Abstract
In recent years, the demand for environmental sustainability has caused a great interest in finding novel polymer materials from natural resources that are both biodegradable and eco-friendly. Natural biodegradable polymers can displace the usage of petroleum-based synthetic polymers due to their renewability, low toxicity, low costs, biocompatibility, and biodegradability. The development of novel starch-based bionanocomposites with improved properties has drawn specific attention recently in many applications, including food, agriculture, packaging, environmental remediation, textile, cosmetic, pharmaceutical, and biomedical fields. This paper discusses starch-based nanocomposites, mainly with nanocellulose, chitin nanoparticles, nanoclay, and carbon-based materials, and their applications in the agriculture, packaging, biomedical, and environment fields. This paper also focused on the lifecycle analysis and degradation of various starch-based nanocomposites.
1. Introduction
In recent days, nanocomposites have gained much attention over traditional composite materials and are widely used in food, packaging, biomedical applications, electronics, energy storage, optics, the automotive industry, bio-sorbants for environmental remediation, textiles, and many other applications [1,2]. Polymer nanocomposites consist of polymer matrices embedded with nanofillers [3]. Petroleum-based polymers are produced in huge amounts globally. Petroleum-based polymers are non-biodegradable, non-renewable, and produce hazardous substances which can threaten human health and the environment [4]. Furthermore, the depletion of these non-renewable petroleum-based fuels demands alternative resources [5].
Thus, biopolymer-based nanocomposites can be a sustainable alternative for petroleum-based nanocomposites in many applications due to their biodegradability, eco-friendliness, renewability, relatively inexpensive, low toxicity, abundancy, and improved thermal, mechanical, physical, barrier, and functional properties [3,4]. Various natural biopolymers, including starch, cellulose, pectin, lignin, chitin/chitosan, alginates, hyaluronic acid, gelatin, terpenes, gelatin, gluten, and polyhydroxyalkanoates (PHAs) from plants, animals, algae, microorganisms and synthetic biopolymers, including polycaprolactone (PCL), poly(butylene succinate) (PBS), poly(lactic-co-glycolic acids) (PLGA), and polylactic acids (PLA), have been used in nanocomposite materials for various applications [1,2,3,6,7,8].
Starch is one of the most abundant natural polymers globally. Starch and its nanocomposites have been extensively studied for their abundance, low cost, ease of processibility, and chemical and physical properties [1,4]. Furthermore, starch can be used in natural or modified form. Native starch has drawbacks, such as poor mechanical properties, high hydrophilicity, and high biodegradability. Thus, researchers are exploring starch modification techniques to improve its properties and develop novel composites [1].
Starch can be modified into nanoparticles and can also undergo various physical (milling, blending with other polymers, extrusion, plasticizers, etc.) and chemical (substitution, graft co-polymerization, cross-linking, oxidation, etherification, esterification, dual modification, etc.) modifications to produce materials with novel properties [9,10,11,12].
Starch can be reinforced with starch nanoparticle/starch nanocrystals and nano polymers such as nanoclay (montmorillonites [MMTs], halloysites nanotubes [HNTs]), carbon nanotubes (CNTs), and nanofibers and nanowhiskers (cellulose, chitin) and metal and metal oxides (TiO2 NPs, ZnO NPs, etc.) to achieve desirable properties and produce potential green sustainable nanocomposite materials [4,7,13]. The addition of nanofillers and additives with antioxidant and antimicrobial properties has been shown to improve or minimally affect biodegradation of starch-based nanocomposites [5,14,15]. Lifecycle assessments on starch and starch-based composites ensure their lower environmental impact and sustainable alternative for petrochemical-based polymers [16,17,18].
This review mainly discusses the starch-based nanocomposites in regard to starch and its nanostructures, various starch-based nanocomposites mainly reinforced with nano polymers, such as nanoclay, carbon-based materials, nanocellulose, and chitin NPs), and their applications, particularly in the fields of agriculture, packaging, biomedicine, and the environment. Moreover, this paper highlights the lifecycle analysis and degradation of various starch-based nanocomposites in order to analyze their environmental impact.
2. Starch
Starch is a polysaccharide and is renewable, inexpensive, biodegradable, and readily available. Starch contains two polymers (glucans) known as amylose (10–30%) and amylopectin (70–90%). Amylose is a linear chain of D-glucose units linked by the α-(1,4) glycosylic bonds, while amylopectin is a highly branched and high molecular weight chain composed of D-glucose repeating units linked by α-(1,4) glycosylic bonds and α-(1,6) glycosidic bonds. The amylopectin chain contains 10–60 glucose units, and the side chains consist of 15–45 glucose units with about 5% of α-(1,6) branching points [6,7]. Amylose and amylopectin are radially arranged in an alternating concentric (amorphous and semi-crystalline) ring in starch granules. Amylopectin is radially arranged in granules and contributes to its crystalline nature (double helices region), and single helices amylose is randomly distributed among amylopectin clusters. Amylose and the branching point of amylopectin form the amorphous region [19,20,21]. Figure 1 illustrates the structure of the starch granule and the chemical structure of amylopectin and amylose.
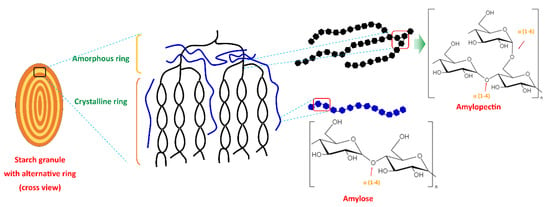
Figure 1.
Starch granule structure and the chemical structure of amylopectin and amylose.
Starch is a primary energy source in plants, which is stored in various parts, including the roots, tubers, seeds, and stems [6]. Various plant sources, such as corn, potato, wheat, cassava, rice, corn, barley, rye, millet, peas, mung beans, lentils, arrowroot, sago, sorghum, banana, yam, and many others, are utilized to obtain starch [22,23,24].
Starches from different sources show variation in their chemical composition (α-glucans, moisture, lipids, proteins, and phosphorylated residues), the structure of glucan components (amylose and amylose), and starch granule size and shape due to genetic and environmental factors [25,26].
Starch granules’ size and shape can vary with the content, structure, and arrangement of amylose and amylopectin [25]. Starch granules are found in various sizes ranging from 2–150 µm and packed with amylose and amylopectin content. Regular starch granules contain amylose in the range of 15–30% but can be varied in the range of 0–78%. Waxy starch contains lower or no amylose, whereas high-amylose starch consists of more than 50% amylose [7,23]. Table 1 shows the amylose contents of various starch sources.

Table 1.
Amylose and amylopectin contents of starch from various sources.
Starch-based hydrogel is formed via gelatinization of starch during heating with excess water and followed by three-dimensional network formation by retrogradation [37]. Gelatinization of starch is an irreversible process that occurs through the absorption of water and disruption of the crystalline structure of starch granules by hydrogen bond breakage, swelling, the disintegration of starch granules, leaching of amylose that increases viscosity and solubilization of starch molecules [32,35,37].
Amylose and amylopectin content, amylopectin structure (molar mass or chain length), and starch granule size influence the chemical, physical, optical/transparency, and functional properties (water uptake, swelling, gelatinization, pasting [pasting viscosity and temperature], retrogradation, and susceptibility to enzymatic hydrolysis of starch [7,20,23,36,38].
Amylopectin contributes to water absorption, swelling, and pasting of starch granules, whereas amylose hinders the swelling property in the presence of lipids, thus preventing gelatinization power [32,38]. Furthermore, short-chain amylopectin showed better swelling power than that of long-chain amylopectin, indicating that starch with higher crystallinity reduces the swelling power [38]. Smaller granule size increases hydration, thus increasing the swelling, viscosity, and gelatinization properties [26].
Amylose content is negatively correlated with swelling power, gelatinization temperature, and the enthalpy of gelatinization required to disrupt the crystalline structure [35]. Waxy starch has a higher degree of crystallinity and higher gelatinization temperature than starch with high amylose content [31,35]. Amylose in starch has a high tendency for retrogradation due to its linear structure. However, the retrogradation properties of starch are mainly determined by the degree of crystallinity and gelatinization temperature than the amylose content [35].
Amylose–amylopectin ratio also influences thermal, mechanical, and barrier properties. Basiak et al. [23] reported that potato starch, containing lower amylose (20%) than that of wheat (25%) and corn (27%) starch, exhibited greater mechanical properties and lower water solubility, water vapor, and oxygen permeability. Other than that, optical properties were influenced by the amylose/amylopectin ratio: the potato (lower amylose) film was transparent, whereas corn and wheat films were opalescent.
However, applications of starch have been limited due to their poor performance, such as through their brittleness, high water sensitivity, poor gas and moisture barrier, susceptibility to retrogradation, high viscosity, and limited solubility [13,39]. Therefore, plasticizers, chemical modifiers, and incorporating nanofillers, such as starch nanoparticles, nanoparticles, nanoclay, nanofibers, and others, have been used to improve the properties of starch [39].
3. Nanomaterials and Nanocomposites
Nanomaterials are referred to as materials which have at least one of their dimensions less than 100 nm. Based on the definition, a thin film with <100 nm thickness is a nanomaterial as one of the dimensions is nanometric. Likewise, nanomaterials such as nanofibers, nanowires, and nanorods have two dimensions on the nanoscale, whereas quantum dots, nanoparticles, dendrimers, and fullerene have three dimensions in the nanometer range (Figure 2) [40].
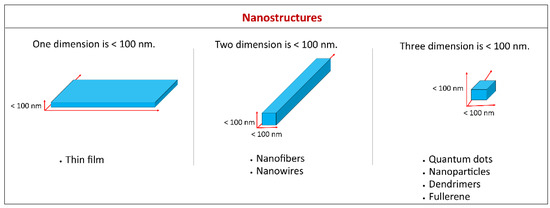
Figure 2.
Examples of various types of nanomaterials based on the number of dimensions in the nanometer range.
Nanomaterials can be classified based on dimensionality (number of dimensions with a length larger than 100 nm), as shown in Figure 3: 0D, 1D, 2D, and 3D. Zero dimension (0D), including spheres, hollow spheres, clusters, quantum dots, and metals, have no dimension of particles larger than 100 nm, i.e., all dimensions in the nanoscale. One-dimensional (1D) nanomaterials, such as nanorods, nanowires, nanofibers, and nanotubes, have one dimension, not in the nanoscale (>100 nm) and the other two are in the nanoscale, whereas two-dimensional (2D) nanomaterials, including thin film, nanocoatings, nanoplates, and nanolayers, have two dimensions, not in nanoscale and another one in nanoscale. Three-dimensional (3D) is the combination of nanocrystals in different directions which have various dimensions above 100 nm. Figure 3 depicts the classification of nanomaterials based on dimensionality [40,41,42].
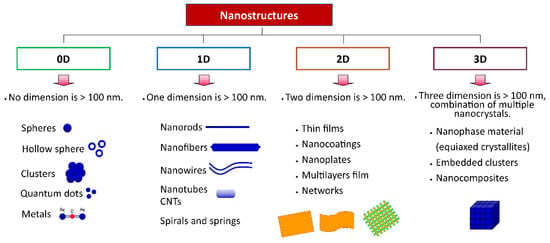
Figure 3.
Classification of materials based on dimensionality.
Nanomaterials can be synthesized by two approaches: top-down and bottom-up approaches (Figure 4). In the top-down method, the bulk material is restructured into nanomaterials using mechanical grinding/milling, ball milling, polishing, lithography, and other means. While in the bottom-up method, nanomaterials are assembled from atomic range particles/molecules or nanoclusters through the sol–gel method, spinning, molecular self-assembly, pyrolysis and condensation, vapor phase deposition, and other methods [40,41].
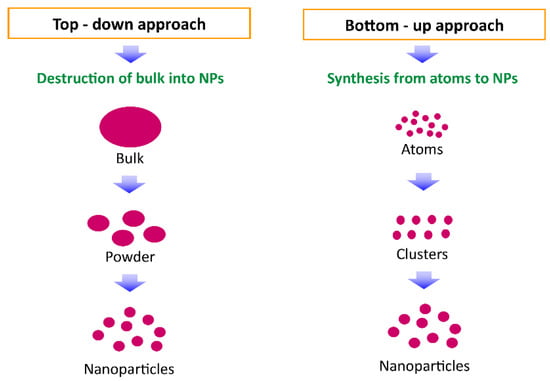
Figure 4.
Nanoparticle synthesis methods: top-down and bottom-up approach.
Composite materials consist of two or more dissimilar materials, which are composed of two major constituents: (1) a matrix as a continuous phase (polymer, ceramic, or metal) and (2) reinforcement materials as an un-continuous phase. Bionanocomposites are composite materials that are composed of biopolymers and particles with at least one dimension in the nanometer range (1–100 nm). Bionanocomposites can also be referred to as green composites or biohybrids, or bioplastics [3,43].
Nanocomposites can be classified into three categories based on the morphology of reinforced nanoparticles: (1) particulate/iso-dimensional (silica, metal NPs, metal oxides), (2) layered (monolayered clays, layered double hydroxides), and (3) elongated (cellulose nanofibrils [CNF], carbon nanotubes [CNTs]) nanoparticles [3,44]. Particulate reinforcements are used to enhance resistance to flammability and reduce permeability and cost, whereas layered reinforcements are used for their superior mechanical behavior [43]. Furthermore, based on the degree of dispersion of particles in the matrix, layered nanocomposites have three subclasses, including intercalated, exfoliated, and flocculated/phase-separated nanocomposites (micro-composites) [3,6,43]. Flocculated/phase-separated nanocomposites are formed without a partition between individual layers due to the particle–particle interactions, polymer chains are intercalated between sheets of layered nanoparticles in intercalated nanocomposites, and exfoliated nanocomposites are formed by partition between individual layers (Figure 5) [43].
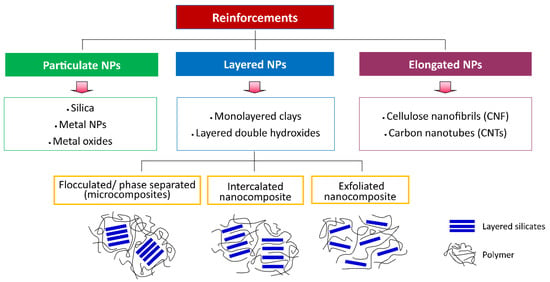
Figure 5.
Classification of the nanocomposites.
4. Starch Nanoparticles (SNPs)
Starch nanoparticles (SNPs) are mainly synthesized by the methods of hydrolysis (acid or enzymatic), regeneration, and physical treatments (milling, high-pressure homogenization, gamma radiation, and ultra-sonication) [45].
SNPs are mainly used as fillers in a polymer matrix to improve their reinforcing effect and mechanical and barrier properties [13]. Nanoparticles have a large surface area/volume ratio, allowing a great interaction capacity, which makes them potential reinforcement materials [46]. SNPs are non-toxic and can be used to prepare nanocomposite, absorbent, carrier (encapsulation), and emulsion stabilizers for food and non-food applications [45,47,48].
Santana et al. [46] reported the SNP obtained from ultrasound showed a significantly higher yield than SNP synthesized by acid hydrolysis. In addition, incorporating SNPs reduced the water vapor permeability of starch film [46]. Lin et al. [49] prepared debranched starch nanoparticles (DSNPs) by reverse emulsification using debranched waxy corn starch (98% of amylopectin), which showed a higher crystallinity and melting temperature than that of native waxy corn starch. Furthermore, the addition of debranched starch nanoparticles (5 wt.%) into corn starch films improved the tensile strength by 85.9% and decreased water vapor permeability and the oxygen transmission rate by 30.94% and 79.31%, respectively.
In another study, starch NPs prepared by acid hydrolysis containing Ag NPs showed good antibacterial activity against Staphylococcus aureus, Salmonella typhi, and Escherichia coli which has the potential to be used as a coating material for food packaging [50].
5. Starch-Based Nanocomposites
Native starch or thermoplastic starch (TPS) has poor mechanical properties (fragility/brittleness), low thermal stability, hydrophilicity, high water vapor permeation, poor resistance to external factors (humidity, tearing, picking, etc.), and a lack of compatibility with hydrophobic polymers [7,12,51]. Therefore, starch is blended with other natural and synthetic polymers or incorporated with various nanomaterials to enhance the physical, mechanical, and barrier properties [7]. Compared with bulk materials, nanoparticles have a surface area/volume ratio and possess unique physical, mechanical, optical, magnetic, electrical, and other properties [42]. Hence, recently, bionanocomposites can be a promising material to enhance mechanical and barrier properties [52]. Starch reinforced with nanofillers, including nanocellulose, chitin nanoparticle, nanoclay, and carbon-based materials, are discussed below.
5.1. Starch/Nanocellulose Composite
Cellulose is the primary component of the plant cell wall and can be extracted from plants, invertebrates, marine animals, algae, fungi, and bacteria [53]. It is the most abundant natural polymer and is popular for its mechanical properties, reinforcement capabilities, low density, renewability, low toxicity, and biodegradability [54]. Cellulose is the polymer of D-glucose units linked by β-(1,4)-glycosidic bonds, and higher hydroxyl groups (-OH and -CH2-OH) at equatorial positions give higher stability (Figure 6) [55]. Cellulose fibres are formed with strong inter and intramolecular hydrogen bonds and aggregate with highly ordered (crystalline) and disordered regions (amorphous) [56]. Nanocellulose is a nanostructure of cellulose and has drawn much attention over the past years due to its excellent characteristics, including its high aspect ratio (length to diameter), improved mechanical and thermal properties, crystallinity, flexibility, renewability, abundance, biocompatibility, and biodegradability [55,57].
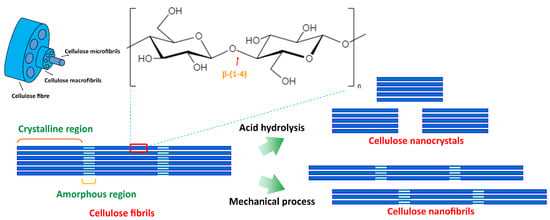
Figure 6.
Cellulose chemical structure and schematic diagram of the formation of cellulose nanocrystals and cellulose nanofibrils.
Nanocellulose can be produced by top-down and bottom-down processes (Figure 6) [53,54] using various techniques, including enzymatic techniques, chemical hydrolysis, and mechanical treatments, including high-pressure homogenization, grinding, cryo-crushing, micro-fluidization, and high-intensity ultrasonication [46,53,54]. These synthetic techniques and conditions influence the dimensions, composition, and properties of nanocellulose. Nanocellulose can be generated in three forms: (1) cellulose nanofibrils (CNFs) and (2) cellulose nanocrystals (CNCs) from woods and other lignocellulosic materials using a top-down process, and (3) bacterial cellulose (BC) from the biosynthesis of bacteria using a bottom-to-top process. Figure 7 summarizes the three forms of cellulose and synthesis methods [53,55].

Figure 7.
Types of nanocelluloses.
Nanocellulose is widely used in various applications, such as biomedical engineering, the automotive industry, electronics, food packaging, cosmetics, construction, textiles, wood adhesives, and wastewater treatment applications [53,57].
Othman et al. [58] prepared the corn starch (CS) film reinforced with nanocellulose fiber (NCF) and thymol, a compound extracted from the essential oil of thyme, which has antioxidant and antimicrobial properties. They reported that adding 1.5% of NCF improved the thermal stability, mechanical, and barrier (water vapor and oxygen) properties of corn starch film. The CS/NCF/thymol composite reported improved thermal stability and flexibility. However, a significant reduction was observed with tensile strength, Young’s modulus, and barrier properties [58]. In another study, starch from an unripe plantain bananas reinforced with cellulose nanofibers from banana peels improved the mechanical and water vapor barrier properties [59]. Starch/CNC nanocomposites were reported to improve the tensile strength (2.8 to 17.4 MPa), Young’s modulus (112 to 520 MPa), and water barrier properties, as well as reduce the water solubility (26.6 to 18.5%) and contact angle 38.2 to 96.3° [60].
5.2. Starch/Chitin Nanoparticles Composites
Chitin is the second most abundant natural polysaccharide next to cellulose and is found in the shell of crustaceans (crab, lobster, and shrimp), the exoskeleton of arthropods, molluscan shells of squid, mushrooms, the cell wall of algae and fungi (yeast and mold). Chitin is composed of N-acetyl-2-amido-2-deoxy-β-D-glucose (N-acetylglucosamine) units linked with a β-(1,4)-glycosidic bond, in which acetamide groups (−NHCOCH3) consists at the C2 of cellulose monomer. Chitosan is derived from the alkaline deacetylation of chitin (Figure 8). Chitin crystals are found in three forms: α-chitins (which contain antiparallel cellulose chains), β-chitins (parallel cellulose chains), and γ-chitin (among three chains, two of them are in the same direction, and one is in the opposite direction) [61,62,63].
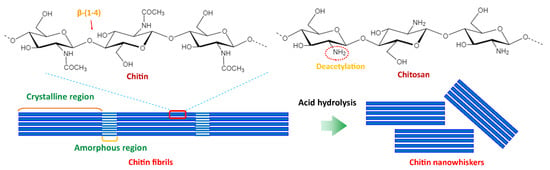
Figure 8.
Chemical structure of chitin and schematic diagram of the formation of chitin nanowhiskers.
Chitin nanomaterial can be prepared through top-down and bottom-up approaches. Chitin fibrils consist of amorphous and crystalline regions and thus can be converted into three types of nano-chitins in a top-down approach: nanocrystals (via acid hydrolysis), nanofibers (via mechanical treatments), and nanowhiskers (consecutive acid hydrolysis at a high temperature and mechanical treatments) [39,62].
Nano-chitin has been widely studied for its high aspect ratio, high surface area, good mechanical properties, lightweight/low density, good chemical stability, renewability, non-toxicity, and antibacterial properties, and it is used in biomedicine, packaging, water treatment, green electronics, cosmetics, and many other applications [61,63].
A combination of chitin nanofibers and starch nanoparticles showed higher emulsion stability over a range of pHs and temperatures and can be used as an emulsion stabilizer in various products, such as food, paint, coating, cosmetics, and pharmaceuticals [48].
Chang et al. [64] reported chitin nanoparticles (CNPs) exhibited lower crystallinity than chitin whiskers. At a low level of CNPs, tensile strength, storage modulus, glass transition temperature, and water vapor barrier properties of plasticized potato starch/CNPs nanocomposite due to good interfacial interaction between CNPs’ nanofiller and starch matrix.
By adding 5 wt.% chitin nanofibers (CNF) obtained from the fungus Mucor indicus, Young’s modulus and the tensile strength of TPS were enhanced by 239% and 216%, respectively, and moisture absorption was reduced from 51% to 38%. However, the addition of CNF at a higher level increased moisture absorption and reduced the mechanical properties of TPS [39]. In another study, Heidari et al. [61] reported that CNF/TPS nanocomposite films were more permeable to water vapor than pure CNF film. CNF at higher levels lowers the dispersion of nanofiller and tends to agglomerate, which leads to poor water vapor barrier and mechanical properties. In addition to that, the presence of excessive NH2 groups at the CNF surface may increase the affinity to water, thereby increasing water absorption [39,61].
5.3. Starch/Nanoclay Nanocomposites
Clay is a polymer composite of two-dimensional layered mineral silicates. The single layer is formed by the edge-linked octahedral sheet of aluminum or magnesium oxide sandwiched between two tetrahedral silicate sheets. As shown in Figure 6, three types of polymer-based nanocomposites can be obtained based on the polymer and silicate layers. Silicate clay is characterized by important physical properties, such as a cation exchange capacity and specific surface area [65,66]. Polymer/nanoclay composites are used in the automotive industry, aeronautical industry, packaging, flame-resistant materials, biomedical applications, and wastewater treatment [67,68]. Nanoclays can be categorized into several classes: smectite, chlorite, kaolinite, illite, and halloysite [68].
Plate-like montmorillonite (MMT) (smectite), a multilayer-aluminosilicates, has been widely studied as a reinforcing material in polymers due to its excellent cation exchange capacity, swelling behavior, and large surface area [68,69]. MMT also improved the thermal stability, mechanical, optical, and barrier properties, even at their lower concentration [70].
Mohan et al. [15] reported that the incorporation of MMT nanoclay into corn starch-based film resulted in a significant reduction in water absorption (by 22%), moisture uptake (40%), oxygen permeation (30%), and swelling thickness (31%) in comparison to corn starch film. Furthermore, the concentration of MMT nanoclay determines the structure of the nanocomposite. X-ray diffraction (XRD) analysis revealed that the intercalated nanoclay structure forms at a higher concentration (>2%), whereas the exfoliated structure forms at a lower concentration in the polymer matrix [15]. In another study, MMT addition was also shown to improve the tensile strength and biodegradability in cross-linked PLA/maleated TPS nanocomposite [71]. Biodegradable nanocomposites fabricated from cross-linked wheat starch (CLWS)/sodium montmorillonite (Na-MMT)/TiO2 NPs showed an exfoliated structure. Incorporating Na-MMT and TiO2 NPs reduced the water vapor permeability and water solubility of the CLWS film, whereas thermal stability, tensile strength, and Young’s modulus were increased. TiO2 NPs showed better UV-blocking properties than Na-MMT [69]. Maize starch/glycerol (20%)/Na-MMT (10%) nanocomposite also showed intercalated structures and improved tensile properties [66].
Iamareerat et al. [72] prepared nanocomposite film with plasticized cassava starch incorporated with sodium-bentonite and cinnamon essential oil. The addition of sodium-bentonite nanoclay (0.5–0.75%) decreased the water vapor permeability in plasticized cassava starch with 2% glycerol film. Further addition of cinnamon essential oil into the CS/glycerol (2%)/sodium-bentonite (0.75%) showed better antibacterial activity and significantly inhibited microbial growth in pork meatballs, despite the increase in water vapor permeability.
Halloysites clay nanotubes (HNTs), aluminosilicate hollow cylinders, have a lower hydroxyl group on the surface than other silicates such as MMT, making them a promising reinforcement material for polymers [68,73]. Furthermore, HNTs exhibit exfoliated structures due to their high aspect ratio [73]. Dang et al. [73] revealed that the addition of modified or unmodified HNTs into the TPS/poly(butylene adipate-co-terephthalate) (PBAT) blend improved the thermal and mechanical properties without loss of ductility of the plasticized wheat starch matrix [74]. Another investigation on PVA/starch/glycerol/HNTs nanocomposites revealed that their hydrophobic nature and biodegradability decreased with the addition of HNTs [75].
5.4. Starch/Carbonaceous Nanocomposites
Fullerenes, diamonds, carbon nanotubes (CNTs), graphene, and their derivatives are common carbon allotropes used in carbon-based nanocomposites [76].
CNTs found in two forms, single-walled (SWCNT) or multi-walled carbon nanotubes (MWCNT), have been widely studied as reinforcing fillers for TPS nanocomposite films [77]. CNTs have a larger surface area, excellent electrical conductivity, mechanical and thermal properties and they also have a higher volume-to-area ratio compared to that of other nanoparticles and they are widely used in various biomedical applications, environmental pollution control, sensing and detection, the automobile industry, and secondary food packaging. Direct contact food packaging materials are limited by their migration and potential toxicity [76,78,79].
Electrically conductive biocomposite films have gained popularity in various electronic, biomedical, and food packaging applications [22]. Potato starch-based film reinforced with MWCNT and ionic surfactants (sodium cholate, SC; cetyltrimethylammonium bromide, CTAB) decreased the contact angle and showed improved antioxidant properties (30.2 and 12% of scavenging activity, respectively) due to the presence of MWCNT. Surfactant SC showed better dispersibility of MWCNT in a potato starch matrix with improved mechanical properties and crystallinity [22].
Starch plasticized with ionic liquids reduces the retrogradation resulting in increased film stability and it has the potential use in ionically conducting solid polymers. The addition of nanofiller MWCNT at 0.5 wt.% in starch plasticized with ionic liquid, 1-ethyl-3-methylimidazolium acetate ([emim+][Ac−]) significantly increased the tensile strength by 327%, Young’s modulus by 2484%, and elongation at break 82% (from 30 to 69%). Moreover, electrical conductivity was increased with MWCNT content (wt.%) and reached a maximum (56.3 S/m) at 5 wt.% MWCNT. MWCNT/starch plasticized with [emim+][Ac−] showed electroconductive properties because of its ionic nature of ionic liquids and the excellent electrical conductivity of MWCNT [77]. A starch–iodine complex matrix reinforced with a small amount of MWCNT (0.055%) reduced the water vapor permeability by 43% [78].
Graphene is a two-dimensional material arranged in a hexagonal lattice. Plasticized starch incorporated with reduced graphene oxide (rGO), a derivative of graphene, exhibited increased conductivity and dielectric properties, which could make it a potential candidate for producing sustainable bio-friendly electronic devices [80].
Investigation of poly(lactic acid) (PLA)/thermoplastic starch (TPS)/graphene nanoplatelets (GNP) blends revealed that the addition of GNP increased the crystallinity of the PLA/TPS blend, and the maximum crystallinity (68.39%) was observed with PLA (70%)/TPS (30%)/GNP (1%). Further increases in GNP resulted in the reduction of compatibility [81].
6. Applications of Biodegradable Starch-Based Nanocomposites
Biodegradable starch-based nanocomposites have been used in agriculture, packaging, biomedical, environment, and many other fields (Figure 9).
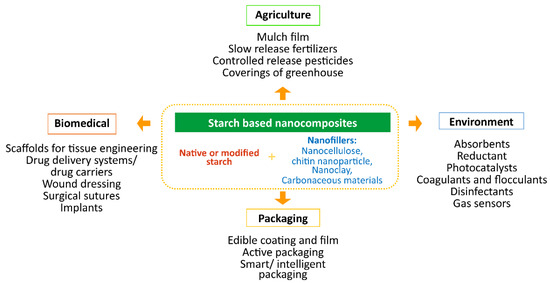
Figure 9.
Applications of starch-based nanocomposites.
6.1. Agriculture
In recent years, biodegradable films have been developed for agricultural purposes, particularly for mulching applications, the coverings of a greenhouse, and the controlled/slow release of agrochemicals such as fertilizers and pesticides [82,83,84].
Agricultural mulches are used to prevent the hindrance caused by the weeds’ growth, maintain soil wetness, and regulate soil temperature [85]. Interaction with water (water vapor permeability, contact angle, and water solubility/resistance) and environmental factors (thermal stability) are important parameters in mulch films. Mulch films must have a very low water vapor permeability to maintain the soil moisture by reducing the water loss by evaporation. Since mulch films are exposed to outdoor conditions, improving the thermal stability is therefore essential [83,86].
Pesticides protect the crop from pests, pathogens, weeds, and insects by destroying, attacking, mitigating, or repelling activity, whereas fertilizers are essential in agriculture to increase crop yield. However, in conventional applications, the efficiency of reaching their target sites is relatively low as they are hindered by immobilization, erosion, volatilization, leaching, surface runoff, or scavenging by soil. In addition, water is also an essential factor in crop growth and driving off fertilizers. Therefore, management of nutrient/pesticide active compounds and water loss is essential for crop production. To reduce the loss and improve their utilization efficiency, slow-release fertilizers or controlled-release pesticides with improved water retention and water holding capacity can be formulated by incorporating nanomaterial into biopolymers [82,84,87,88].
Merino et al. [83] investigated the water and light interaction with corn starch-based mulch film. The study revealed that the addition of chitosan/bentonite nanofiller into native and oxidized thermoplastic corn starch improved the water resistance, radiometric, and antibacterial properties without having a significant effect on the water vapor permeation and mechanical properties [83]. In another study, Merino et al. [86] reported that the addition of bentonite/chitosan into both matrixes, native and oxidized thermoplastic corn starch, increased the crystallinity (3.0 and 3.4%) and slightly increased thermal stability in comparison to the addition of natural bentonite.
Superabsorbent hydrogels are widely used in bi-functional (retain and supply water and nutrient over a long period) slow-release fertilizers due to their water retention properties. The addition of natural char nanoparticles (NCNPs) into corn starch-g-poly(AA-co-AAm) encapsulated urea provided high biodegradability and improved the soil water-retention capacity along with the slow release of urea [84]. Chitosan (CS)/sago starch (ST)/nano zeolite (NZ) nanocomposite released 64% of phosphorus and 41.93% of urea after 14 days and increased the water retention capacity. Furthermore, CS/ST/NZ nanocomposites showed better growth indexes in Philodendron spp. compared to the direct application of urea, suggesting the efficacy of nanocomposites in slow-release fertilizer formulation [88]. Urea encapsulated with starch (10%)/PVA (5%) with crosslinker acrylic acid (2%) and citric acid (2%) showed higher nitrogen-releasing efficiency, 70.10 and 50.74%, respectively, as well as improved growth factors in spinach plants [89]. Modified starch (esterified with dicarboxylic acid chloride)/organobentonite-based composites regulate the effective controlled release of encapsulated pesticide atrazine [90].
6.2. Packaging
Food packaging protects food from humidity, high/low temperatures, and other physiological factors and aids in food quality monitoring and control in the food supply chain and during storage (gas sensors, electronic nose) [91]. Starch has been used in food packaging applications because of its strong mechanical properties, transparent/translucent appearance, and tasteless and flavorless characteristics [69]. Brittleness and poor water vapor barrier properties limit their applications. Nanoparticle reinforcement can improve the mechanical properties, hydrophobicity, water vapor and oxygen barrier, UV barrier, thermal properties, and other functional properties (antioxidant, antimicrobial, etc.) of starch which makes nanoparticles a potent material for edible film/coating, active and intelligent/smart packaging for protecting or maintaining and monitoring the quality of food materials [91,92,93].
Organic or inorganic nanofillers have been widely studied for food packaging applications, whereas organic nanofillers include nanoclay (MMTs, HNTs), natural biopolymers (chitosan, cellulose), and natural antimicrobial agents (nisin), and inorganic nanofillers includes metals (Ag, Au, Cu), and metal oxides (ZnO, TiO2, Ag2O, MgO, CuO, SnO2) [44,52,91].
The suitability of a film for packaging materials is mainly assessed by water vapor and oxygen barrier properties and good heat salability [94]. Furthermore, a film with improved mechanical strength and flexibility protects against shock and other physical damage. TiO2 NPs reinforcement in potato starch-based composite films led to a reduction in water solubility, moisture uptake, and water vapor permeability, and an increment of UV barrier properties and tensile strength of the film, showing its potential for food packaging [92]. Na-MMT and TiO2 NPs reduce the hydrophilicity and improve mechanical, water vapor, and UV barrier properties in cross-linked wheat starch, which makes them a suitable material for food packaging [69]. UV barrier packaging film from starch/kefiran/ZnO NPs showed improved tensile strength, Young’s modulus, and thermal stability (increased melting temperature), which are beneficial to the packaging system [95]. Starch NPs/Ag NPs showed increased antibacterial activity against Staphylococcus aureus, Salmonella typhi, and Escherichia coli and can be used as an antibacterial food coating material [50]. Linseed polyol increased the contact angle, water absorption capacity, thermal stability, and biodegradation of polyvinyl alcohol/corn starch film. Further addition of Ag NPs showed antimicrobial behavior against Proteus mirabilis, Candida albicans, Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, Klebsiella pneumoniae, among others, which shows the potential applications in antimicrobial packaging [96]. Poly(ethyl methacrylate)-co-starch (PEMA-co-starch)/graphene oxide/Ag NPs (2 wt.%) nanocomposite film showed improved thermal stability, chemical resistance, tensile strength, oxygen barrier properties, and antimicrobial properties against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis [97].
Plasticised corn starch films reinforced with nanocellulose improved the mechanical strength, flexibility, and water vapor and oxygen barrier properties that have a beneficial effect on reducing the oxidation of oil during storage. This film showed good heat salability, which further prevents oxygen and water vapor transmission. Moreover, the storage study ensures that this plasticized corn starch-based nanocomposite can be used as an alternative packaging material for storing edible oils at ambient conditions (27 ± 3 °C temperature, 65 ± 5% RH) for more than three months without affecting the oil quality in terms of rancidity, viscosity, and color [94].
Starch from potato, wheat, and corn blended with carboxyl methylcellulose (CMC)/Na-MMT has potential applications in food packaging [98]. Cellulose nanocrystals (CNC) obtained from sugarcane bagasse blending with starch improved mechanical, water resistance, and water barrier properties and decreased surface hydrophilicity (contact angle > 90°), which makes this starch/CNC nanocomposite a potential food packaging material [60]. Heidari et al. [61] developed edible food packaging using chitin nanofibers (CNF)/TPS nanocomposite.
TPS/MMT/carvacrol essential oil showed biocidal effects against Escherichia coli due to the synergistic antibacterial effect of carvacrol essential oil and MMT suggesting the applications in antimicrobial packaging [99]. Packaging material fabricated with sweet potato starch (SPS)/MMT/thyme essential oil (TEO) was studied by Issa et al. [100]. They reported that the addition of MMT improved the mechanical and water barrier properties of SPS, whereas biodegradability decreased. However, incorporating TEO decreased the tensile strength, elongation, Young’s modulus, and water barrier with improved biodegradability in SPS/MMT. The nanocomposite made from cassava starch/glycerol (2%)/Na-bentonite (0.75%)/cinnamon essential oil (2.5%) exhibited antibacterial activity against Escherichia coli, Salmonella typhimurium, and Staphylococcus aureus, and significantly inhibited the microbial growth in pork meatballs stored under ambient and refrigeration conditions [72].
The addition of potassium sorbate, a commonly used preservative, into starch/nanoclay films controlled the migration of sorbate, resulting in the retention of antimicrobial activity for a long period [101]. Chen et al. [102] also developed a controlled-release active film from starch/polyvinyl alcohol (PVA) incorporated with cinnamaldehyde and microfibrillated cellulose (MFC). The addition of MFC was found to improve the tensile strength, crystallinity, hydrophobicity, and antimicrobial activity (against S. putrefaciens) with reduced flexibility. The oxygen and water vapor permeability reduced at 1.0 and 2.5% MFC but increased at higher concentrations. In addition, MFC, at 1 and 7.5%, controlled the release of cinnamaldehyde.
Smart packaging materials for monitoring the spoilage of milk packed in a bottle were developed by incorporating pH indicators, including bromocresol green (BG) and methyl orange (MO), into a starch/nanoclay nanocomposite [93]. Further nanometals (TiO2, SnO2, Ag2O, MgO, ZnO, CuO) can be used in gas sensors to monitor food quality [91].
6.3. Biomedical
Biodegradable polymers, including starch-based bionanocomposites, are widely used as scaffolds for tissue engineering, drug delivery systems/drug carriers, wound dressing, surgical sutures, and implants due to their mechanical properties, biocompatibility, biodegradability, and also the generation of non-toxic, biodegradable products [103,104,105].
Biopolymers in the repair of healing tissues accelerate treatment processes and eliminate implant removal surgery. Furthermore, implant materials and their biodegradable products must be non-cytotoxic and biocompatible [105]. Incorporating bioactive beta-tricalcium phosphate (β-TCP) nanoparticles (at 10%) into thermoplastic starch (TPS) drastically improved the mechanical properties and showed excellent biocompatibility with no cytotoxic effect for bone tissue engineering materials [105]. Waghmare et al. [106] fabricated starch-based nanofibrous scaffolds by electrospinning for wound healing applications.
Hydroxyapatite has been used widely in biomedical applications due to its biocompatibility and osteoconductive (cell regeneration process) properties. However, brittleness and lack of flexibility limit the applications. The combination of hydroxyapatite with starch materials can reduce brittleness, and the polar nature of starch encourages a good adhesion between starch and hydroxyapatite. Sadjadi et al. [107] synthesized a nanocomposite from starch/nano-hydroxyapatite, which possesses mechanical and biological properties identical to natural bone.
Abdel-Halim and Al-Deyab [108] reported that Ag NPs/starch/polyacrylamide nanocomposite hydrogel showed antimicrobial activity against fungi (Aspergillus flavus and Candida albicans) and bacteria (Staphylococcus aureus and Escherichia coli). PVA/starch incorporated with Ag NPs synthesized from green methods (Diospyros lotus fruit extract) has the potential to be used in wound dressing as it shows increased swelling and moisture retention capacity and reduced water vapor transmission that prevents the wound from dehydration and better antimicrobial activity against Escherichia coli and Staphylococcus aureus [109].
The ternary blend was developed by mixing polylactic acid (PLA)/starch (S)/poly-ε-caprolactone (PCL) with nano-hydroxyapatite (nHAp) for controlled release of antibacterial triclosan. The incorporation of nHA (3%) improved the hydrolytic hydrophilicity, hydrolytic degradation, antibacterial activity (against Escherichia coli and Staphylococcus aureus), and drug release of PLA/S/PCL film. An increase in nHA content (1–7%) improved the biodegradation (13–10 months), and the antibacterial triclosan release rate of PLA/S/PCL/nHA film at 37 °C in buffer solution was increased (0.12–0.18 μg/mL every day), which is in the range of MIC of triclosan (0.025–1 μg/mL). Furthermore, the degradation and release time of PLA/S/PCL/nHA (3 wt.%) nanocomposite showed similar profiles that ensure continuous drug release during the application [110]. Mallakpour and khodadadzadeh [111] also developed starch/MWCNT modified with glucose (MWCNT-G) nanocomposites for slow release of zolpidem drug delivery. Gao et al. [112] developed spherical core-shell Ag/starch NPs using green synthesis for slow-released nano silver as an antibacterial material which can be used in pharmaceutical and biomedical applications.
Nezami et al. [113] fabricated pH-sensitive magnetic nanocomposite hydrogel using graft copolymerization of itaconic acid (IA) and starch in the presence of magnetic Fe3O4 NPs (St-IA/Fe3O4) for the controlled-release of guaifenesin (GFN) with low cytotoxicity. A nanocomposite with magnetic Fe3O4 NPs at 0.83% significantly enhanced the drug release from 54.1 to 90.4% within 24 h in pH 7.4 [113].
Starch-based-fluorescent organic nanoparticles (FONs) reported high water dispersibility and excellent biocompatibility (cell viability was 99.69% at the concentration of FONs 100 µg/mL after 24 h). Thus, FONs are a promising candidate for biomedical applications that can be potentially used as fluorescence probes and carriers for delivering biologically active components [114].
6.4. Environment
Extensive agricultural and industrial practices lead to the accumulation of various contaminants, including heavy metals and metalloids (Cr6+, Hg2+, Zn2+, Pb2+, Co2+, Cd2+, Cu2+, etc.), dyes, organic substances (pesticides, herbicides, fertilizers, aliphatic and aromatic hydrocarbons, volatile organic compounds [VOCs], oil spills), pathogenic microbes (virus, bacteria, fungi), and toxic gases (nitrogen oxides, SO2, CO) in water, soil, and air [115].
Starch-based nanocomposites with various nanofillers, including metal (Ag, Au, and Pd NPs), bimetal (Ag/Au), metal oxides (TiO2, ZnO, Fe2O3, MnO2), nanoscale zero-valent iron (nZVI) (Fe⁰), carbonaceous materials (CNTs [SWCNTs and MWCNTs], graphene, graphene oxide), nanoclays (MMTs, HNTs, bentonite), and polymers (chitin, cellulose nanowhiskers) are used in materials as recyclable and reusable filters, absorbents, reductants, photocatalysts, coagulants and flocculants, disinfectants, and gas sensors to detect or remediate contaminants, such as dyes, heavy metals ions (As, Pb2+, Cr6+, Cu2+, Cd2+, Hg2+, Ni2+, Co2+, etc.), various aromatic derivatives, fertilizers (urea), and other organic pollutants [116,117,118,119,120,121,122,123,124].
Green synthesis of Ag/Au bimetallic nanocomposite using graft copolymer hydroxyethyl starch-g-poly(acrylamide-co-acrylic acid) reported catalytic activities that involve the reduction of 4-nitrophenol to 4-aminophenol and degradation of azo dyes (congo red, Sudan-1, and methyl orange) by cleavage of −N = N-bond thus can be used in water treatment [122]. Gomes et al. [125] analyzed a starch/cellulose nanowhiskers hydrogel composite and highlighted the outstanding capacity for methylene blue dye removal.
Starch-graft-poly(acrylamide) (PAM)/graphene oxide (GO)/hydroxyapatite NPs (nHAp) nanocomposite was developed as a recyclable adsorbent for efficient removal of malachite green (MG) and other cationic dye from aqueous solution. The introduction of nHAp improved the biocompatibility of the PAM/GO composite, whereas the biodegradability, porosity, water content, and water uptake decreased with increasing nHAp content. Adsorption capacity increased with agitation time, pH, nHAp content, and initial dye concentration, and the optimum conditions were 60 min, pH 10, 5% nHAp, and 100 mg/L. PAM/GO and nHAp at 1–5 wt.% reported excellent porosity (31–11%), degradability (41–11% after 15 days), the maximum adsorption capacity of 297 mg/g, excellent regeneration capacity after five consecutive adsorption-desorption cycles of dye with high removal efficiency (77–86%) [126].
Adsorption is a basic principle of mechanism in targeted drug delivery, controlled release of pharmaceutically active compounds, and treatment of chemical water pollution [11]. The degree of the time dependency of kinetic coefficient (kobs) and the influencing factors (pH, temperature, initial concentration of tetracycline) are important to explore the suitability of materials in adsorption-based applications. Monodispersed starch stabilized magnetite nanoparticle (MSM) showed 70% absorption of antibiotic tetracycline within the first 5 min and reached 90% after 1 h. The degree of the time dependency of the kinetic coefficient (kobs) had a negative correlation with the initial tetracycline concentration [11].
Chitin nanowhiskers (CNW) are better nano-adsorbents due to their high surface/volume ratio and abundant hydroxyl and acetamide functional groups on the surface [63]. MMT is hydrophilic and has a high specific area [127]. The bean starch/Na-MMT nanocomposite showed high absorption capacity for heavy metals Ni2+ (97.1% at pH 4.5, initial concentration of 100 ppm) and Co2+ (78.03% at pH 6, initial concentration of 140) in comparison to the starch matrix (72 and 74.2%, respectively) [116]. Yang et al. [123] studied the material nZVI loaded on biochar stabilized by starch to remediate Cr6+.
Enzyme immobilization is an emerging technology for environmental remediation which gives many advantages over free enzymes, which include the efficiency and stability of catalytic enzymes and their enhanced recovery and reusability [128]. Further, the immobilized enzyme can be used as biosensors and biocatalysts to degrade dye from textile, leather, coloring, and printing industries [129]. Immobilized peroxidase on polymer/Fe3O4 magnetic NPs has been successfully used to remediate wastewater containing different dyes in the textile industry [128]. Immobilized phenoloxidases other than peroxidase, including laccase and tyrosinase, are also used to degrade dyes and phenolic pollutants, and lipases are used to remediate oily wastewater [130]. Mehde [131] reported that magnetic NPs/tannic acid/starch/cross-linked enzyme aggregates-peroxidase are used to remove different types of dyes, such as methylene blue, Congo red, indigo carmine, and malachite green.
6.5. Other Applications
Plasticized starch/reduced graphene oxide (rGO) nanocomposites with improved conductivity and dielectric properties can be used in bio-friendly flexible electronic devices [80]. The maize starch/glycerol (20%)/Na-MMT (10%) nanocomposite showed improved tensile properties, which can be used in lightweight architectural constructions [66]. Starch-based nanocomposites can also be used in lithium batteries, fuel cells, dye-sensitized solar cells, and electrically conductive biocomposite film for various other purposes [22,77].
Table 2 summarizes the studies reported on various biodegradable starch-based nanocomposites in regard to their applications and properties.

Table 2.
Starch-based nanocomposites using various biodegradable polymers in regard to their applications and properties.
7. Lifecycle Analysis of Nanocomposites
With increasing fossil depletion and environmental concerns, sustainable biobased materials have gained increasing interest. For biobased materials to be sustainable, preparation and processing should have limited environmental impacts [132].
The environmental credentials of bionanocomposites are evaluated by assessing their material production, product manufacturing, and product end-of-life. Many tools, including environmental impact analysis (EIA), life cycle analysis (LCA), material flow analysis (MFA), and ecological footprint (EF), are used for analyzing the environmental impacts of materials and manufacturing processes [133]. Life cycle assessment (LCA) is the most widely accepted method to assess environmental impact [134]. LCA is a science-based tool to comparatively analyze the environmental impacts of product systems concerning the extraction of raw materials, manufacturing, the use of final products, and their disposal [133,135,136].
The international organization for standardization (ISO) standardized the LCA via ISO 14040 series [134]. The two most commonly used methods are “cradle to grave” and “cradle to gate” [134,135]. The “cradle to gate” system covers all the steps from raw material extraction and energy to product conversion and delivery at the factory gate, whereas “cradle to grave” covers all phases of the lifecycle of a product, i.e., includes all steps of “cradle to gate” and usage and disposal phase [134]. LCA can be investigated through several environmental impact categories, such as global warming, ozone depletion, acidification, eutrophication, resource depletion (fossil fuel), ecotoxicity, human toxicity, photo oxidant formation, smog air, etc. [136,137,138]. Thus it is difficult to compare the results between studies [138]. Furthermore, there are only very few mentions in the literature about the environmental performance of nanomaterials based on LCA methods which also has some limitations, including a lack of life cycle inventory data and characterization factors for NMs’ emissions [139,140]. Figure 10 depicts the simplified framework for the LCA of nanocomposite materials.
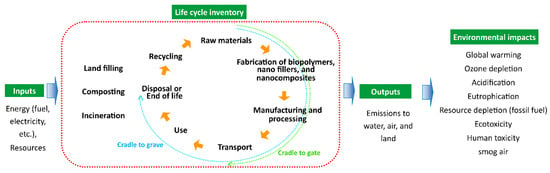
Figure 10.
A general framework for the LCA of nanocomposite materials.
This section covers the environmental profile of starch-based nanocomposites in comparison to nonconventional counterparts. The environmental impacts of starch-based composites production with PBS, PLA/PBAT, PHB, PLA, PBS/fiber, and recycled-PLA were greatly varied: non-renewable energy use (NREU) (33–72 MJ/kg, when using virgin starch), eutrophication (1.2–1.9 g P eq./kg), greenhouse gas (GHG) emissions (1.8–3.7 kg CO2 eq./kg) and agricultural land use (0.3–1.3 m2yr/kg) (Table 3). Compared to petrochemical polymers, LDPE and PP, virgin starch-based polymers reduced GHG emissions (up to 80%, except starch/PBS, starch/PLA/PBAT) and NREU (up to 60%) but increased eutrophication potential (up to 400%) and agricultural land use. Furthermore, reclaimed starch from wastewater instead of virgin starch reduced environmental impacts [141].
The microwave-assisted technique can be an environmentally friendly alternative for glucose-reduced and starch-stabilized Ag NPs production [137].
LeCorre et al. [132] compared the sustainability of extraction of nanofillers’ starch nanocrystals (SNC) and organically modified nanoclay montmorillonite (OMMT). Though global warming and acidification potential indicators of SNC were higher than those of OMMT, SNC has more positive impacts than OMMT, which contributes to non-renewable energy and mineral depletion.
The choice of starch sources and plasticizers influences the environmental impacts displayed by the production of composites. Corn starch/glycerol exhibited the lowest impact on the ecosystem, human health, and resources [142].

Table 3.
Environmental impacts of starch polymer and nanofiller compared with LDPE polymer (Functional unit = 1 kg).
Table 3.
Environmental impacts of starch polymer and nanofiller compared with LDPE polymer (Functional unit = 1 kg).
| Impact Category | Ozone Depletion (kg CFC-11 eq.) | Global Warming Potential/Greenhouse Gas Emissions (kg CO2 eq.) | Smog (kg O3 eq.) | Acidification (kg SO2 eq.) | Eutrophication (kg N eq.) | Human Toxicity, Carcinogen (CTUh) | Human Toxicity, Noncarcinogen (CTUh) | Respiratory Effects (kg PM2.5 eq.) | Ecotoxicity (CTUe) | Water Consumption (Kg) | Agricultural Land Use (m2yr/kg) | Fossil Fuel Depletion/Non-Renewable Energy Use (MJ Surplus) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PE waste management | 1.28 × 10−5 | 3.82 × 103 | 5.77 × 10 | 1.39 × 10 c | 1.05 × 10−4 | [143] | |||||||
| Starch-based polymers production with PBS, PLA/PBAT, PHB, PLA, PBS/fiber, and recycled-PLA | 1.8–3.7 | 1.2–1.9 d | 0.3–1.3 | 33–72 | [141] | ||||||||
| Starch-stabilized Ag NPs manufacturing via microwave-assisted heating | 1.24 × 10−7 | 8.44 × 10−2 | 2.37 × 10−1 | 2.51 × 10−1 | 1.21 × 10−1 | 4.44 × 10−6 | 8.02 × 10−4 | 6.41 × 102 | 5.85 × 105 | 7.08 × 102 | [137] | ||
| Starch nanofiller preparation using various process | 0.00 | 7.95–13.07 | 0.5–0.6 a | 8.78–15.51 b | 0.16–0.23 | 0.9–0.16 e | 2216.99–3747.76 f | 0.02 | 33.15–115.82 h | 16–19 | [132] | ||
| Nanofiller OMMT | 1.52 | 1.139 g | 40.079 | [144] |
a, kg/NOx eq.; b, H+ moles eq.; c, kg PO43- eq.; d, g P eq./kg; e, kg benzene eq.; f, kg toluene eq.; g, g PM; h, kg 2,4-D eq. PM2.5, particulate matter of size under 2.5 µm; 2,4-D, 2,4-dichlorophenoxyacetic acid used as a herbicide and pesticide.
8. Biodegradation of Starch
Based on ASTM, biodegradable is defined as ‘capable of undergoing decomposition into carbon dioxide, methane, water, inorganic compounds, or biomass in which the predominant mechanism is the enzymatic action of microorganisms that can be measured by standard tests, in a specified period, reflecting available disposal condition’ [44]. Biodegradable polymers play a critical role in environmental sustainability as they take part in the natural cycle “from nature to nature” [145]. With regard to biopolymer, to be certified as a biodegradable material, 90% of its mass should be decomposed in composting conditions within 90 days [146].
The type, nature, concentration, chemical modification, and antimicrobial properties of nanofiller, biodegradation test methods, and parameters, including temperature, moisture, humidity, pH, quantity and type of microorganisms, etc., can influence the biodegradability of nanocomposites [15,145,147].
Starch modification and incorporation of nanomaterials as nanofiller have been shown to alter biodegradability. For example, the biodegradability of starch increased with the addition of MMT at lower concentrations because of increased hydrophilicity that permits the microorganisms to enter into the polymer. In contrast, chemical modification of starch, nanofillers such as TiO2, graphene oxide, etc., reduce the biodegradability of starch-based nanocomposite because of their antioxidant potential [5,14,15,148].
Crosslinked nanocomposite film produced from thermoplastic corn starch crosslinked with oxidized sucrose and reinforced with cellulose nanofibrils from a pineapple leaf was reported to have a 30% weight loss rate after 30 days of burial, much lower than that of thermoplastic starch (80%) [5]. Crosslinking thermoplastic starch is hard to decompose due to the formation of acetal/hemiacetals and reduction of hydrophilicity and water permeability of nanocomposite, which decrease the attraction and permeability of microorganisms into the polymer matrix [5].
The addition of MMT into sweet potato starch (SPS) hindered biodegradability in soil burial tests due to the strong hydrogen bond between the hydroxyl groups of SPS and MMT and decreased water solubility that prevents water diffusion into the film [100]. However, the effect of MMT on biodegradability is concentration dependent. In corn starch-based film, adding MMT nanoclay at a lower concentration (1–3%) delayed the biodegradation rate (22–23 days for complete degradation), which may be attributed to the formation of the exfoliated structure at a lower concentration of MMT, which ensures good interaction between MMT and the polymer matrix. The biodegradability was increased at a higher level (>3%) of MMT due to agglomeration [15].
The cationic starch-based film incorporated with MMT and nanocrystalline cellulose degrade faster than the pure cationic starch film in composting at 58 °C, which may be attributed to hydrophilic nanocrystalline cellulose [127]. Thyme essential oil (TEO) and MMT incorporation have also been shown to increase biodegradation in SPS/MMT nanocomposites [100].
Incorporating fibrous TiO2 (0.01 and 0.05 wt.%) in maize starch/PVA composite films improved the tensile strength, water vapor, and UV barrier properties with little effect on biodegradability in soil [146]. The addition of nanoclay fillers delays the biodegradation of corn starch when buried in a microbiological medium of pure Micrococcus luteus culture at room temperature for 30 days [15]. Incorporating antimicrobial Ag NPs into starch/PVA composite film reduced its biodegradability [14].
The addition of CaCO3 in starch/polyethylhexylacrylate (PEHA)/PVA composite film improved the tensile strength, thermal stability, chemical resistance, and antimicrobial properties, which can be suitable for packaging. Starch/PEHA/PVA/CaCO3 degraded by 65% after 15 days in activated sludge water [149]. Food packaging material prepared from poly(ethyl methacrylate)-co-starch/graphene oxide/AgNPs showed only a 4.5% biodegradation in active sludge water after 180 days [97].
Poly(lactic acid) (PLA)/thermoplastic cassava starch (TPCS)/graphene nanoplatelets (GRH) nanocomposite film showed a lower biodegradation rate than PLA film in vermiculite (0.11 to 0.06 d−1) and compost media (0.09 to 0.08 d−1) [148].
In slow-release fertilizer formulation, the incorporation of natural char nanoparticles (NCNPs) into corn starch-g-poly (acrylic acid-co-acrylamide)/urea composite increased the degradation rate (23.9% after 30 days in soil), which may be attributed to the increment in water absorbance that promotes the soil microorganisms to enter into the polymer matrix [84].
The biodegradability of nanocomposite film polylactic acid/starch/poly-ε-caprolactone/nano hydroxyapatite (nHAp) was increased with the nHAp content [110]. Hosseinzadeh and Ramin [126] reported that the degradability of starch-graft-poly(acrylamide) (PAM)/graphene oxide (GO) nanocomposite decreased with increasing nHAp addition in buffer solution due to the higher crystallinity, compressive strength, and elastic modulus of nanocomposite film.
In vitro degradation tests performed in a simulated body fluid (SBF) showed that thermoplastic starch (TPS)/beta-tricalcium phosphate (β-TCP) NPs degraded 51% after 28 days, higher than that of TPS (47%) [105]. Table 4 summarizes the recent findings about the biodegradability of different starch-based biopolymers.

Table 4.
Biodegradability of different starch-based biopolymers.
9. Conclusions and Future Perspectives
In summary, starch is a natural polymer with outstanding biocompatible characteristics and can be used as both a matrix and reinforcement material for the development of new bionanocomposites. Starch nanoparticles and other nanofillers, including nanocellulose, chitin NPs, nanoclay (MMT, HNTs, bentonites), carbon nanoparticles (MWCNTs, SWCNTs, graphene, graphene oxides), metal and metal oxides (Ag NPs, TiO2, ZnO, CaCO3, etc.), have been widely used for the creation of new starch-based bionanocomposites and are promising candidates for various industrial applications.
The excellent biocompatibility, complete degradability without toxic residues, low cost, wide availability, and renewability of starch-based nanocomposites would open up many applications in agriculture, packaging, environmental remediation, biomedicine, and many other fields. Some of the reported applications are edible food coating, active and intelligent food packaging, controlled/slow-released pesticides and fertilizers, mulch films, drug carriers (controlled/target specific), wound healing, scaffolds in tissue engineering, absorbents, filters, catalysts, or disinfectants for environmental remediation, electronic devices, lightweight architectural constructions, stabilizers in food and paints such as non-food applications, and many others.
Modification of starch or reinforcement with other materials to form a nanocomposite may alter biodegradability. Therefore, regarding the biodegradability of starch-based nanocomposites is important for them to be claimed as being biodegradable materials. Life cycle assessment of starch-based biocomposite materials for their respective applications provides critical information regarding the environmental and ecological benefits of the materials over fossil-based synthetic polymers for developing sustainable nanocomposites. However, only few studies have focused on life cycle assessment. Therefore, further studies on life cycle assessment of starch-based nanocomposites needs to be investigated. Nanomaterials can also enter the human body through inhalation, contact, and ingestion, which can lead to their accumulation in the human body, Therefore, further investigations on toxicity and risk factor analysis are necessary to find the most suitable starch-based nanocomposite materials.
Author Contributions
Writing—original draft, A.G., P.T., S.M., P.G.P., P.E. and T.M. Review and editing, A.G., P.T., S.M., P.G.P., A.M., O.M. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva Lactuca Algae Based Chitosan Bio-Composites for Bioremediation of Cd(II) Ions. J. Bioresour. Bioprod. 2021, 6, 223–242. [Google Scholar] [CrossRef]
- Puiggalí, J.; Katsarava, R. Chapter 7—Bionanocomposites. In Clay-Polymer Nanocomposites; Jlassi, K., Chehimi, M.M., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–272. ISBN 978-0-323-46153-5. [Google Scholar]
- Madhumitha, G.; Fowsiya, J.; Mohana Roopan, S.; Thakur, V.K. Recent Advances in Starch–Clay Nanocomposites. Int. J. Polym. Anal. Charact. 2018, 23, 331–345. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Geethamma, V.G.; Gopi, S.; Thomas, M.G.; Kunaver, M.; Huskić, M.; Kalarikkal, N.; Volova, T.; Rouxel, D.; Thomas, S. Thermal, Biodegradation and Theoretical Perspectives on Nanoscale Confinement in Starch/Cellulose Nanocomposite Modified via Green Crosslinker. Int. J. Biol. Macromol. 2019, 134, 781–790. [Google Scholar] [CrossRef]
- Arora, B.; Bhatia, R.; Attri, P. 28—Bionanocomposites: Green Materials for a Sustainable Future. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Wilmington, NC, USA, 2018; pp. 699–712. ISBN 978-0-12-811033-1. [Google Scholar]
- García, N.L.; Famá, L.; D’Accorso, N.B.; Goyanes, S. Biodegradable Starch Nanocomposites. In Eco-friendly Polymer Nanocomposites: Processing and Properties; Thakur, V.K., Thakur, M.K., Eds.; Advanced Structured Materials; Springer: New Delhi, India, 2015; pp. 17–77. ISBN 978-81-322-2470-9. [Google Scholar]
- Mohammad, F.; Arfin, T.; Bwatanglang, I.B.; Al-lohedan, H.A. Starch-Based Nanocomposites: Types and Industrial Applications. In Bio-Based Polymers and Nanocomposites: Preparation, Processing, Properties & Performance; Sanyang, M.L., Jawaid, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 157–181. ISBN 978-3-030-05825-8. [Google Scholar]
- BeMiller, J.N. Chapter 5—Physical Modification of Starch. In Starch in Food, 2nd ed.; Sjöö, M., Nilsson, L., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2018; pp. 223–253. ISBN 978-0-08-100868-3. [Google Scholar]
- Gunawardene, O.H.P.; Gunathilake, C.A.; Amaraweera, A.P.S.M.; Fernando, N.M.L.; Manipura, A.; Manamperi, W.A.; Kulatunga, K.M.A.K.; Rajapaksha, S.M.; Gamage, A.; Dassanayake, R.S.; et al. Removal of Pb(II) Ions from Aqueous Solution Using Modified Starch. J. Compos. Sci. 2021, 5, 46. [Google Scholar] [CrossRef]
- Okoli, C.P.; Ofomaja, A.E. Degree of Time Dependency of Kinetic Coefficient as a Function of Adsorbate Concentration; New Insights from Adsorption of Tetracycline onto Monodispersed Starch-Stabilized Magnetic Nanocomposite. J. Environ. Manag. 2018, 218, 139–147. [Google Scholar] [CrossRef]
- Zarski, A.; Bajer, K.; Kapuśniak, J. Review of the Most Important Methods of Improving the Processing Properties of Starch toward Non-Food Applications. Polymers 2021, 13, 832. [Google Scholar] [CrossRef]
- Le Corre, D.; Angellier-Coussy, H. Preparation and Application of Starch Nanoparticles for Nanocomposites: A Review. React. Funct. Polym. 2014, 85, 97–120. [Google Scholar] [CrossRef]
- Cano, A.I.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Biodegradation Behavior of Starch-PVA Films as Affected by the Incorporation of Different Antimicrobials. Polym. Degrad. Stab. 2016, 132, 11–20. [Google Scholar] [CrossRef]
- Mohan, T.; Devchand, K.; Kanny, K. Barrier and Biodegradable Properties of Corn Starch-Derived Biopolymer Film Filled with Nanoclay Fillers. J. Plast. Film Sheeting 2017, 33, 309–336. [Google Scholar] [CrossRef]
- Venkatesh, G.; Nyflött, Å.; Bonnerup, C.; Lestelius, M. An Economic-Environmental Analysis of Selected Barrier-Coating Materials Used in Packaging Food Products: A Swedish Case Study. Env. Dev. Sustain. 2018, 20, 1483–1497. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P. Application of Life Cycle Assessment for Starch and Starch Blends. In Starch-Based Polymeric Materials and Nanocomposites; Ahmed, J., Tiwari, B.K., Imam, S.H., Rao, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-0-429-10818-1. [Google Scholar]
- Kakadellis, S.; Harris, Z.M. Don’t Scrap the Waste: The Need for Broader System Boundaries in Bioplastic Food Packaging Life-Cycle Assessment—A Critical Review. J. Clean. Prod. 2020, 274, 122831. [Google Scholar] [CrossRef]
- Bertolini, A. (Ed.) Starches: Characterization, Properties, and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-0-429-14172-0. [Google Scholar]
- Govindaraju, I.; Zhuo, G.-Y.; Chakraborty, I.; Melanthota, S.K.; Mal, S.S.; Sarmah, B.; Baruah, V.J.; Mahato, K.K.; Mazumder, N. Investigation of Structural and Physico-Chemical Properties of Rice Starch with Varied Amylose Content: A Combined Microscopy, Spectroscopy, and Thermal Study. Food Hydrocoll. 2022, 122, 107093. [Google Scholar] [CrossRef]
- Pérez, S.; Baldwin, P.M.; Gallant, D.J. Chapter 5—Structural Features of Starch Granules I. In Starch, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Food Science and Technology; Academic Press: San Diego, CA, USA, 2009; pp. 149–192. ISBN 978-0-12-746275-2. [Google Scholar]
- Alves, Z.; Abreu, B.; Ferreira, N.M.; Marques, E.F.; Nunes, C.; Ferreira, P. Enhancing the Dispersibility of Multiwalled Carbon Nanotubes within Starch-Based Films by the Use of Ionic Surfactants. Carbohydr. Polym. 2021, 273, 118531. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of Starch Type on the Physico-Chemical Properties of Edible Films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Vijayakumar, R.P. Synthesis of Polystyrene/Starch/CNT Composite and Study on Its Biodegradability. J. Polym. Res. 2020, 27, 187. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and Functionality of Starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The Structural Characteristics of Starches and Their Functional Properties. CyTA—J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Extraction and Characterization of Arrowroot (Maranta Arundinaceae L.) Starch and Its Application in Edible Films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Barbosa, L.S.; Ramos, I.G.; Coelho, R.E.; Druzian, J.I. The Important Role of Crystallinity and Amylose Ratio in Thermal Stability of Starches. J. Anal. Calorim. 2018, 131, 2555–2567. [Google Scholar] [CrossRef]
- Li, Z.; Guo, K.; Lin, L.; He, W.; Zhang, L.; Wei, C. Comparison of Physicochemical Properties of Starches from Flesh and Peel of Green Banana Fruit. Molecules 2018, 23, 2312. [Google Scholar] [CrossRef] [PubMed]
- Thanyapanich, N.; Jimtaisong, A.; Rawdkuen, S. Functional Properties of Banana Starch (Musa Spp.) and Its Utilization in Cosmetics. Molecules 2021, 26, 3637. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Höchstötter, A.; Jekle, M.; Arendt, E.; Becker, T. Physicochemical and Morphological Characterization of Different Starches with Variable Amylose/Amylopectin Ratio. Food Hydrocoll. 2013, 32, 52–63. [Google Scholar] [CrossRef]
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Alimi, B.A. Progress in Research and Applications of Cassava Flour and Starch: A Review. J. Food Sci. Technol. 2019, 56, 2799–2813. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Hou, J.; Yang, N.; Zhang, Y.; Chen, H.; Zhang, Z.; Shen, Y.; Huang, S.; Guo, S. Insight on the Changes of Cassava and Potato Starch Granules during Gelatinization. Int. J. Biol. Macromol. 2019, 126, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, L.; Qu, J.; Blennow, A.; Hansen, A.R.; Wu, Y.; Guo, D.; Liu, X. Amylose Content and Specific Fine Structures Affect Lamellar Structure and Digestibility of Maize Starches. Food Hydrocoll. 2020, 108, 105994. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, P.; Sui, Z.; Bao, J. Physicochemical Properties of Starches from Diverse Rice Cultivars Varying in Apparent Amylose Content and Gelatinisation Temperature Combinations. Food Chem. 2015, 172, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yang, J.; Hong, Y.; Liu, G.; Zheng, J.; Gu, Z.; Zhang, P. Impact of Amylose Content on Starch Physicochemical Properties in Transgenic Sweet Potato. Carbohydr. Polym. 2015, 122, 417–427. [Google Scholar] [CrossRef]
- Biduski, B.; da Silva, W.M.F.; Colussi, R.; Halal, S.L.; De, M.E.; Lim, L.-T.; Dias, Á.R.G.; da Zavareze, E.R. Starch Hydrogels: The Influence of the Amylose Content and Gelatinization Method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Isono, N.; Noda, T. Relationship of Granule Size Distribution and Amylopectin Structure with Pasting, Thermal, and Retrogradation Properties in Wheat Starch. J. Agric. Food Chem. 2010, 58, 1180–1188. [Google Scholar] [CrossRef]
- Bahrami, B.; Behzad, T.; Salehinik, F.; Zamani, A.; Heidarian, P. Incorporation of Extracted Mucor Indicus Fungus Chitin Nanofibers into Starch Biopolymer: Morphological, Physical, and Mechanical Evaluation. Starch—Stärke 2021, 73, 2000218. [Google Scholar] [CrossRef]
- Ngô, C.; Van de Voorde, M.H. Nanomaterials: Doing More with Less. In Nanotechnology in a Nutshell: From Simple to Complex Systems; Ngô, C., Van de Voorde, M., Eds.; Atlantis Press: Paris, France, 2014; pp. 55–70. ISBN 978-94-6239-012-6. [Google Scholar]
- Saleh, T.A. Nanomaterials: Classification, Properties, and Environmental Toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Singh, V.; Yadav, P.; Mishra, V. Recent Advances on Classification, Properties, Synthesis, and Characterization of Nanomaterials. In Green Synthesis of Nanomaterials for Bioenergy Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 83–97. ISBN 978-1-119-57678-5. [Google Scholar]
- Zafar, R.; Zia, K.M.; Tabasum, S.; Jabeen, F.; Noreen, A.; Zuber, M. Polysaccharide Based Bionanocomposites, Properties and Applications: A Review. Int. J. Biol. Macromol. 2016, 92, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Turan, D.; Gunes, G.; Kilic, A. Perspectives of Bio-Nanocomposites for Food Packaging Applications. In Bionanocomposites for Packaging Applications; Jawaid, M., Swain, S.K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–32. ISBN 978-3-319-67319-6. [Google Scholar]
- Sandhu, K.S.; Nain, V. Starch Nanoparticles: Their Preparation and Applications. In Plant Biotechnology: Recent Advancements and Developments; Gahlawat, S.K., Salar, R.K., Siwach, P., Duhan, J.S., Kumar, S., Kaur, P., Eds.; Springer: Singapore, 2017; pp. 213–232. ISBN 978-981-10-4732-9. [Google Scholar]
- Santana, J.S.; de Carvalho Costa, É.K.; Rodrigues, P.R.; Correia, P.R.C.; Cruz, R.S.; Druzian, J.I. Morphological, Barrier, and Mechanical Properties of Cassava Starch Films Reinforced with Cellulose and Starch Nanoparticles. J. Appl. Polym. Sci. 2019, 136, 47001. [Google Scholar] [CrossRef]
- Campelo, P.H.; Sant’Ana, A.S.; Pedrosa Silva Clerici, M.T. Starch Nanoparticles: Production Methods, Structure, and Properties for Food Applications. Curr. Opin. Food Sci. 2020, 33, 136–140. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Tarté, R.; Acevedo, N.C. Synergistic Effects of Starch Nanoparticles and Chitin Nanofibers on the Stability of Oil-in-Water Pickering Emulsions. Food Chem. 2021, 363, 130301. [Google Scholar] [CrossRef]
- Lin, Q.; Ji, N.; Li, M.; Dai, L.; Xu, X.; Xiong, L.; Sun, Q. Fabrication of Debranched Starch Nanoparticles via Reverse Emulsification for Improvement of Functional Properties of Corn Starch Films. Food Hydrocoll. 2020, 104, 105760. [Google Scholar] [CrossRef]
- Amirsoleimani, M.; Khalilzadeh, M.A.; Sadeghifar, F.; Sadeghifar, H. Surface Modification of Nanosatrch Using Nano Silver: A Potential Antibacterial for Food Package Coating. J. Food Sci. Technol. 2018, 55, 899–904. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Khachatryan, K.; Konieczna-Molenda, A.; Grzesiakowska, A.; KuchtaGładysz, M.; Kawecka, A.; Grzebieniarz, W.; Nowak, N. The Functional and Application Possibilities of Starch/Chitosan Polymer Composites Modified by Graphene Oxide. Int. J. Mol. Sci. 2022, 23, 5956. [Google Scholar] [CrossRef]
- Othman, S.H. Bio-Nanocomposite Materials for Food Packaging Applications: Types of Biopolymer and Nano-Sized Filler. Agric. Agric. Sci. Procedia 2014, 2, 296–303. [Google Scholar] [CrossRef]
- Nasir, M.; Hashim, R.; Sulaiman, O.; Asim, M. 11—Nanocellulose: Preparation Methods and Applications. In Cellulose-Reinforced Nanofibre Composites; Jawaid, M., Boufi, S., Hps, A.K., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Cambridge, UK, 2017; pp. 261–276. ISBN 978-0-08-100957-4. [Google Scholar]
- Dufresne, A. Nanocellulose: A New Ageless Bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; Sirohi, R. Nanocellulose-Based Products for Sustainable Applications-Recent Trends and Possibilities. Rev. Env. Sci. Biotechnol. 2020, 19, 779–806. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and Application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H.; Nordin, N.; Azman, N.A.A.; Tawakkal, I.S.M.A.; Basha, R.K. Effects of Nanocellulose Fiber and Thymol on Mechanical, Thermal, and Barrier Properties of Corn Starch Films. Int. J. Biol. Macromol. 2021, 183, 1352–1361. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; do Sobral, P.J.A.; Menegalli, F.C. Nanocomposites Based on Banana Starch Reinforced with Cellulose Nanofibers Isolated from Banana Peels. J. Colloid Interface Sci. 2017, 505, 154–167. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A. Water Barrier Properties of Starch Films Reinforced with Cellulose Nanocrystals Obtained from Sugarcane Bagasse. Carbohydr. Polym. 2014, 110, 53–61. [Google Scholar] [CrossRef]
- Heidari, M.; Khomeiri, M.; Yousefi, H.; Rafieian, M.; Kashiri, M. Chitin Nanofiber-Based Nanocomposites Containing Biodegradable Polymers for Food Packaging Applications. J. Consum. Prot. Food Saf. 2021, 16, 237–246. [Google Scholar] [CrossRef]
- Thomas, M.S.; Koshy, R.R.; Mary, S.K.; Thomas, S.; Pothan, L.A. Starch, Chitin and Chitosan Based Composites and Nanocomposites; SpringerBriefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-03157-2. [Google Scholar]
- Yang, X.; Liu, J.; Pei, Y.; Zheng, X.; Tang, K. Recent Progress in Preparation and Application of Nano-Chitin Materials. Energy Environ. Mater. 2020, 3, 492–515. [Google Scholar] [CrossRef]
- Chang, P.R.; Jian, R.; Yu, J.; Ma, X. Starch-Based Composites Reinforced with Novel Chitin Nanoparticles. Carbohydr. Polym. 2010, 80, 420–425. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-Layered Silicate Nanocomposites: Preparation, Properties and Uses of a New Class of Materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Mansour, G.; Zoumaki, M.; Marinopoulou, A.; Raphaelides, S.N.; Tzetzis, D.; Zoumakis, N. Investigation on the Effects of Glycerol and Clay Contents on the Structure and Mechanical Properties of Maize Starch Nanocomposite Films. Starch—Stärke 2020, 72, 1900166. [Google Scholar] [CrossRef]
- Abulyazied, D.E.; Ene, A. An Investigative Study on the Progress of Nanoclay-Reinforced Polymers: Preparation, Properties, and Applications: A Review. Polymers 2021, 13, 4401. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A Review of the Synthesis and Applications of Polymer–Nanoclay Composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef]
- Yousefi, A.R.; Savadkoohi, B.; Zahedi, Y.; Hatami, M.; Ako, K. Fabrication and Characterization of Hybrid Sodium Montmorillonite/TiO2 Reinforced Cross-Linked Wheat Starch-Based Nanocomposites. Int. J. Biol. Macromol. 2019, 131, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Garrido-Miranda, K.A.; Pereira, E.D. Release of Essential Oil Constituent from Thermoplastic Starch/Layered Silicate Bionanocomposite Film as a Potential Active Packaging Material. Eur. Polym. J. 2018, 109, 64–71. [Google Scholar] [CrossRef]
- Shayan, M.; Azizi, H.; Ghasemi, I.; Karrabi, M. Effect of Modified Starch and Nanoclay Particles on Biodegradability and Mechanical Properties of Cross-Linked Poly Lactic Acid. Carbohydr. Polym. 2015, 124, 237–244. [Google Scholar] [CrossRef]
- Iamareerat, B.; Singh, M.; Sadiq, M.B.; Anal, A.K. Reinforced Cassava Starch Based Edible Film Incorporated with Essential Oil and Sodium Bentonite Nanoclay as Food Packaging Material. J. Food Sci. Technol. 2018, 55, 1953–1959. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R.; Pollet, E.; Avérous, L. Morphology and Properties of Thermoplastic Starch Blended with Biodegradable Polyester and Filled with Halloysite Nanoclay. Carbohydr. Polym. 2020, 242, 116392. [Google Scholar] [CrossRef]
- Schmitt, H.; Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P. Preparation and Properties of Novel Melt-Blended Halloysite Nanotubes/Wheat Starch Nanocomposites. Carbohydr. Polym. 2012, 89, 920–927. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Biodegradable and Water Resistant Poly(Vinyl) Alcohol (PVA)/Starch (ST)/Glycerol (GL)/Halloysite Nanotube (HNT) Nanocomposite Films for Sustainable Food Packaging. Front. Mater. 2019, 6, 58. [Google Scholar] [CrossRef]
- Ambika; Singh, P.P. Advances in Carbon Nanomaterial-Based Green Nanocomposites. In Emerging Carbon-Based Nanocomposites for Environmental Applications; Mishra, A.K., Hussain, C.M., Mishra, S.B., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 175–201. ISBN 978-1-119-55488-2. [Google Scholar]
- Domene-López, D.; Delgado-Marín, J.J.; García-Quesada, J.C.; Martín-Gullón, I.; Montalbán, M.G. Electroconductive Starch/Multi-Walled Carbon Nanotube Films Plasticized by 1-Ethyl-3-Methylimidazolium Acetate. Carbohydr. Polym. 2020, 229, 115545. [Google Scholar] [CrossRef] [PubMed]
- Famá, L.; Rojo, P.G.; Bernal, C.; Goyanes, S. Biodegradable Starch Based Nanocomposites with Low Water Vapor Permeability and High Storage Modulus. Carbohydr. Polym. 2012, 87, 1989–1993. [Google Scholar] [CrossRef]
- Xu, D. Carbon Nanotubes (CNTs) Composite Materials and Food Packaging. In Composites Materials for Food Packaging; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 235–249. ISBN 978-1-119-16024-3. [Google Scholar]
- Mollik, S.I.; Alam, R.B.; Islam, M.R. Significantly Improved Dielectric Properties of Bio-Compatible Starch/Reduced Graphene Oxide Nanocomposites. Synth. Met. 2021, 271, 116624. [Google Scholar] [CrossRef]
- Solati, M.; Saeidi, A.; Ghasemi, I. The Effect of Graphene Nanoplatelets on Dynamic Properties, Crystallization, and Morphology of a Biodegradable Blend of Poly(Lactic Acid)/Thermoplastic Starch. Iran Polym. J. 2019, 28, 649–658. [Google Scholar] [CrossRef]
- Kalia, A.; Sharma, S.P.; Kaur, H.; Kaur, H. Chapter 5—Novel Nanocomposite-Based Controlled-Release Fertilizer and Pesticide Formulations: Prospects and Challenges. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Abd-Elsalam, K.A., Ed.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 99–134. ISBN 978-0-12-821354-4. [Google Scholar]
- Merino, D.; Gutiérrez, T.J.; Mansilla, A.Y.; Casalongué, C.A.; Alvarez, V.A. Critical Evaluation of Starch-Based Antibacterial Nanocomposites as Agricultural Mulch Films: Study on Their Interactions with Water and Light. ACS Sustain. Chem. Eng. 2018, 6, 15662–15672. [Google Scholar] [CrossRef]
- Salimi, M.; Motamedi, E.; Motesharezedeh, B.; Hosseini, H.M.; Alikhani, H.A. Starch-g-Poly(Acrylic Acid-Co-Acrylamide) Composites Reinforced with Natural Char Nanoparticles toward Environmentally Benign Slow-Release Urea Fertilizers. J. Environ. Chem. Eng. 2020, 8, 103765. [Google Scholar] [CrossRef]
- Gamage, A.; Liyanapathiranage, A.; Manamperi, A.; Gunathilake, C.; Mani, S.; Merah, O.; Madhujith, T. Applications of Starch Biopolymers for a Sustainable Modern Agriculture. Sustainability 2022, 14, 6085. [Google Scholar] [CrossRef]
- Merino, D.; Gutiérrez, T.J.; Alvarez, V.A. Structural and Thermal Properties of Agricultural Mulch Films Based on Native and Oxidized Corn Starch Nanocomposites. Starch—Stärke 2019, 71, 1800341. [Google Scholar] [CrossRef]
- Pereira, E.I.; Giroto, A.S.; Bortolin, A.; Yamamoto, C.F.; Marconcini, J.M.; de Campos Bernardi, A.C.; Ribeiro, C. Perspectives in Nanocomposites for the Slow and Controlled Release of Agrochemicals: Fertilizers and Pesticides. In Nanotechnologies in Food and Agriculture; Rai, M., Ribeiro, C., Mattoso, L., Duran, N., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 241–265. ISBN 978-3-319-14024-7. [Google Scholar]
- Pimsen, R.; Porrawatkul, P.; Nuengmatcha, P.; Ramasoot, S.; Chanthai, S. Efficiency Enhancement of Slow Release of Fertilizer Using Nanozeolite–Chitosan/Sago Starch-Based Biopolymer Composite. J. Coat. Technol. Res. 2021, 18, 1321–1332. [Google Scholar] [CrossRef]
- Zafar, N.; Niazi, M.B.K.; Sher, F.; Khalid, U.; Jahan, Z.; Shah, G.A.; Zia, M. Starch and Polyvinyl Alcohol Encapsulated Biodegradable Nanocomposites for Environment Friendly Slow Release of Urea Fertilizer. Chem. Eng. J. Adv. 2021, 7, 100123. [Google Scholar] [CrossRef]
- Jain, S.K.; Dutta, A.; Kumar, J.; Shakil, N.A. Preparation and Characterization of Dicarboxylic Acid Modified Starch-Clay Composites as Carriers for Pesticide Delivery. Arab. J. Chem. 2020, 13, 7990–8002. [Google Scholar] [CrossRef]
- Galstyan, V.; Bhandari, M.P.; Sberveglieri, V.; Sberveglieri, G.; Comini, E. Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging. Chemosensors 2018, 6, 16. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A.A. Modification of Physicochemical and Thermal Properties of Starch Films by Incorporation of TiO2 Nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef]
- Pirsa, S.; Karimi Sani, I.; Khodayvandi, S. Design and Fabrication of Starch-Nano Clay Composite Films Loaded with Methyl Orange and Bromocresol Green for Determination of Spoilage in Milk Package. Polym. Adv. Technol. 2018, 29, 2750–2758. [Google Scholar] [CrossRef]
- Patil, S.; Bharimalla, A.K.; Nadanathangam, V.; Dhakane-Lad, J.; Mahapatra, A.; Jagajanantha, P.; Saxena, S. Nanocellulose Reinforced Corn Starch-Based Biocomposite Films: Composite Optimization, Characterization and Storage Studies. Food Packag. Shelf Life 2022, 33, 100860. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Shahabi-Ghahfarrokhi, I.; Goudarzi, V. Preparation of UV-Protective Starch/Kefiran/ZnO Nanocomposite as a Packaging Film: Characterization. Food Packag. Shelf Life 2018, 16, 103–111. [Google Scholar] [CrossRef]
- Sharmin, E.; Kafyah, M.T.; Alzaydi, A.A.; Fatani, A.A.; Hazazzi, F.A.; Babgi, S.K.; Alqarhi, N.M.; Sindi, A.A.H.; Akram, D.; Alam, M.; et al. Synthesis and Characterization of Polyvinyl Alcohol/Corn Starch/Linseed Polyol-Based Hydrogel Loaded with Biosynthesized Silver Nanoparticles. Int. J. Biol. Macromol. 2020, 163, 2236–2247. [Google Scholar] [CrossRef]
- Mohanty, F.; Swain, S.K. Nano Silver Embedded Starch Hybrid Graphene Oxide Sandwiched Poly(Ethylmethacrylate) for Packaging Application. Nano-Struct. Nano-Objects 2019, 18, 100300. [Google Scholar] [CrossRef]
- Jha, P.; Dharmalingam, K.; Nishizu, T.; Katsuno, N.; Anandalakshmi, R. Effect of Amylose–Amylopectin Ratios on Physical, Mechanical, and Thermal Properties of Starch-Based Bionanocomposite Films Incorporated with CMC and Nanoclay. Starch—Stärke 2020, 72, 1900121. [Google Scholar] [CrossRef]
- de Souza, A.G.; dos Santos, N.M.A.; da Silva Torin, R.F.; dos Santos Rosa, D. Synergic Antimicrobial Properties of Carvacrol Essential Oil and Montmorillonite in Biodegradable Starch Films. Int. J. Biol. Macromol. 2020, 164, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Issa, A.T.; Schimmel, K.A.; Worku, M.; Shahbazi, A.; Ibrahim, S.A.; Tahergorabi, R. Sweet Potato Starch-Based Nanocomposites: Development, Characterization, and Biodegradability. Starch—Stärke 2018, 70, 1700273. [Google Scholar] [CrossRef]
- Barzegar, H.; Azizi, M.H.; Barzegar, M.; Hamidi-Esfahani, Z. Effect of Potassium Sorbate on Antimicrobial and Physical Properties of Starch–Clay Nanocomposite Films. Carbohydr. Polym. 2014, 110, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, Z.; Das, R.; Jiang, Q. Starch-Based Carbon Nanotubes and Graphene: Preparation, Properties and Applications. ES Food Agrofor. 2020, 2, 13–21. [Google Scholar] [CrossRef]
- Gomes, M.E.; Godinho, J.S.; Tchalamov, D.; Cunha, A.M.; Reis, R.L. Alternative Tissue Engineering Scaffolds Based on Starch: Processing Methodologies, Morphology, Degradation and Mechanical Properties. Mater. Sci. Eng. C 2002, 20, 19–26. [Google Scholar] [CrossRef]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-Based Completely Biodegradable Polymer Materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Taherimehr, M.; Bagheri, R.; Taherimehr, M. In-Vitro Evaluation of Thermoplastic Starch/ Beta-Tricalcium Phosphate Nano-Biocomposite in Bone Tissue Engineering. Ceram. Int. 2021, 47, 15458–15463. [Google Scholar] [CrossRef]
- Waghmare, V.S.; Wadke, P.R.; Dyawanapelly, S.; Deshpande, A.; Jain, R.; Dandekar, P. Starch Based Nanofibrous Scaffolds for Wound Healing Applications. Bioact. Mater. 2018, 3, 255–266. [Google Scholar] [CrossRef]
- Sadjadi, M.S.; Meskinfam, M.; Jazdarreh, H. Hydroxyapatite—Starch Nano Biocomposites Synthesis and Characterization. Int. J. Nano Dimens. 2010, 1, 57–63. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Al-Deyab, S.S. Antimicrobial Activity of Silver/Starch/Polyacrylamide Nanocomposite. Int. J. Biol. Macromol. 2014, 68, 33–38. [Google Scholar] [CrossRef]
- Batool, S.; Hussain, Z.; Niazi, M.B.K.; Liaqat, U.; Afzal, M. Biogenic Synthesis of Silver Nanoparticles and Evaluation of Physical and Antimicrobial Properties of Ag/PVA/Starch Nanocomposites Hydrogel Membranes for Wound Dressing Application. J. Drug Deliv. Sci. Technol. 2019, 52, 403–414. [Google Scholar] [CrossRef]
- Davachi, S.M.; Shiroud Heidari, B.; Hejazi, I.; Seyfi, J.; Oliaei, E.; Farzaneh, A.; Rashedi, H. Interface Modified Polylactic Acid/Starch/Poly ε-Caprolactone Antibacterial Nanocomposite Blends for Medical Applications. Carbohydr. Polym. 2017, 155, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Khodadadzadeh, L. Ultrasonic-Assisted Fabrication of Starch/MWCNT-Glucose Nanocomposites for Drug Delivery. Ultrason. Sonochem. 2018, 40, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wei, L.; Yan, H.; Xu, B. Green Synthesis and Characteristic of Core-Shell Structure Silver/Starch Nanoparticles. Mater. Lett. 2011, 65, 2963–2965. [Google Scholar] [CrossRef]
- Nezami, S.; Sadeghi, M.; Mohajerani, H. A Novel PH-Sensitive and Magnetic Starch-Based Nanocomposite Hydrogel as a Controlled Drug Delivery System for Wound Healing. Polym. Degrad. Stab. 2020, 179, 109255. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, D.; Liu, M.; Fu, L.; Wan, Q.; Mao, L.; Dai, Y.; Wen, Y.; Zhang, X.; Wei, Y. Room Temperature Preparation of Fluorescent Starch Nanoparticles from Starch-Dopamine Conjugates and Their Biological Applications. Mater. Sci. Eng. C 2018, 82, 204–209. [Google Scholar] [CrossRef]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef]
- García-Padilla, Á.; Moreno-Sader, K.A.; Realpe, Á.; Acevedo-Morantes, M.; Soares, J.B.P. Evaluation of Adsorption Capacities of Nanocomposites Prepared from Bean Starch and Montmorillonite. Sustain. Chem. Pharm. 2020, 17, 100292. [Google Scholar] [CrossRef]
- Mallakpour, S.; Nouruzi, N. Application of Vitamin B1-Coated Carbon Nanotubes for the Production of Starch Nanocomposites with Enhanced Structural, Optical, Thermal and Cd(II) Adsorption Properties. J. Polym. Environ. 2018, 26, 2954–2963. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, Cellulose, Pectin, Gum, Alginate, Chitin and Chitosan Derived (Nano)Materials for Sustainable Water Treatment: A Review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Orooji, Y.; Nezafat, Z.; Nasrollahzadeh, M.; Kamali, T.A. Polysaccharide-Based (Nano)Materials for Cr(VI) Removal. Int. J. Biol. Macromol. 2021, 188, 950–973. [Google Scholar] [CrossRef] [PubMed]
- del Orta, M.M.; Martín, J.; Santos, J.L.; Aparicio, I.; Medina-Carrasco, S.; Alonso, E. Biopolymer-Clay Nanocomposites as Novel and Ecofriendly Adsorbents for Environmental Remediation. Appl. Clay Sci. 2020, 198, 105838. [Google Scholar] [CrossRef]
- Russo, T.; Fucile, P.; Giacometti, R.; Sannino, F. Sustainable Removal of Contaminants by Biopolymers: A Novel Approach for Wastewater Treatment. Current State and Future Perspectives. Processes 2021, 9, 719. [Google Scholar] [CrossRef]
- Tripathy, T.; Kolya, H.; Jana, S.; Senapati, M. Green Synthesis of Ag-Au Bimetallic Nanocomposites Using a Biodegradable Synthetic Graft Copolymer; Hydroxyethyl Starch-g-Poly (Acrylamide-Co-Acrylic Acid) and Evaluation of Their Catalytic Activities. Eur. Polym. J. 2017, 87, 113–123. [Google Scholar] [CrossRef]
- Yang, C.; Ge, C.; Li, X.; Li, L.; Wang, B.; Lin, A.; Yang, W. Does Soluble Starch Improve the Removal of Cr(VI) by NZVI Loaded on Biochar? Ecotoxicol. Environ. Saf. 2021, 208, 111552. [Google Scholar] [CrossRef]
- Adeola, A.O.; Nomngongo, P.N. Advanced Polymeric Nanocomposites for Water Treatment Applications: A Holistic Perspective. Polymers 2022, 14, 2462. [Google Scholar] [CrossRef]
- Gomes, R.F.; de Azevedo, A.C.N.; Pereira, A.G.B.; Muniz, E.C.; Fajardo, A.R.; Rodrigues, F.H.A. Fast Dye Removal from Water by Starch-Based Nanocomposites. J. Colloid Interface Sci. 2015, 454, 200–209. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Ramin, S. Fabrication of Starch-Graft-Poly(Acrylamide)/Graphene Oxide/Hydroxyapatite Nanocomposite Hydrogel Adsorbent for Removal of Malachite Green Dye from Aqueous Solution. Int. J. Biol. Macromol. 2018, 106, 101–115. [Google Scholar] [CrossRef]
- Vaezi, K.; Asadpour, G.; Sharifi, S.H. Bio Nanocomposites Based on Cationic Starch Reinforced with Montmorillonite and Cellulose Nanocrystals: Fundamental Properties and Biodegradability Study. Int. J. Biol. Macromol. 2020, 146, 374–386. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Ali, S.S.; Matter, I.A.; Elsamahy, T.; Mahmoud, Y.A. Chapter Twenty—Enzymes Immobilization onto Magnetic Nanoparticles to Improve Industrial and Environmental Applications. In Methods in Enzymology; Kumar, C.V., Ed.; Nanoarmoring of Enzymes with Carbon Nanotubes and Magnetic Nanoparticles; Academic Press: Boca Raton, FL, USA, 2020; Volume 630, pp. 481–502. [Google Scholar]
- Sharmeen, S.; Rahman, S.; Islam, M.; Islam, S.; Shahruzzaman; Mallik, A.K.; Haque, P.; Rahman, M.M. 11—Application of Polysaccharides in Enzyme Immobilization. In Functional Polysaccharides for Biomedical Applications; Maiti, S., Jana, S., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 357–395. ISBN 978-0-08-102555-0. [Google Scholar]
- Liu, D.-M.; Dong, C. Recent Advances in Nano-Carrier Immobilized Enzymes and Their Applications. Process. Biochem. 2020, 92, 464–475. [Google Scholar] [CrossRef]
- Mehde, A.A. Development of Magnetic Cross-Linked Peroxidase Aggregates on Starch as Enhancement Template and Their Application for Decolorization. Int. J. Biol. Macromol. 2019, 131, 721–733. [Google Scholar] [CrossRef] [PubMed]
- LeCorre, D.; Hohenthal, C.; Dufresne, A.; Bras, J. Comparative Sustainability Assessment of Starch Nanocrystals. J. Polym. Environ. 2013, 21, 71–80. [Google Scholar] [CrossRef]
- Finnveden, G.; Moberg, Å. Environmental Systems Analysis Tools—An Overview. J. Clean. Prod. 2005, 13, 1165–1173. [Google Scholar] [CrossRef]
- Shen, L.; Patel, M.K. Life Cycle Assessment of Polysaccharide Materials: A Review. J. Polym. Environ. 2008, 16, 154–167. [Google Scholar] [CrossRef]
- Foroughi, F.; Rezvani Ghomi, E.; Morshedi Dehaghi, F.; Borayek, R.; Ramakrishna, S. A Review on the Life Cycle Assessment of Cellulose: From Properties to the Potential of Making It a Low Carbon Material. Materials 2021, 14, 714. [Google Scholar] [CrossRef] [PubMed]
- Klöpffer, W.; Grahl, B. Life Cycle Assessment (LCA): A Guide to Best Practice; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 978-3-527-65564-9. [Google Scholar]
- Bafana, A.; Kumar, S.V.; Temizel-Sekeryan, S.; Dahoumane, S.A.; Haselbach, L.; Jeffryes, C.S. Evaluating Microwave-Synthesized Silver Nanoparticles from Silver Nitrate with Life Cycle Assessment Techniques. Sci. Total Environ. 2018, 636, 936–943. [Google Scholar] [CrossRef]
- Bishop, G.; Styles, D.; Lens, P.N.L. Environmental Performance Comparison of Bioplastics and Petrochemical Plastics: A Review of Life Cycle Assessment (LCA) Methodological Decisions. Resour. Conserv. Recycl. 2021, 168, 105451. [Google Scholar] [CrossRef]
- Miseljic, M.; Olsen, S.I. LCA of Nanomaterials. In Life Cycle Assessment: Theory and Practice; Hauschild, M.Z., Rosenbaum, R.K., Olsen, S.I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 817–833. ISBN 978-3-319-56475-3. [Google Scholar]
- Nizam, N.U.M.; Hanafiah, M.M.; Woon, K.S. A Content Review of Life Cycle Assessment of Nanomaterials: Current Practices, Challenges, and Future Prospects. Nanomaterials 2021, 11, 3324. [Google Scholar] [CrossRef]
- Broeren, M.L.M.; Kuling, L.; Worrell, E.; Shen, L. Environmental Impact Assessment of Six Starch Plastics Focusing on Wastewater-Derived Starch and Additives. Resour. Conserv. Recycl. 2017, 127, 246–255. [Google Scholar] [CrossRef]
- Rojas-Bringas, P.M.; De-la-Torre, G.E.; Torres, F.G. Influence of the Source of Starch and Plasticizers on the Environmental Burden of Starch-Brazil Nut Fiber Biocomposite Production: A Life Cycle Assessment Approach. Sci. Total Environ. 2021, 769, 144869. [Google Scholar] [CrossRef]
- Aryan, Y.; Yadav, P.; Samadder, S.R. Life Cycle Assessment of the Existing and Proposed Plastic Waste Management Options in India: A Case Study. J. Clean. Prod. 2019, 211, 1268–1283. [Google Scholar] [CrossRef]
- Joshi, S. Can Nanotechnology Improve the Sustainability of Biobased Products? J. Ind. Ecol. 2008, 12, 474–489. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent Progress in Biodegradable Polymers and Nanocomposite-Based Packaging Materials for Sustainable Environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Kochkina, N.E.; Butikova, O.A. Effect of Fibrous TiO2 Filler on the Structural, Mechanical, Barrier and Optical Characteristics of Biodegradable Maize Starch/PVA Composite Films. Int. J. Biol. Macromol. 2019, 139, 431–439. [Google Scholar] [CrossRef]
- Glaskova-Kuzmina, T.; Starkova, O.; Gaidukovs, S.; Platnieks, O.; Gaidukova, G. Durability of Biodegradable Polymer Nanocomposites. Polymers 2021, 13, 3375. [Google Scholar] [CrossRef] [PubMed]
- Bher, A.; Unalan, I.U.; Auras, R.; Rubino, M.; Schvezov, C.E. Graphene Modifies the Biodegradation of Poly(Lactic Acid)-Thermoplastic Cassava Starch Reactive Blend Films. Polym. Degrad. Stab. 2019, 164, 187–197. [Google Scholar] [CrossRef]
- Prusty, K.; Swain, S.K. Nano CaCO3 Imprinted Starch Hybrid Polyethylhexylacrylate\polyvinylalcohol Nanocomposite Thin Films. Carbohydr. Polym. 2016, 139, 90–98. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).