhani, M.; Khodaei, M.; Jafari Nodoushan, M. Electro-Conductive 3D Printed Polycaprolactone/Gold Nanoparticles Nanocomposite Scaffolds for Myocardial Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2022, 132, 105271. [Google Scholar] [CrossRef] [PubMed]
- El-Morsy, M.A.; Afifi, M.; Ahmed, M.K.; Awwad, N.S.; Ibrahium, H.A.; Alqahtani, M.S. Electrospun Nanofibrous Scaffolds of Polycaprolactone Containing Binary Ions of Pd/Vanadate Doped Hydroxyapatite for Biomedical Applications. J. Drug Deliv. Sci. Technol. 2022, 70, 103153. [Google Scholar] [CrossRef]
- Altun, E.; Ahmed, J.; Onur Aydogdu, M.; Harker, A.; Edirisinghe, M. The Effect of Solvent and Pressure on Polycaprolactone Solutions for Particle and Fibre Formation. Eur. Polym. J. 2022, 173, 111300. [Google Scholar] [CrossRef]
- Salehi, A.O.M.; Keshel, S.H.; Rafienia, M.; Nourbakhsh, M.S.; Baradaran-Rafii, A. Promoting Keratocyte Stem like Cell Proliferation and Differentiation by Aligned Polycaprolactone-Silk Fibroin Fibers Containing Aloe Vera. Biomater. Adv. 2022, 137, 212840. [Google Scholar] [CrossRef]
- Rogina, A. Electrospinning Process: Versatile Preparation Method for Biodegradable and Natural Polymers and Biocomposite Systems Applied in Tissue Engineering and Drug Delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Alecu, A.E.; Costea, C.C.; Surdu, V.A.; Voicu, G.; Jinga, S.I.; Busuioc, C. Processing of Calcium Magnesium Silicates by the Sol-Gel Route. Gels 2022, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.; Aparicio, M.; Jitianu, A. Handbook of Sol-Gel Science and Technology: Processing, Characterization and Applications; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Mitchell, G.R. Electrospinning: Principles, Practice and Possibilities; Royal Society of Chemistry: London, UK, 2015. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproduce In Vivo Surface-Structure Changes in Bioactive Glass-Ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Yamaguchi, S. The Use of Simulated Body Fluid (SBF) for Assessing Materials Bioactivity in the Context of Tissue Engineering: Review and Challenges. Biomimetics 2020, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Bafandeha, M.R.; Mojarrabianb, H.M.; Doostmohammadic, A. Poly (Vinyl Alcohol)/Chitosan/Akermanite Nanofibrous Scaffolds Prepared by Electrospinning. J. Macromol. Sci. B 2019, 58, 749–759. [Google Scholar] [CrossRef]
- Jinga, S.I.; Zamfirescu, A.I.; Voicu, G.; Enculescu, M.; Evanghelidis, A.; Busuioc, C. PCL-ZnO/TiO2/HAp Electrospun Composite Fibres with Applications in Tissue Engineering. Polymers 2019, 11, 1793. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Mohamadi, Z. Acidic Ionic Liquids Catalyst in Homo and Graft Polymerization of ε-Caprolactone. Colloid. Polym. Sci. 2013, 291, 1999–2005. [Google Scholar] [CrossRef]
- Jinga, S.I.; Costea, C.C.; Zamfirescu, A.I.; Banciu, A.; Banciu, D.D.; Busuioc, C. Composite Fibre Networks Based on Polycaprolactone and Bioactive Glass-Ceramics for Tissue Engineering Applications. Polymers 2020, 12, 1806. [Google Scholar] [CrossRef]
- Negrea, R.; Busuioc, C.; Constantinoiu, I.; Miu, D.; Enache, C.; Iordache, F.; Jinga, S.I. Akermanite Based Coatings Grown by Pulsed Laser Deposition for Metallic Implants Employed in Orthopaedics. Surf. Coat. Technol. 2019, 357, 1015–1026. [Google Scholar] [CrossRef]
- Schitea, R.I.; Nitu, A.; Ciobota, A.A.; Munteanu, A.L.; David, I.M.; Miu, D.; Raileanu, M.; Bacalum, M.; Busuioc, C. Pulsed Laser Deposition Derived Bioactive Glass-Ceramic Coatings for Enhancing the Biocompatibility of Scaffolding Materials. Materials 2020, 13, 2615. [Google Scholar] [CrossRef]
- Prefac, G.A.; Milea, M.L.; Vadureanu, A.M.; Muraru, S.; Dobrin, D.I.; Isopencu, G.O.; Jinga, S.I.; Raileanu, M.; Bacalum, M.; Busuioc, C. CeO2 Containing Thin Films as Bioactive Coatings for Orthopaedic Implants. Coatings 2020, 10, 642. [Google Scholar] [CrossRef]
- Ho, C.C.; Fang, H.Y.; Wang, B.; Huang, T.H.; Shie, M.Y. The Effects of Biodentine/Polycaprolactone Three-Dimensional-Scaffold with Odontogenesis Properties on Human Dental Pulp Cells. Int. Endod. J. 2018, 51, e291–e300. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.G.; Wei, J.; Shin, J.V.; Wu, Y.R.; Su, J.; Park, Y.S.; Shin, J.W. Enhanced Biocompatibility and Osteogenic Potential of Mesoporous Magnesium Silicate/Polycaprolactone/Wheat Protein Composite Scaffolds. Int. J. Nanomedicine 2018, 13, 1107–1117. [Google Scholar] [CrossRef]
- Shkarina, S.; Shkarin, R.; Weinhardt, V.; Melnik, E.; Vacun, G.; Kluger, P.J.; Loza, K.; Epple, M.; Ivlev, S.I.; Baumbach, T.; et al. 3D Biodegradable Scaffolds of Polycaprolactone with Silicate-Containing Hydroxyapatite Microparticles for Bone Tissue Engineering: High-Resolution Tomography and in Vitro study. Sci. Rep. 2018, 8, 8907. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Hsu, T.T.; Huang, T.H.; Lin, C.Y.; Shie, M.Y. Fabrication and Characterization of Polycaprolactone and Tricalcium Phosphate Composites for Tissue Engineering Applications. J. Dent. Sci. 2017, 12, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Chhabra, H.; Soni, V.P.; Bellare, J.R. Enhanced Mechanical Strength and Biocompatibility of Electrospun Polycaprolactone-Gelatin Scaffold with Surface Deposited Nano-Hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 2376–2385. [Google Scholar] [CrossRef]
- Salgado, C.L.; Sanchez, E.M.S.; Zavaglia, C.A.C.; Granja, P.L. Biocompatibility and Biodegradation of Polycaprolactone-Sebacic Acid Blended Gels. J. Biomed. Mater. Res. A 2012, 100A, 243–251. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef]
- Coombes, A.G.A.; Rizzi, S.C.; Williamson, M.; Barralet, J.E.; Downes, S.; Wallace, W.A. Precipitation Casting of Polycaprolactone for Applications in Tissue Engineering and Drug Delivery. Biomaterials 2004, 25, 315–325. [Google Scholar] [CrossRef]



| No. | Sample Code | PCL (%) | D (%) | A (%) | M (%) |
|---|---|---|---|---|---|
| 1 | PCL | 16 | - | - | - |
| 2 | PCL-D-5% | 5 | - | - | |
| 3 | PCL-D-10% | 10 | - | - | |
| 4 | PCL-A-5% | - | 5 | - | |
| 5 | PCL-A-10% | - | 10 | - | |
| 6 | PCL-M-5% | - | - | 5 | |
| 7 | PCL-M-10% | - | - | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busuioc, C.; Alecu, A.-E.; Costea, C.-C.; Beregoi, M.; Bacalum, M.; Raileanu, M.; Jinga, S.-I.; Deleanu, I.-M. Composite Fibers Based on Polycaprolactone and Calcium Magnesium Silicate Powders for Tissue Engineering Applications. Polymers 2022, 14, 4611. https://doi.org/10.3390/polym14214611
Busuioc C, Alecu A-E, Costea C-C, Beregoi M, Bacalum M, Raileanu M, Jinga S-I, Deleanu I-M. Composite Fibers Based on Polycaprolactone and Calcium Magnesium Silicate Powders for Tissue Engineering Applications. Polymers. 2022; 14(21):4611. https://doi.org/10.3390/polym14214611
Chicago/Turabian StyleBusuioc, Cristina, Andrada-Elena Alecu, Claudiu-Constantin Costea, Mihaela Beregoi, Mihaela Bacalum, Mina Raileanu, Sorin-Ion Jinga, and Iuliana-Mihaela Deleanu. 2022. "Composite Fibers Based on Polycaprolactone and Calcium Magnesium Silicate Powders for Tissue Engineering Applications" Polymers 14, no. 21: 4611. https://doi.org/10.3390/polym14214611
APA StyleBusuioc, C., Alecu, A.-E., Costea, C.-C., Beregoi, M., Bacalum, M., Raileanu, M., Jinga, S.-I., & Deleanu, I.-M. (2022). Composite Fibers Based on Polycaprolactone and Calcium Magnesium Silicate Powders for Tissue Engineering Applications. Polymers, 14(21), 4611. https://doi.org/10.3390/polym14214611








