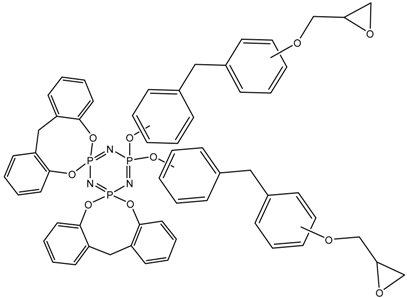
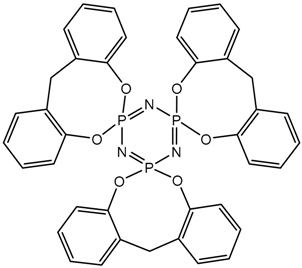
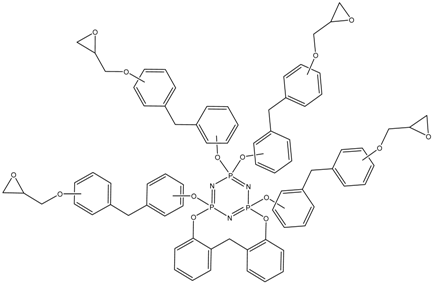
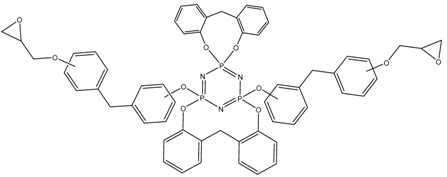
Abstract
Organophosphazenes are of interest due to the combination of increased mechanical and thermal properties of polymer materials obtained with their use, however, they are characterized by a complex multi-stage synthesis. Moreover, the high viscosity of phosphazene-containing epoxy resins (PhER) makes their processing difficult. To simplify the synthesis of PhER, a one-step method was developed, and bisphenol F was chosen, which also provided a decrease in viscosity. In the current study, PhER were formed by a one-stage interaction of hexachlorocyclotriphosphazene (HCP) with bisphenol F isomers and epichlorohydrin in the presence of alkali, which was a mixture of epoxycyclophosphazenes (ECPh) with a functionality from 1 to 4 according to the results of MALDI-TOF analysis. Conventional epoxy resins based on bisphenol F, also formed during the process, showed high mechanical properties and glass transition temperature, and the reactivity of the obtained resins is similar to the base epoxy resins based on bisphenols A and F. Cured PhER had higher or the same mechanical properties compared to base epoxy resins based on bisphenol A and F, and a glass transition temperature comparable to base epoxy resins based on bisphenol F: glass transition temperature (Tg) up to 174.5 °C, tensile strength up to 74.5 MPa, tensile modulus up to 2050 MPa, tensile elongation at break up to 6.22%, flexural strength up to 146.6 MPa, flexural modulus up to 3630 MPa, flexural elongation at break up to 9.15%, and Izod impact strength up to 4.01 kJ/m2. Analysis of the composition of the obtained PhER was carried out by 1H and 31P NMR spectroscopy, MALDI-TOF mass spectrometry, X-ray fluorescence elemental analysis, and contained up to 3.9% phosphorus and from 1.3% to 4.2% chlorine. The temperature profile of the viscosity of the resulting epoxy resins was determined, and the viscosity at 25 °C ranged from 20,000 to 450,000 Pa·s, depending on the ratio of reagents. The resins studied in this work can be cured with conventional curing agents and, with a low content of the phosphazene fraction, can act as modifiers for traditional epoxy resins, being compatible with them, to increase impact strength and elasticity while maintaining the rest of the main mechanical and processing properties, and can be used as a resin component for composite materials, adhesives, and paints.
1. Introduction
Epoxy resins are one of the most common thermosetting polymers, having a wide range of applications, such as: coatings, adhesives, casting compounds, resins, and binders for composite materials in various areas, from household to critical in aircraft, rocket, mechanical engineering, and electronics and electrical engineering [1].
At the same time, a number of thermoplastics and other types of thermosetting polymers are used for the same applications. A new generation of binders is being developed with enhanced mechanical properties, thermal and fire resistance, such as: bismaleimides, polyimides, and cyanate ethers [2]. However, they are inferior to epoxides in cost, manufacturability, and in some cases in mechanical properties and toxicity during processing [2,3].
With their advantages, base epoxy resins also have a number of disadvantages, as they often have low and unstable performance characteristics, in particular, combustibility and relatively low heat resistance [4]. An effective way to increase the heat resistance of epoxy matrices is to modify them with polyfunctional resins that crosslink and form a joint mesh structure with base resins. Examples of such resins are triglycidyl-p-aminophenol, tetraglycidyl-4,4′-methylenedianiline, and epoxidized novolaks [4,5].
The problem of flammability of epoxy resins is more complex and can be solved using various additives and/or components:
- Halogenated epoxy resins
- Additive-type fire retardant
- Halogen-free organoelement compounds
The traditional way to reduce flammability is to use halogen-containing epoxy resins, in particular based on brominated bisphenol A. However, these types of resins release toxic gases when in contact with a flame, which limits their use in areas that involve human contact [6,7].
The modern global trend is the use of halogen-free polymer materials as additives to improve the fire resistance of polymeric materials [6]. A cheap and common option is the use of inorganic additives, such as aluminum or magnesium hydroxides, polyphosphates, and red phosphorus, but they significantly reduce the mechanical properties and transparency of materials and degrade the technological properties important for processing [6,8].
Organophosphates proved to be more functional. They are better combined with the base polymer, but at the same time they can act as a plasticizer and reduce heat resistance [9].
Functional organoelement compounds combine the advantages of non-toxicity, preservation of the properties of the base polymer, and heat resistance, capable of forming a common three-dimensional structure with the base epoxy resin [7,10]. An example of such modifiers are glycidyl esters of phosphorus acids, with a decrease in flammability, which increase the mechanical and adhesive strength of the material [10,11]. However, they are not well-compatible with base epoxy resins [11,12]. Additionally, the disadvantage of phosphorus-containing fire retardants is their lower heat resistance compared to modified epoxy bases [6,7,13,14], which limits their use in engineering plastics and high-temperature binders.
One of the promising classes of compounds that can meet the growing needs of high-tech industries are phosphazenes. The main chain of organophosphazenes consists of alternating phosphorus and nitrogen atoms, and at the phosphorus atom there are organic radicals introduced by substitution of a halogen in halophosphazenes. The nature of organic substituents, usually introduced by the reaction of nucleophilic substitution of chlorine, can vary widely and determines the properties of the final polymer or oligomer.
There are two main synthetic approaches that make it possible to obtain functional phosphazenes capable of forming covalent bonds with epoxy matrices:
- (1)
- Synthesis of organophosphazenes with reactive epoxy groups for addition to the epoxy component [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40].
- (2)
- Synthesis of organophosphazenes with reactive amino groups for use as a hardener or its component [41,42,43,44,45,46,47,48,49].
Epoxyphosphazenes are well-compatible with the epoxy matrix and at the same time improve its mechanical properties and heat resistance, probably due to the formation of a special three-dimensional polymer network at the junction nodes, where phosphazene cycles are located [50].
Previously, the synthesis of epoxyphosphazenes was primarily scientific due to the complexity of scaling and the large number of intermediate steps [15,16,17,18,19,21,25,27,28,30,31,32,33]. At present, a number of works have appeared that present fairly simple synthesis methods, with a level of manufacturability close to the production processes of basic epoxy resins [20,22,23,24,26,29,34,37]. Some of them, such as alkoxyphosphazenes, are thermally unstable [51]. Thus, the synthesis of aromatic organophosphazenes is preferred. Examples of such processes are one-stage methods for obtaining: (a) phosphazene-containing epoxy resins based on bisphenol A [52,53] and (b) based on resorcinol [54]. The resulting PhER consisted mainly of tetra- and penta-epoxyphosphazenes and an organic epoxy monomer, which is essentially an active diluent that reduces the viscosity and average functionality to a level acceptable for further processing, and these PhER demonstrated increased mechanical properties and heat resistance. Due to the fact that the viscosity of such resins at ambient temperature is more than 200 Pa∙s, which is a value close to the processing limit, studies were carried out to obtain PhER based on bisphenol A and phenol, including the one-stage method, with the purpose of reducing viscosity and increasing the phosphorus content to reduce flammability [50,55].
In order to obtain PhER with a lower viscosity, which ensures the processability of processing, and a higher phosphorus content, for a potential reduction in flammability, in comparison with PhER described in [52,53,54,55], while maintaining their advantages in mechanical properties, PhER were synthesized by direct interaction of HCP, bisphenol F, and epichlorohydrin. To assess the impact on the physical properties of the use of bisphenol F and the content of the phosphazene fraction, the analysis of the composition of the obtained phosphazene-containing epoxy resins and tests for mechanical properties, glass transition temperature, and viscosity were carried out.
2. Materials and Methods
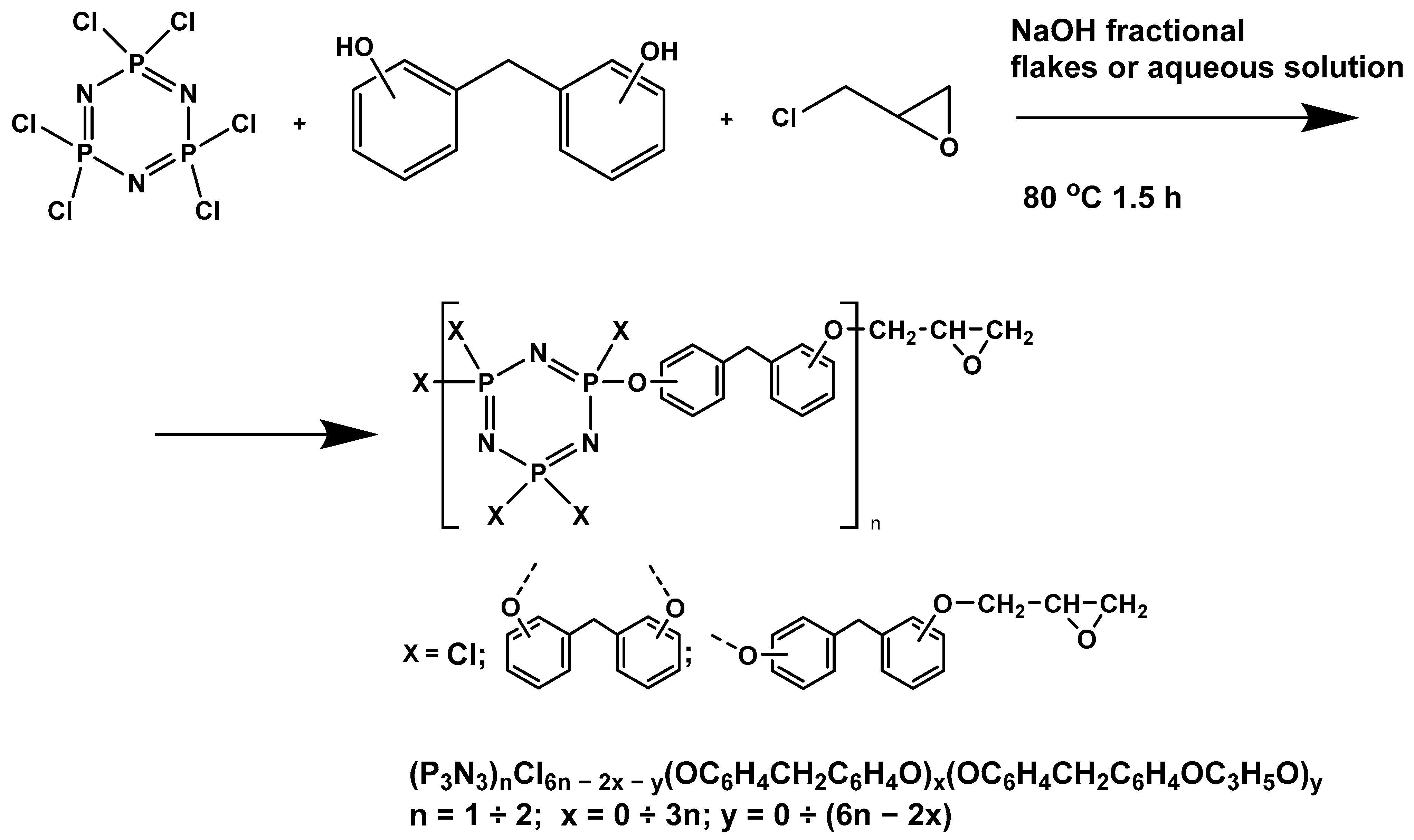
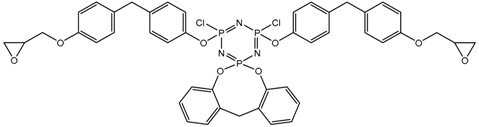
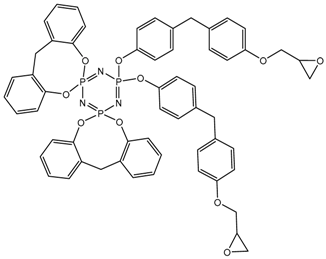
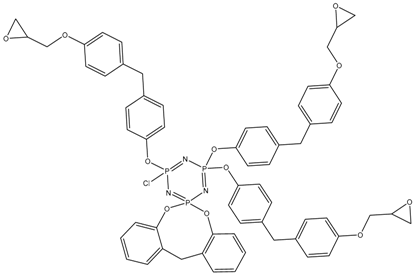
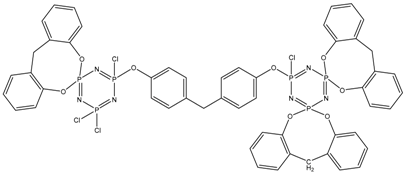
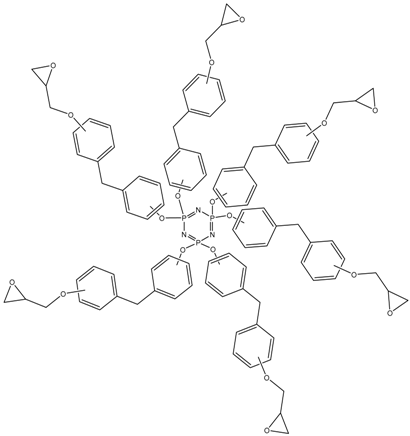
In this article, PhER were synthesized by a single-stage interaction of hexachlorocyclotriphosphazene with an isomer mixture of bisphenol F, in the medium of epichlorohydrin, which acts both as a reagent and as a solvent (Figure 1). The epoxy group formed by the catalyst and the HCl acceptor NaOH was used in one case in solid form, and in the other in the form of an aqueous solution.
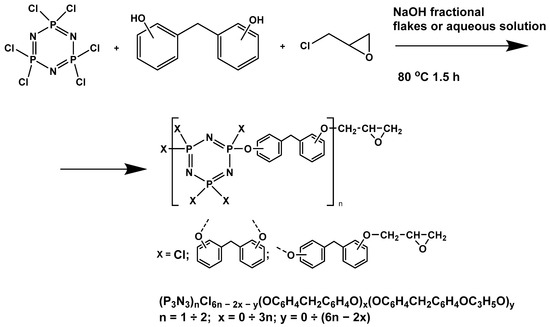
Figure 1.
Single-stage synthesis of phosphazene-containing epoxy resins by direct interaction of hexachlorocyclotriphosphazene (HCP) and bisphenol F in epichlorohydrin medium (this work).
2.1. Materials
Hexachlorocyclotriphosphazene (HCP), a white crystalline substance (Tm.p. = 113.0 °C; 31P NMR spectrum, singlet with δP = 19.9 ppm), was obtained by the method in [56].
Bisphenol F (BPF), a white powder (mixture of isomers 2,2′-, 2,4′-, and 4,4′-dioxydiphenyldimethylmethane, Tm.p. = 162–164 °C) (Manufacturer: Hangzhou Sartort Biopharma Co., Ltd., Hangzhou, China).
Epichlorohydrin (ECH) is a colorless liquid, the content of the main substance is 99.8%, and it was used after preliminary distillation, Тb.p. = 116 °С (Manufacturer: Solvay, Tavaux, France).
Potassium hydroxide, in the form of white flakes (the content of the main substance is 90%), was used without purification. The water content was determined by acid-base titration and was about 10% (Manufacturer: JSC “Caustic” Volgograd, Russia).
Acetone is a colorless liquid, and the content of the main substance is 99.8%. It was used as a solvent during isolation without preliminary purification (Supplier: Component-Reaktiv LLC, Moscow, Russia).
Diglycidyl ether of bisphenol A (DGEBA) is a clear-cloudy viscous liquid at room temperature. Commercial-grade KER 828 was used without pre-treatment (Manufacturer: Kumho P & B Chemicals, Inc., Seoul, South Korea).
Diglycidyl ether of bisphenol F (DGEBF) is a clear-cloudy viscous liquid at room temperature. The commercial brand Ipox er 1016 was used without preliminary purification (Supplier: NORTEX LLC, Moscow, Russia).
Diaminodiphenylsulfone (DDS) (Aradur 9664-1) is a fine powder with a particle size smaller than 64 μm and Тm.p. = 175 °С (Manufacturer: Huntsman Corporation, Spain).
2.2. Synthetic Methods
2.2.1. Synthesis of Phosphazene-Containing Resins Based on Bisphenol F (PNA-BPF) with Batch Introduction of Solid Alkali NaOH
HCP, BPF, and epichlorohydrin were loaded into a 2 L batch reactor equipped with a mechanical stirrer and a direct condenser cooled by recycled water in HCP:BPF ratios from 1:8 to 1:24 with a constant excess of 16-fold ECH (Table 1).

Table 1.
The amounts of initial reagents for the synthesis of PhER.
The reaction mixture was heated with stirring to a temperature of 45–50 °C and kept at this temperature until complete dissolution of the solid reagents. Then, the mixture was heated to 77 °C, after which the first portion of sodium hydroxide was loaded in the form of a powder, then at intervals of 5 min, but taking into account that the temperature of the reaction mixture should not exceed 85 °C, the rest of the sodium hydroxide was loaded in equal portions over 1/8 of the total. Sodium hydroxide was taken in a stoichiometric amount. After loading the entire amount of sodium hydroxide, the reaction was carried out for 50 min at a temperature of 80 °C, distilling off the epichlorohydrin–water mixture in a vacuum.
At the end of the reaction, from the resulting suspension, ECH was distilled off on a rotary evaporator, and then the resulting mixture of resin and salt was dissolved in an excess of acetone, and the solution was subsequently filtered from the precipitate through a paper filter, after which acetone was distilled off from the filtrate on a rotary evaporator. The product was a yellow viscous liquid. The yield was 91.2–94.3%.
2.2.2. Synthesis of Phosphazene-Containing Resins Based on Bisphenol F (PNA-BPF-S) with Batch Introduction of NaOH Alkali Solution
The synthesis of PNA-BPF-S was carried out in exactly the same way as the synthesis of PNA-BPF, except that the alkali was loaded in portions in the form of a solution (Table 1). The yield was 76%.
2.3. Methods of Analysis
2.3.1. Epoxy Group Content
The epoxy group content was determined by back acid-base titration in acetone according to GOST R 56752-2015 (ISO 3001:1999).
2.3.2. Elemental Analysis
Elemental analysis was carried out by X-ray fluorescence spectrometry with calibration according to the method of fundamental parameters on an X-ray fluorescence spectrometer, ARL PFX-101 (Thermo Fisher Scientific (Ecublens) SARL, Ecublens, Switzerland).
2.3.3. Nuclear Magnetic Resonance Spectroscopy
31P NMR spectroscopy was performed on a Bruker AV600 at a working purity of 242 MHz. 1H NMR spectroscopy was carried out on a Bruker AV600 instrument at a working purity of 600 MHz. Deuterochloroform was used as a solvent.
2.3.4. Mass Spectrometric Analysis of MALDI-TOF
Mass spectrometric analysis of MALDI-TOF was carried out on a Bruker Auto Flex II instrument.
2.3.5. Rheology
The temperature profile of viscosity was measured on an MCR 302 rheometer (Anton Paar, Graz, Austria). The measurements were carried out in the continuous shear mode with the measuring plate–plate geometry, shear rate of 10 s−1, and gap of 1 mm. Top plate diameter was 25 mm.
2.4. Method of Curing
The calculated amount of hardener, DDS, was added to the epoxy resin, based on the stoichiometric ratio. The resulting composition was stirred and degassed on an oil bath rotor at 125 °C under a vacuum of 100 mm Hg for 20–40 min until the mixture was completely homogenized. The molds were degreased with acetone before pouring and then treated with an anti-adhesive liquid. All samples were cured at 180 °C for 8 h.
2.5. Methods of Testing
2.5.1. Tensile Testing
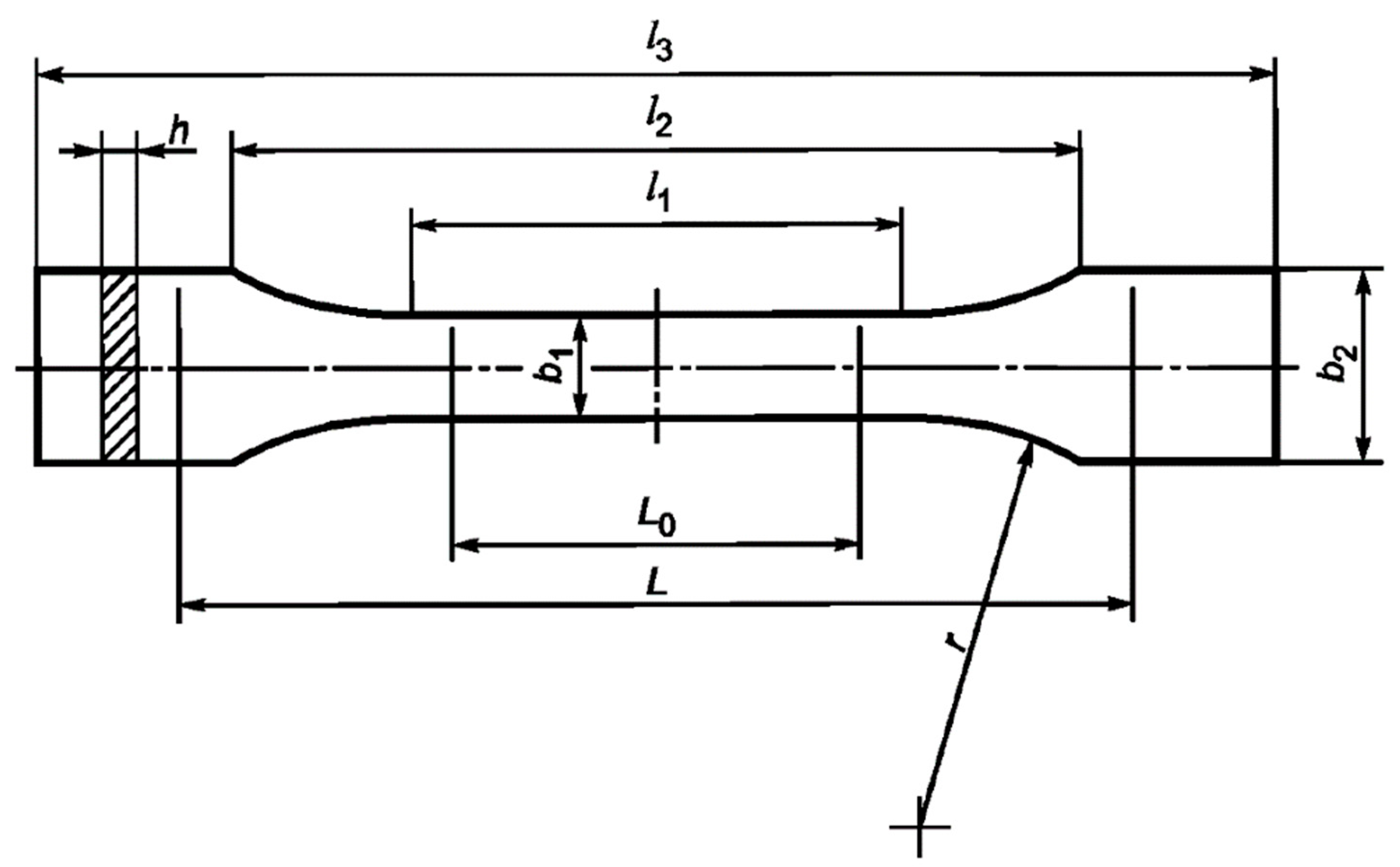
Tensile tests were carried out on a 50ST (Tinius Olsen, Redhill, United Kingdom) universal electromechanical tensile testing machine according to GOST 11262-2017 (ISO 527-2:2012). Samples of the cured binder were cut from a 4 mm-thick plate on a milling machine in accordance with the paragraph 6.1. standard (Figure 2). The stretching speed was 10 mm/min. From the data obtained, the values of tensile strength, tensile modulus, and elongation were calculated.
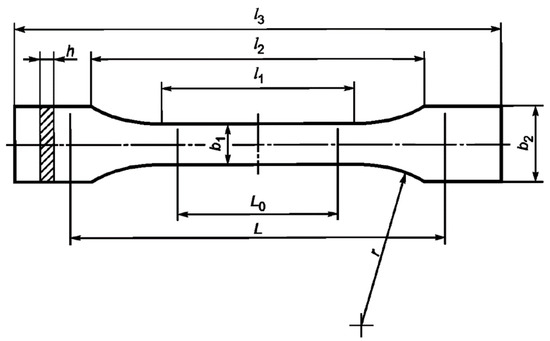
Figure 2.
Tensile test sample. Dimensions, in mm: l1 = 80 ± 2, l2 = 109.3 ± 3.2, l3 = 170, L0 = 75 ± 0.5, L = 115 ± 1, b1 = 10 ± 0.2, b2 = 20 ± 0.2, h = 4 ± 0.2, r = 24 ± 1.
2.5.2. Flexural Testing
Flexural tests were carried out on a 50ST electromechanical universal tensile testing machine (Tinius Olsen, Redhill, United Kingdom) according to GOST R 56810-2015 (ASTM D790-10) at a speed of 0.85 mm/min. Samples of the cured binder were cut from a 3.6 mm-thick plate on a milling machine in accordance with the paragraph 6.1. standard. The samples were bars with an aspect ratio of length × width × thickness equal to 80 × 9.8 × 3.6 mm. Span-to-depth ratio was 64:3.6 mm. From the data obtained, the values of flexural strength and flexural modulus were calculated.
2.5.3. Izod Impact Test
Izod impact tests were carried out on an Izod-Charpy K 1053 machine (ATS FAAR, Cassina De’ Pecchi, Italy) according to GOST 19109-2017 (ISO 180:2000), “Plastics. Izod impact strength method”, which is a modified ISO 180:2000 standard. The samples were bars with an aspect ratio of length × width × thickness equal to 80 × 9.8 × 3.6 mm.
2.5.4. Glass Transition Temperature
The glass transition temperature was determined on a DSC 204 F1 Phoenix differential scanning calorimeter (Netzsch, Selb, Germany) according to GOST R 55135-2012 (ISO 11357-2:1999). Under the dynamic heating mode, the temperature range was 40–270 °C, and the heating rate was 10 °C/min. All tests were carried out under a nitrogen atmosphere with a flow rate of 60–100 mL/min. The weight of the samples was 5–10 mg.
The device was calibrated for temperature and enthalpy for standard metals gallium, indium, tin, bismuth, and zinc.
The analysis of parameters and characteristic points was carried out in the Proteus thermal analysis software.
3. Results and Discussion
3.1. Synthesis and Study of PNA-BPF and PNA-BPF-S
Analysis of the composition and structure of the obtained PhER was carried out using MALDI-TOF spectrometry and 31P NMR spectroscopy.
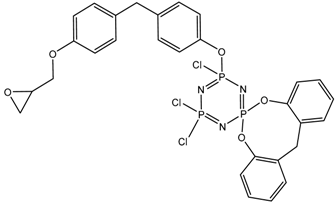
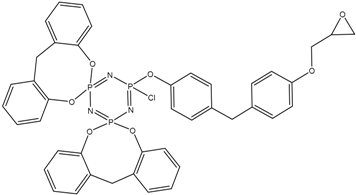
MALDI-TOF analysis showed the presence in the products of compounds with m/z values and possible structures, as presented in Table 2.

Table 2.
Possible structures and formulas of the main compounds formed during the synthesis of PNA-BPF and PNA-BPF-S.
The formation of VII-membered spirocyclic fragments at the phosphorus atom with the participation of the 2,2-isomer of bisphenol F in almost every component of the product mixture is noteworthy. This leads to a decrease in the average functionality of the mixture in comparison with epoxyphosphazenes based on bisphenol A and resorcinol, which can favorably affect the pot life of the resins.
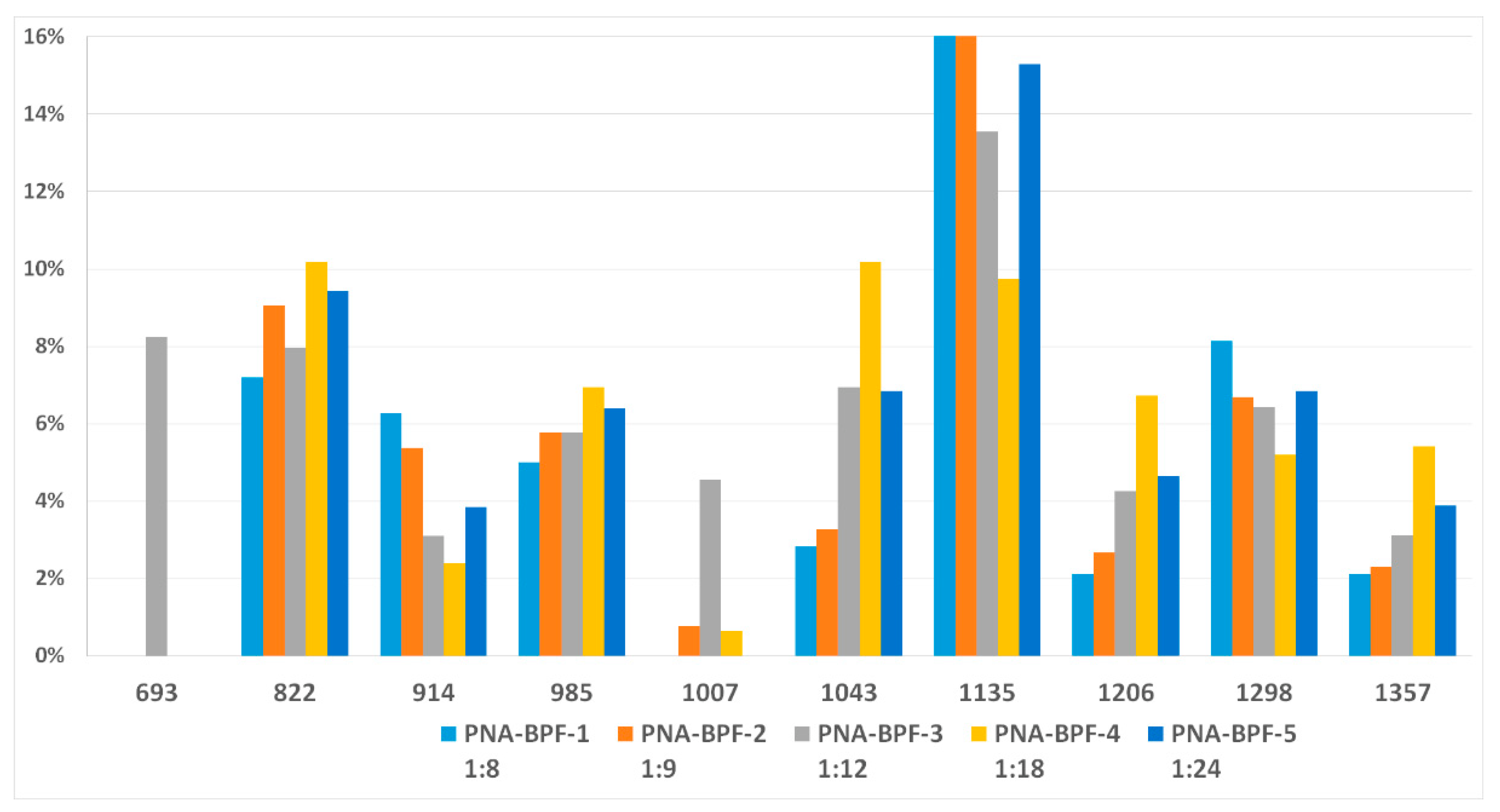
MALDI-TOF analysis is not an unambiguously quantitative method of analysis without confirmation of its results by other methods, however it can be used to determine the qualitative presence of compounds and trends in their formation depending on the conditions. According to the results of MALDI-TOF, presented in Table 3 and Table 4 and Figure 3 and Figure 4, there are pronounced extreme dependencies of the formation of compounds with structures I and V with peaks at a ratio of 1:12. A less pronounced increase in the formation of compounds VI, VIII, and X, and a decline for compounds III, VII, and IX, were observed with peaks at a ratio of 1:18. The amounts of compounds II and IV fluctuate without unambiguous dependencies. Extreme values of the formation of substances are typical for synthesis 4 with a ratio of 1:18.

Table 3.
The main compounds formed during the synthesis of PNA-BPF and PNA-BPF-S, the share of which is more than 4% in at least one of the experiments.

Table 4.
Possible structures and formulas of hexa-substituted phosphazenes formed during the synthesis of PNA-BPF.
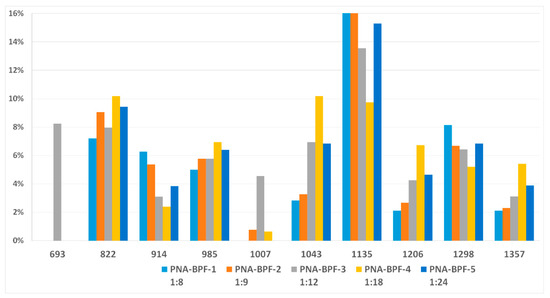
Figure 3.
The content of compounds I–X (Table 2) in samples PNA-BPF-1 ÷ 5 ((1)–(5), respectively).
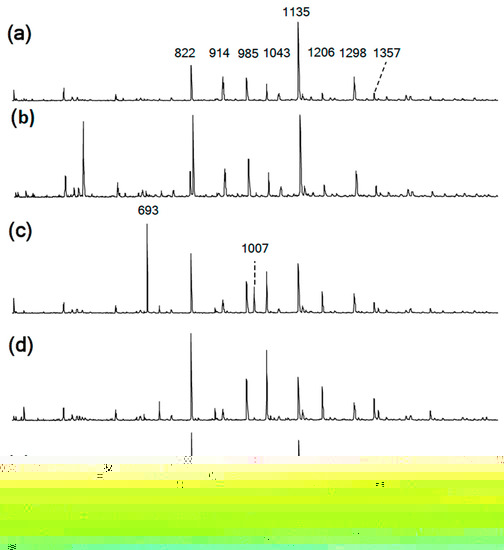
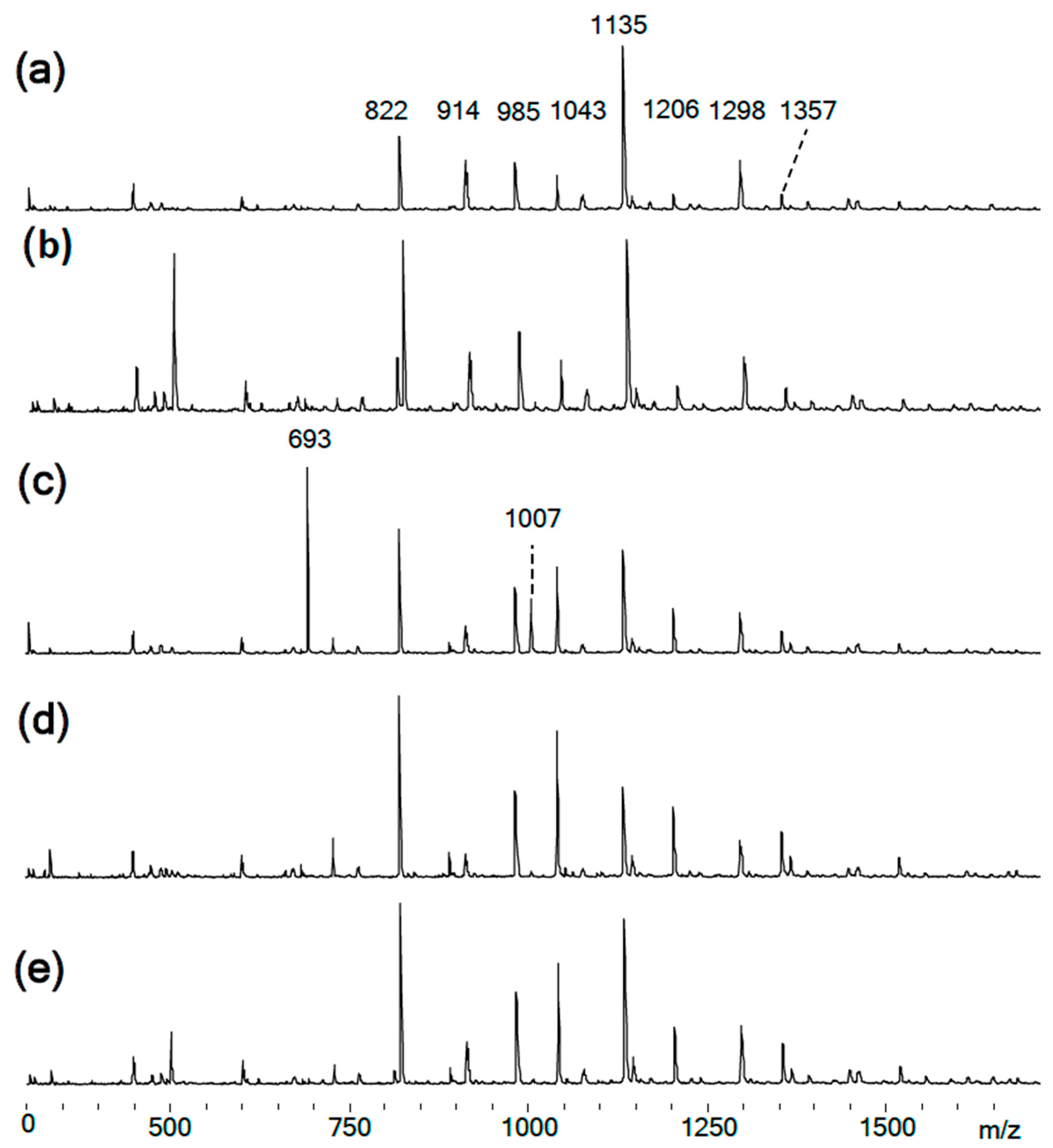
Figure 4.
(a–e) MALDI-TOF spectra of the obtained PhER syntheses of PNA-BPF-1 ÷ 5, respectively.
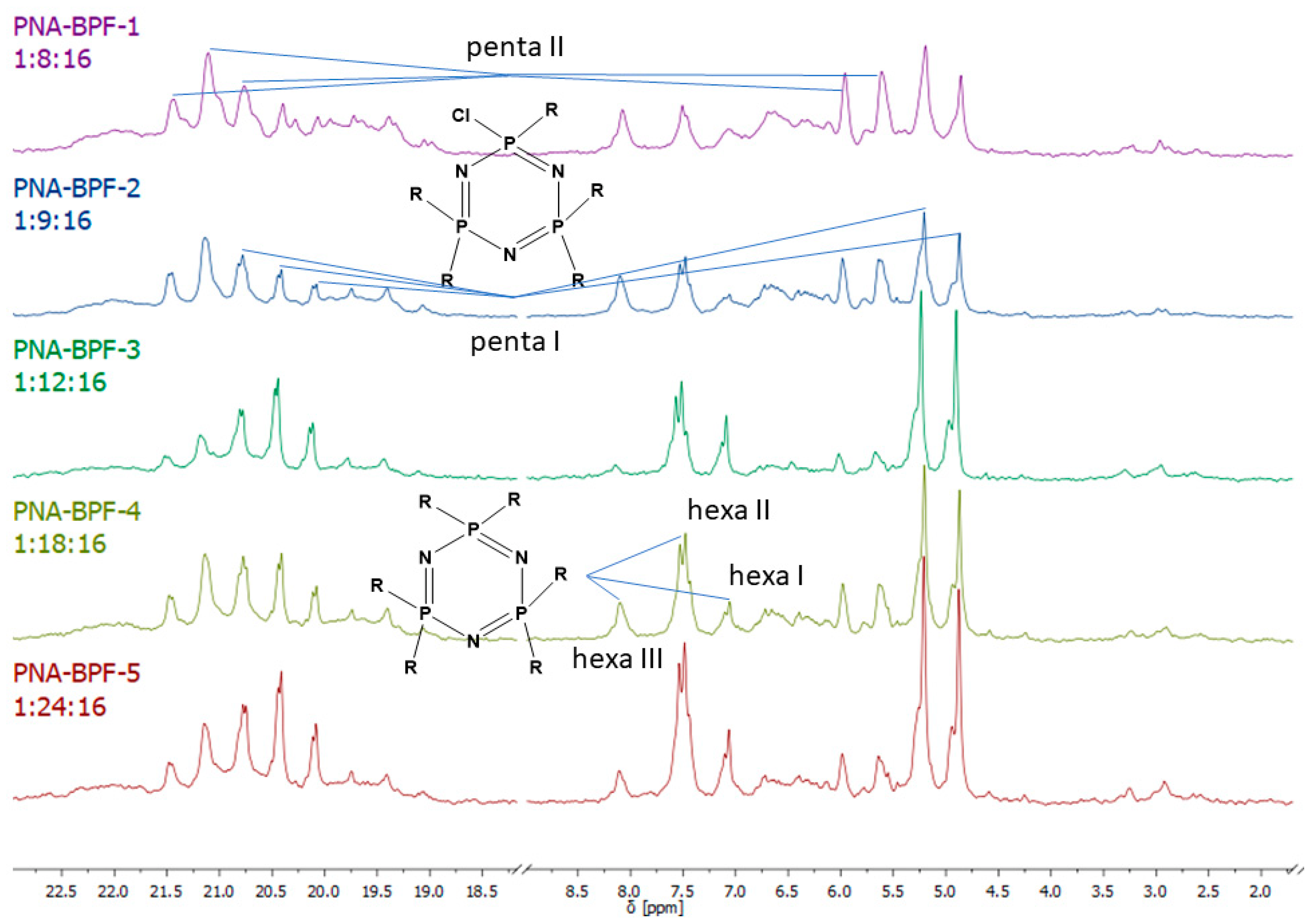
31P NMR spectra of the final product (Figure 5) indicate incomplete substitution and the formation of a mixture of tetra-, penta-, and hexa-derivatives of hexachlorocyclo-triphosphazene with a predominance of penta-derivatives. Due to the presence of singlet signals in the region of 7.07, 7.51, and 8.08 ppm, it can be assumed that several types of hexa-substituted triphosphazenes are formed (Table 4). However, due to steric difficulties, the formation of compounds XIV, XV, and XVI is unlikely; therefore, the three signals mentioned above probably correspond to compounds XI, XII, and XIII. The presence of compound XIII is also confirmed by MALDI-TOF analysis and corresponds to compound VI. As the BPF excess increases, the yield of hexa-substituted derivatives with a singlet signal of 8.08 ppm decreases, and the yield of hexa-substituted derivatives of the structure increases with signals at 7.51 and 7.07. The penta-derivative penta I corresponds to the doublet (4.88–5.21 ppm) and triplet (20.08–20.75 ppm) systems. The penta-derivative penta II corresponds to the doublet (5.64–5.99 ppm) and triplet (20.75–21.48 ppm) systems. In the penta-derivatives penta I and penta II, the triplet peaks in the region of 20.78 ppm overlap, which is confirmed by the values of the integral areas—the integral area under the peak with overlap is equal to the sum of the areas under the peaks in the region at 21.48 and 20.08 ppm. A tetrasubstituted product corresponds to a triplet (2.96 ppm) and a doublet (19.41–19.74 ppm) signal system. Derivatives of hexa I, II, III, and penta I and II may not be individual substances, but a mixture of derivatives with the same degree of substitution, but different substituents—epoxy, spirocyclic, and bridging—which produce signals in one or close intervals, which can lead to their overlap.
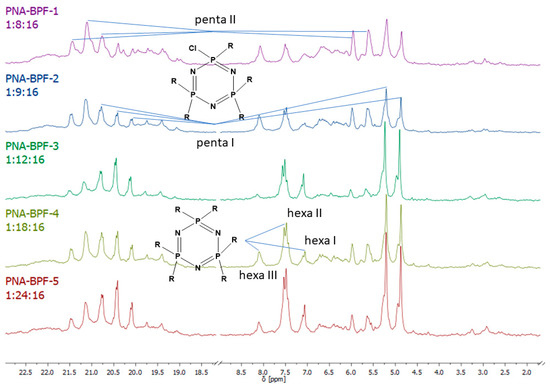
Figure 5.
31P NMR spectra of PNA-BPF.
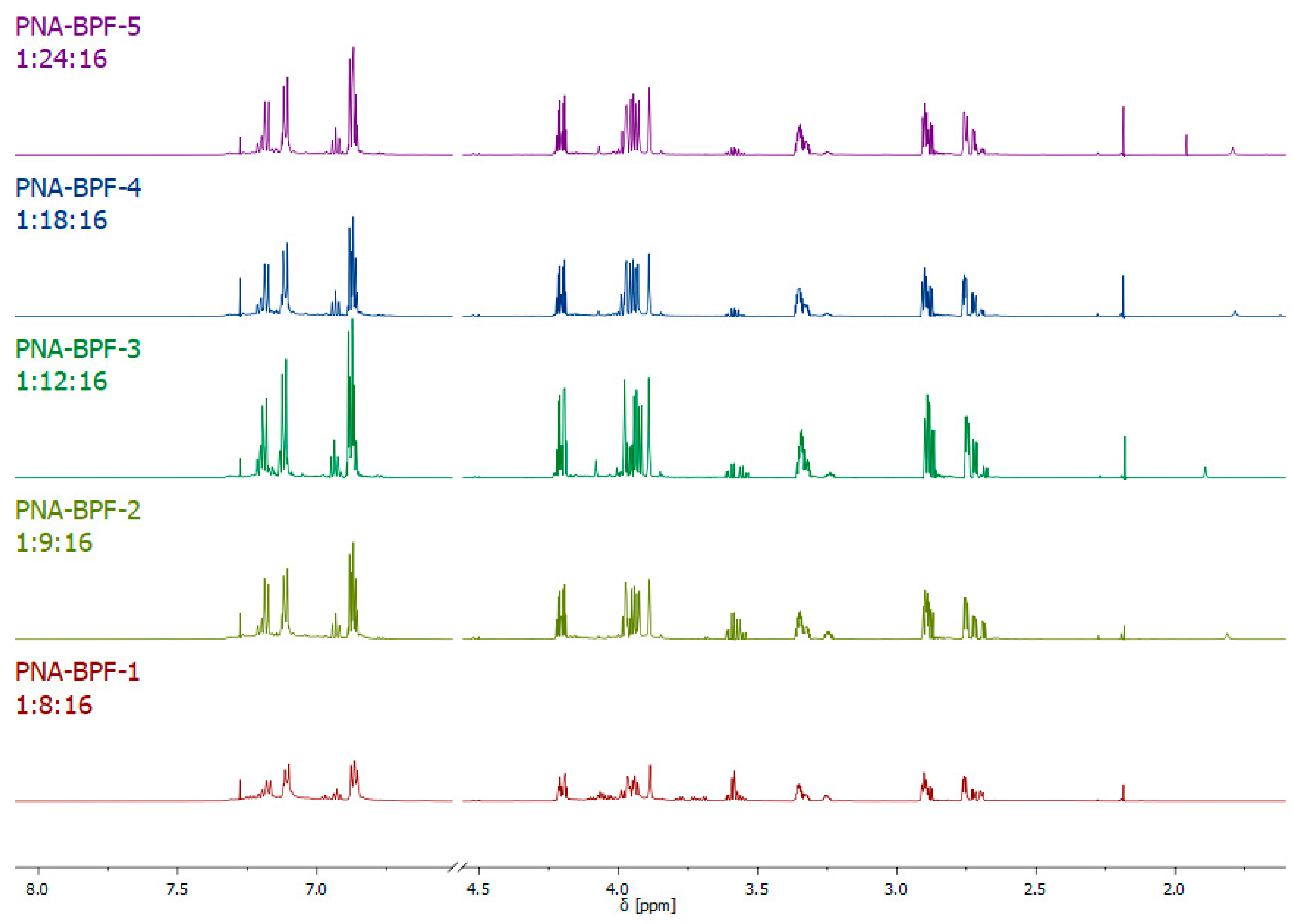
1H NMR spectra of the obtained epoxy oligomers (Figure 6) are similar to the spectra for dianic epoxy resins (industrial grades ED-20, KER-828, and their analogues); however, the signals in the region of 3.55–3.95 ppm indicate the presence of a small amount of glycol end groups formed as a result of the hydrolysis of epoxy groups, apparently caused by the presence of residual alkali in the reaction medium, which was not completely consumed due to incomplete substitution of chlorine atoms in hexachlorocyclotriphosphazene. The results of the integration of signal systems are consistent with theoretical calculations.
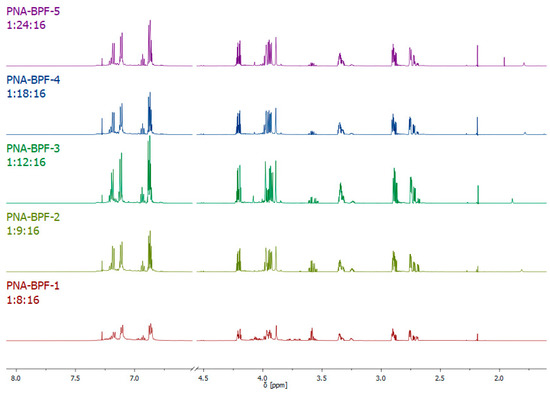
Figure 6.
1H NMR spectra of PNA-BPF.
Table 5 shows the PhER yields depending on the ratios of the initial reagents. The values of the yields of syntheses 1–5 are close and are associated with losses during filtration from salts during the isolation process. The lower yield in the synthesis of PNA-BPF-S-1 is associated with the formation of sparingly soluble oligomers, which were deposited on the filter along with the salt during filtration, which also affected the deviation in the epoxy number.

Table 5.
Yield of the obtained PhER.
Table 6 presents the technological characteristics of various base and phosphazene-containing epoxy resins. Among all pairs of base and phosphazene-containing epoxy resins based on the same diphenols, there is a pattern of increase in viscosity and decrease in the epoxy number in PhER. An exception can be considered PhER obtained by the interaction of HCP with BPA and phenol. By using phenol, this type of resin reduces the viscosity and increases the phosphorus content, which can improve the fire resistance. However, at the same time, the epoxy number drops significantly for these resins, which will reduce the crosslink density during curing, and may worsen the mechanical properties. The described pattern is observed in the case of PhER based on BPF; first, when epoxyphosphazenes appear in the composition, the mechanical properties increase compared to the base resin, then, with an increase in the proportion of the phosphazene fraction and a decrease in the epoxy number, the mechanical properties either remain at the same level, or fall (Table 7 and Table 8).

Table 6.
Comparison of technological characteristics of base and phosphazene-containing epoxy resins based on basic diphenols and the content of phosphorus and chlorine between different PhER. The content of P and Cl wt.% in PhER obtained in this work, determined by X-ray fluorescence spectrometry.

Table 7.
Tensile and impact properties and glass transition temperature of the cured epoxy resins compared to base epoxy resins.

Table 8.
Flexural properties of the cured epoxy resins compared to base epoxy resins.
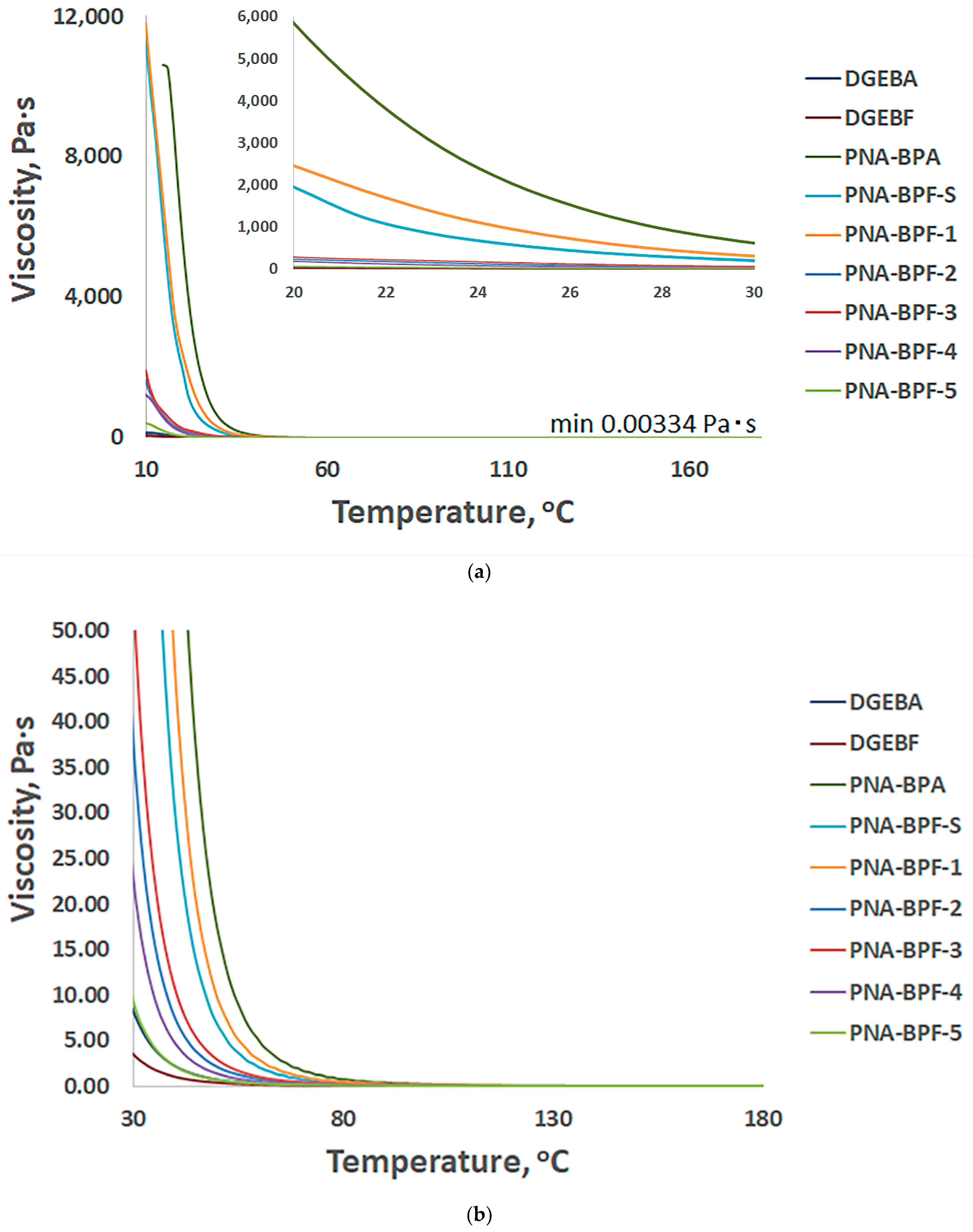
Figure 7 shows the temperature dependence of the viscosity of PhER compared to base epoxy resins. With a decrease in the higher molecular weight phosphazene component and a change in the substituent from bisphenol A to bisphenol F, when receiving PhER (Table 6), the viscosity also naturally decreases.
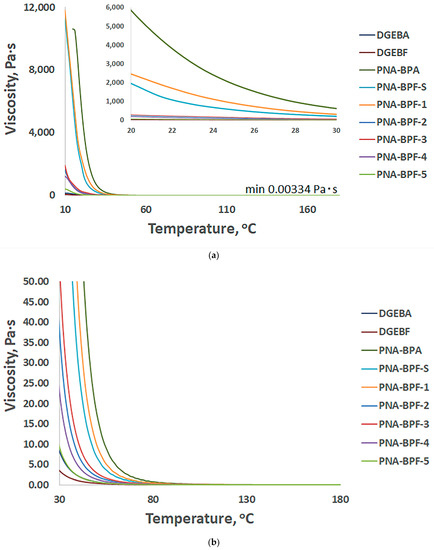
Figure 7.
Temperature profile of viscosity versus temperature of obtained PhER, (a) from 10 to 180 °C and from 22 to 26 °C, and (b) from 30 to 170 °C, respectively. PNA-BPA—epoxy resin obtained by the method in [52] at a ratio of HCP:BPA equal to 1:9.
3.2. Testing of Cured Compositions PNA-BPF and PNA-BPF-S
With an increase in the degree of substitution along with an increase in the excess of bisphenol, which is described by the interpretation of 31P NMR spectra (Figure 5), and the overall increase in the content of phosphazene, the dynamics of changes in the physical and mechanical properties of cured epoxy resins with diaminodiphenyl sulfone at 180 °C for 8 h are also complexly correlated. Extreme dependencies of the formation of a number of compounds and fluctuations in their content, which is described in Section 3.1 when interpreting the MALDI-TOF spectra, could affect the fluctuations in the values of mechanical properties (Table 7 and Table 8).
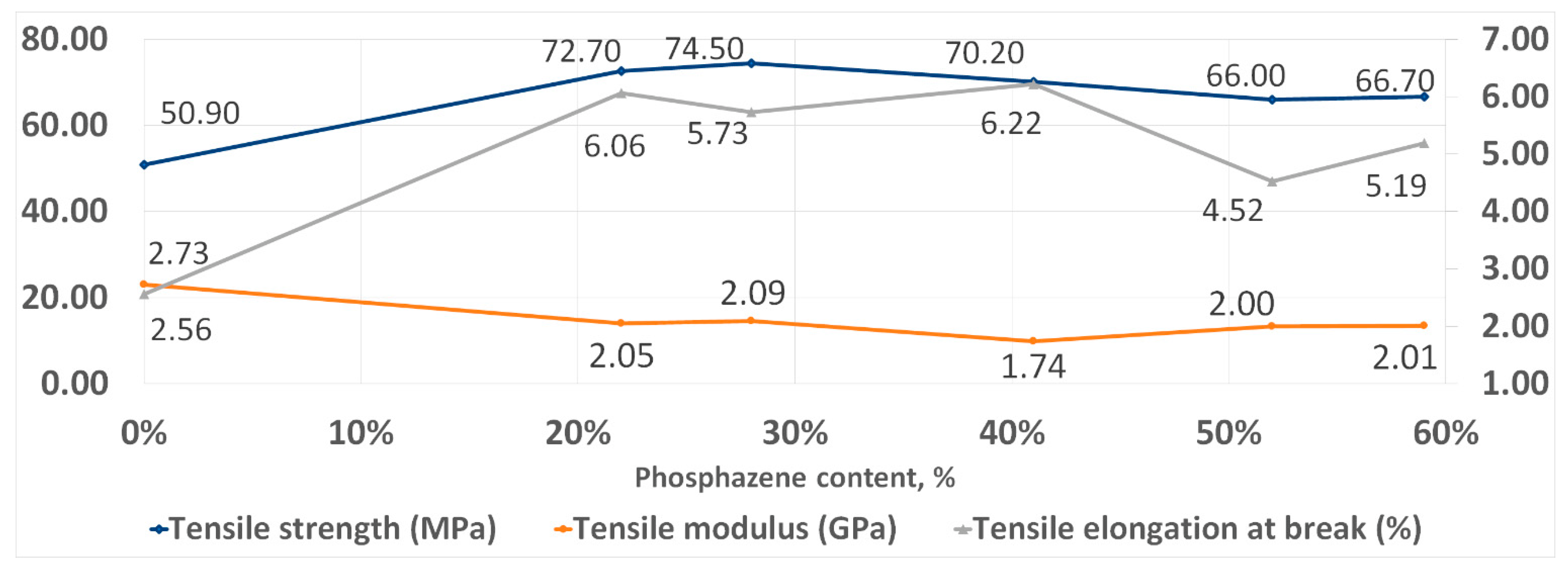
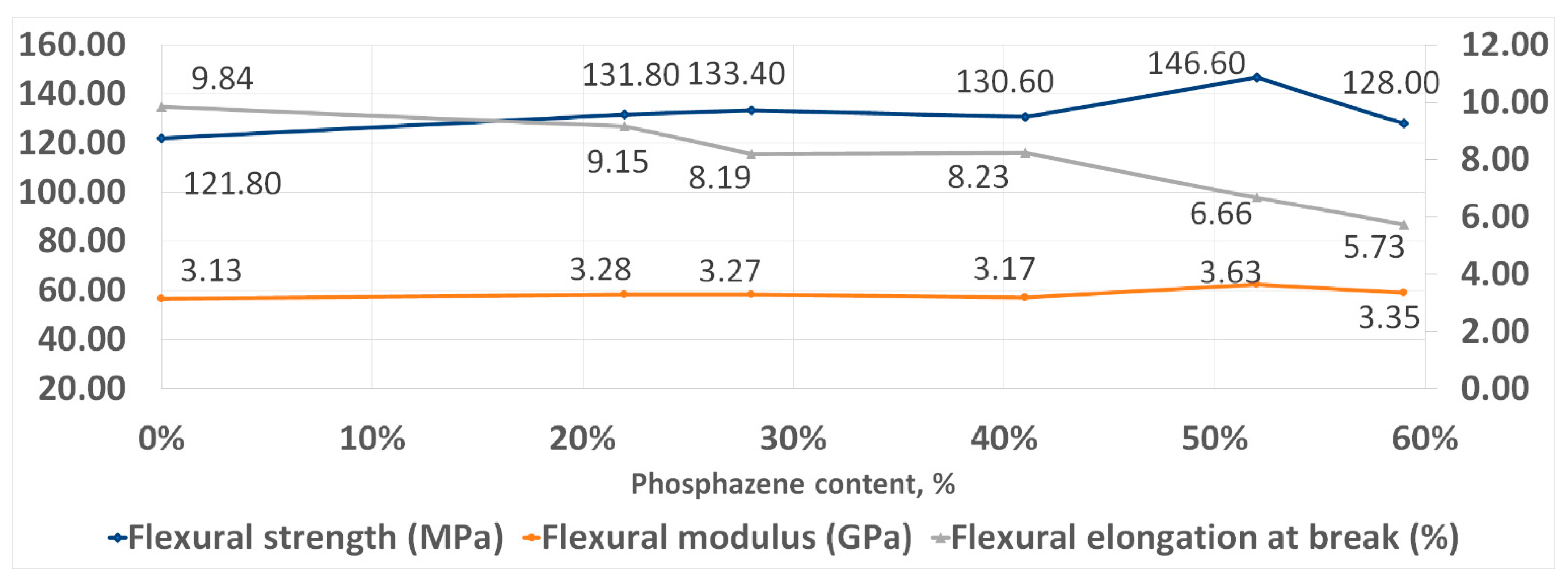
With an increase in the phosphazene fraction, first, an increase in the strength and elasticity of the cured PhER during tensile testing was observed, then a tendency to a decrease in tensile modulus (Figure 8). In flexural tests, an increase in strength was also observed, while the trends in elasticity and flexural modulus were different to tensile tests: the flexural elongation at break continuously decreased with increasing content of the phosphazene fraction, while flexural modulus remained unchanged (Figure 9).
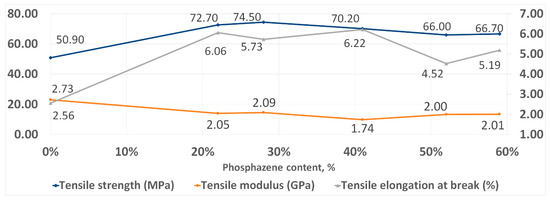
Figure 8.
Tensile strength properties of cured resins.
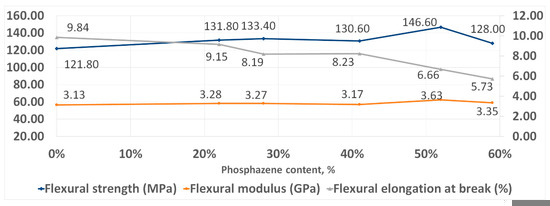
Figure 9.
Flexural strength properties of cured resins.
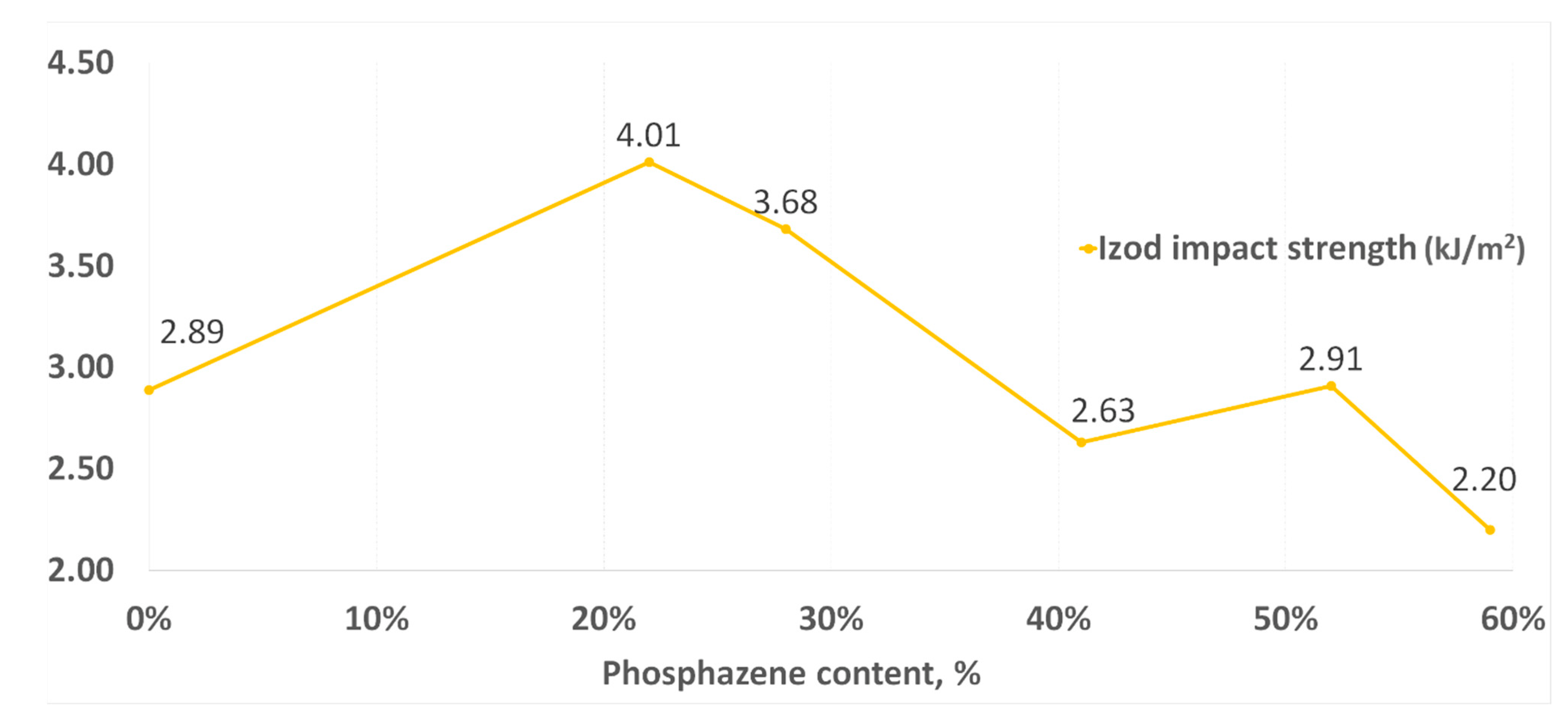
Similar dependencies were also observed when testing for impact strength (Figure 10). With a decrease in the phosphazene fraction, the impact resistance increased to its maximum in the synthesis of PNA-BPF-5, but at the same time it decreased again for the DGEBF epoxy resin, which does not contain epoxyphosphazenes. Based on this, one can talk about the existing modifying property of epoxyphosphazenes based on bisphenol F with respect to the base DGEBF resin. The modifying effect is most likely maximal at the content of the phosphazene fraction in the range of 10–25%. A similar dependence was observed earlier in the study of the properties of PhER based on bisphenol A in [58].
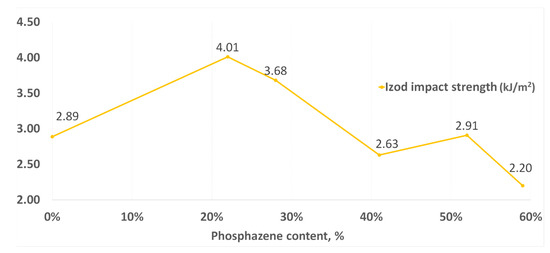
Figure 10.
Impact strength according to Izod.
Supplementary Materials show graphs of percentage changes in the mechanical properties of neat and phosphazene-containing resins based on bisphenol F relative to DGEBA.
A different kind of dependence was observed for the glass transition temperature. Its values decreased compared to the DGEBF neat resin (Table 8), with a minimum content of the phosphazene fraction in PNA-BPF-5, after which they passed through a maximum in PNA-BPF-3 with indicators at the level of base epoxy resins, and then with an increased PhER content, continued to drop significantly, which was not observed for PhER of other types [58].
It is likely that with a further decrease in the content of the phosphazene fraction, the glass transition temperature will resume growth, which may coincide with the maxima of the values of impact strength, elasticity in tension and flexion, and viscosity, while maintaining or some increase in the tensile and flexural properties, according to comparison with the base resin DGEBF, which was observed for all experiments during synthesis on the solid alkali.
Despite the sharply different composition of PNA-BPF-4, corresponding to a phosphazene content of 28%, this was not reflected in the physical and mechanical properties. A sharp change in properties was observed immediately at the lowest phosphazene content of 22%, then the properties either remained at the same level or a tendency to decrease was observed. This may be due to a double modifying effect of the phosphazene content: factor 1 is an increase in the total phosphazene content, and factor 2 is a change in the ratio of the various epoxyphosphazenes (compounds I–X) formed depending on the proportions of the initial reagents. Comprehensively, we can speak of an important effect on the improvement of the properties of compounds II and VII, which constitute the main part for all syntheses. For the remaining compounds, more pronounced fluctuations and extreme dependencies in content were observed with an increase in the proportion of phosphazene, which could affect the general trend of a gradual or significant decrease in properties with an increase in the proportion of phosphazene after an initial sharp increase. The difficulty of assessing factor 2 lies in the need to obtain individual substances formed during the synthesis, and for this it is necessary to develop a method for separating the resulting mixture of epoxyphosphazene resins into individual substances, after which it will be possible to evaluate the modifying effect of the content of each epoxyphosphazene. This paper presents a comprehensive assessment of the effect of the phosphazene content in the production of PhER by a one-stage method, which may be of interest from the perspective of commercial production. Based on the results obtained during the tests, to find the best combination of properties, it is worth studying PhER with a content of 10–25% obtained by this method.
4. Conclusions
The phosphazene-containing epoxy resins obtained in this work have the content of epoxy groups of 15.2–22.8%, and phosphorus of 1.5–3.9%. With a decrease in the content of HCP in the initial reagents, an increase in the mechanical properties of the cured resulting epoxy resins was observed. The most successful in terms of their performance were samples with a ratio of initial reagents HCP:BPF:ECH of 1:12:16–1:24:16. They demonstrated an increase in impact strength by 40–80%, relative elongation at fracture in tension and in flexion by 50–100%, while maintaining glass transition temperature and tensile and flexural strength compared to base epoxy resins based on bisphenols A and F. These PhER can be cured with conventional hardeners to obtain materials with mechanical properties and heat resistance above the base commercial grades of epoxy resins and potentially lower combustibility due to the content of phosphorus.
Thus, phosphazene-containing epoxy resins based on bisphenol F, with a low content of the phosphazene fraction, can act as modifiers for traditional epoxy resins, being compatible with them, to increase impact resistance and elasticity while maintaining other basic mechanical and technological characteristics, and can be used as a binder component for composite materials, adhesives, and paints.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym14214547/s1. Figure S1. Flexural properties; Figure S2. Impact strength; Figure S3. Tensile properties.
Author Contributions
Conceptualization, I.S.S.; formal analysis, I.V.T. and I.S.S.; investigation, I.V.T., A.V.O. and R.S.B.; methodology, I.V.T. and I.S.S.; project administration, I.V.T.; writing—original draft, I.V.T.; writing—review and editing, V.V.K. and I.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Russian Science Foundation under grant 22-73-10242.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The corresponding author can be approached for the availability of data.
Acknowledgments
We thank Korokhin R.A., Korotkov R.F., Zhidal V.C., Kulemza D.S. and Shutov V.V. for technical assistance in the characterization of materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and Application of Epoxy Resins: A Review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Dodiuk, H.; Goodman, S. Handbook of Thermoset Plastics; William Andrew: San Diego, CA, USA, 2014; Volume 254, ISBN 978-1-4557-3107-7. [Google Scholar]
- Biron, M. Thermosets and Composites: Material Selection, Applications, Manufacturing, and Cost Analysis, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; p. 526. [Google Scholar]
- Flick, E.W. Epoxy Resins, Curing Agents, Compounds, and Modifiers: An Industrial Guide; William Andrew: San Diego, USA, 2012; ISBN 978-0-8155-1708-5. [Google Scholar]
- Petrie, E.M. Epoxy Adhesive Formulations; McGraw Hill Professional: New York, NY, USA, 2005; ISBN 978-0-07-158908-6. [Google Scholar]
- Visakh, P.M.; Arao, Y. Flame Retardants: Polymer Blends, Composites and Nanocomposites. Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-03467-6. [Google Scholar]
- Lu, S.-Y.; Hamerton, I. Recent Developments in the Chemistry of Halogen-Free Flame Retardant Polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Green, J. A Review of Phosphorus-Containing Flame Retardants. J. Fire Sci. 1992, 10, 470–487. [Google Scholar] [CrossRef]
- Rodriguez, F.; Cohen, C.; Ober, C.K.; Archer, L.A. Principles of Polymer Systems, 6th ed.; CRC Press: Boca Raton, FL, USA, 2014; p. 775. ISBN 978-1-4822-2378-1. [Google Scholar]
- Амирoва, Л.М. Фoсфoрсoдержащие и Металлкooрдинирoванные Эпoксидные Пoлимерные Материалы; Дoктoр Химических наук: Казань, Рoссия, 2004; 372p. [Google Scholar]
- Amirova, L.; Andrianova, K. Gradient Polymeric Materials Based on Poorly Compatible Epoxy Oligomers. J. Appl. Polym. Sci. 2006, 102, 96–103. [Google Scholar] [CrossRef]
- Amirova, L.; Stroganov, V.; Sakhabieva, E. Optical Materials the Based on Low Flammable Epoxy Resins. Int. J. Polym. Mater. 2000, 47, 43–60. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Price, D. Advances in Fire Retardant Materials; Elsevier: Amsterdam, The Netherlands, 2008; p. 616. [Google Scholar]
- Salmeia, K.A.; Gaan, S. An Overview of Some Recent Advances in DOPO-Derivatives: Chemistry and Flame Retardant Applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Fantin, G.; Medici, A.; Fogagnolo, M.; Pedrini, P.; Gleria, M.; Bertani, R.; Facchin, G. Functionalization of Poly(Organophosphazenes)—III. Synthesis of Phosphazene Materials Containing Carbon-Carbon Double Bonds and Epoxide Groups. Eur. Polym. J. 1993, 29, 1571–1579. [Google Scholar] [CrossRef]
- Allcock, H.; Nelson, C.; Coggio, W. Photoinitiated Graft Poly(Organophosphazenes): Functionalized Immobilization Substrates for the Binding of Amines, Proteins, and Metals. Chem. Mater. 1994, 6, 516–524. [Google Scholar] [CrossRef]
- Chen-yang, Y.W.; Lee, H.-F.; Yuan, C. Flame-Retardant Phosphate and Cyclotriphosphazene-Containing Epoxy Resin: Synthesis and Properties. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 972–981. [Google Scholar] [CrossRef]
- Bertani, R.; Boscolo-Boscoletto, A.; Dintcheva, N.; Ghedini, E.; Gleria, M.; La Mantia, F.; Pace, G.; Pannocchia, P.; Sassi, A.; Scaffaro, R.; et al. New Phosphazene-Based Chain Extenders Containing Allyl and Epoxide Groups. Des. Monomers Polym. 2003, 6, 245–266. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; La Mantia, F.P.; Magagnini, P.; Acierno, D.; Gleria, M.; Bertani, R. Effect of Adding New Phosphazene Compounds to Poly(Butylene Terephthalate)/Polyamide Blends. I: Preliminary Study in a Batch Mixer. Polym. Degrad. Stab. 2005, 90, 234–243. [Google Scholar] [CrossRef]
- el gouri, M.; el Bachiri, A.; Hegazi, S.E.; Rafik, M.; Elharfi, A. Thermal Degradation of a Reactive Flame Retardant Based on Cyclotriphosphazene and Its Blend with DGEBA Epoxy Resin. Polym. Degrad. Stab. 2009, 94, 2101–2106. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X. Synthesis, Characterization, Thermal Properties and Flame Retardancy of a Novel Nonflammable Phosphazene-Based Epoxy Resin. Polym. Degrad. Stab. 2009, 94, 617–624. [Google Scholar] [CrossRef]
- el gouri, M.; Cherkaoui, O.; Ziraoui, R.; Elharfi, A. Physico-Chemical Study of DGEBA Epoxy Resin Flame Retarded with an: Ecological Flame Retardant Based on Cyclotriphosphazene. J. Mater. Environ. Sci. 2010, 1, 157–162. [Google Scholar]
- el gouri, M.; el Bachiri, A.; Hegazi, S.E.; Rafik, M.; Elharfi, A. Fireproofing Amelioration of Epoxy Resin Material by Way a Reactive Flame Retardant Based on Cyclophosphazene. Phys. Chem. News 2010, 56, 128–137. [Google Scholar]
- el gouri, M.; Hegazi, S.E.; Rafik, M.; Elharfi, A. Synthesis and Thermal Degradation of Phosphazene Containing the Epoxy Group. Ann. De Chim. Sci. Des Mater. 2010, 35, 27–39. [Google Scholar] [CrossRef]
- Liu, F.; Wei, H.; Huang, X.; Zhang, J.; Zhou, Y.; Tang, X. Preparation and Properties of Novel Inherent Flame-Retardant Cyclotriphosphazene-Containing Epoxy Resins. J. Macromol. Sci. Part B 2010, 49, 1002–1011. [Google Scholar] [CrossRef]
- el gouri, M.; el Bachiri, A.; Hegazi, S.E.; Ziraoui, R.; Rafik, M.; Elharfi, A. A Phosphazene Compound Multipurpose Application -Composite Material Precursor and Reactive Flame Retardant for Epoxy Resin Materials. J. Mater. Environ. Sci. 2011, 2, 319–334. [Google Scholar]
- Gu, X.; Huang, X.; Wei, H.; Tang, X. Synthesis of Novel Epoxy-Group Modified Phosphazene-Containing Nanotube and Its Reinforcing Effect in Epoxy Resin. Eur. Polym. J. 2011, 47, 903–910. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Wu, D. Novel Cyclolinear Cyclotriphosphazene-Linked Epoxy Resin for Halogen-Free Fire Resistance: Synthesis, Characterization, and Flammability Characteristics. Ind. Eng. Chem. Res. 2012, 51, 15064–15074. [Google Scholar] [CrossRef]
- el gouri, M.; Elharfi, A. Chemical Modification of Hexachlorocyclotriphosphazene—Preparation of Flame Retardants and Ecological Flame Retardant Polymers. J. Mater. Environ. Sci. 2012, 3, 17–33. [Google Scholar]
- Liu, J.; Tang, J.; Wang, X.; Wu, D. Synthesis, Characterization and Curing Properties of a Novel Cyclolinear Phosphazene-Based Epoxy Resin for Halogen-Free Flame Retardancy and High Performance. RSC Adv. 2012, 2, 5789. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Wu, D. Novel Spirocyclic Phosphazene-Based Epoxy Resin for Halogen-Free Fire Resistance: Synthesis, Curing Behaviors, and Flammability Characteristics. ACS Appl. Mater. Interfaces 2012, 4, 4047–4061. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Wang, X.; Wu, D. Fabrication of Spirocyclic Phosphazene Epoxy-Based Nanocomposites with Graphene via Exfoliation of Graphite Platelets and Thermal Curing for Enhancement of Mechanical and Conductive Properties. Ind. Eng. Chem. Res. 2013, 52, 10160–10171. [Google Scholar] [CrossRef]
- Huang, X.; Wei, W.; Wei, H.; Li, Y.; Gu, X.; Tang, X. Preparation of Heat-Moisture Resistant Epoxy Resin Based on Phosphazene. J. Appl. Polym. Sci. 2013, 130, 249–255. [Google Scholar] [CrossRef]
- el gouri, M.; Mansouri, A.; Elgouri, R.; Hadik, N.; Cherkaoui, O.; Outzourhit, A.; Elharfi, A. Physical Behaviour of Epoxy Resin Material Flame Retarded with a Reactive Flame Retardant Based on Cyclophosphazene. J. Mater. Environ. Sci. 2014, 5, 400–407. [Google Scholar]
- Lu, L.; Chen, Y.; Wang, S.; Yang, S.; Dong, X. Preparation and Flame Retardancy of MMT Pattern Synergy Intumescent Flame-Retardant Epoxy Resin. Gaofenzi Cailiao Kexue Yu Gongcheng/Polym. Mater. Sci. Eng. 2014, 30, 139–144. [Google Scholar]
- Xu, G.-R.; Xu, M.-J.; Li, B. Synthesis and Characterization of a Novel Epoxy Resin Based on Cyclotriphosphazene and Its Thermal Degradation and Flammability Performance. Polym. Degrad. Stab. 2014, 109, 240–248. [Google Scholar] [CrossRef]
- Huan, L.; Wang, X.; Wu, D. Novel Cyclotriphosphazene-Based Epoxy Compound and Its Application in Halogen-Free Epoxy Thermosetting Systems: Synthesis, Curing Behaviors, and Flame Retardancy. Polym. Degrad. Stab. 2014, 103, 96–112. [Google Scholar] [CrossRef]
- Lakshmikandhan, T.; Sethuraman, K.; Chandramohan, A.; Alagar, M. Development of Phosphazene Imine-Modified Epoxy Composites for Low Dielectric, Antibacterial Activity, and UV Shielding Applications. Polym. Compos. 2017, 38, E24–E33. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Synthesis of a Novel Linear Polyphosphazene-Based Epoxy Resin and Its Application in Halogen-Free Flame-Resistant Thermosetting Systems. Polym. Degrad. Stab. 2005, 118, 45–58. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, J.; Wang, X.; Shao, Z.; Zhao, D.; Zeng, X. Thermal decomposition kinetics and flame retardance of phosphazene-containing epoxy resin. Polym. Mater. Sci. Eng. 2016, 32, 54–58. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamamoto, T.; Itoh, S.; Harakawa, K.; Kajiwara, M. Mechanical properties of epoxy resins cured by various amines. Zair. J. Soc. Mater. Sci. Jpn. 1988, 37, 454–459. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takahashi, K.; Kon, Y.; Harakawa, K. Curing of Epoxy Resin with Phosphazene Derivatives. Kobunshi Ronbunshu 1988, 45, 851–856. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takahashi, K.; Kon, Y.; Kobayashi, K. Tensile Behavior and Heat Resistance of Epoxy Resin Cured with Phosphazene Derivatives. Kobunshi Ronbunshu 1989, 46, 177–181. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikawa, N.; Komori, T.; Yoon, H.-S. Curing of an Epoxy Resin with P3N3(NH2)2 (OCH2CF3)4 and Its Mechanical Properties. Kobunshi Ronbunshu 1990, 47, 727–734. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikawa, N.; Yoon, H.-S. Mechanical Properties of Epoxy Resins Cured with P3N3(NH2)2 (OC6H4Cl)4. Kobunshi Ronbunshu 1990, 47, 757–762. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikawa, N.; Yoon, H.-S. Resistance of Epoxy Resins Cured with Trichloro-Tridimethylamino-Cyclotriphosphazene against Chemical Substances. Zair. J. Soc. Mater. Sci. Jpn. 1990, 39, 1001–1006. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikawa, N.; Kohno, T.; Yoon, H.-S. Water Resistance of an Epoxy Resin Cured with P3N3Cl3(N(CH3)2)3 and the Effect of Glass Flake Reinforcement. Zair. J. Soc. Mater. Sci. Jpn. 1991, 40, 458–463. [Google Scholar] [CrossRef]
- Takahashi, K.; Nakashima, J.; Ishiguro, S. Mechanical Properties of Trifunctional Epoxy Resin with Phosphazene Derivatives. Kobunshi Ronbunshu 1994, 51, 717–723. [Google Scholar] [CrossRef]
- Chen, Y.M.; Liao, Y.L.; Lin, J.J. Synergistic Effect of Silicate Clay and Phosphazene-Oxyalkyleneamines on Thermal Stability of Cured Epoxies. J. Colloid Interface Sci. 2010, 343, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Terekhov, I.V.; Filatov, S.N.; Chistyakov, E.M.; Borisov, R.S.; Kireev, V.V. Synthesis of Oligomeric Epoxycyclotriphosphazenes and Their Properties as Reactive Flame-Retardants for Epoxy Resins. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 544–554. [Google Scholar] [CrossRef]
- Allcock, H. Phosphorus-Nitrogen Compounds, Cyclic, Linear and High Polymeric System; Elsevier: Amsterdam, The Netherlands, 1972; ISBN 978-0-12-050560-9. [Google Scholar]
- Sirotin, I.S.; Bilichenko, Y.V.; Brigadnov, K.A.; Kireev, V.V.; Prudskov, B.M.; Borisov, R.S. Single-Stage Synthesis of Phosphazene-Containing Epoxy Oligomers. Polym. Sci. Ser. B 2014, 56, 471–476. [Google Scholar] [CrossRef]
- Brigadnov, K.A.; Bilichenko, Y.V.; Polyakov, V.A.; Borisov, R.S.; Gusev, K.I.; Rudakova, T.A.; Filatov, S.N.; Kireev, V.V. Epoxy Oligomers Modified with Epoxyphosphazenes. Polym. Sci. Ser. B 2016, 58, 549–555. [Google Scholar] [CrossRef]
- Sarychev, I.A.; Sirotin, I.S.; Borisov, R.S.; Mu, J.; Sokolskaya, I.B.; Bilichenko, J.V.; Filatov, S.N.; Kireev, V.V. Synthesis of Resorcinol-Based Phosphazene-Containing Epoxy Oligomers. Polymers 2019, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Kireev, V.V.; Bilichenko, Y.V.; Borisov, R.S.; Mu, J.; Kuznetsov, D.A.; Eroshenko, A.V.; Filatov, S.N.; Sirotin, I.S. Synthesis of Bisphenol A Based Phosphazene-Containing Epoxy Resin with Reduced Viscosity. Polymers 2019, 11, 1914. [Google Scholar] [CrossRef]
- Sirotin, I.S.; Bilichenko, Y.V.; Suraeva, O.V.; Solodukhin, A.N.; Kireev, V.V. Synthesis of Oligomeric Chlorophosphazenes in the Presence of ZnCl2. Polym. Sci. Ser. B 2013, 55, 63–68. [Google Scholar] [CrossRef]
- Simonov-Emel’yanov, I.D.; Apeksimov, N.V.; Kochergina, L.M.; Bilichenko, Y.V.; Kireev, V.V.; Brigadnov, K.A.; Sirotin, I.S.; Filatov, S.N. Rheological and Rheokinetic Properties of Phosphazene-Containing Epoxy Oligomers. Polym. Sci. Ser. B 2016, 58, 168–172. [Google Scholar] [CrossRef]
- Onuchin, D.V.; Sirotin, I.S.; Sarychev, I.A.; Bornosuz, N.V.; Kireev, V.V.; Gorbunova, I.Y.; Gorbatkina, Y.A. Physicochemical Properties of Epoxy Composites Modified with Epoxyphosphazene. Polym. Sci. Ser. B 2019, 61, 286–293. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).