Bio-Based Porous Aerogel with Bionic Structure and Hydrophobic Polymer Coating for Efficient Absorption of Oil/Organic Liquids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Procedure of Carbon Aerogels
2.3. Modification of Carbon Aerogels
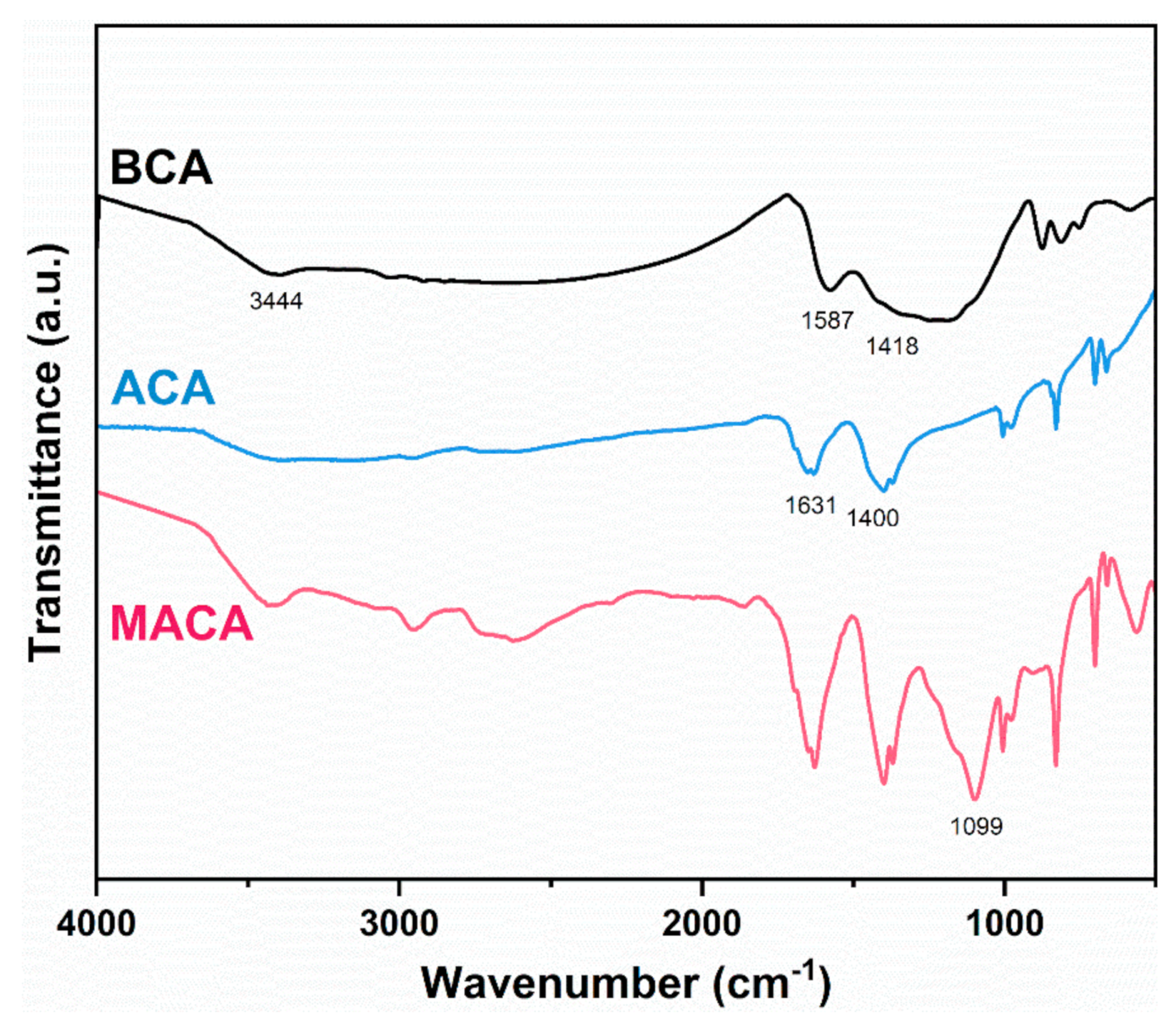
2.4. Characterization
2.5. Oil and Organic Solvent Absorption Experiments
3. Results and Discussion
3.1. Preparation Schematic of Polymer/DE-Modified Porous Material
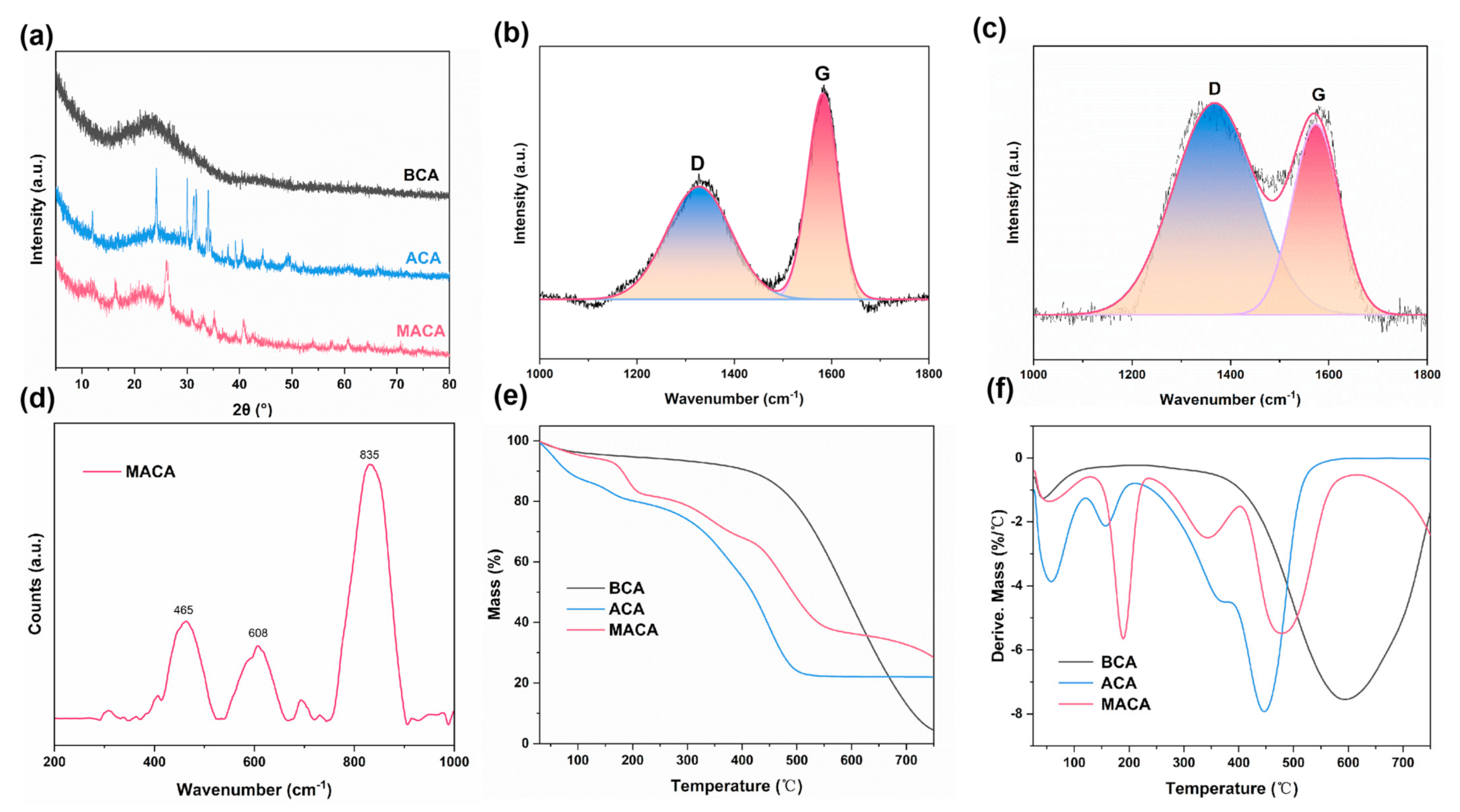
3.2. Micro-Morphology and Structure Analyses
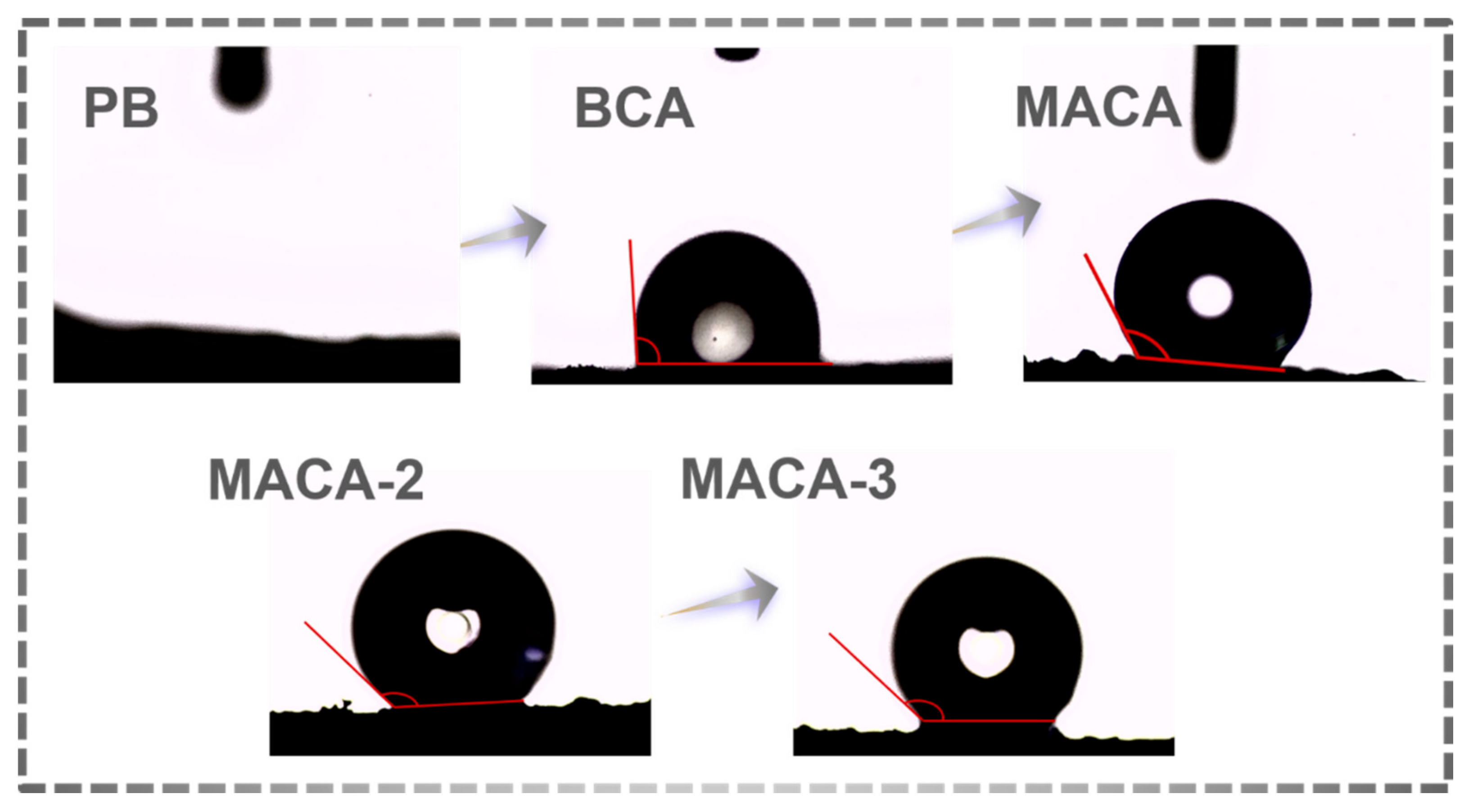
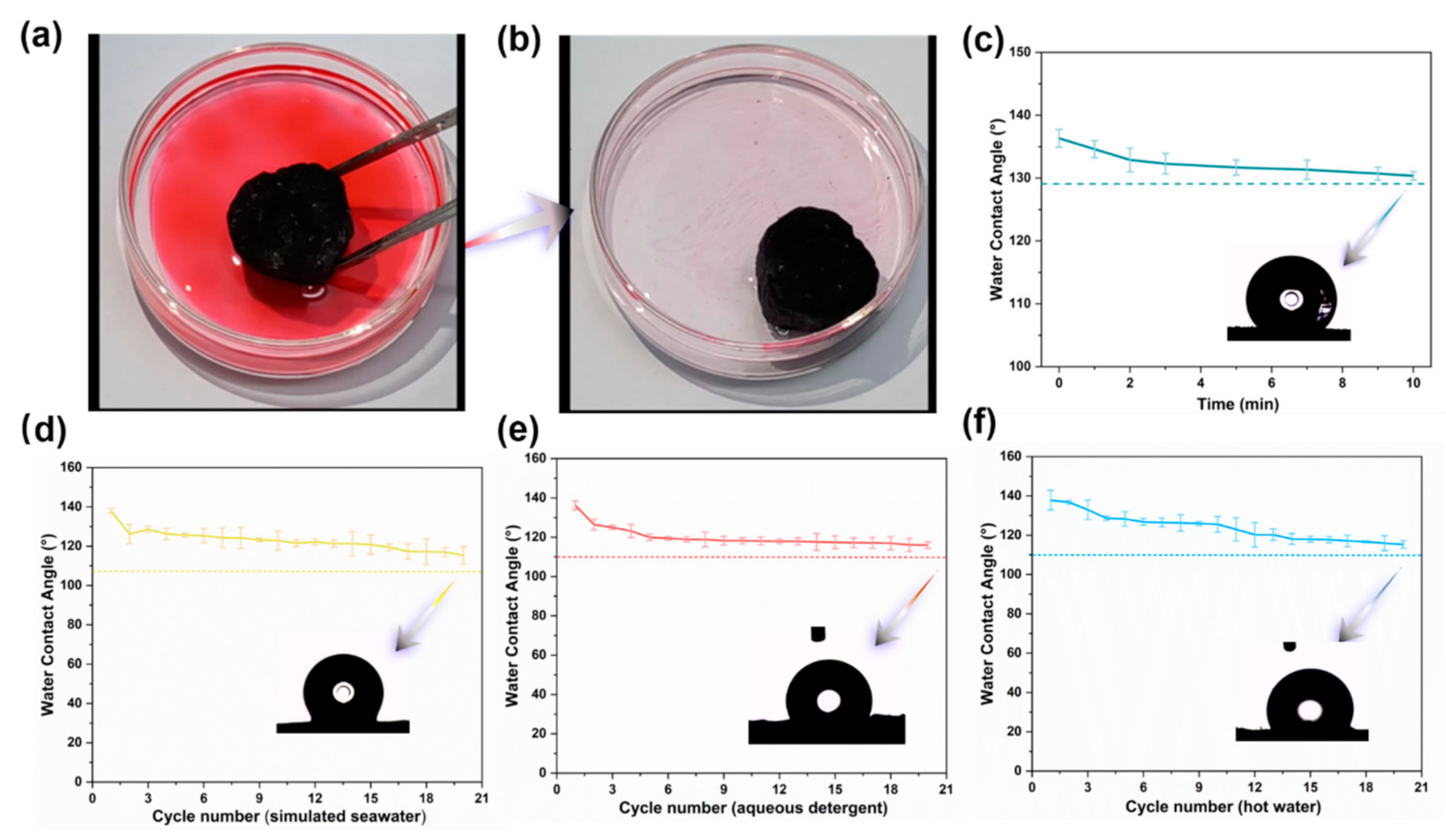
3.3. Characterization of Hydrophobicity
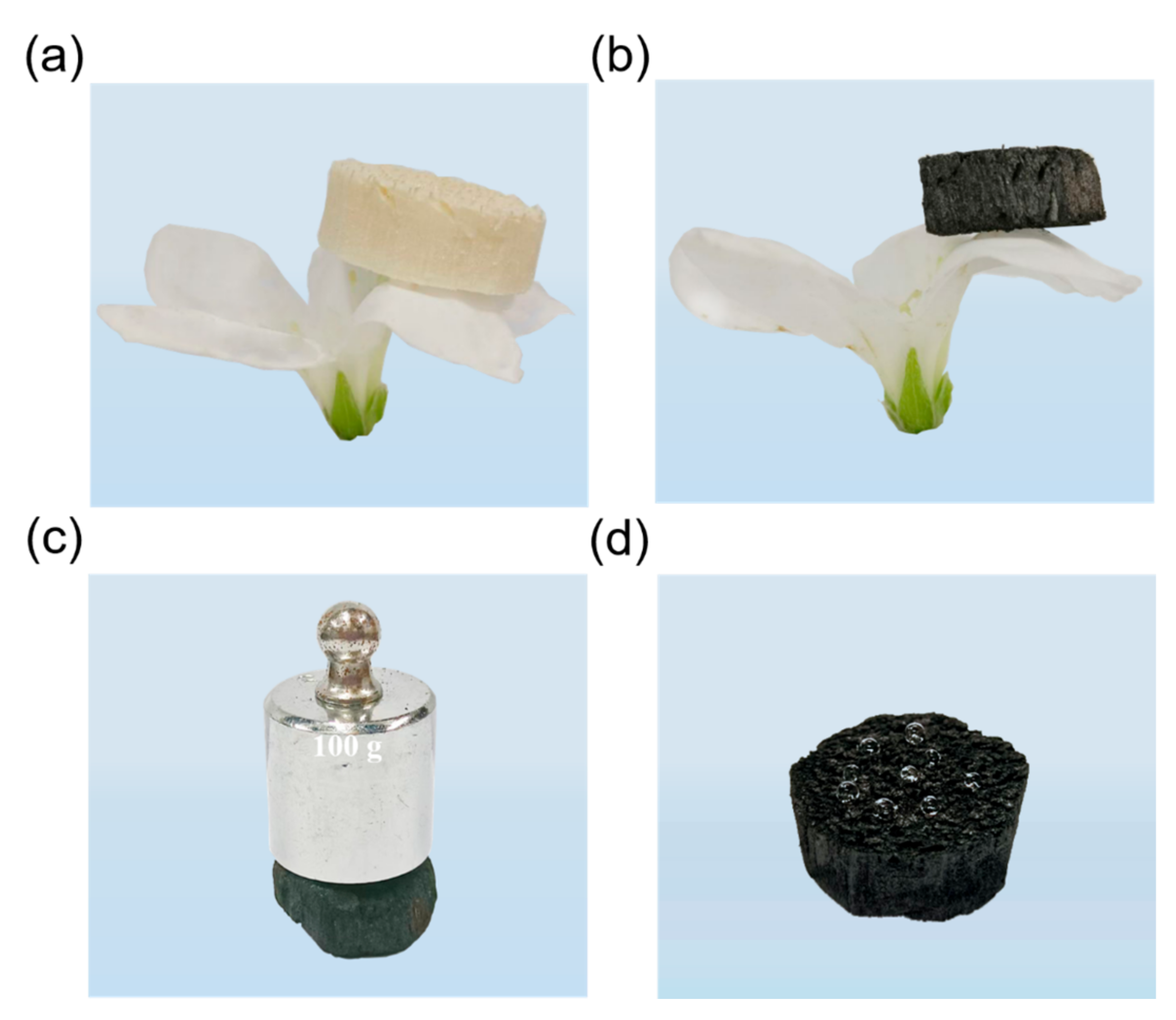
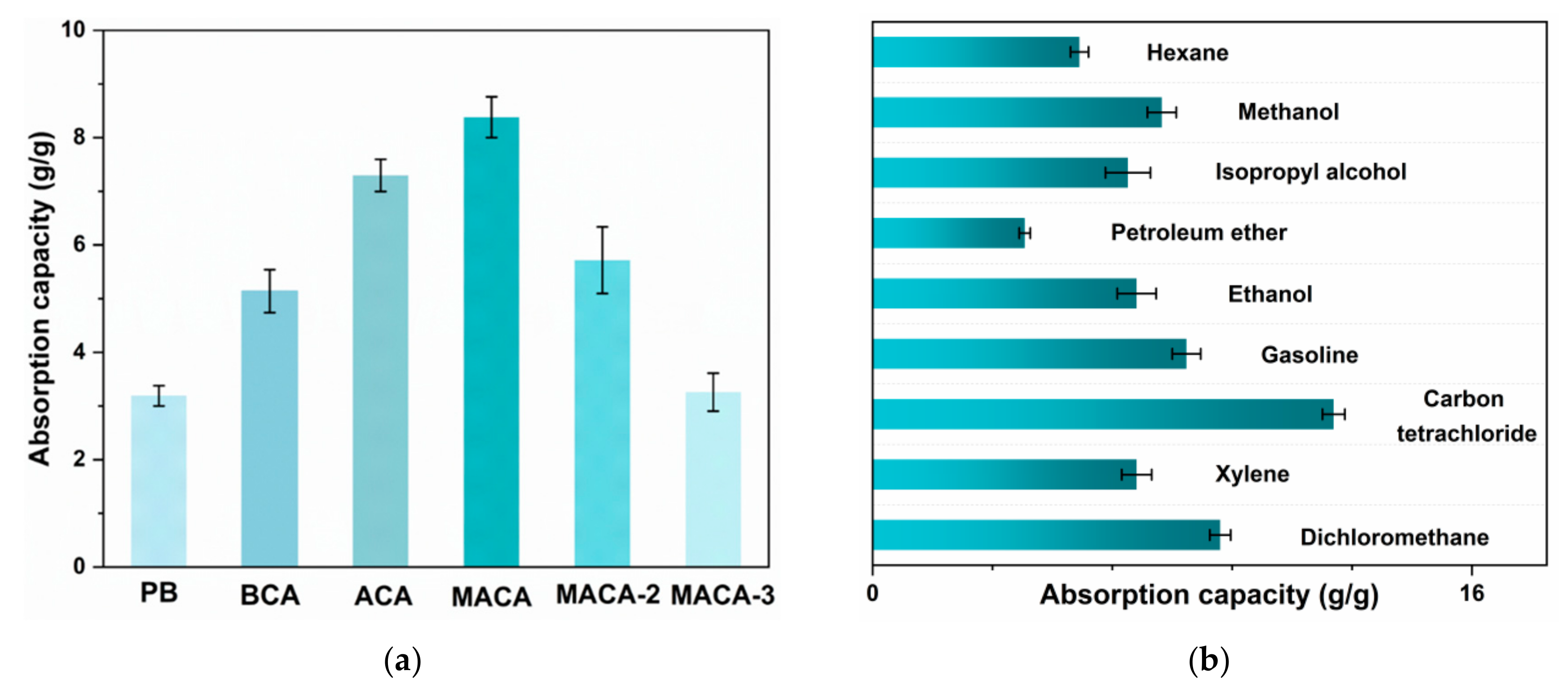
3.4. Oil/Organic Solvents Absorption Performance of Aerogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, P.; Cai, Q.; Lin, W.; Chen, B.; Zhang, B. Offshore oil spill response practices and emerging challenges. Mar. Pollut. Bull. 2016, 110, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, W.; Wan, Z.; Li, S.; Huang, T.; Fei, Y. Oil spills from global tankers: Status review and future governance. J. Clean. Prod. 2019, 227, 20–32. [Google Scholar] [CrossRef]
- Weng, D.; Song, L.; Li, W.; Yan, J.; Chen, L.; Liu, Y. Review on synthesis of three-dimensional graphene skeletons and their absorption performance for oily wastewater. Environ. Sci. Pollut. R. 2021, 28, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous materials for oil spill cleanup: A review of synthesis and absorbing properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]
- Bayat, A.; Aghamiri, S.F.; Moheb, A.; Vakili-Nezhaad, G.R. Oil Spill Cleanup from Sea Water by Sorbent Materials. Chem. Eng. Technol. 2005, 28, 1525–1528. [Google Scholar] [CrossRef]
- Doshi, B.; Sillanpaa, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Bandura, L.; Woszuk, A.; Kołodyńska, D.; Franus, W. Application of Mineral Sorbents for Removal of Petroleum Substances: A Review. Minerals 2017, 7, 37. [Google Scholar] [CrossRef]
- Bastani, D.; Safekordi, A.A.; Alihosseini, A.; Taghikhani, V. Study of oil sorption by expanded perlite at 298.15K. Sep. Purif. Technol. 2006, 52, 295–300. [Google Scholar] [CrossRef]
- Annunciado, T.R.; Sydenstricker, T.H.; Amico, S.C. Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar. Pollut. Bull. 2005, 50, 1340–1346. [Google Scholar] [CrossRef]
- Radetic, M.M.; Jocic, D.M.; Jovancic, P.M.; Petrovic, Z.L.; Thomas, H.F. Recycled wool-based nonwoven material as an oil sorbent. Environ. Sci. Technol. 2003, 37, 1008–1012. [Google Scholar] [CrossRef]
- Choi, S.J.; Kwon, T.H.; Im, H.; Moon, D.I.; Baek, D.J.; Seol, M.L.; Duarte, J.P.; Choi, Y.K. A polydimethylsiloxane (PDMS) sponge for the selective absorption of oil from water. ACS Appl. Mater. Inter. 2011, 3, 4552–4556. [Google Scholar] [CrossRef]
- Thai, Q.B.; Nguyen, S.T.; Ho, D.K.; Tran, T.D.; Huynh, D.M.; Do, N.H.N.; Luu, T.P.; Le, P.K.; Le, D.K.; Phan-Thien, N.; et al. Cellulose-based aerogels from sugarcane bagasse for oil spill-cleaning and heat insulation applications. Carbohyd. Polym. 2020, 228, 115365. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lei, S.; Xi, J.; Ye, D.; Hu, W.; Song, L.; Hu, Y.; Cai, W.; Gui, Z. Bio-based multifunctional carbon aerogels from sugarcane residue for organic solvents adsorption and solar-thermal-driven oil removal. Chem. Eng. J. 2021, 426, 129580. [Google Scholar] [CrossRef]
- Lv, L.-Y.; Cao, C.-F.; Qu, Y.-X.; Zhang, G.-D.; Zhao, L.; Cao, K.; Song, P.; Tang, L.-C. Smart fire-warning materials and sensors: Design principle, performances, and applications. Mater. Sci. Eng. R. 2022, 150, 100690. [Google Scholar] [CrossRef]
- Yang, W.-J.; Yuen, A.C.Y.; Li, A.; Lin, B.; Chen, T.B.Y.; Yang, W.; Lu, H.-D.; Yeoh, G.H. Recent progress in bio-based aerogel absorbents for oil/water separation. Cellulose 2019, 26, 6449–6476. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, J.; Ge, S.; Jiang, C.; Guo, T.; Peng, T.; Huang, T.; Xie, L. Biocompatible, hydrophobic and resilience graphene/chitosan composite aerogel for efficient oil−water separation. Surf. Coat. Technol. 2020, 385, 125361. [Google Scholar] [CrossRef]
- Ma, Q.; Cheng, H.; Fane, A.G.; Wang, R.; Zhang, H. Recent Development of Advanced Materials with Special Wettability for Selective Oil/Water Separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef]
- Pakdel, E.; Wang, J.; Kashi, S.; Sun, L.; Wang, X. Advances in photocatalytic self-cleaning, superhydrophobic and electromagnetic interference shielding textile treatments. Adv. Colloid. Interface 2020, 277, 102116. [Google Scholar] [CrossRef]
- Hu, W.J.; Xia, Q.Q.; Pan, H.T.; Chen, H.Y.; Qu, Y.X.; Chen, Z.Y.; Zhang, G.D.; Zhao, L.; Gong, L.X.; Xue, C.G.; et al. Green and Rapid Preparation of Fluorosilicone Rubber Foam Materials with Tunable Chemical Resistance for Efficient Oil-Water Separation. Polymers 2022, 14, 1628. [Google Scholar] [CrossRef]
- Pakdel, E.; Wang, J.; Varley, R.; Wang, X. Recycled carbon fiber nonwoven functionalized with fluorine-free superhydrophobic PDMS/ZIF-8 coating for efficient oil-water separation. J. Environ. Chem. Eng. 2021, 9, 106329. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Rodak, D.E. Is the lotus leaf superhydrophobic? Appl. Phys. Lett. 2005, 86, 144101. [Google Scholar] [CrossRef]
- Zhang, G.D.; Wu, Z.H.; Xia, Q.Q.; Qu, Y.X.; Pan, H.T.; Hu, W.J.; Zhao, L.; Cao, K.; Chen, E.Y.; Yuan, Z.; et al. Ultrafast Flame-Induced Pyrolysis of Poly(dimethylsiloxane) Foam Materials toward Exceptional Superhydrophobic Surfaces and Reliable Mechanical Robustness. ACS Appl. Mater. Interfaces 2021, 13, 23161–23172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, M.; Lin, C.; Li, F.; Aladejana, J.T.; Hong, J.; Zhao, G.; Qin, Z.; Zhu, X.; Zhang, W.; et al. Solar-assisted high-efficient cleanup of viscous crude oil spill using an ink-modified plant fiber sponge. J. Hazard. Mater. 2022, 432, 128740. [Google Scholar] [CrossRef]
- Zamparas, M.; Tzivras, D.; Dracopoulos, V.; Ioannides, T. Application of Sorbents for Oil Spill Cleanup Focusing on Natural-Based Modified Materials: A Review. Molecules 2020, 25, 4522. [Google Scholar] [CrossRef] [PubMed]
- Vukčević, M.M.; Kalijadis, A.M.; Vasiljević, T.M.; Babić, B.M.; Laušević, Z.V.; Laušević, M.D. Production of activated carbon derived from waste hemp (Cannabis sativa) fibers and its performance in pesticide adsorption. Micropor. Mesopor. Mater. 2015, 214, 156–165. [Google Scholar] [CrossRef]
- Jin, H.; Capareda, S.; Chang, Z.; Gao, J.; Xu, Y.; Zhang, J. Biochar pyrolytically produced from municipal solid wastes for aqueous As(V) removal: Adsorption property and its improvement with KOH activation. Bioresource Technol. 2014, 169, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Luo, Z.; Ning, H.; Zhou, X.; Yuan, B. Efficient flame-retardant biomass aerogel endowed with graphene oxide interconnected networks for ultrasensitive fire warning. Mater. Lett. 2022, 318, 132237. [Google Scholar] [CrossRef]
- Cao, C.; Yuan, B. Thermally induced fire early warning aerogel with efficient thermal isolation and flame-retardant properties. Polym. Adv. Technol. 2021, 32, 2159–2168. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S.; Mehala, R.; Sharumitha, T.J.; Surendhar, D. Enhanced adsorptive removal of sulfamethoxazole from water using biochar derived from hydrothermal carbonization of sugarcane bagasse. J. Hazard. Mater. 2021, 407, 124825. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhang, T.; Guo, Q.; Qiu, F.; Yang, D.; Ou, Z. Recyclable biomass carbon@SiO2@MnO2 aerogel with hierarchical structures for fast and selective oil-water separation. Chem. Eng. J. 2018, 351, 622–630. [Google Scholar] [CrossRef]
- Cao, C.-F.; Wang, P.-H.; Zhang, J.-W.; Guo, K.-Y.; Li, Y.; Xia, Q.-Q.; Zhang, G.-D.; Zhao, L.; Chen, H.; Wang, L.; et al. One-step and green synthesis of lightweight, mechanically flexible and flame-retardant polydimethylsiloxane foam nanocomposites via surface-assembling ultralow content of graphene derivative. Chem. Eng. J. 2020, 393, 124724. [Google Scholar] [CrossRef]
- Guo, K.-Y.; Wu, Q.; Mao, M.; Chen, H.; Zhang, G.-D.; Zhao, L.; Gao, J.-F.; Song, P.; Tang, L.-C. Water-based hybrid coatings toward mechanically flexible, super-hydrophobic and flame-retardant polyurethane foam nanocomposites with high-efficiency and reliable fire alarm response. Compos. Part B-Eng. 2020, 193, 108017. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Zhang, J.-W.; Cao, C.-F.; Guo, K.-Y.; Zhao, L.; Zhang, G.-D.; Gao, J.-F.; Tang, L.-C. Temperature-responsive resistance sensitivity controlled by L-ascorbic acid and silane co-functionalization in flame-retardant GO network for efficient fire early-warning response. Chem. Eng. J. 2020, 386, 123894. [Google Scholar] [CrossRef]
- Mao, M.; Yu, K.-X.; Cao, C.-F.; Gong, L.-X.; Zhang, G.-D.; Zhao, L.; Song, P.; Gao, J.-F.; Tang, L.-C. Facile and green fabrication of flame-retardant Ti3C2Tx MXene networks for ultrafast, reusable and weather-resistant fire warning. Chem. Eng. J. 2022, 427, 131615. [Google Scholar] [CrossRef]
- Yu, Z.-R.; Mao, M.; Li, S.-N.; Xia, Q.-Q.; Cao, C.-F.; Zhao, L.; Zhang, G.-D.; Zheng, Z.-J.; Gao, J.-F.; Tang, L.-C. Facile and green synthesis of mechanically flexible and flame-retardant clay/graphene oxide nanoribbon interconnected networks for fire safety and prevention. Chem. Eng. J. 2021, 405, 126620. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, Y.; Chen, G.; Yang, F.; Zhang, H.; Cao, C.; Zuo, B. Nacre-like graphene oxide paper bonded with boric acid for fire early-warning sensor. J. Hazard. Mater. 2021, 403, 123645. [Google Scholar] [CrossRef]
- Zou, B.; Qiu, S.; Ren, X.; Zhou, Y.; Zhou, F.; Xu, Z.; Zhao, Z.; Song, L.; Hu, Y.; Gong, X. Combination of black phosphorus nanosheets and MCNTs via phosphoruscarbon bonds for reducing the flammability of air stable epoxy resin nanocomposites. J. Hazard. Mater. 2020, 383, 121069. [Google Scholar] [CrossRef]
- Bedin, K.C.; Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. KOH-activated carbon prepared from sucrose spherical carbon: Adsorption equilibrium, kinetic and thermodynamic studies for Methylene Blue removal. Chem. Eng. J. 2016, 286, 476–484. [Google Scholar] [CrossRef]
- Jing, Z.; Ding, J.; Zhang, T.; Yang, D.; Qiu, F.; Chen, Q.; Xu, J. Flexible, versatility and superhydrophobic biomass carbon aerogels derived from corn bracts for efficient oil/water separation. Food Bioprod. Process. 2019, 115, 134–142. [Google Scholar] [CrossRef]
- Guo, B.-F.; Wang, P.-H.; Cao, C.-F.; Qu, Z.-H.; Lv, L.-Y.; Zhang, G.-D.; Gong, L.-X.; Song, P.; Gao, J.-F.; Mai, Y.-W.; et al. Restricted assembly of ultralow loading of graphene oxide for lightweight, mechanically flexible and flame retardant polydimethylsiloxane foam composites. Compos. Part B-Eng. 2022, 247, 110290. [Google Scholar] [CrossRef]
- Allahbakhsh, A.; Bahramian, A.R. Self-assembled and pyrolyzed carbon aerogels: An overview of their preparation mechanisms, properties and applications. Nanoscale 2015, 7, 14139–14158. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Zhu, F.; You, L.; Shen, X.; Li, S. Removal of organic solvents/oils using carbon aerogels derived from waste durian shell. J. Taiwan Inst. Chem. Eng. 2017, 78, 351–358. [Google Scholar] [CrossRef]
- Sarıcı-Özdemir, Ç.; Önal, Y. Synthesis of new activated carbons produced from polymer waste. Fuller. Nanotub. Carbon Nanostructures 2018, 26, 451–457. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Z.; Pan, Z.; Wang, D. Mohr’s salt assisted KOH activation strategy to customize S-doped hierarchical carbon frameworks enabling satisfactory rate performance of supercapacitors. J. Alloys Compd. 2021, 876, 160203. [Google Scholar] [CrossRef]
- Ferreira, P.; Carvalho, A.; Correia, T.R.; Antunes, B.P.; Correia, I.J.; Alves, P. Functionalization of polydimethylsiloxane membranes to be used in the production of voice prostheses. Sci. Technol. Adv. Mater. 2013, 14, 055006. [Google Scholar] [CrossRef]
- Yuan, B.; Hu, Y.; Chen, X.; Shi, Y.; Niu, Y.; Zhang, Y.; He, S.; Dai, H. Dual modification of graphene by polymeric flame retardant and Ni(OH)2 nanosheets for improving flame retardancy of polypropylene. Compos. Part A-Sci. Manuf. 2017, 100, 106–117. [Google Scholar] [CrossRef]
- Zou, B.; Qiu, S.; Zhou, Y.; Qian, Z.; Xu, Z.; Wang, J.; Xiao, Y.; Liao, C.; Yang, W.; Han, L.; et al. Photothermal-healing, and record thermal stability and fire safety black phosphorus–boron hybrid nanocomposites: Mechanism of phosphorus fixation effects and charring inspired by cell walls. J. Mater. Chem. A 2022, 10, 14423–14434. [Google Scholar] [CrossRef]
- Liu, T.; Huang, M.; Li, X.; Wang, C.; Gui, C.-X.; Yu, Z.-Z. Highly compressible anisotropic graphene aerogels fabricated by directional freezing for efficient absorption of organic liquids. Carbon 2016, 100, 456–464. [Google Scholar] [CrossRef]
- Nine, M.J.; Cole, M.A.; Johnson, L.; Tran, D.N.; Losic, D. Robust Superhydrophobic Graphene-Based Composite Coatings with Self-Cleaning and Corrosion Barrier Properties. ACS Appl. Mater. Interfaces 2015, 7, 28482–28493. [Google Scholar] [CrossRef]
- Ong, C.C.; Sundera Murthe, S.; Mohamed, N.M.; Perumal, V.; Mohamed Saheed, M.S. Nanoscaled Surface Modification of Poly(dimethylsiloxane) Using Carbon Nanotubes for Enhanced Oil and Organic Solvent Absorption. ACS Omega 2018, 3, 15907–15915. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Tandon, P.; Gupta, V.D. Phonon dispersion in poly(dimethylsilane). J. Organomet. Chem. 2006, 691, 2902–2908. [Google Scholar] [CrossRef]
- Nour, M.; Berean, K.; Balendhran, S.; Ou, J.Z.; Du Plessis, J.; McSweeney, C.; Bhaskaran, M.; Sriram, S.; Kalantar-zadeh, K. CNT/PDMS composite membranes for H2 and CH4 gas separation. Int. J. Hydrogen Energy 2013, 38, 10494–10501. [Google Scholar] [CrossRef]
- Wang, W.; Wen, C.; Liu, T.; Li, C.; Liu, H.; Liu, E.; Xu, M. Emissions of PM10 from the co-combustion of high-Ca pyrolyzed biochar and high-Si coal under air and oxyfuel atmosphere. Proc. Combust. Inst. 2021, 38, 4091–4099. [Google Scholar] [CrossRef]
- Chen, L.; Wen, C.; Wang, W.; Liu, T.; Liu, E.; Liu, H.; Li, Z. Combustion behaviour of biochars thermally pretreated via torrefaction, slow pyrolysis, or hydrothermal carbonisation and co-fired with pulverised coal. Renew. Energy 2020, 161, 867–877. [Google Scholar] [CrossRef]
- Ma, J.; Yu, F.; Zhou, L.; Jin, L.; Yang, M.; Luan, J.; Tang, Y.; Fan, H.; Yuan, Z.; Chen, J. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces 2012, 4, 5749–5760. [Google Scholar]
- Pan, F.; Chen, T.; Cai, M.; Wu, F.; You, Z.; Li, J. Fabrication of large-surface-area graphitized carbons by potassium hydroxide-promoted catalytic graphitization. Mater. Res. Bull. 2021, 140, 111333. [Google Scholar] [CrossRef]
- Kiciński, W.; Norek, M.; Bystrzejewski, M. Monolithic porous graphitic carbons obtained through catalytic graphitization of carbon xerogels. J. Phys. Chem. Solids 2013, 74, 101–109. [Google Scholar] [CrossRef]
- Jiménez, R.; García, X.; López, T.; Gordon, A.L. Catalytic combustion of soot. Effects of added alkali metals on CaO–MgO physical mixtures. Fuel Process. Technol. 2008, 89, 1160–1168. [Google Scholar] [CrossRef]
- Gupta, P.; Verma, C.; Maji, P.K. Flame retardant and thermally insulating clay based aerogel facilitated by cellulose nanofibers. J. Supercrit. Fluid 2019, 152, 104537. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Optimization of microwave preparation conditions for activated carbon from Albizia lebbeck seed pods for methylene blue dye adsorption. J. Anal. Appl. Pyrol. 2014, 105, 199–208. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I.P. Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Methods 2018, 2, 1800173. [Google Scholar] [CrossRef]
- Bae, Y.S.; Yazaydin, A.O.; Snurr, R.Q. Evaluation of the BET method for determining surface areas of MOFs and zeolites that contain ultra-micropores. Langmuir 2010, 26, 5475–5483. [Google Scholar] [CrossRef]
- Ladavos, A.K.; Katsoulidis, A.P.; Iosifidis, A.; Triantafyllidis, K.S.; Pinnavaia, T.J.; Pomonis, P.J. The BET equation, the inflection points of N2 adsorption isotherms and the estimation of specific surface area of porous solids. Micropor. Mesopor. Mater. 2012, 151, 126–133. [Google Scholar] [CrossRef]
- Hussein, M.; Amer, A.A.; Sawsan, I.I. Oil spill sorption using carbonized pith bagasse: 1. Preparation and characterization of carbonized pith bagasse. J. Anal. Appl. Pyrol. 2008, 82, 205–211. [Google Scholar] [CrossRef]



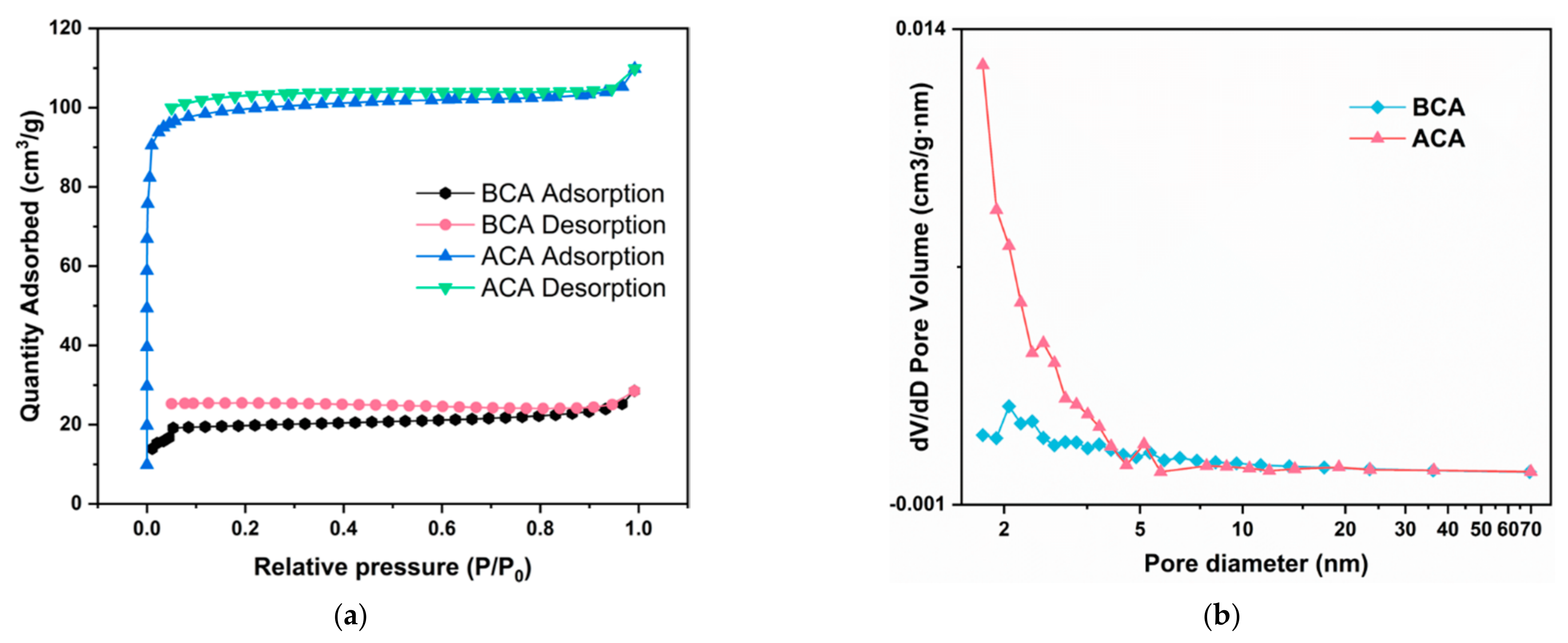


| Sample | SBET 1 | SMicro 2 | SMeso 3 | VTotal 4 | VMicro 5 | VMaso 6 | DBJH 7 |
|---|---|---|---|---|---|---|---|
| BCA | 60.99 | 52.30 | 8.70 | 0.04 | 0.03 | 0.01 | 8.98 |
| ACA | 304.37 | 274.12 | 30.25 | 0.16 | 0.14 | 0.02 | 4.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wu, Y.; Tao, H.; Yuan, B. Bio-Based Porous Aerogel with Bionic Structure and Hydrophobic Polymer Coating for Efficient Absorption of Oil/Organic Liquids. Polymers 2022, 14, 4579. https://doi.org/10.3390/polym14214579
Huang Y, Wu Y, Tao H, Yuan B. Bio-Based Porous Aerogel with Bionic Structure and Hydrophobic Polymer Coating for Efficient Absorption of Oil/Organic Liquids. Polymers. 2022; 14(21):4579. https://doi.org/10.3390/polym14214579
Chicago/Turabian StyleHuang, Yi, Yucheng Wu, Hao Tao, and Bihe Yuan. 2022. "Bio-Based Porous Aerogel with Bionic Structure and Hydrophobic Polymer Coating for Efficient Absorption of Oil/Organic Liquids" Polymers 14, no. 21: 4579. https://doi.org/10.3390/polym14214579
APA StyleHuang, Y., Wu, Y., Tao, H., & Yuan, B. (2022). Bio-Based Porous Aerogel with Bionic Structure and Hydrophobic Polymer Coating for Efficient Absorption of Oil/Organic Liquids. Polymers, 14(21), 4579. https://doi.org/10.3390/polym14214579






