Functional Separators for Long-Life and Safe Li Metal Batteries: A Minireview
Abstract
1. Introduction
2. Mechanically Strengthened Separator Fabrication
3. Functional Separator Construction towards Regulated Li Ion Deposition
4. Flame-Retardant Separator Design

5. Summary and Outlook
- (1)
- Most of the reported functional separators have been developed with top-down strategies such as the tap-casting method, which leads to a lack of control over the thickness and the homogeneous distribution of different components. More elaborate bottom-up strategies should be designed to develop uniform and homogenous separators.
- (2)
- To minimize sacrifice in both volumetric and gravitation energy density, the thickness of the modification layer should be controlled at <1 µm (<5% in thickness compared to commercially separator such as Celgard 2325) and its density should be reduced as low as possible. In this regard, novel nanostructured nanomaterials, especially 2D graphene and boron nitride, are good options.
- (3)
- To avoid peeling of the modification layer from the separator during Li plating/stripping cycles, molecular and structural designs to improve the flexibility and elasticity of the modification layer, as well as its adhesion on the separator, are highly desirable.
- (4)
- To help eliminate battery thermal runaway caused by Li dendrites and improve battery safety, the thermal conductivity of the separator should be further improved for better battery heat dissipation; few studies have focused on this.
- (5)
- The “activation” of functional separators should be understood in depth. Some of the designed separators are not electrochemically inert, and they may absorb Li+, or even react with electrolyte or Li metal electrodes, especially under complex electrochemical conditions. These “activations” (absorption and reaction) together with the influence on battery performance should be carefully studied.
- (6)
- The performance of the designed separators should be evaluated under practical conditions. The areal current density and capacity for Li|Li symmetric cells and Li metal full cells should be higher than 3 mA cm−2 and 3 mAh cm−2, respectively, because the area capacity of commercial energy-type LMBs is >3 mAh cm−2 and the discharging/charging current is >3 mA cm−2 based on 1 C rate. The electrolyte amount should be controlled at <10 µL mAh−1 (requirement of lean electrolyte), and the capacity ratio of Li metal anode and cathode should be <5 to maintain high volumetric energy density.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, e1800561. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Kaur, J.; Wuest, M.; Wuest, F. In situ click chemistry generation of cyclooxygenase-2 inhibitors. Nat. Commun. 2017, 8, 1. [Google Scholar] [CrossRef]
- Wu, J.; Lin, C.; Liang, Q.; Zhou, G.; Liu, J.; Liang, G.; Wang, M.; Li, B.; Hu, L.; Ciucci, F.; et al. Sodium-rich NASICON-structured cathodes for boosting the energy density and lifespan of sodium-free-anode sodium metal batteries. InfoMat 2022, 4, e12288. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- An, H.; Roh, Y.; Jo, Y.; Lee, H.; Lim, M.; Lee, M.; Lee, Y.M.; Lee, H. Separator dependency on cycling stability of lithium metal batteries under practical conditions. Energy Environ. Mater. 2022. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, C.; Li, H.; Du, J.; Xiong, R. Deep neural network-driven in-situ detection and quantification of lithium plating on anodes in commercial lithium-ion batteries. EcoMat 2022, e12280. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing lithium metal batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Q.; Bi, Y.; Cai, M.; Dunn, B.; Glossmann, T.; Liu, J.; Osaka, T.; Sugiura, R.; Wu, B.; et al. Understanding and applying coulombic efficiency in lithium metal batteries. Nat. Energy 2020, 5, 561–568. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wu, C.; Pang, W.K.; Mao, J.; Guo, Z. Constructing nitrided interfaces for stabilizing Li metal electrodes in liquid electrolytes. Chem. Sci. 2021, 12, 8945–8966. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Archer, L.A. Stabilizing metal battery anodes through the design of solid electrolyte interphases. Joule 2021, 5, 1119–1142. [Google Scholar] [CrossRef]
- Yu, Z.; Cui, Y.; Bao, Z. Design Principles of Artificial Solid Electrolyte Interphases for Lithium-Metal Anodes. Cell Rep. Phys. Sci. 2020, 1, 100119. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhao, L.; Fan, Q.; Zeng, X.; Liu, S.; Pang, W.K.; He, Y.B.; Guo, Z. Lithium metal electrode with increased air stability and robust solid electrolyte interphase realized by silane coupling agent modification. Adv. Mater. 2021, 33, e2008133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Zhang, Z.; Chen, X.; Lie, W.; He, Y.B.; Zhou, Z.; Xia, G.; Guo, Z. Building artificial solid-electrolyte interphase with uniform intermolecular ionic bonds toward dendrite-free lithium metal anodes. Adv. Funct. Mater. 2020, 30, 2002414. [Google Scholar] [CrossRef]
- Teng, W.; Wu, J.; Liang, Q.; Deng, J.; Xu, Y.; Liu, Q.; Wang, B.; Ma, T.; Nan, D.; Liu, J.; et al. Designing advanced liquid electrolytes for alkali metal batteries: Principles, progress, and perspectives. Energy Environ. Mater. 2022. [Google Scholar] [CrossRef]
- Qin, K.; Holguin, K.; Mohammadiroudbari, M.; Huang, J.; Kim, E.Y.S.; Hall, R.; Luo, C. Strategies in structure and electrolyte design for high-performance lithium metal batteries. Adv. Funct. Mater. 2021, 31, 2009694. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Li, B.; Bouwer, J.C.; Davey, K.; Lu, J.; Guo, Z. Non-flammable ester electrolyte with boosted stability against Li for high-performance Li metal batteries. Angew. Chem. Int. Ed. 2022, 61, e202206682. [Google Scholar] [CrossRef]
- Lee, H.; Ren, X.; Niu, C.; Yu, L.; Engelhard, M.H.; Cho, I.; Ryou, M.H.; Jin, H.S.; Kim, H.T.; Liu, J.; et al. Suppressing lithium dendrite growth by metallic coating on a separator. Adv. Funct. Mater. 2017, 27, 1704391. [Google Scholar] [CrossRef]
- Foroozan, T.; Soto, F.A.; Yurkiv, V.; Sharifi-Asl, S.; Deivanayagam, R.; Huang, Z.; Rojaee, R.; Mashayek, F.; Balbuena, P.B.; Shahbazian-Yassar, R. Synergistic effect of graphene oxide for impeding the dendritic plating of Li. Adv. Funct. Mater. 2018, 28, 1705917. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Shi, C.; Liang, G.; Lu, Z.; Fu, R.; Wu, D. Two-dimensional molecular brush-functionalized porous bilayer composite separators toward ultrastable high-current density lithium metal anodes. Nat. Commun. 2019, 10, 1363. [Google Scholar] [CrossRef]
- Zheng, S.; Mo, L.; Chen, K.; Chen, A.L.; Zhang, X.; Fan, X.; Lai, F.; Wei, Q.; Miao, Y.E.; Liu, T.; et al. Precise control of Li+ directed transport via electronegative polymer brushes on polyolefin separators for dendrite-free lithium deposition. Adv. Funct. Mater. 2022, 32, 2201430. [Google Scholar] [CrossRef]
- Guo, Y.; Niu, P.; Liu, Y.; Ouyang, Y.; Li, D.; Zhai, T.; Li, H.; Cui, Y. An autotransferable g-C3N4 Li+-modulating layer toward stable lithium anodes. Adv. Mater. 2019, 31, e1900342. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, L.; Liu, D.; Liao, M.; Chen, J.; Yuan, K.; Xiang, J.; Li, Z.; Huang, Y. Elevated lithium ion regulation by a “Natural Silk” modified separator for high-performance lithium metal anode. Adv. Funct. Mater. 2021, 31, 2100537. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wei, S.; Li, S.; Li, Q.; Lu, Y. Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Adv. Energy Mater. 2018, 8, 1702657. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Zou, G.; Liu, D.; Peng, Q. lithiation MXene derivative skeletons for wide-temperature lithium metal anodes. Adv. Funct. Mater. 2021, 31, 202101180. [Google Scholar] [CrossRef]
- Qian, X.; Fan, X.; Peng, Y.; Xue, P.; Sun, C.; Shi, X.; Lai, C.; Liang, J. Polysiloxane cross-linked mechanically stable MXene-based lithium host for ultrastable lithium metal anodes with ultrahigh current densities and capacities. Adv. Funct. Mater. 2020, 31, 2008044. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Lei, D.; Lv, W.; Zhao, Q.; Ni, B.; Liu, Y.; Li, B.; Kang, F.; He, Y.B. spherical Li deposited inside 3D Cu skeleton as anode with ultrastable performance. ACS Appl. Mater. Interfaces 2018, 10, 20244–20249. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, H.; Zhang, Q.; Liu, Y.; Chen, J.; Guo, Z. Recent advances in 3D graphene architectures and their composites for energy storage applications. Small 2019, 15, e1803858. [Google Scholar] [CrossRef]
- Kim, P.J.; Pol, V.G. High performance lithium metal batteries enabled by surface tailoring of polypropylene separator with a polydopamine/graphene layer. Adv. Energy Mater. 2018, 8, 1802665. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, R.; Xu, C.; Ruan, C.; Edström, K.; Strømme, M.; Nyholm, L. Conducting polymer paper-derived separators for lithium metal batteries. Energy Storage Mater. 2018, 13, 283–292. [Google Scholar] [CrossRef]
- Oh, J.; Jo, H.; Lee, H.; Kim, H.-T.; Lee, Y.M.; Ryou, M.H. Polydopamine-treated three-dimensional carbon fiber-coated separator for achieving high-performance lithium metal batteries. J. Power Sources 2019, 430, 130–136. [Google Scholar] [CrossRef]
- Yu, B.; Chen, D.; Wang, Z.; Qi, F.; Zhang, X.; Wang, X.; Hu, Y.; Wang, B.; Zhang, W.; Chen, Y.; et al. Mo2C quantum dots@graphene functionalized separator toward high-current-density lithium metal anodes for ultrastable Li-S batteries. Chem. Eng. J. 2020, 399, 125837. [Google Scholar] [CrossRef]
- Wen, K.; Liu, L.; Chen, S.; Zhang, S. A bidirectional growth mechanism for a stable lithium anode by a platinum nanolayer sputtered on a polypropylene separator. RSC Adv. 2018, 8, 13034–13039. [Google Scholar] [CrossRef]
- Liu, M.; Deng, N.; Ju, J.; Wang, L.; Wang, G.; Ma, Y.; Kang, W.; Yan, J. Silver nanoparticle-doped 3D porous carbon nanofibers as separator coating for stable lithium metal anodes. ACS Appl. Mater. Interfaces 2019, 11, 17843–17852. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, D.; Yuen, P.Y.; Liu, K.; Xie, J.; Dauskardt, R.H.; Cui, Y. An artificial solid electrolyte interphase with high li-ion conductivity, mechanical strength, and flexibility for stable lithium metal anodes. Adv. Mater. 2017, 29, 1605531. [Google Scholar] [CrossRef]
- Huo, H.; Li, X.; Chen, Y.; Liang, J.; Deng, S.; Gao, X.; Doyle-Davis, K.; Li, R.; Guo, X.; Shen, Y.; et al. Bifunctional composite separator with a solid-state-battery strategy for dendrite-free lithium metal batteries. Energy Storage Mater. 2020, 29, 361–366. [Google Scholar] [CrossRef]
- Wu, H.; Zhuo, D.; Kong, D.; Cui, Y. Improving battery safety by early detection of internal shorting with a bifunctional separator. Nat. Commun. 2014, 5, 5193. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Wang, Z.; Shi, L.; Zhao, Y.; Yuan, S. Dual-Scale Al2O3 particles coating for high-performance separator and lithium metal anode. Energy Technol. 2020, 8, 1901429. [Google Scholar] [CrossRef]
- Monroe, C.; Newman, J. Dendrite Growth in lithium/polymer systems a propagation model for liquid electrolytes under galvanostatic conditions. J. Electrochem. Soc. 2003, 150, A1377–A1384. [Google Scholar] [CrossRef]
- Liang, J.; Chen, Q.; Liao, X.; Yao, P.; Zhu, B.; Lv, G.; Wang, X.; Chen, X.; Zhu, J. A nano-shield design for deparators to resist dendrite formation in lithium-metal batteries. Angew. Chem. Int. Ed. 2020, 59, 6561–6566. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Kim, H.M.; Shin, S.; Sun, Y.-K. Designing a high-performance lithium-sulfur batteries based on layered double hydroxides-carbon nanotubes composite cathode and a dual-functional graphene-polypropylene-Al2O3 separator. Adv. Funct. Mater. 2018, 28, 1704294. [Google Scholar] [CrossRef]
- Hao, X.; Zhu, J.; Jiang, X.; Wu, H.; Qiao, J.; Sun, W.; Wang, Z.; Sun, K. Ultrastrong polyoxyzole nanofiber membranes for dendrite-proof and heat-resistant battery separators. Nano Lett. 2016, 16, 2981–2987. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, J.; Yaghoobnejad Asl, H.; Manthiram, A. A 3D lithiophilic Mo2N-modified carbon nanofiber architecture for dendrite-free lithium-metal anodes in a full cell. Adv. Mater. 2019, 31, e1904537. [Google Scholar] [CrossRef] [PubMed]
- Adair, K.R.; Iqbal, M.; Wang, C.; Zhao, Y.; Banis, M.N.; Li, R.; Zhang, L.; Yang, R.; Lu, S.; Sun, X. Towards high performance Li metal batteries: Nanoscale surface modification of 3D metal hosts for pre-stored Li metal anodes. Nano Energy 2018, 54, 375–382. [Google Scholar] [CrossRef]
- Pei, F.; Fu, A.; Ye, W.; Peng, J.; Fang, X.; Wang, M.S.; Zheng, N. Robust lithium metal anodes realized by lithiophilic 3D porous current collectors for constructing high-energy lithium-sulfur batteries. ACS Nano 2019, 13, 8337–8346. [Google Scholar] [CrossRef]
- Yang, C.; Yao, Y.; He, S.; Xie, H.; Hitz, E.; Hu, L. Ultrafine silver nanoparticles for seeded lithium deposition toward stable lithium metal anode. Adv. Mater. 2017, 29, 1702714. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, J.; Chen, X.; Zhang, X.D.; Li, J.Y.; Huang, L.B.; Zhang, X.; Shi, J.L.; Yin, Y.X.; Zhang, Q.; et al. Uniform nucleation of lithium in 3D current collectors via bromide intermediates for stable cycling lithium metal batteries. J. Am. Chem. Soc. 2018, 140, 18051–18057. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Pan, G.; Ai, C.; Deng, S.; Liu, S.; Liu, Q.; Wang, X.; Xia, X.; Tu, J. Ordered lithiophilic sites to regulate Li plating/stripping behavior for superior lithium metal anodes. J. Mater. Chem. A 2019, 7, 21794–21801. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Y.X.; Li, J.Y.; Guo, Y.G.; Wan, L.J. Ladderlike carbon nanoarrays on 3D conducting skeletons enable uniform lithium nucleation for stable lithium metal anodes. Chem. Commun. 2018, 54, 5330–5333. [Google Scholar] [CrossRef]
- Xie, J.; Wan, J.; Lee, H.R.; Yan, K.; Li, Y.; Shi, F.; Huang, W.; Pei, A.; Chen, G.; Subbaraman, R.; et al. Engineering stable interfaces for three-dimensional lithium metal anodes. Sci. Adv. 2018, 4, eaat5168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiong, S.; Wang, J.; Jiao, X.; Li, S.; Zhang, C.; Song, Z.; Song, J. Dendrite-free lithium metal anode enabled by separator engineering via uniform loading of lithiophilic nucleation sites. Energy Storage Mater. 2019, 19, 24–30. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.-R.; Hou, T.-Z.; Li, B.-Q.; Cheng, X.-B.; Zhang, R.; Zhang, Q. Lithiophilicity chemistry of heteroatom-doped carbon to guide uniform lithium nucleation in lithium metal anodes. Sci. Adv. 2019, 5, eaau7728. [Google Scholar] [CrossRef] [PubMed]
- Balach, J.; Jaumann, T.; Klose, M.; Oswald, S.; Eckert, J.; Giebeler, L. Improved cycling stability of lithium-sulfur batteries using a polypropylene-supported nitrogen-doped mesoporous carbon hybrid separator as polysulfide adsorbent. J. Power Sources 2016, 303, 317–324. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, Z.; Wang, L.; Nie, H.; Zhong, M.; Lai, Q.; Xu, X.; Zhang, L.; Huang, S. A lightweight TiO2/Graphene interlayer, applied as a highly effective polysulfide absorbent for fast, long-life lithium-sulfur batteries. Adv. Mater. 2015, 27, 2891–2898. [Google Scholar] [CrossRef]
- Shen, F.; Wang, K.; Yin, Y.; Shi, L.; Zeng, D.; Han, X. PAN/PI functional double-layer coating for dendrite-free lithium metal anodes. J. Mater. Chem. A 2020, 8, 6183–6189. [Google Scholar] [CrossRef]
- Baginska, M.; Blaiszik, B.J.; Merriman, R.J.; Sottos, N.R.; Moore, J.S.; White, S.R. Autonomic shutdown of lithium-Ion batteries using thermoresponsive microspheres. Adv. Energy Mater. 2012, 2, 583–590. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, W.-Y.; Kim, K.J.; Yu, J.-S.; Kim, Y.-J. Shutdown-functionalized nonwoven separator with improved thermal and electrochemical properties for lithium-ion batteries. J. Power Sources 2016, 305, 225–232. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Wang, S.; Cao, Y.; Yang, H.; Ai, X.; Zhong, F. A polyethylene microsphere-coated separator with rapid thermal shutdown function for lithium-ion batteries. J. Energy Chem. 2020, 44, 33–40. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, Z.; Wei, S.; Ruan, S.; Shen, C.; Turng, L.-S. Thermosensitive polyacrylonitrile/polyethylene oxide/polyacrylonitrile membrane separators for prompt and safer thermal lithium-ion battery shutdown. J. Electrochem. Soc. 2020, 167, 020509. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, Y.; Sun, S.; Zhang, L.; Li, S.; Liu, X.; Xu, Z.; Xu, S. Tri-layer nonwoven membrane with shutdown property and high robustness as a high-safety lithium ion battery separator. J. Membr. Sci. 2018, 565, 50–60. [Google Scholar] [CrossRef]
- Gong, W.; Wei, S.; Ruan, S.; Shen, C. Electrospun coaxial PPESK/PVDF fibrous membranes with thermal shutdown property used for lithium-ion batteries. Mater. Lett. 2019, 244, 126–129. [Google Scholar] [CrossRef]
- Jiang, X.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. A novel bifunctional thermo-sensitive poly(lactic acid)@poly(butylene succinate) core-shell fibrous separator prepared by a coaxial electrospinning route for safe lithium-ion batteries. J. Mater. Chem. A 2017, 5, 23238–23242. [Google Scholar] [CrossRef]
- Sun, G.; Kong, L.; Liu, B.; Niu, H.; Zhang, M.; Tian, G.; Qi, S.; Wu, D. Ultrahigh-strength, nonflammable and high-wettability separators based on novel polyimide-core@polybenzimidazole-sheath nanofibers for advanced and safe lithium-ion batteries. J. Membr. Sci. 2019, 582, 132–139. [Google Scholar] [CrossRef]
- Sun, G.; Dong, G.; Kong, L.; Yan, X.; Tian, G.; Qi, S.; Wu, D. Robust polyimide nanofibrous membrane with porous-layer-coated morphology by in situ self-bonding and micro-crosslinking for lithium-ion battery separator. Nanoscale 2018, 10, 22439–22447. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Xiao, K.; Yu, J.; Yang, J.; Ding, B. Thermostable and nonflammable silica-polyetherimide-polyurethane nanofibrous separators for high power lithium ion batteries. J. Mater. Chem. A 2015, 3, 10551–10558. [Google Scholar] [CrossRef]
- Woo, J.-J.; Nam, S.H.; Seo, S.-J.; Yun, S.-H.; Kim, W.B.; Xu, T.; Moon, S.-H. A flame retarding separator with improved thermal stability for safe lithium-ion batteries. Electrochem. Commun. 2013, 35, 68–71. [Google Scholar] [CrossRef]
- Yeon, D.; Lee, Y.; Ryou, M.-H.; Lee, Y.M. New flame-retardant composite separators based on metal hydroxides for lithium-ion batteries. Electrochim. Acta 2015, 157, 282–289. [Google Scholar] [CrossRef]
- Liu, K.; Liu, W.; Qiu, Y.; Kong, B.; Sun, Y.; Chen, Z.; Zhuo, D.; Lin, D.; Cui, Y. Electrospun core-shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries. Sci. Adv. 2017, 3, e1601978. [Google Scholar] [CrossRef]
- Kim, S.; Han, T.; Jeong, J.; Lee, H.; Ryou, M.-H.; Lee, Y.M. A flame-retardant composite polymer electrolyte for lithium-ion polymer batteries. Electrochim. Acta 2017, 241, 553–559. [Google Scholar] [CrossRef]
- Zeng, G.; Zhao, J.; Feng, C.; Chen, D.; Meng, Y.; Boateng, B.; Lu, N.; He, W. Flame-retardant bilayer separator with multifaceted van der waals interaction for lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 26402–26411. [Google Scholar] [CrossRef] [PubMed]
- Yim, T.; Park, M.S.; Woo, S.G.; Kwon, H.K.; Yoo, J.K.; Jung, Y.S.; Kim, K.J.; Yu, J.S.; Kim, Y.J. Self-extinguishing lithium ion batteries based on internally embedded fire-Extinguishing microcapsules with temperature-responsiveness. Nano Lett. 2015, 15, 5059–5067. [Google Scholar] [CrossRef] [PubMed]
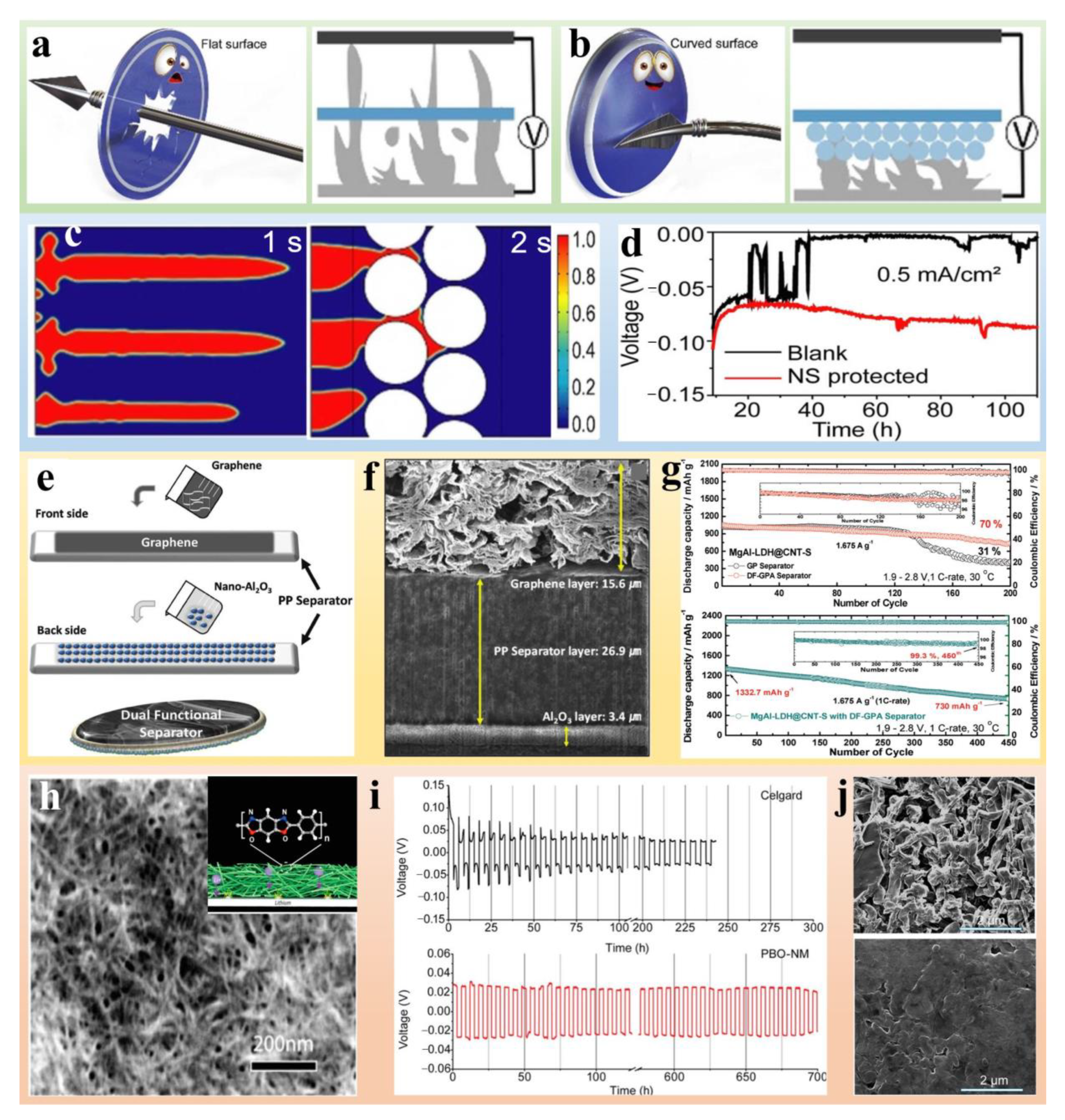

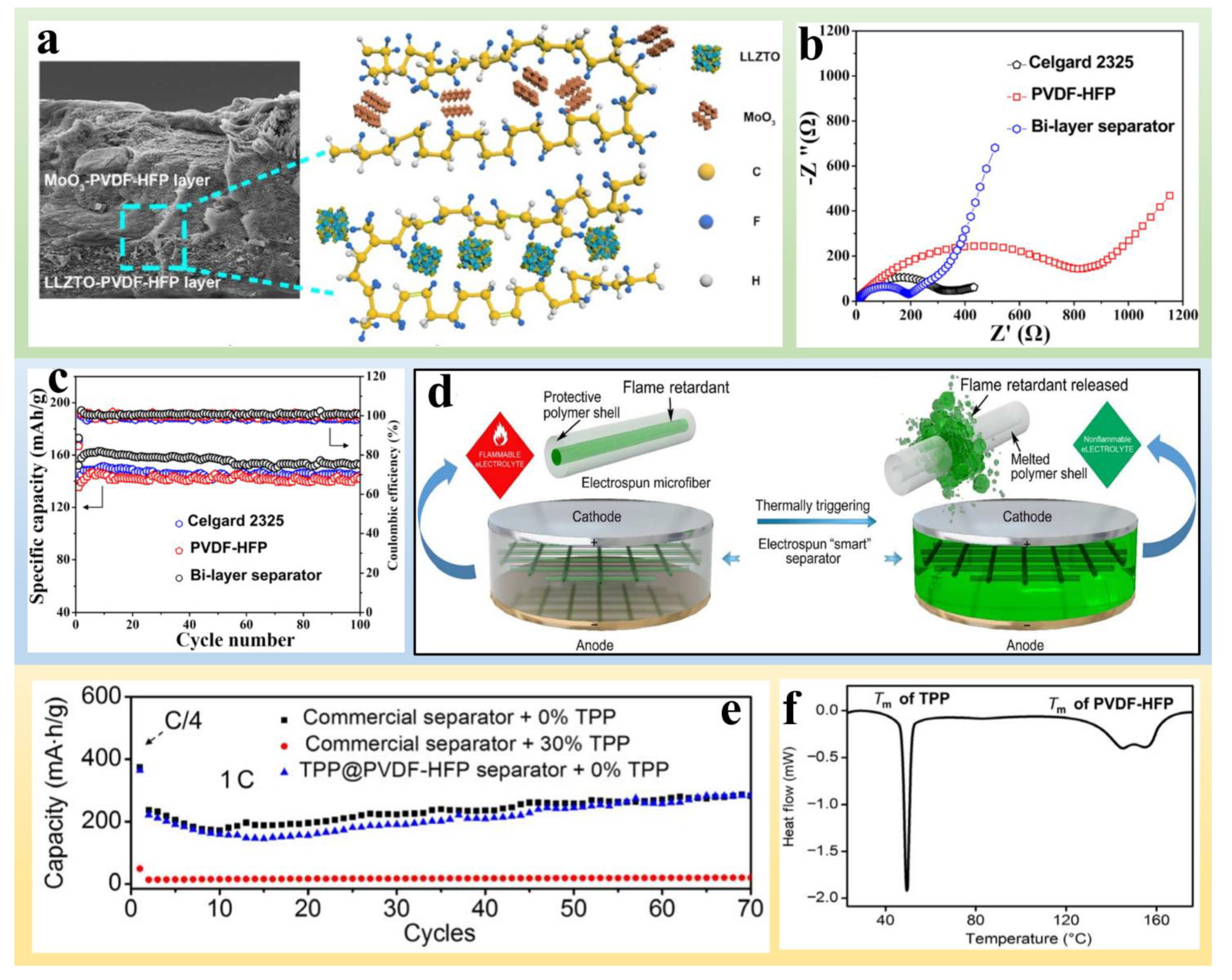
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhao, Y.; Yang, Y.; Wang, Z.; Yang, Q.; Deng, J. Functional Separators for Long-Life and Safe Li Metal Batteries: A Minireview. Polymers 2022, 14, 4546. https://doi.org/10.3390/polym14214546
Li Y, Zhao Y, Yang Y, Wang Z, Yang Q, Deng J. Functional Separators for Long-Life and Safe Li Metal Batteries: A Minireview. Polymers. 2022; 14(21):4546. https://doi.org/10.3390/polym14214546
Chicago/Turabian StyleLi, Yanyan, Yu Zhao, Yong Yang, Zhijie Wang, Qin Yang, and Jiaojiao Deng. 2022. "Functional Separators for Long-Life and Safe Li Metal Batteries: A Minireview" Polymers 14, no. 21: 4546. https://doi.org/10.3390/polym14214546
APA StyleLi, Y., Zhao, Y., Yang, Y., Wang, Z., Yang, Q., & Deng, J. (2022). Functional Separators for Long-Life and Safe Li Metal Batteries: A Minireview. Polymers, 14(21), 4546. https://doi.org/10.3390/polym14214546







