Functional Polymer Materials for Advanced Lithium Metal Batteries: A Review and Perspective
Abstract
:1. Introduction
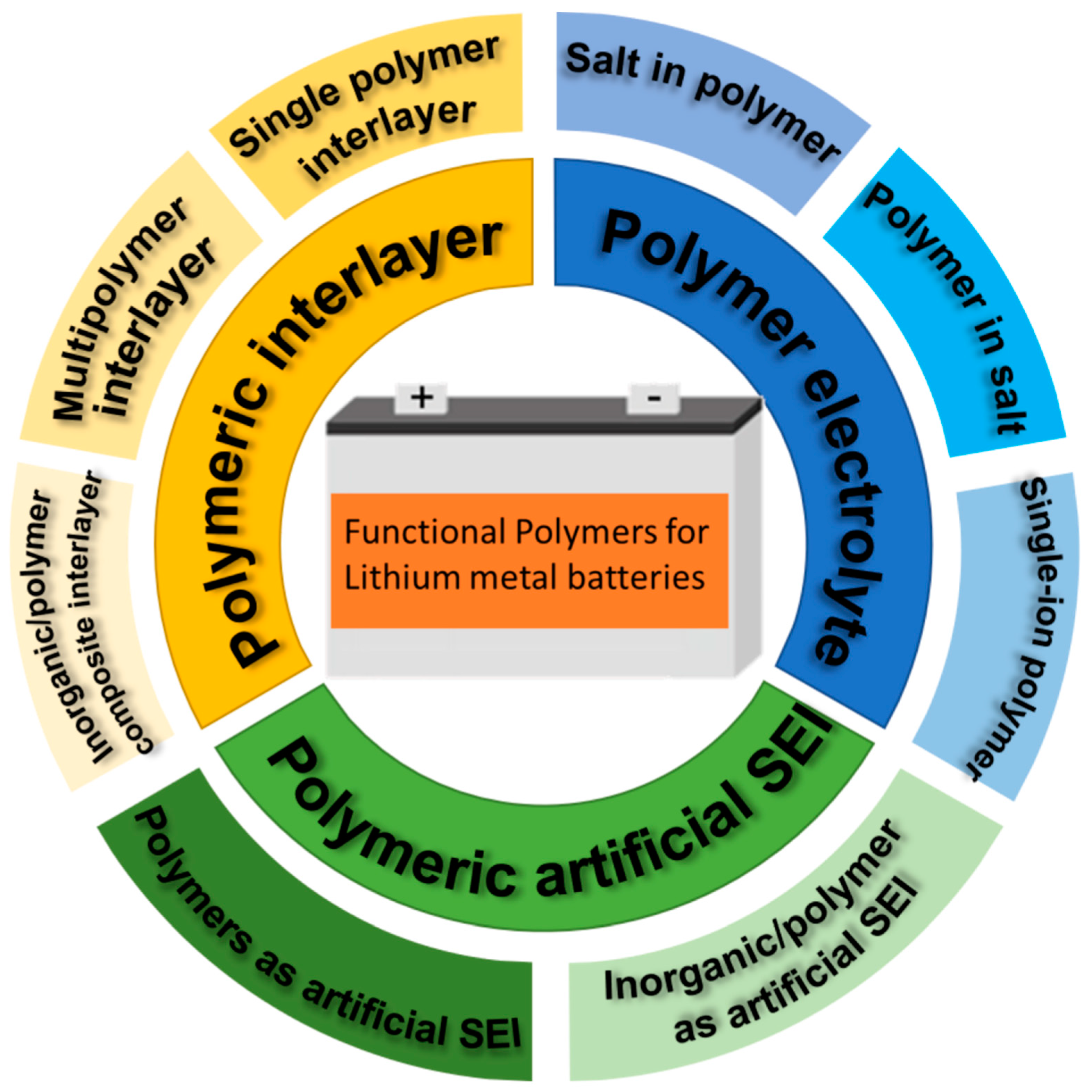
2. Polymeric Artificial SEI
2.1. Polymers as Artificial SEI
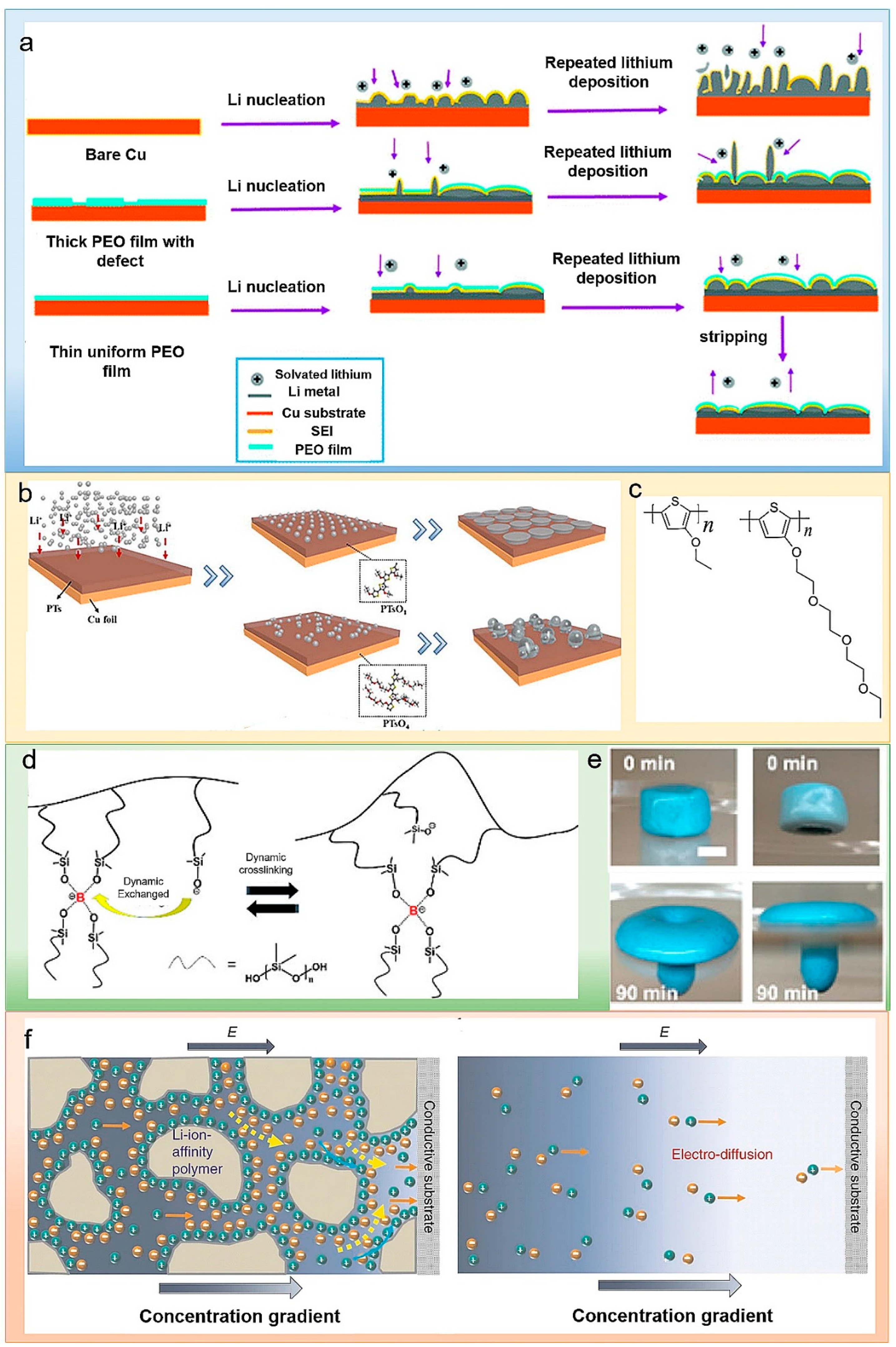

2.2. Inorganic/Polymer Composites as Artificial SEI
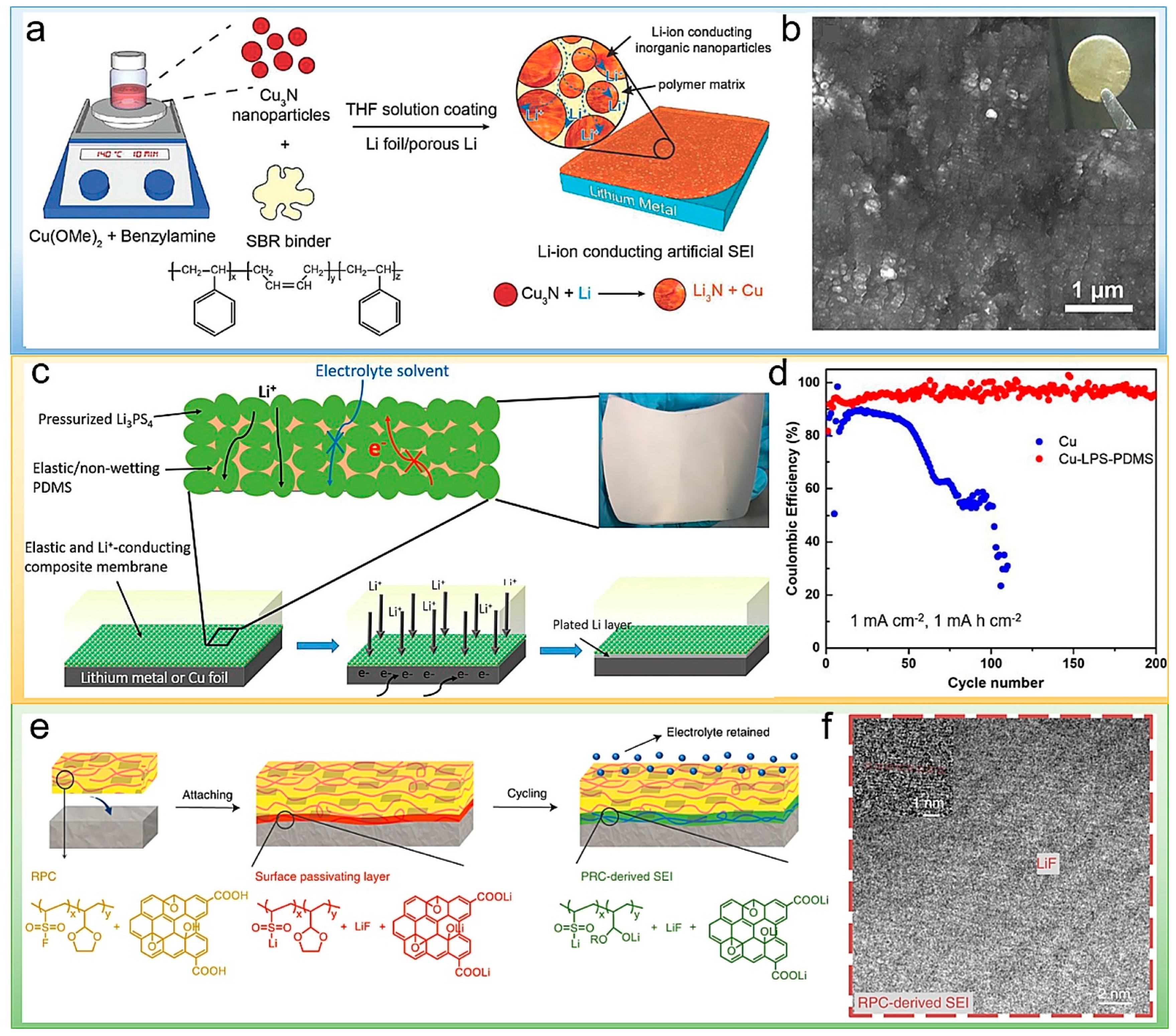
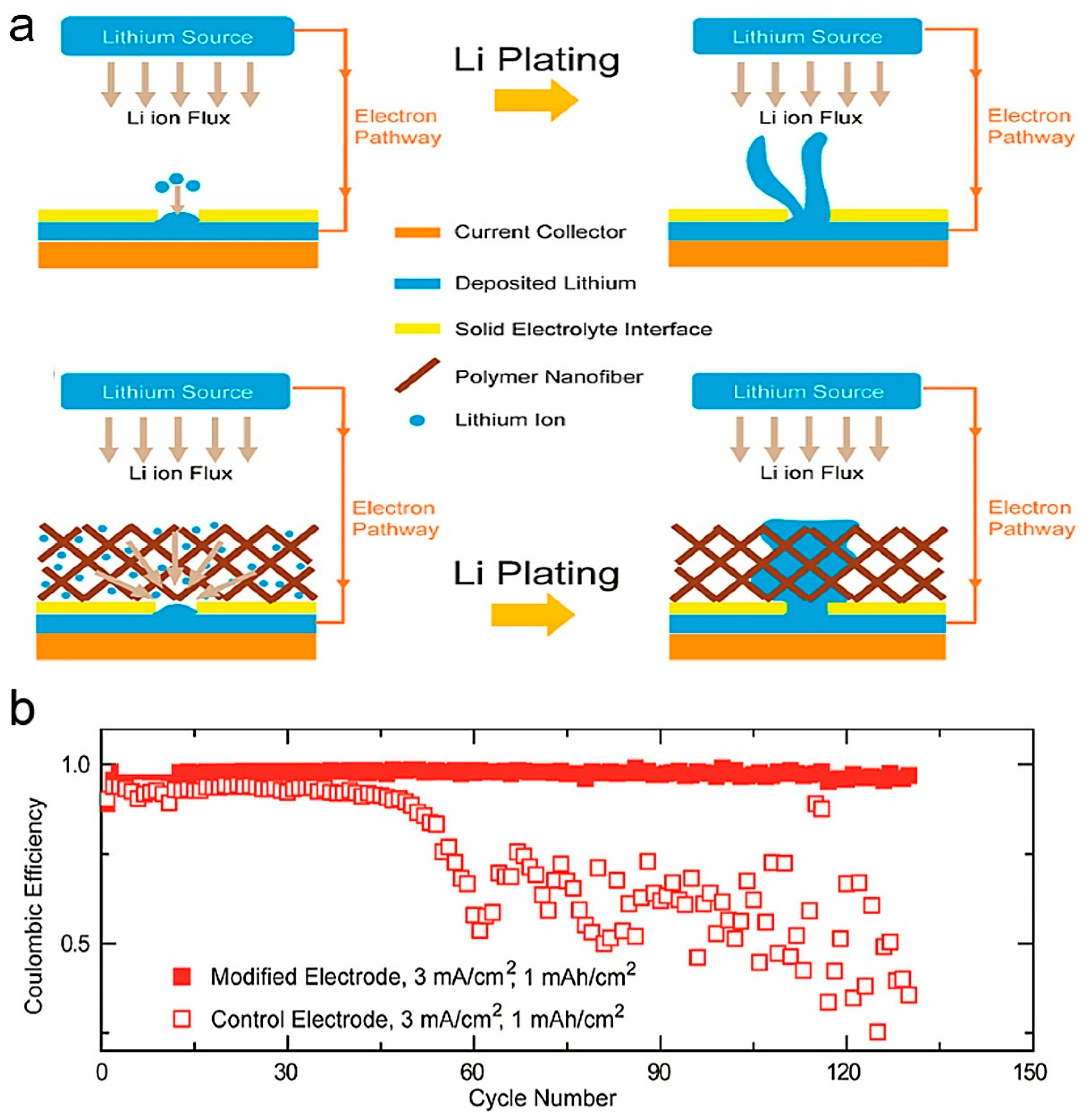
3. Polymeric Functional Interlayers in LMBs
3.1. Single-Polymer Interlayers
3.2. Multipolymer Interlayer
3.3. Inorganic/Polymer Composite Interlayer
4. Polymer Electrolyte
4.1. Salt in Polymer Electrolyte

4.2. Polymer-in-Salt Electrolyte

4.3. Single-Ion Polymer Electrolyte
5. Conclusions and Perspectives
- (1)
- For polymeric artificial SEI and interlayers, multifunctional polymers should be synthesized to concurrently achieve rapid Li+ transportation, high mechanical modulus, and good compatibility with lithium metal anode. The functional layers should possess optimized thickness: for one thing to minimize the mass of inactive components in LMB and for another to assure moderate mechanical strength.
- (2)
- For polymer electrolyte, a high Li+ conductivity is a prerequisite for its proper function. A prospective approach is decoupling Li+ conduction from the polymer’s segmental motion. The thickness of polymer electrolyte should also be well adjusted to balance the Li+ ionic conductivity and mechanical property. Moreover, it is urgent to enhance the compatibility of polymer electrolytes with highly reductive lithium metal anode and oxidative high-voltage cathode.
- (3)
- Exploring facile and low-cost synthetic procedures for functional polymers is of great significance for mass production.
- (4)
- Advanced characterization tools must be used to observe the evolution of ASEIs during cycling to guide the design of better coatings. Advanced characterization techniques should be utilized to in situ monitor lithium metal deposition at electrode interfaces, thus unveiling the mechanism of Li metal nucleation/growth control by functional polymers.
- (5)
- Battery testing at practical conditions is also required to facilitate practical applications of functional polymers in LMBs. Cell fabrication parameters such as the amount of electrolyte used, cathode mass loading and external pressure applied could affect the electrochemical performances of LMBs. Although a massive electrolyte could sustain a longer lifespan, it sacrifices the energy density of the battery cell; high cathode mass loading is necessary for achieving high energy density of batteries, yet the derived high internal resistance should be of careful consideration; a certain external pressure is favorable for inducing more uniform Li deposition and thus boosting the cycle life [103], while too high external pressure is infeasible in practical scenarios. Therefore, battery testing should be performed with moderate electrolyte amount, cathode mass loading as well as external pressure to more rationally evaluate the functional polymer-enhanced electrochemical performances in LMBs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PEO | polyethylene oxide |
| PVDF | polyvinylidene fluoride |
| AG | agarose |
| PTs | polythiophene derivative |
| SP | silly putty |
| PDMS | polydimethylsiloxane |
| PDA | polydopamine |
| PAA | polyacrylic acid |
| MPS | 3-methacryloxypropyltrimethoxysilane |
| PTFE | polytetrafluoroethylene |
| PEDOT | poly(3,4-ethylene dioxythiophene) |
| PEG | poly(ethylene glycol) |
| DSN | dynamic single-ion conductive network |
| PTMEG | poly(tetramethylene ether glycol) |
| P(SF-DOL) | poly(vinylsulfonyl fluoride-ran-2-vinyl-1,3-dioxolane) |
| PECA | poly(2-chloroethyl acrylate) |
| PAN | polyacrylonitrile |
| PVA | polyvinyl alcohol |
| PMMA | polymethyl methacrylate |
| PP | polypropylene |
| β-PVDF | β-phase polyvinylidene fluoride |
| PMF | poly-melamine-formalde-hyde |
| PPN | polar polymer network |
| PEGDA | polyethylene glycol diacrylate |
| f-PTC-PD | ferroelectric terpolymer-polydopamine |
| POSS | polyoctaammonium |
| PTEGDMA | polytriglycol dimethacrylate |
| CTS | chitosan |
| P(EO-co-PO) | poly(ethylene oxide-co-epoxypropane) |
| PDMSDGE | poly(dimethylsiloxane) diglycidyl ether |
| PMIA | poly(m-phenylene dicarboxamide) |
| PAM | polyacrylamide |
| PVDF-HFP | poly(vinylidene-co-hexafluoropropylene) |
| PEG | polyethylene glycol |
| PEGMA | Poly(ethylene glycol) methacrylate |
| PMA | poly(N-methyl-malonic amide) |
| PETEA | pentaerythritol tetraacrylate |
| PDAAli | poly(3,3-dimethacrylic acid lithium) |
| PI | polyimide |
| PEC | poly(ethylene carbonate) |
| PEGDM | polyethylene glycol methyl ether dimethacrylate |
| PC | propylene carbonate |
| PES | poly(arylene ether sulfone) |
| BC | bacterial cellulose |
References
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 1–4. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries: 50 years of advances to address the next 20 years of climate issues. Nano Lett. 2020, 20, 8435–8437. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Yan, K.; Lu, Z.; Lee, H.W.; Xiong, F.; Hsu, P.-C.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Xu, R.; Cheng, X.-B.; Yan, C.; Zhang, X.-Q.; Xiao, Y.; Zhao, C.-Z.; Huang, J.-Q.; Zhang, Q. Artificial interphases for highly stable lithium metal anode. Matter 2019, 1, 317–344. [Google Scholar] [CrossRef]
- Wei, S.; Cheng, Z.; Nath, P.; Tikekar, M.D.; Li, G.; Archer, L.A. Stabilizing electrochemical interfaces in viscoelastic liquid electrolytes. Sci. Adv. 2018, 4, eaao6243. [Google Scholar] [CrossRef]
- Wu, J.; Ihsan-Ul-Haq, M.; Chen, Y.; Kim, J.-K. Understanding solid electrolyte interphases: Advanced characterization techniques and theoretical simulations. Nano Energy 2021, 89, 106489. [Google Scholar] [CrossRef]
- Meyerson, M.L.; Papa, P.E.; Heller, A.; Mullins, C.B. Recent developments in dendrite-free lithium-metal deposition through tailoring of micro-and nanoscale artificial coatings. ACS Nano 2020, 15, 29–46. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Xu, W.; Xiao, J.; Cao, X.; Liu, J. Lithium metal anodes with nonaqueous electrolytes. Chem. Rev. 2020, 120, 13312–13348. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Murugesan, V.; Han, K.S.; Jiang, X.; Cao, Y.; Xiao, L.; Ai, X.; Yang, H.; Zhang, J.-G.; Sushko, M.L. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries. Nat. Energy 2018, 3, 674–681. [Google Scholar] [CrossRef]
- Zheng, J.; Ji, G.; Fan, X.; Chen, J.; Li, Q.; Wang, H.; Yang, Y.; DeMella, K.C.; Raghavan, S.R.; Wang, C. High-fluorinated electrolytes for Li–S batteries. Adv. Energy Mater. 2019, 9, 1803774. [Google Scholar] [CrossRef]
- Boz, B.; Dev, T.; Salvadori, A.; Schaefer, J.L. Electrolyte and electrode designs for enhanced ion transport properties to enable high performance lithium batteries. J. Electrochem. Soc. 2021, 168, 090501. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, R.; Zhang, D.; Liu, J.; Liu, F.; Cui, J.; Lin, Z.; Wu, J.; Zhu, M. Constructing Li-rich artificial SEI layer in alloy–polymer composite electrolyte to achieve high ionic conductivity for all-solid-state lithium metal batteries. Adv. Mater. 2021, 33, 2004711. [Google Scholar] [CrossRef]
- Chen, C.; Liang, Q.; Wang, G.; Liu, D.; Xiong, X. Grain-boundary-rich artificial SEI layer for high-rate lithium metal anodes. Adv. Funct. Mater. 2022, 32, 2107249. [Google Scholar] [CrossRef]
- Kim, P.J.; Pol, V.G. High performance lithium metal batteries enabled by surface tailoring of polypropylene separator with a polydopamine/graphene layer. Adv. Energy Mater. 2018, 8, 1802665. [Google Scholar] [CrossRef]
- Wu, J.; Chen, X.; Fan, W.; Li, X.; Mai, Y.-W.; Chen, Y. Rationally designed alloy-based phases for highly reversible alkali metal batteries. Energy Storage Mater. 2022, 48, 223–243. [Google Scholar] [CrossRef]
- Chiu, L.-L.; Chung, S.-H. A poly (ethylene oxide)/lithium bis (trifluoromethanesulfonyl) imide-coated polypropylene membrane for a high-loading lithium–sulfur battery. Polymers 2021, 13, 535. [Google Scholar] [CrossRef]
- Fu, K.; Gong, Y.; Liu, B.; Zhu, Y.; Xu, S.; Yao, Y.; Luo, W.; Wang, C.; Lacey, S.D.; Dai, J. Toward garnet electrolyte–based Li metal batteries: An ultrathin, highly effective, artificial solid-state electrolyte/metallic Li interface. Sci. Adv. 2017, 3, e1601659. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Hou, S.; Wang, P.; He, X.; Piao, N.; Chen, J.; Fan, X.; Wang, C. Solid-state electrolyte design for lithium dendrite suppression. Adv. Mater. 2020, 32, 2002741. [Google Scholar] [CrossRef] [PubMed]
- Yun, Q.; He, Y.B.; Lv, W.; Zhao, Y.; Li, B.; Kang, F.; Yang, Q.H. Chemical dealloying derived 3D porous current collector for Li metal anodes. Adv. Mater. 2016, 28, 6932–6939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lyu, R.; Lv, W.; Li, H.; Jiang, W.; Li, J.; Gu, S.; Zhou, G.; Huang, Z.; Zhang, Y. A lightweight 3D Cu nanowire network with phosphidation gradient as current collector for high-density nucleation and stable deposition of lithium. Adv. Mater. 2019, 31, 1904991. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, X.; Li, Q.; Yang, H.; Khoshi, M.R.; Xu, Y.; Hwang, S.; Chen, L.; Ji, X.; Yang, C. Electrolyte design for LiF-rich solid–electrolyte interfaces to enable high-performance microsized alloy anodes for batteries. Nat. Energy 2020, 5, 386–397. [Google Scholar] [CrossRef]
- Wu, B.; Lochala, J.; Taverne, T.; Xiao, J. The interplay between solid electrolyte interface (SEI) and dendritic lithium growth. Nano Energy 2017, 40, 34–41. [Google Scholar] [CrossRef]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Electrode–electrolyte interfaces in lithium-based batteries. Energ. Environ. Sci. 2018, 11, 527–543. [Google Scholar] [CrossRef]
- Yan, C.; Xu, R.; Xiao, Y.; Ding, J.F.; Xu, L.; Li, B.Q.; Huang, J.Q. Toward critical electrode/electrolyte interfaces in rechargeable batteries. Adv. Funct. Mater. 2020, 30, 1909887. [Google Scholar] [CrossRef]
- Assegie, A.A.; Cheng, J.H.; Kuo, L.M.; Su, W.N.; Hwang, B.J. Polyethylene oxide film coating enhances lithium cycling efficiency of an anode-free lithium-metal battery. Nanoscale 2018, 10, 6125–6138. [Google Scholar] [CrossRef]
- Dong, H.; Xiao, X.; Jin, C.; Wang, X.; Tang, P.; Wang, C.; Yin, Y.; Wang, D.; Yang, S.; Wu, C. High lithium-ion conductivity polymer film to suppress dendrites in Li metal batteries. J. Power Sources 2019, 423, 72–79. [Google Scholar] [CrossRef]
- Zhang, S.J.; Gao, Z.G.; Wang, W.W.; Lu, Y.Q.; Deng, Y.P.; You, J.H.; Li, J.T.; Zhou, Y.; Huang, L.; Zhou, X.D.; et al. A natural biopolymer film as a robust protective layer to effectively stabilize lithium-metal anodes. Small 2018, 14, 1801054. [Google Scholar] [CrossRef] [PubMed]
- Wickramaarachchi, K.; Sundaram, M.M.; Henry, D.J.; Gao, X. Alginate biopolymer effect on the electrodeposition of manganese dioxide on electrodes for supercapacitors. ACS Appl. Energ. Mater. 2021, 4, 7040–7051. [Google Scholar] [CrossRef]
- Ramkumar, R.; Minakshi, M. Fabrication of ultrathin CoMoO4 nanosheets modified with chitosan and their improved performance in energy storage device. Dalton Trans. 2015, 44, 6158–6168. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, R.; Sundaram, M.M. Electrochemical synthesis of polyaniline cross-linked NiMoO 4 nanofibre dendrites for energy storage devices. New J. Chem. 2016, 40, 7456–7464. [Google Scholar] [CrossRef]
- Fan, Y.; Zhao, Y.; Li, S.; Liu, Y.; Lv, Y.; Zhu, Y.; Xiang, R.; Maruyama, S.; Zhang, H.; Zhang, Q. Altering polythiophene derivative substrates to explore the mechanism of heterogeneous lithium nucleation for dendrite-free lithium metal anodes. J. Energy Chem. 2021, 59, 63–68. [Google Scholar] [CrossRef]
- Zheng, G.Y.; Wang, C.; Pei, A.; Lopez, J.; Shi, F.F.; Chen, Z.; Sendek, A.D.; Lee, H.W.; Lu, Z.D.; Schneider, H.; et al. High-performance lithium metal negative electrode with a soft and flowable polymer coating. ACS Energy Lett. 2016, 1, 1247–1255. [Google Scholar] [CrossRef]
- Liu, K.; Pei, A.; Lee, H.R.; Kong, B.; Liu, N.; Lin, D.; Liu, Y.; Liu, C.; Hsu, P.-C.; Bao, Z.; et al. Lithium metal anodes with an adaptive “solid-liquid” interfacial protective layer. J. Am. Chem. Soc. 2017, 139, 4815–4820. [Google Scholar] [CrossRef]
- Ma, L.; Fu, C.; Li, L.; Mayilvahanan, K.S.; Watkins, T.; Perdue, B.R.; Zavadil, K.R.; Helms, B.A. Nanoporous polymer films with a high cation transference number stabilize lithium metal anodes in light-weight batteries for electrified transportation. Nano Lett. 2019, 19, 1387–1394. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.; Li, Y.C.; Huang, Q.; Mallouk, T.E.; Wang, D. Interfacial chemistry regulation via a skin-grafting strategy enables high-performance lithium-metal batteries. J. Am. Chem. Soc. 2017, 139, 15288–15291. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Wang, J.; Liang, J.; Wang, C.; Sun, Q.; Lin, X.; Adair, K.R.; Luo, J.; Wang, D.; et al. A novel organic "polyurea" thin film for ultralong-life lithium-metal anodes via molecular-layer deposition. Adv. Mater. 2019, 31, e1806541. [Google Scholar] [CrossRef]
- Luo, J.; Fang, C.C.; Wu, N.L. High polarity poly(vinylidene difluoride) thin coating for dendrite-free and high-performance lithium metal anodes. Adv. Energy Mater. 2018, 8, 1701482. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, F.-L.; Guan, Y.-P.; Jin, Z.-Q.; Huang, Y.-Q.; Yao, M.; Wang, W.-K.; Wang, A.-B. Poly (dimethylsiloxane) modified lithium anode for enhanced performance of lithium-sulfur batteries. Energy Storage Mater. 2018, 13, 151–159. [Google Scholar] [CrossRef]
- An, Y.L.; Zhang, Z.; Fei, H.F.; Xu, X.Y.; Xiong, S.L.; Feng, J.K.; Ci, L.J. Lithium metal protection enabled by in-situ olefin polymerization for high-performance secondary lithium sulfur batteries. J. Power Sources 2017, 363, 193–198. [Google Scholar] [CrossRef]
- Wondimkun, Z.T.; Tegegne, W.A.; Shi-Kai, J.; Huang, C.J.; Sahalie, N.A.; Weret, M.A.; Hsu, J.Y.; Hsieh, P.L.; Huang, Y.S.; Wu, S.H.; et al. Highly-lithiophilic Ag@PDA-GO film to suppress dendrite formation on Cu substrate in anode-free lithium metal batteries. Energy Storage Mater. 2021, 35, 334–344. [Google Scholar] [CrossRef]
- Li, N.W.; Shi, Y.; Yin, Y.X.; Zeng, X.X.; Li, J.Y.; Li, C.J.; Wan, L.J.; Wen, R.; Guo, Y.G. A flexible solid electrolyte interphase layer for long-life lithium metal anodes. Angew. Chem. Int. Edit. 2018, 57, 1505. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhao, L.; Fan, Q.; Zeng, X.; Liu, S.; Pang, W.K.; He, Y.B.; Guo, Z. Lithium metal electrode with increased air stability and robust solid electrolyte interphase realized by silane coupling agent modification. Adv. Mater. 2021, 33, 2008133. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Liu, B.Y.; Jiang, F.; Zhang, Y.; Xie, H.; Hitz, E.; Hu, L.B. Garnet/polymer hybrid ion-conducting protective layer for stable lithium metal anode. Nano Res. 2017, 10, 4256–4265. [Google Scholar] [CrossRef]
- Fu, C.; Battaglia, C. Polymer–inorganic nanocomposite coating with high ionic conductivity and transference number for a stable lithium metal anode. ACS Appl. Mater. Inter. 2020, 12, 41620–41626. [Google Scholar] [CrossRef]
- Sun, S.H.; Myung, S.; Kim, G.; Lee, D.; Son, H.; Jang, M.; Park, E.; Son, B.; Jung, Y.G.; Paik, U.; et al. Facile ex situ formation of a LiF-polymer composite layer as an artificial SEI layer on Li metal by simple roll-press processing for carbonate electrolyte-based Li metal batteries. J. Mater. Chem. A 2020, 8, 17229–17237. [Google Scholar] [CrossRef]
- Kim, J.H.; Woo, H.S.; Kim, W.K.; Ryu, K.H.; Kim, D.W. Improved cycling performance of lithium–oxygen cells by use of a lithium electrode protected with conductive polymer and aluminum fluoride. ACS Appl. Mater. Inter. 2016, 8, 32300–32306. [Google Scholar] [CrossRef]
- Yu, Z.A.; Mackanic, D.G.; Michaels, W.; Lee, M.; Pei, A.; Feng, D.W.; Zhang, Q.H.; Tsao, Y.C.; Amanchukwu, C.V.; Yan, X.Z.; et al. A Dynamic, electrolyte-blocking, and single-ion-conductive network for stable lithium-metal anodes. Joule 2019, 3, 2761–2776. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Yuen, P.Y.; Liu, K.; Xie, J.; Dauskardt, R.H.; Cui, Y. An artificial solid electrolyte interphase with high Li-ion conductivity, mechanical strength, and flexibility for stable lithium metal anodes. Adv. Mater. 2017, 29, 1605531. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, S.; Dong, S.; Li, W.; Li, H.; Cui, G.; Chen, L. Poly (ethyl α-cyanoacrylate)-based artificial solid electrolyte interphase layer for enhanced interface stability of Li metal anodes. Chem. Mater. 2017, 29, 4682–4689. [Google Scholar] [CrossRef]
- Tu, Z.; Choudhury, S.; Zachman, M.J.; Wei, S.; Zhang, K.; Kourkoutis, L.F.; Archer, L.A. Designing artificial solid-electrolyte interphases for single-ion and high-efficiency transport in batteries. Joule 2017, 1, 394–406. [Google Scholar] [CrossRef]
- Pang, Q.; Zhou, L.; Nazar, L.F. Elastic and Li-ion–percolating hybrid membrane stabilizes Li metal plating. Proc. Natl. Acad. Sci. USA 2018, 115, 12389–12394. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, H.; Liu, Y.; Huang, S.; Zhou, T.; Yang, X.; Wang, B.; Zhang, W.; Ming, H.; Xiang, Y. A scalable bio-inspired polydopamine-Cu ion interfacial layer for high-performance lithium metal anode. Nano Res. 2019, 12, 2919–2924. [Google Scholar] [CrossRef]
- Jiang, Z.; Jin, L.; Han, Z.; Hu, W.; Zeng, Z.; Sun, Y.; Xie, J. Facile generation of polymer-alloy hybrid layers for dendrite-free lithium-metal anodes with improved moisture stability. Angew. Chem. Int. Edit. 2019, 58, 11374–11378. [Google Scholar] [CrossRef]
- Ma, L.; Kim, M.S.; Archer, L.A. Stable artificial solid electrolyte interphases for lithium batteries. Chem. Mater. 2017, 29, 4181–4189. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, G.Y.; Tang, L.; Wu, J.J.; Guo, B.K.; Zhu, M.; Wu, C.; Dou, S.X.; Wu, M.H. Stable lithium metal anodes enabled by inorganic/organic double-layered alloy and polymer coating. J. Mater. Chem. A 2019, 7, 25369–25376. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, Z.; Gray, J.L.; He, X.; Wang, D.; Chen, T.; Huang, Q.; Li, Y.C.; Wang, H.; Kim, S.H.; et al. Polymer-inorganic solid-electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 2019, 18, 384–389. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, G.; Gao, Y.; Wang, D.; Huang, Q.; Wang, D. Stable Li metal anode by a hybrid lithium polysulfidophosphate/polymer cross-linking film. ACS Energy Lett. 2019, 4, 1271–1278. [Google Scholar] [CrossRef]
- Liang, Z.; Zheng, G.; Liu, C.; Liu, N.; Li, W.; Yan, K.; Yao, H.; Hsu, P.C.; Chu, S.; Cui, Y. Polymer nanofiber-guided uniform lithium deposition for battery electrodes. Nano Lett. 2015, 15, 2910–2916. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xiong, X.; Lin, Z.; Zheng, J.; Fenghua, Z.; Li, Y.; Liu, Y.; Yang, C.; Tang, Y.; Liu, M. Uniform Li deposition regulated via three-dimensional polyvinyl alcohol nanofiber networks for effective Li metal anodes. Nanoscale 2018, 10, 10018–10024. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zong, W.; Zhou, G.; Fan, X.; Miao, Y.-E. Radical-functionalized polymer nanofiber composite separator for ultra-stable dendritic-free lithium metal batteries. Compos. Commun. 2021, 25, 100696. [Google Scholar] [CrossRef]
- Hwang, C.; Song, W.J.; Song, G.; Wu, Y.; Lee, S.; Son, H.B.; Kim, J.; Liu, N.; Park, S.; Song, H.K. A three-dimensional nano-web scaffold of ferroelectric beta-PVDF fibers for lithium metal plating and stripping. ACS Appl. Mater. Inter. 2020, 12, 29235–29241. [Google Scholar] [CrossRef]
- Fan, L.; Zhuang, H.L.; Zhang, W.; Fu, Y.; Liao, Z.; Lu, Y. Stable lithium electrodeposition at ultra-high current densities enabled by 3D PMF/Li composite anode. Adv. Energy Mater. 2018, 8, 1703360. [Google Scholar] [CrossRef]
- Pan, R.; Xu, X.; Sun, R.; Wang, Z.; Lindh, J.; Edstrom, K.; Stromme, M.; Nyholm, L. Nanocellulose modified polyethylene separators for lithium metal batteries. Small 2018, 14, e1704371. [Google Scholar] [CrossRef]
- Bae, J.; Qian, Y.; Li, Y.; Zhou, X.; Goodenough, J.B.; Yu, G. Polar polymer–solvent interaction derived favorable interphase for stable lithium metal batteries. Energy Environ. Sci. 2019, 12, 3319–3327. [Google Scholar] [CrossRef]
- Ryu, J.; Han, D.-Y.; Hong, D.; Park, S. A polymeric separator membrane with chemoresistance and high Li-ion flux for high-energy-density lithium metal batteries. Energy Storage Mater. 2022, 45, 941–951. [Google Scholar] [CrossRef]
- Wang, Y.N.; Shi, L.Y.; Zhou, H.L.; Wang, Z.Y.; Li, R.; Zhu, J.F.; Qiu, Z.F.; Zhao, Y.; Zhang, M.H.; Yuan, S. Polyethylene separators modified by ultrathin hybrid films enhancing lithium ion transport performance and Li-metal anode stability. Electrochim. Acta 2018, 259, 386–394. [Google Scholar] [CrossRef]
- Shen, L.D.; Liu, X.; Dong, J.; Zhang, Y.T.; Xu, C.X.; Lai, C.; Zhang, S.Q. Functional lithiophilic polymer modified separator for dendrite-free and pulverization-free lithium metal batteries. J. Energy Chem. 2021, 52, 262–268. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Tennenbaum, M.J.; Althouse, Z.; Ma, Y.; He, Y.; Wu, Y.; Wu, T.H.; Mathur, A.; Chen, P.; et al. Polypropylene carbonate-based adaptive buffer layer for stable interfaces of dolid polymer lithium metal batteries. ACS Appl. Mater. Inter. 2019, 11, 27906–27912. [Google Scholar] [CrossRef]
- Subramani, R.; Pham, M.N.; Lin, Y.H.; Hsieh, C.T.; Lee, Y.L.; Jan, J.S.; Chiu, C.C.; Teng, H. Design of networked solid-state polymer as artificial interlayer and solid polymer electrolyte for lithium metal batteries. Chem. Eng. J. 2021, 431, 133442. [Google Scholar] [CrossRef]
- Liu, H.; Peng, D.; Xu, T.; Cai, K.; Sun, K.; Wang, Z. Porous conductive interlayer for dendrite-free lithium metal battery. J. Energy Chem. 2021, 53, 412–418. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Shi, C.; Liang, G.; Lu, Z.; Fu, R.; Wu, D. Two-dimensional molecular brush-functionalized porous bilayer composite separators toward ultrastable high-current density lithium metal anodes. Nat. Commun. 2019, 10, 1363. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, X.Q.; Cheng, X.B.; Peng, H.J.; Zhao, C.Z.; Yan, C.; Huang, J.Q. Artificial soft-rigid protective layer for dendrite-free lithium metal anode. Adv. Funct. Mater. 2018, 28, 1705838. [Google Scholar] [CrossRef]
- Huo, H.; Li, X.; Chen, Y.; Liang, J.; Deng, S.; Gao, X.; Doyle-Davis, K.; Li, R.; Guo, X.; Shen, Y.; et al. Bifunctional composite separator with a solid-state-battery strategy for dendrite-free lithium metal batteries. Energy Storage Mater. 2020, 29, 361–366. [Google Scholar] [CrossRef]
- Yan, M.; Liang, J.Y.; Zuo, T.T.; Yin, Y.X.; Xin, S.; Tan, S.J.; Guo, Y.G.; Wan, L.J. Stabilizing polymer–lithium interface in a rechargeable solid battery. Adv. Funct. Mater. 2020, 30, 1908047. [Google Scholar] [CrossRef]
- Chiu, L.-L.; Chung, S.-H. Composite gel-polymer electrolyte for high-loading polysulfide cathodes. J. Mater. Chem. A 2022, 10, 13719–13726. [Google Scholar] [CrossRef]
- Wu, J.Y.; Rao, Z.X.; Cheng, Z.X.; Yuan, L.X.; Li, Z.; Huang, Y.H. Ultrathin, flexible polymer electrolyte for cost-effective fabrication of all-solid-state lithium metal batteries. Adv. Energy Mater. 2019, 9, 1902767. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, H.W.; Qie, B.Y.; Cheng, Q.; Li, A.J.; Borovilas, J.; Xu, B.Q.; Shi, C.M.; Jin, T.W.; Liao, X.B.; et al. Rechargeable solid-state lithium metal batteries with vertically aligned ceramic nanoparticle/polymer composite electrolyte. Nano Energy 2019, 60, 205–212. [Google Scholar] [CrossRef]
- Pan, K.; Zhang, L.; Qian, W.; Wu, X.; Dong, K.; Zhang, H.; Zhang, S. A flexible ceramic/polymer hybrid solid electrolyte for solid-state lithium metal batteries. Adv. Mater. 2020, 32, e2000399. [Google Scholar] [CrossRef]
- Zhou, T.; Zhao, Y.; Choi, J.W.; Coskun, A. Ionic liquid functionalized gel polymer electrolytes for stable lithium metal batteries. Angew. Chem. Int. Edit. 2021, 133, 22973–22978. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Pu, Y.; Li, Y.; Xin, S.; Li, X.; Chen, J.; Goodenough, J.B. Double-layer polymer electrolyte for high-voltage all-solid-state rechargeable batteries. Adv. Mater. 2019, 31, e1805574. [Google Scholar] [CrossRef]
- Zhou, D.; Tkacheva, A.; Tang, X.; Sun, B.; Shanmukaraj, D.; Li, P.; Zhang, F.; Armand, M.; Wang, G. Stable conversion chemistry-based lithium metal batteries enabled by hierarchical multifunctional polymer electrolytes with near-single ion conduction. Angew. Chem. Int. Edit. 2019, 58, 6001–6006. [Google Scholar] [CrossRef]
- Jaumaux, P.; Liu, Q.; Zhou, D.; Xu, X.; Wang, T.; Wang, Y.; Kang, F.; Li, B.; Wang, G. Deep-eutectic-solvent-based self-healing polymer electrolyte for safe and long-life lithium-metal batteries. Angew. Chem. Int. Edit. 2020, 59, 9134–9142. [Google Scholar] [CrossRef]
- Wan, J.; Xie, J.; Kong, X.; Liu, Z.; Liu, K.; Shi, F.; Pei, A.; Chen, H.; Chen, W.; Chen, J. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat. Nanotechnol. 2019, 14, 705–711. [Google Scholar] [CrossRef]
- Angell, C.; Liu, C.; Sanchez, E. Rubbery solid electrolytes with dominant cationic transport and high ambient conductivity. Nature 1993, 362, 137–139. [Google Scholar] [CrossRef]
- Liu, W.; Yi, C.; Li, L.; Liu, S.; Gui, Q.; Ba, D.; Li, Y.; Peng, D.; Liu, J. Designing polymer-in-salt electrolyte and fully infiltrated 3D electrode for integrated solid-state lithium batteries. Angew. Chem. Int. Edit. 2021, 133, 13041–13050. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, Y.; Bai, Y.; An, M.; Chen, G.; Li, W.; Li, C.; Zhou, Y. A rational design of solid polymer electrolyte with high salt concentration for lithium battery. J. Power Sources 2018, 407, 23–30. [Google Scholar] [CrossRef]
- He, Z.J.; Fan, L.Z. Poly (ethylene carbonate)-based electrolytes with high concentration Li salt for all-solid-state lithium batteries. Rare Met. 2018, 37, 488–496. [Google Scholar] [CrossRef]
- Wu, H.; Gao, P.; Jia, H.; Zou, L.; Zhang, L.; Cao, X.; Engelhard, M.H.; Bowden, M.E.; Ding, M.S.; Hu, J. A polymer-in-salt electrolyte with enhanced oxidative stability for lithium metal polymer batteries. ACS Appl. Mater. Inter. 2021, 13, 31583–31593. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fan, L.Z. Dendrite-free Li metal deposition in all-solid-state lithium sulfur batteries with polymer-in-salt polysiloxane electrolyte. Energy Storage Mater. 2018, 15, 37–45. [Google Scholar] [CrossRef]
- Feng, Y.; Tan, R.; Zhao, Y.; Gao, R.; Yang, L.; Yang, J.; Li, H.; Zhou, G.; Chen, H.; Pan, F. Insight into fast ion migration kinetics of a new hybrid single Li-ion conductor based on aluminate complexes for solid-state Li-ion batteries. Nanoscale 2018, 10, 5975–5984. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Vechambre, C.; Viau, L.; Mehdi, A.; Fontaine, O.; Mourad, E.; Monge, S.; Chenal, J.M.; Chazeau, L.; Vioux, A. Single-ion conductor nanocomposite organic–inorganic hybrid membranes for lithium batteries. J. Mater. Chem. A 2014, 2, 12162–12165. [Google Scholar] [CrossRef]
- Cao, C.; Li, Y.; Feng, Y.Y.; Peng, C.; Li, Z.Y.; Feng, W. A solid-state single-ion polymer electrolyte with ultrahigh ionic conductivity for dendrite-free lithium metal batteries. Energy Storage Mater. 2019, 19, 401–407. [Google Scholar] [CrossRef]
- Porcarelli, L.; Shaplov, A.S.; Bella, F.; Nair, J.R.; Mecerreyes, D.; Gerbaldi, C. Single-ion conducting polymer electrolytes for lithium metal polymer batteries that operate at ambient temperature. ACS Energy Lett. 2016, 1, 678–682. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Kim, G.-T.; Shi, J.; Paillard, E.; Judeinstein, P.; Lyonnard, S.; Bresser, D.; Iojoiu, C. Nanostructured multi-block copolymer single-ion conductors for safer high-performance lithium batteries. Energ. Environ. Sci. 2018, 11, 3298–3309. [Google Scholar] [CrossRef]
- Borzutzki, K.; Thienenkamp, J.; Diehl, M.; Winter, M.; Brunklaus, G. Fluorinated polysulfonamide based single ion conducting room temperature applicable gel-type polymer electrolytes for lithium ion batteries. J. Mater. Chem. A 2019, 7, 188–201. [Google Scholar] [CrossRef]
- Ding, C.; Fu, X.; Li, H.; Yang, J.; Lan, J.L.; Yu, Y.; Zhong, W.H.; Yang, X. An ultrarobust composite gel electrolyte stabilizing ion deposition for long-life lithium metal batteries. Adv. Funct. Mater. 2019, 29, 1904547. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Gao, H.; Hang, J.X.; Li, C.; Liu, P.B. MOF-derived ionic conductor enhancing polymer electrolytes with superior electrochemical performances for all solid lithium metal batteries. J. Membr. Sci. 2020, 598, 117800. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, L.; Zhang, H.; Xu, H.; Dong, P.; Zhang, Y.; Long, D. Hexagonal rodlike Cu-MOF-74-derived filler-reinforced composite polymer electrolyte for high-performance solid-state lithium batteries. ACS Appl. Energy Mater. 2022, 5, 1095–1105. [Google Scholar] [CrossRef]
- Niu, C.; Lee, H.; Chen, S.; Li, Q.; Du, J.; Xu, W.; Zhang, J.-G.; Whittingham, M.S.; Xiao, J.; Liu, J. High-energy lithium metal pouch cells with limited anode swelling and long stable cycles. Nat. Energy 2019, 4, 551–559. [Google Scholar] [CrossRef] [Green Version]

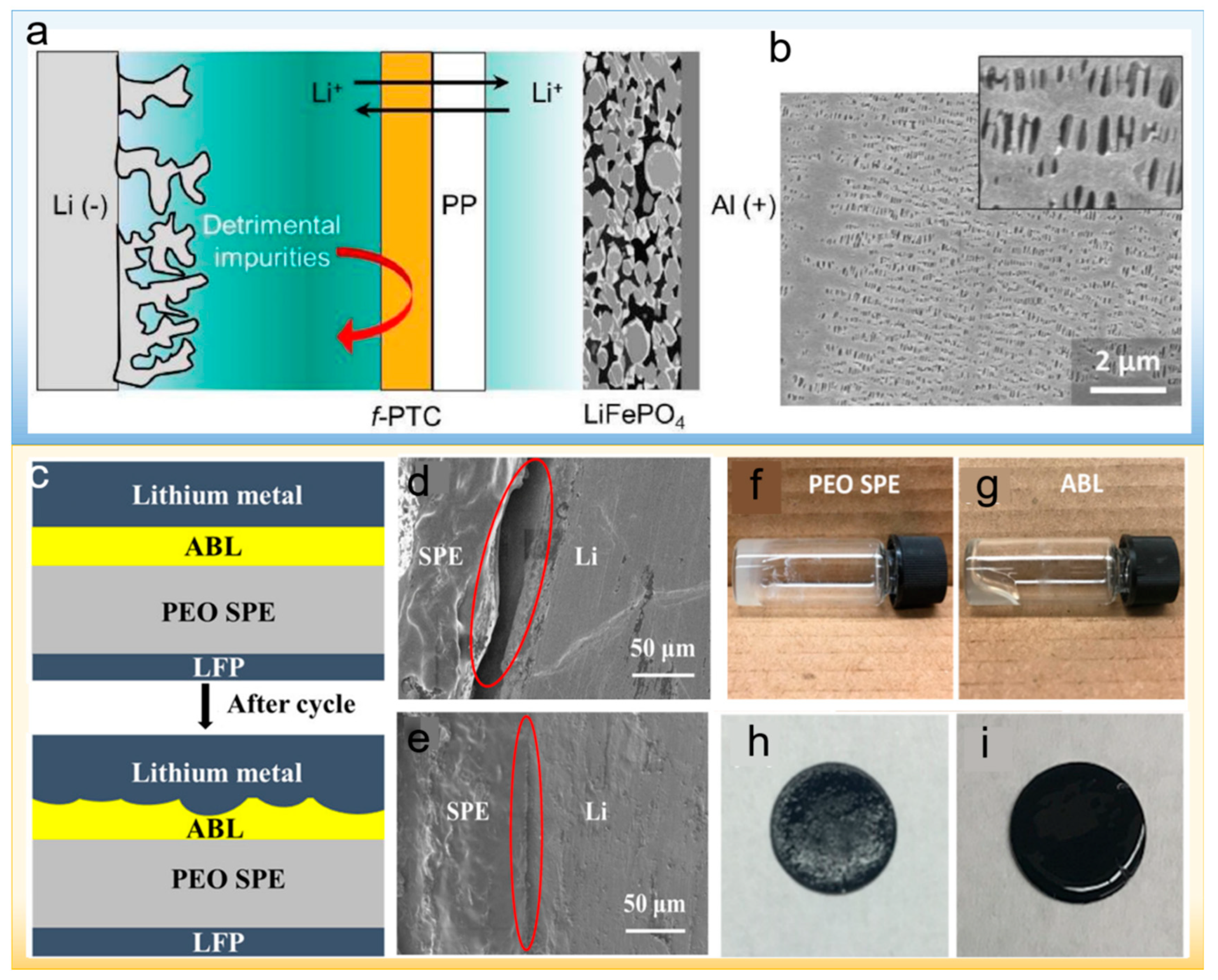
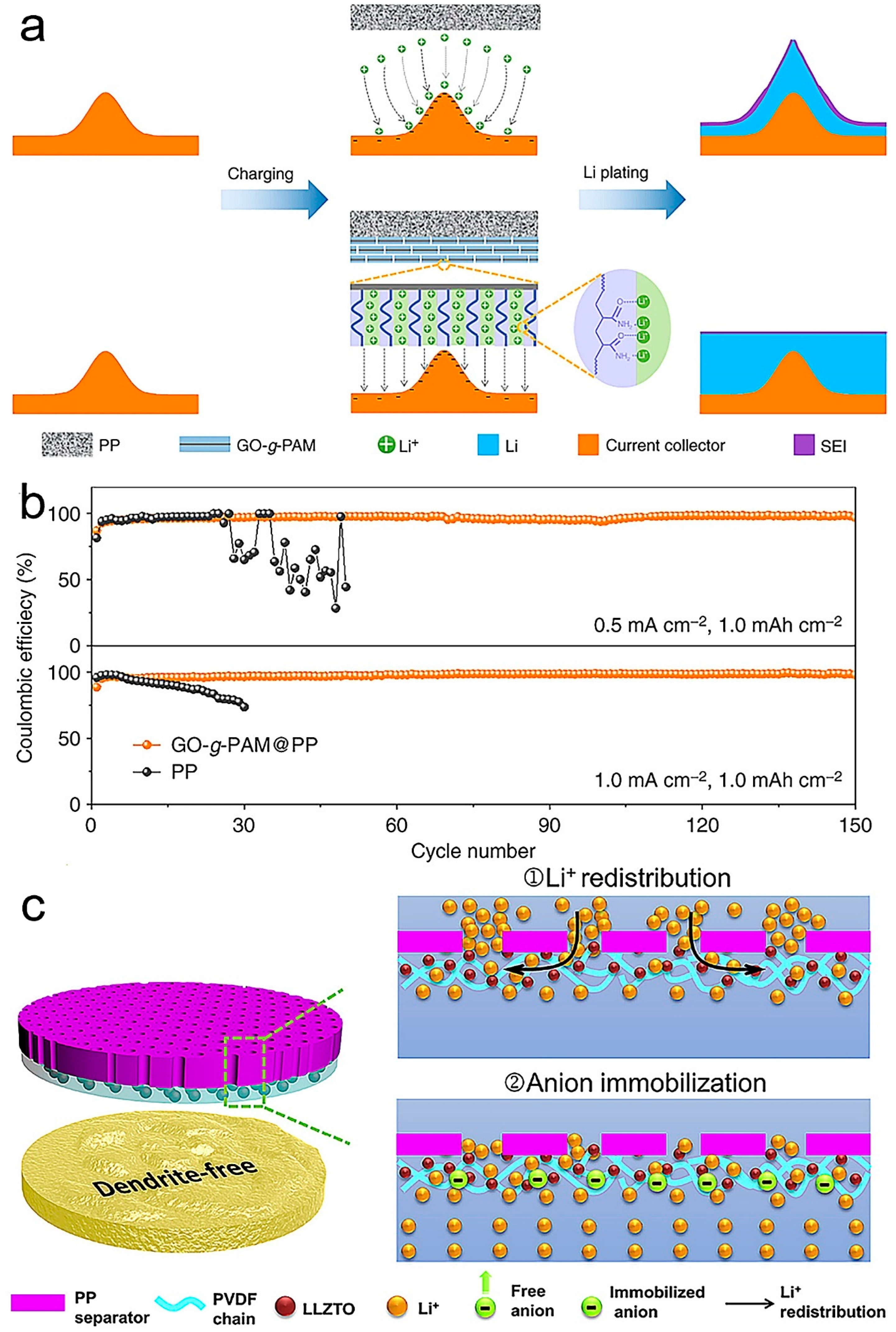

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Ren, X.; Hu, L.; Teng, W.; Wang, X.; Wu, G.; Liu, J.; Nan, D.; Yu, X. Functional Polymer Materials for Advanced Lithium Metal Batteries: A Review and Perspective. Polymers 2022, 14, 3452. https://doi.org/10.3390/polym14173452
Ma T, Ren X, Hu L, Teng W, Wang X, Wu G, Liu J, Nan D, Yu X. Functional Polymer Materials for Advanced Lithium Metal Batteries: A Review and Perspective. Polymers. 2022; 14(17):3452. https://doi.org/10.3390/polym14173452
Chicago/Turabian StyleMa, Ting, Xiuyun Ren, Liang Hu, Wanming Teng, Xiaohu Wang, Guanglei Wu, Jun Liu, Ding Nan, and Xiaoliang Yu. 2022. "Functional Polymer Materials for Advanced Lithium Metal Batteries: A Review and Perspective" Polymers 14, no. 17: 3452. https://doi.org/10.3390/polym14173452
APA StyleMa, T., Ren, X., Hu, L., Teng, W., Wang, X., Wu, G., Liu, J., Nan, D., & Yu, X. (2022). Functional Polymer Materials for Advanced Lithium Metal Batteries: A Review and Perspective. Polymers, 14(17), 3452. https://doi.org/10.3390/polym14173452








