Abstract
Silver nanoparticles (AgNPs) are used in a wide range of applications, and the size control and stability of the nanoparticles are crucial aspects in their applications. In the present study, cyclized poly(ethylene glycol) (c-PEG) with various molecular weights, along with linear PEG with hydroxy chain ends (HO–PEG–OH) and methoxy chain ends (MeO–PEG–OMe) were applied for the Tollens’ synthesis of AgNPs. The particle size was significantly affected by the topology and end groups of PEG. For example, the size determined by TEM was 40 ± 7 nm for HO–PEG5k–OH, 21 ± 4 nm for c-PEG5k, and 48 ± 9 nm for MeO–PEG5k–OMe when the molar ratio of PEG to AgNO3 (ω) was 44. The stability of AgNPs was also drastically improved by cyclization; the relative UV–Vis absorption intensity (A/A0 × 100%) at λmax to determine the proportion of persisting AgNPs in an aqueous NaCl solution (37.5 mM) was 58% for HO–PEG5k–OH, 80% for c-PEG5k, and 40% for MeO–PEG5k–OMe, despite the fact that AgNPs with c-PEG5k were much smaller than those with HO–PEG5k–OH and MeO–PEG5k–OMe.
1. Introduction
Metal nanoparticles have been widely used in catalysis [1], bio-sensors [2], optics [3], electronic components [4], and biomedical treatments [5] in recent years. Due to the surface plasmon resonance (SPR) interaction between light and the surface of metal nanoparticles, various intrinsic particle properties such as size and shape affect the color and dispersion statuses of the colloidal solution [6]. In order to preserve their properties, the stability of the nanoparticles is a crucial aspect which has to be taken into account. Generally, nanoparticles are kept in solution. since their agglomeration is facilitated in the dry state, due to the presence of capillary forces. However, the stability of nanoparticles in solution is often disturbed by a range of factors such as light [7], heat [8], salt [9], and pH [10], resulting in aggregation of nanoparticles or changes in the color of the colloidal solution. In order to contain these phenomena, small molecules or polymers are dispersed into the colloidal solution, which are then adsorbed onto the particle surface. While molecules such as sodium citrate [11] and cetyltriethylammonium bromide (CTAB) [12] can stabilize particles from agglomeration as a result of electrostatic repulsion through the presence of negative and positive charges, respectively, incorporated in their structures, neutral polymers such as poly(ethylene glycol) (PEG) and poly(vinyl pyrrolidone) (PVP) mainly create steric hindrance in order to protect the particle surface, which is reported to be more effective than the charge stabilization mechanism [13]. On the other hand, polymers equipped with charges in their backbone structure, such as poly(sodium acrylate), can combine both stabilization mechanisms [14].
In the last decade, PEG with a long alkoxy chain has received more attention from researchers, due to its water solubility and biocompatibility, which can provide steric hindrance for the preparation of gold nanoparticles (AuNPs) or silver nanoparticles (AgNPs) [15]. In relation to this, PEG coverage on the surface of nanostructures was reported to be crucial for the ligand exchange process [16], and for controlling the shape of nanocrystals [17]. In addition, PEG is also often used to disperse/stabilize AuNPs or AgNPs in immunoassays, thus maintaining the dispersion of the particles and avoiding tracking by the immune system [18]. In particular, the physiological environment is an important factor for in vivo and in vitro experiments, and increasing salt concentration or ionic strength can lead to the aggregation or dissolution of nanoparticles [19]. In general, the addition of NaCl to a metal nanoparticle solution, together with the presence of oxygen, leads to the catalyzed dissolution of the particles, attributed to the corrosive nature of the chloride ions [20]. Furthermore, Li et al. reported the bridging of individual particles by secondary precipitates of AgCl when AgNPs were exposed to chloride-containing solutions [19]. AgNPs incubated in an aqueous solution of PEG with Mn = 8000 Da prevented agglomeration in a NaCl concentration between 10 and 100 mM upon increasing the concentration of PEG from 1 to 5 mg/mL. At a PEG concentration as low as 10–100 nM, steric protection is already initiated [21]. Nevertheless, an increase in salt concentration leads to a deterioration in the stabilizing ability of PEG [22].
On the other hand, the topological effects of polymers are gaining attention, and have become another important parameter of polymers in addition to molecular weight [23] and repeating units [24], in recent years. Many researchers have synthesized and successfully isolated polymers with various topological shapes such as monocyclic, multicyclic, H-shape, etc. [25]. Among all topologies, cyclic polymers without end groups exhibit unique physical/chemical properties such as higher glass transition temperature [26], reduced hydrodynamic volume [27], higher density [28], lower viscosity [29], higher resistance of their micelles towards salt [30] and temperature [31], and higher interfacial activity [32] compared with their respective linear counterparts. In particular, we reported that dispersion stability of AuNPs [33] and AgNPs [34] was drastically enhanced by the physisorption of cyclic PEG. With these previous works in mind, the present research investigates the utilization of PEG in the preparation process of AgNPs, where the influences of the linear and cyclic polymer topologies as well as the chain-end groups on the AgNPs’ size and stability against NaCl were investigated.
2. Materials and Methods
2.1. Materials
Silver nitrate (AgNO3) (<99%) and poly(ethylene glycol) (PEG) of Mn = 2000 Da (HO–PEG3k–OH) were purchased from Sigma Aldrich Co., LLC, St. Louis, MO, USA, while dichloromethane (CH2Cl2), tetrahydrofuran (THF), n-heptane, potassium hydroxide (KOH), sodium hydroxide (NaOH), chlorobenzene, maltose monohydrate, sodium chloride (NaCl), ammonia (28–30%), iodomethane (>99.5%), and PEG of Mn = 4000 Da (HO–PEG5k–OH) and 6000 Da (HO–PEG10k–OH), were obtained from Kanto Chemicals Co., Inc., Tokyo, Japan.
2.2. Instruments
1H NMR (400 MHz) and 13C NMR (100 MHz) were measured in CDCl3 using a JNM-ESC400 instrument (JEOL Ltd., Akishima, Japan) at ambient temperature. SEC was performed at 40 °C in THF (flow rate, 1.0 mL/min), using a Shodex GPC-101 (Showa Denko K.K., Tokyo, Japan) gel permeation chromatography system (Shodex DU-2130 dual pump, Shodex RI-71 reflective index detector, and Shodex ERC-3125SN degasser) equipped with a Shodex KF-G guard column (4.6 mm × 10 mm; pore size, 8 μm) and two Shodex KF-804L columns (8 mm × 300 mm) in series. TEM measurements were performed with a JEM-2010 (JEOL Ltd., Akishima, Japan) operated at 200 kV. The size distribution of AgNPs was determined by the average of 150–200 observed particles. UV–Vis absorption spectra were recorded on a JASCO 4100 spectrophotometer (JASCO Co., Tokyo, Japan) at 25 °C. Relative UV–Vis absorption intensity (A/A0 × 100%) of AgNPs without and with NaCl was compared at λmax (409, 405, and 420 nm for HO–PEG5k–OH, c-PEG5k, and MeO–PEG5k–Ome, respectively). MALDI–TOF mass spectrometry was carried out using a Bruker Daltonics Ultraflex-S at the Open Facility, Hokkaido University, Hokkaido, Japan.
2.3. Synthesis of c-PEG
HO–PEG3k–OH, HO–PEG5k–OH, and HO–PEG10k–OH were cyclized according to a previous report by Cooke et al. [35]. Typically, HO–PEG5k–OH (5.0 g, 1.3 mmol) and TsCl (0.48 g, 2.5 mmol) were dissolved in dry THF (100 mL) and added dropwise to a stirred suspension of KOH (3.3 g) in THF/n-heptane (75/25, 100 mL) through a syringe pump, at 40 °C under N2. The addition was conducted over 144 h at a rate of 0.7 mL/h. After the addition was concluded, the reaction mixture was stirred for a further 24 h at 40 °C. The resulting suspension was filtered and concentrated in vacuo. Thereafter, the residue was redissolved in CH2Cl2 and washed with brine once and with distilled water four times, until neutral pH was observed for the aqueous phase. Thereafter, the combined organic phase was concentrated in vacuo, and purification was conducted by fractionation, where the residue was initially dissolved in CH2Cl2, treated with n-heptane until becoming cloudy, heated to 40 °C, and cooled to 25 °C, followed by decantation of the liquid phase from the precipitate. The supernatant was concentrated in vacuo, and the purification step was conducted for a further three times, to give c-PEG5k (806 mg, 16%) as a colorless/slightly yellow solid. 1H NMR: δ (ppm) 3.61 (s, –OCH2CH2O–). 13C NMR: δ (ppm) 70.7 (–OCH2CH2O–). Mn(SEC) = 3200 Da, Mp(SEC) = 3200 Da, Mw/Mn = 1.06.
2.4. Synthesis of MeO–PEG–OMe
The dimethylation of HO–PEG5k–OH was performed using a previously reported procedure [35]. A suspension of KOH (3.45 g) and iodomethane (2.28 g) in chlorobenzene (20 mL) in a 300 mL three-neck flask was treated dropwise with a solution of HO–PEG5k–OH in chlorobenzene (50 mL), over ~20 min. After the addition was completed, the mixture was stirred at 25 °C for 48 h under Ar. The resulting suspension was diluted with CH2Cl2 and filtered, and the filtrate was concentrated in vacuo. The residue was redissolved in CH2Cl2 and washed with deionized H2O seven times, dried over MgSO4 for 24 h, and concentrated in vacuo to obtain MeO–PEG5k–OMe (3.38 g, 68%) as a white solid. 1H NMR: δ (ppm) 3.60 (s, –OCH2CH2O–), 3.62 (s, –OCH2CH2OCH3), 3.34 (s, –OCH2CH2OCH3). 13C NMR: δ (ppm) 72.0 (–OCH2CH2OCH3), 70.7 (–OCH2CH2O–), 59.1 (s, –OCH2CH2OCH3).
2.5. Synthesis of AgNPs through the Tollens’ Process
AgNPs were prepared according to a reported procedure [36]. Thus, to a mixture of AgNO3 (17 mg, 0.10 mmol), PEG (10–200 mg, 0.22–4.4 mmol), an aqueous solution of ammonia (28–30%, 34 µL) in deionized H2O (90 mL), an aqueous solution of NaOH (10 mL, 0.10 mM) was added, under vigorous stirring. Thereafter, solid maltose monohydrate (360 mg, 1.0 mmol) was added, and the resulting mixture stirred overnight. The prepared AgNPs dispersions were characterized and used without further purification.
3. Results
3.1. Synthesis of c-PEG
Cyclized PEG with a molecular weight (Mn) of 3000, 5000, and 10,000 Da (namely, c-PEG3k, c-PEG5k, and c-PEG10k, respectively,) was obtained in a range between 0.7 and 1.5 g, which is a considerably large amount for cyclic polymers to be produced in a single batch reaction. With increasing molecular weight, the cyclization reaction became more difficult to achieve, which led to a decrease in the yield (Table S1) [35]. In this work, HO–PEG–OH was cyclized via etherification through the modified tosylation reaction, in line with previous reports [37]. Thus, formed cyclic PEG was homogeneous in the chemical structure. Recently, Hirose et al. reported the preparation of cyclic PEG (Mn = 400–1000 Da), where the two terminal groups of the polymer are connected by a C12 chain [38]. Additionally, the cyclization of PEG can also be achieved by performing the Glaser coupling [39] or via click chemistry [40]. However, the mentioned reactions are limited PEG with low Mn or conducted in batch reactions under high dilutions, which often yield a low amount of product (less than 100 mg product per batch), thus rendering them unattractive for our research, since larger amounts of polymer are required for performing sufficient tests. Each single peak of size-exclusion-chromatography (SEC) trace exhibits the high purity of HO–PEG–OH and c-PEG (Figure S1). The lower molecule weight-shift of c-PEG in the SEC traces, reflects the reduced hydrodynamic volume of polymers upon cyclization. For instance, Mp = 3100 of HO–PEG3k–OH was decreased to 2000 upon the polymer topology conversion. Furthermore, matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectra of HO–PEG3k–OH showed a peak at m/z = 2022.74 (DPn = 45, Na+ adduct), whereas the corresponding peak from c-PEG3k was at m/z = 2005.02 (Figure S2); the value of the highest peak in the isotope distribution shifted by a mass unit of 18, due to the net elimination of a water molecule upon cyclization. This was also observed for c-PEG5k (Figure S3), but it was not possible to conduct the same MALDI-TOF mass measurements for c-PEG10k, due to its large Mn. Moreover, 13C nuclear magnetic resonance (NMR) of c-PEG showed the complete disappearance of the carbon atoms adjacent to the terminal hydroxy groups (~61.6 ppm) in HO–PEG–OH, which confirmed the effective elimination of the terminal groups (Figures S4–S6).
3.2. Synthesis of AgNPs
AgNPs were prepared by the Tollens’ method, similar to the report by Kvítek et al., with a modification which consisted of using PEG instead of poly(acrylic acid) as a polymeric stabilizer [36]. In this set of experiments, the molar ratio of PEG to AgNO3 (ω) was varied from 2.2 to 44, while other reaction parameters were kept constant (Table 1). Hydroxy-terminated linear PEG with Mn of 3000, 5000 and 10,000 Da, HO–PEG3k–OH, HO–PEG5k–OH, and HO–PEG10k–OH, respectively, as well as their corresponding cyclic counterparts, c-PEG3k, c-PEG5k, and c-PEG10k, respectively, were used as a polymeric stabilizer.

Table 1.
Summary of the TEM-Measured Size of AgNPs Prepared in the Presence of HO–PEG–OH, c-PEG, and MeO–PEG–OMe with Various Molecular Weight and ω.
In addition, linear PEG (Mn = 5000) with methoxy termini (MeO–PEG5k–OMe) was also tested to investigate the effects of the end groups. AgNPs that were prepared in this work featured a similar particle size spectrum to the observation made by Kvítek et al. at comparable polymer concentrations [36]. Here, PEG was primarily used as a stabilizing agent, while the reducing environment was provided through the use of maltose monohydrate. The pH of the reaction was adjusted to ~11 by using NaOH, which was responsible for the completion of the reaction within minutes. The formed particles were characterized by transmission electron microscopy (TEM) and UV–Vis spectroscopy (Figure 1, Figure 2 and Figures S7–S13).
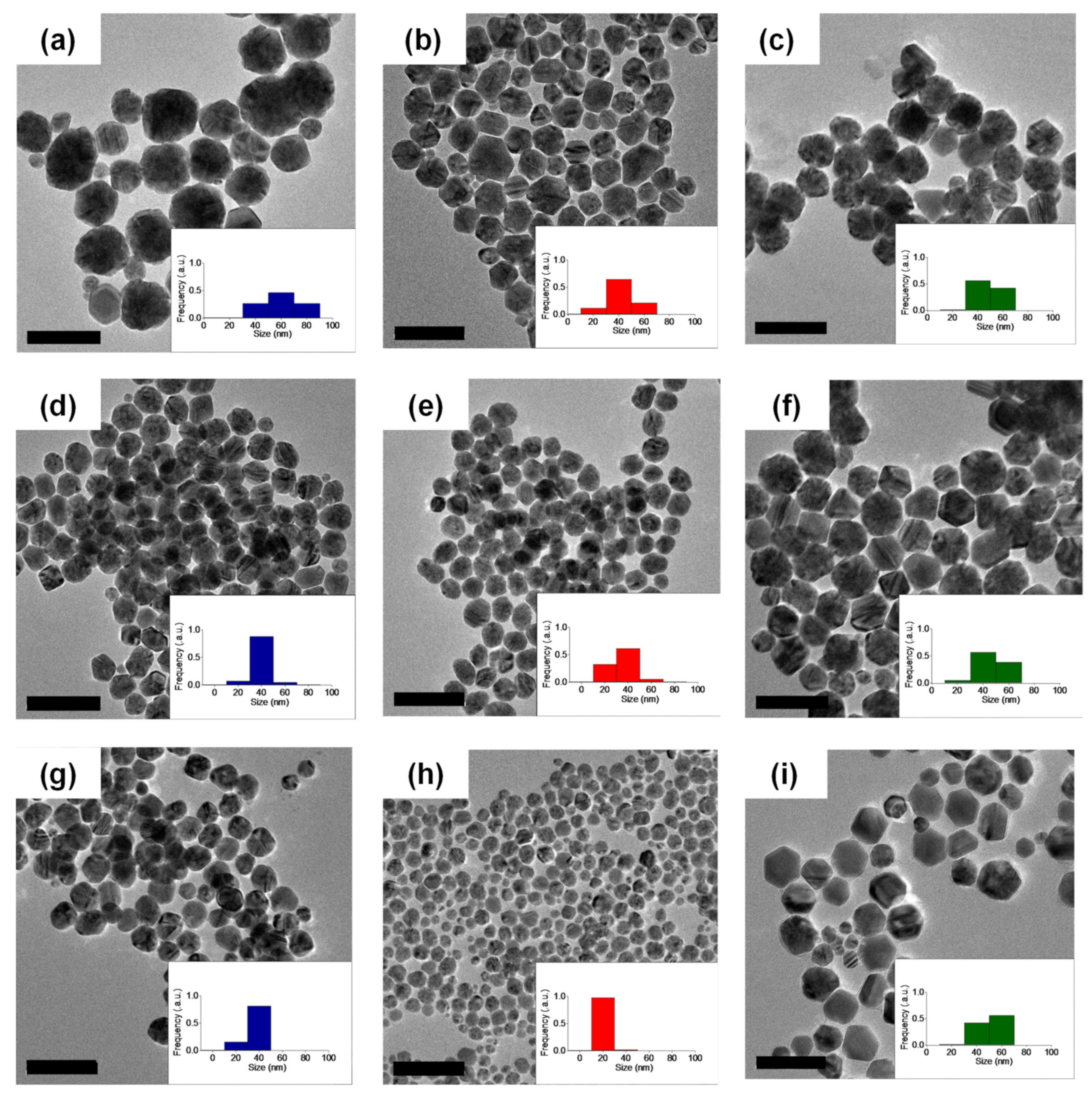
Figure 1.
TEM micrographs of AgNPs prepared in the presence of (a) HO–PEG5k–OH (ω = 2.2), (b) c-PEG5k (ω = 2.2), (c) MeO–PEG5k–OMe (ω = 2.2), (d) HO–PEG5k–OH (ω = 11), (e) c-PEG5k (ω = 11), (f) MeO–PEG5k–OMe (ω = 11), (g) HO–PEG5k–OH (ω = 44), (h) c-PEG5k (ω = 44), and (i) MeO–PEG5k–OMe (ω = 44) (Scale bar: 100 nm).
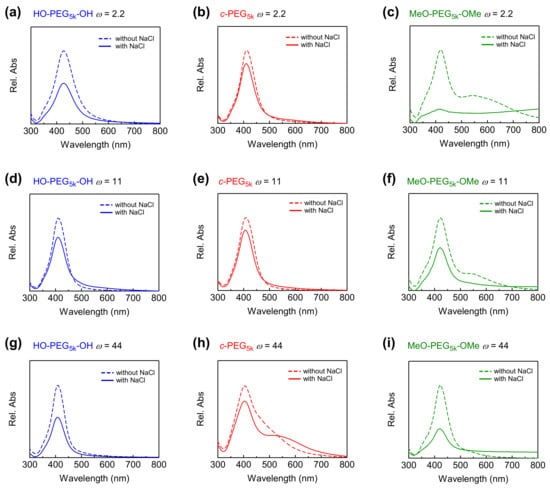
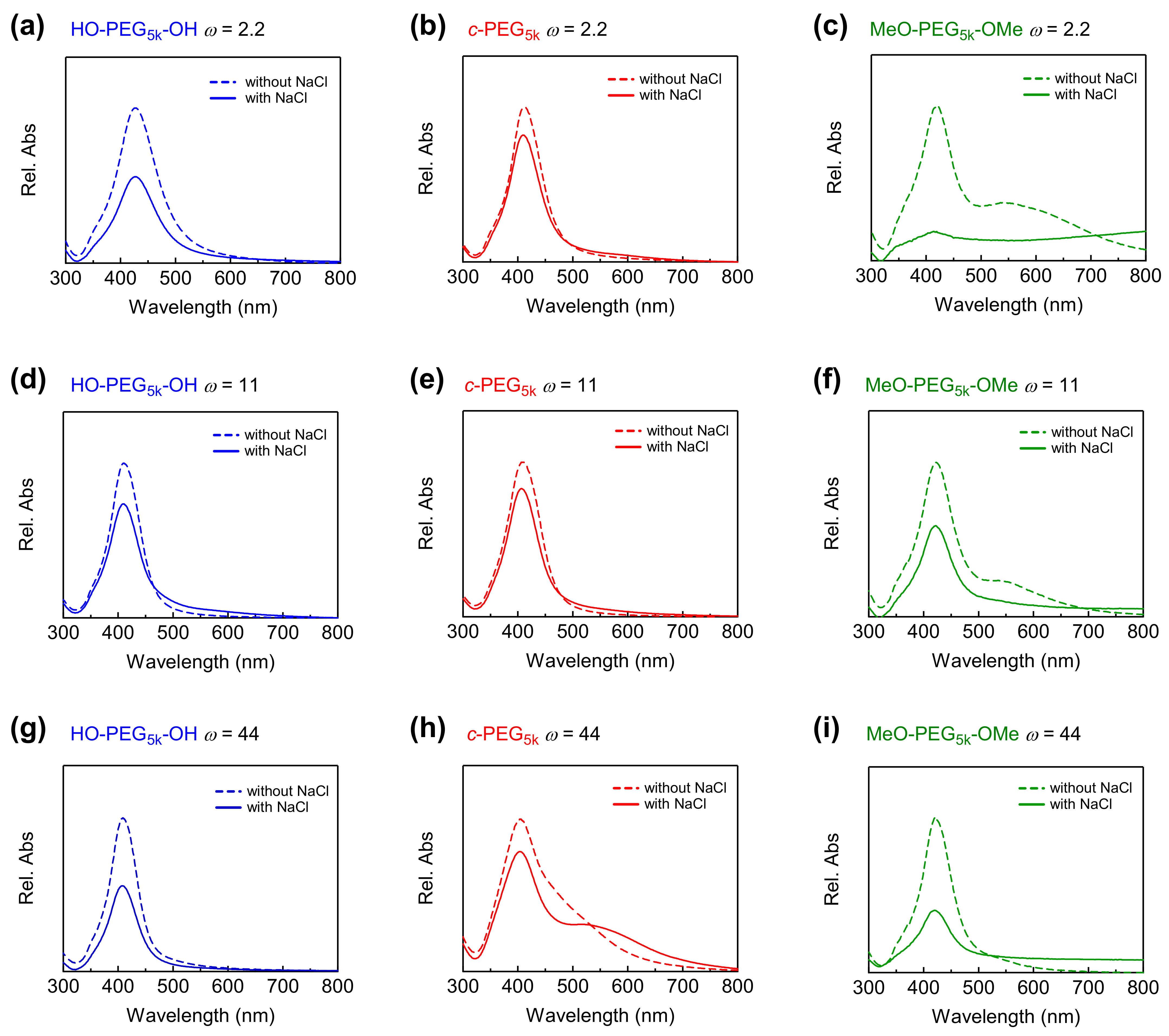
Figure 2.
Relative UV–Vis absorption spectra of AgNPs without NaCl (dashed line) and with 37.5 mM of NaCl (solid line) in the presence of (a) HO–PEG5k–OH, (b) c-PEG5k, (c) MeO–PEG5k–OMe at ω = 2.2, (d) HO–PEG5k–OH, (e) c-PEG5k, (f) MeO–PEG5k–OMe at ω = 11, (g) HO–PEG5k–OH, (h) c-PEG5k, and (i) MeO–PEG5k–OMe at ω = 44.
The particle size obtained from the experiments using c-PEG5k and c-PEG10k was significantly smaller, compared with the cases of corresponding HO–PEG5k–OH and HO–PEG10k–OH, respectively, (Table 1, Figure 1 and Figures S10–S12). For example, at ω = 44, 21 ± 4 nm for c-PEG5k was substantially smaller than 40 ± 7 nm for HO–PEG5k–OH. Nonetheless, the average particle size remained similar when HO–PEG3k–OH and c-PEG3k were used at the respective ω values (Table 1, Figures S7–S9). For example, at ω = 44, 30 ± 7 nm for c-PEG3k was comparable to 33 ± 4 nm for HO–PEG3k–OH. On the other hand, an increase in the ω value using a same type of polymer generally led to a decrease in the average particle size. Taking c-PEG5k as an example, the size decreased from 42 ± 9 nm at ω = 2.2 to 31 ± 4 nm at ω = 11, and eventually to 21 ± 4 nm at ω = 44. These results likely arose from an increase in the potential interaction sites between the silver ions (in the form of [Ag(NH3)]2+) and the PEG chain, suppressing the growth of the nanoparticles. The oxyethylene units of PEG complexed with the Ag+ ions, which were then reduced to form particles [41]. Initially, primary nanoparticles were formed, which underwent coalescence with their neighboring species to form the more stable secondary nanoparticles. In this regard, Shin et al. stated that when the concentration of the polymer is increased, the number of primary AgNPs at close range decreases, thus reducing the possibility of coalescence and eventually yielding small particle sizes [42].
The increase in the ω value meant that it is accompanied by the increase of the number of the hydroxy end groups for HO–PEG–OH, while no hydroxy end groups existed in c-PEG. In order to eliminate the effects of the hydroxy end groups, to determine the strict topology effects, PEG with methoxy end groups (MeO–PEG5k–OMe) was synthesized and subjected to the same AgNPs formation experiment. Interestingly, the size of AgNPs formed in the presence of MeO–PEG5k–OMe was basically unchanged (44–48 nm), despite the ω value (Table 1, Figure 1c,f,i). This result suggested that the increase in the concentration of the hydroxy end groups leads to the decrease in the particle size, and this effect plays a significant role in the nanoparticle size, using HO–PEG–OH. Therefore, the strict topology effects were actually more prominent by comparing the AgNPs sizes of c-PEG5k and MeO–PEG5k–OMe; 21 ± 4 nm for c-PEG5k and 48 ± 9 nm for MeO–PEG5k–OMe at ω = 44 as a representative example. In our opinion, the smaller hydrodynamic volume and the corresponding denser structure of c-PEG, similar to the case of the micelles from amphiphilic block copolymers [43,44], provided more effective shielding of the metal surface from the coalescence of the primary nanoparticles, which led to the reduction of the average particle size.
The size of the AgNPs was also characterized by UV–Vis spectroscopy (Figure 2 and Figure S13, dotted lines). For example, at ω = 44, the absorption maximum (λmax) was found at 409, 405, and 420 nm for HO–PEG5k–OH, c-PEG5k, and MeO–PEG5k–OMe, respectively. The correlation of the mean particle diameter measured by TEM with λmax in the UV−Vis spectrum was reported previously [45], and the present results reasonably matched the report (Figure S14). In the meantime, broadened UV–Vis absorption in the longer wavelengths was observed for some samples such as c-PEG5k at ω = 44 (Figure 2h), MeO–PEG5k–OMe at ω = 2.2 and 11 (Figure 2c,f). According to Tadano et al., the polymers can sometimes cause the interactions (i.e., hydrogen bonding and van der Waals interaction) between the polymer chains, which eventually trigger the agglomeration of the nanoparticles [46]. The extent of the agglomeration, however, is also dependent on the particle size and surface properties [47]. At this point, we have not determined the cause of the broadening trend by ω, the molecular weight, or the topology of the polymer. Nevertheless, this effect also manifested itself in the corresponding UV–Vis spectra, where absorption at higher wavelengths appeared. Dynamic light scattering was also attempted for size characterization. However, no valuable data were obtained, likely due to the low concentration of the particles, which gave relatively weak and disturbed signals, where the appropriate concentration of samples for DLS is normally in the range of 1–10 mg/mL.
3.3. Stability of AgNPs
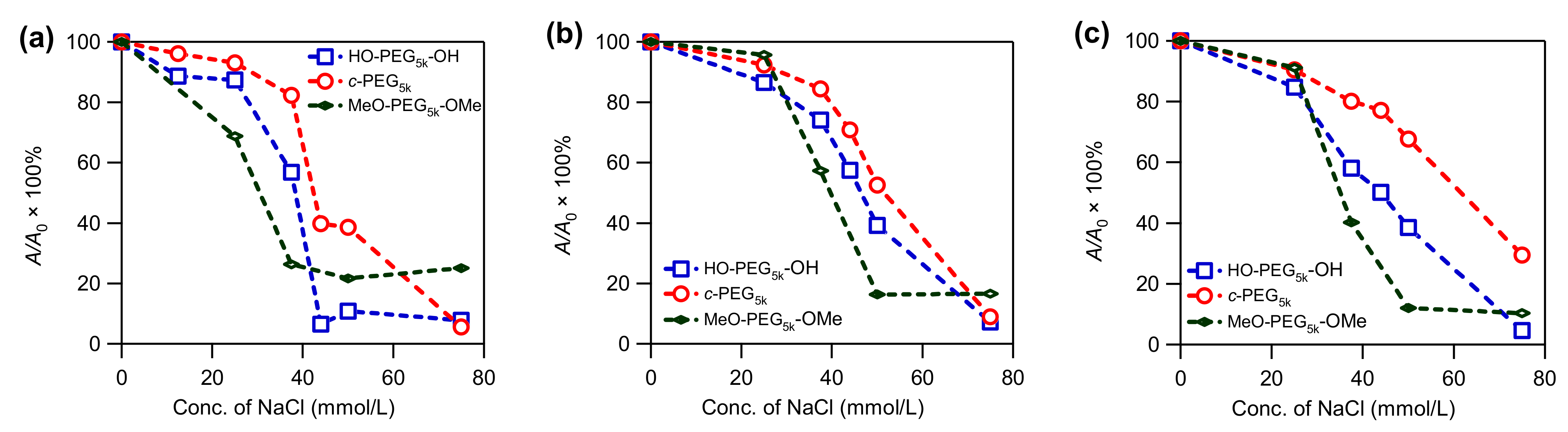
The prepared AgNPs were subjected to stability tests by exposing them to a solution containing various concentrations of NaCl. The effect of the salt on the intensity of the absorption at λmax was monitored by UV–Vis spectroscopy. The AgNPs prepared in the absence of PEG were susceptible to NaCl, and completely lost the absorption at the salt concentration of 37.5 mM, likely due to aggregation and dissolution (Figure S13, solid line). In comparison, AgNPs prepared in the presence of various PEG were more stable. Among them, AgNPs stabilized by c-PEG exhibited a higher resistance, compared with those stabilized by corresponding HO–PEG–OH. This phenomenon was illustrated by the c-PEG-stabilized AgNPs’ ability to preserve the relative UV–Vis absorption intensity (A/A0 × 100%) at λmax more efficiently than their linear counterparts, especially at the NaCl concentration range from 37.5 to 50 mM (Figure 2 and Figure 3 and Figures S15–S18). For example, at ω = 44 with a NaCl concentration of 37.5 mM, the relative absorption for AgNPs with HO–PEG5k–OH (blue) was 58%, while for c-PEG5k (red) it was 80% (Figure 3c). Moreover, for MeO–PEG5k–OMe (green), it was only 40% under the same conditions, suggesting the hydroxy end groups also played an important role in the stabilization against NaCl, due to the PEG and AgNPs surface interactions. It is likely that the hydroxy end groups bound to the surface of the AgNPs and protected them from corrosion with chloride anions. Thus, the pure topology effects which were not caused by the hydroxy end groups, on the stabilization of the AgNPs, can be considered as the difference between c-PEG5k (80%) and MeO–PEG5k–OMe (40%), despite the fact that AgNPs with c-PEG5k were much smaller (21 ± 4 nm) than those with MeO–PEG5k–OMe (48 ± 9 nm). In the meantime, the difference between HO–PEG3k–OH and c-PEG3k was not as significant, except for ω = 44 (Figure S17), suggesting that the molecular weight and the ω value were also essential factors.
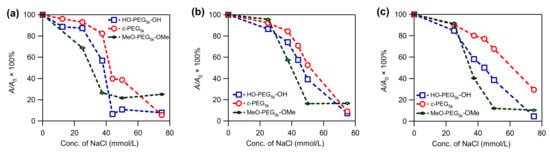
Figure 3.
Plots of relative UV–Vis absorption intensity (A/A0 × 100%) at λmax versus the NaCl concentration for AgNPs prepared in the presence of HO–PEG5k–OH (blue), c-PEG5k (red), and MeO–PEG5k–OMe (green) at (a) ω = 2.2, (b) ω = 11, and (c) ω = 44.
4. Conclusions
In conclusion, it has been shown that c-PEG tends to yield smaller and more narrowly dispersed AgNPs when employed during Tollens’ synthesis in comparison with HO–PEG–OH and MeO–PEG–OMe, where smaller and narrowly dispersed metal nanoparticles are desired in various applications. Furthermore, the AgNPs with c-PEG exhibited superior stabilizing properties against NaCl, despite the smaller size. Specifically, the pure topological conversion resulted in the drastically improved persistence of the UV–Vis absorption intensity (c-PEG5k, 80%; MeO–PEG5k–OMe, 40%). These results clearly indicate that cyclized polymers endow AgNPs with superior stability, compared with their linear counterparts. The enhanced colloidal stability through the use of c-PEG would enable applications within biological systems, where the high salt resistance can be exploited. This advantage is coupled with the biocompatibility of PEG itself, where cyclization requires no chemical functionalization on the repeating units of the polymer chain.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym14214535/s1, Table S1: Molecular weight of PEG samples, Figure S1: SEC traces of PEG samples, Figures S2 and S3: MALDI-TOF spectra of PEG samples, Figures S4–S6: NMR spectra of PEG samples, Figures S7–S12: TEM micrographs of AgNPs, Figure S13: UV–Vis absorption spectra of AgNPs, Figure S14: Correlation between the particle size of AgNPs by TEM analysis and UV-Vis spectroscopy, Figures S15 and S16: UV–Vis absorption spectra of AgNPs. Figures S17 and S18: Plots of relative UV–Vis absorption intensity versus the NaCl concentration.
Author Contributions
Conceptualization, J.E.Q.Q. and T.Y.; methodology, Y.W. and J.E.Q.Q.; formal analysis, Y.W. and J.E.Q.Q.; investigation, Y.W. and J.E.Q.Q.; writing—original draft preparation, J.E.Q.Q.; writing—review and editing, Y.W. and T.Y.; supervision, F.L., T.I., K.T., T.S., S.-i.S. and T.Y.; project administration, T.Y.; funding acquisition, Y.W., J.E.Q.Q. and T.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS Fellowship for Young Scientists (Y.W.), JSPS Program for Leading Graduate Schools (Hokkaido University “Ambitious Leader’s Program”) (Y.W.), Grant-in-Aid for JSPS Fellows (J.E.Q.Q.), JST A-STEP (JPMJTR22TD, T.Y.), Grant-in-Aid for Challenging Research (Pioneering) (22K18334, T.Y.), Grant-in-Aid for Scientific Research (B) (21H01991, T.Y.), The Asahi Glass Foundation (T.Y.), and Ogasawara Toshiaki Memorial Foundation (T.Y.).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kästner, C.; Thünemann, A.F. Catalytic reduction of 4-nitrophenol using silver nanoparticles with adjustable activity. Langmuir 2016, 32, 7383–7391. [Google Scholar] [CrossRef] [PubMed]
- Beqa, L.; Singh, A.K.; Khan, S.A.; Senapati, D.; Arumugam, S.R.; Ray, P.C. Gold nanoparticle-based simple colorimetric and ultrasensitive dynamic light scattering assay for the selective detection of Pb(II) from paints, plastics, and water samples. ACS Appl. Mater. Interfaces 2011, 3, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, A.S.; Kinnan, M.K.; Chumanov, G. Multipole plasmon resonances of submicron silver particles. J. Am. Chem. Soc. 2005, 127, 12444–12445. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.Y.; Li, Z.Y.; Au, L.; Hartland, G.V.; Li, X.D.; Marquez, M.; Xia, Y.N. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.J.; Chen, Y.C.; McLellan, J.M.; Xiong, Y.J.; Li, Z.Y.; Ginger, D.; Xia, Y.N. Synthesis and optical properties of silver nanobars and nanorice. Nano Lett. 2007, 7, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Mittelman, A.M.; Fortner, J.D.; Pennell, K.D. Effects of ultraviolet light on silver nanoparticle mobility and dissolution. Environ. Sci. Nano 2015, 2, 683–691. [Google Scholar] [CrossRef]
- Qu, Y.Q.; Yang, H.B.; Yang, N.; Fan, Y.Z.; Zhu, H.Y.; Zou, G.T. The effect of reaction temperature on the particle size, structure and magnetic properties of coprecipitated CoFe2O4 nanoparticles. Mater. Lett. 2006, 60, 3548–3552. [Google Scholar] [CrossRef]
- Hu, S.Q.; Huang, P.J.J.; Wang, J.X.; Liu, J.W. Dissecting the effect of salt for more sensitive label-free colorimetric detection of DNA using gold nanoparticles. Anal. Chem. 2020, 92, 13354–13360. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Chen, B.; Banfield, J.F. Particle size and pH effects on nanoparticle dissolution. J. Phys. Chem. C 2010, 114, 14876–14884. [Google Scholar] [CrossRef]
- Henglein, A.; Giersig, M. Formation of colloidal silver nanoparticles: Capping action of citrate. J. Phys. Chem. B 1999, 103, 9533–9539. [Google Scholar] [CrossRef]
- Fenger, R.; Fertitta, E.; Kirmse, H.; Thunemann, A.F.; Rademann, K. Size dependent catalysis with CTAB-stabilized gold nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9343–9349. [Google Scholar] [CrossRef]
- Tejamaya, M.; Romer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Hussain, I.; Brust, M.; Papworth, A.J.; Cooper, A.I. Preparation of acrylate-stabilized gold and silver hydrosols and gold-polymer composite films. Langmuir 2003, 19, 4831–4835. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, Y.; Zeng, X.; Zeng, Y.; Wang, Y. The role of poly(ethylene glycol) in the formation of silver nanoparticles. J. Colloid Interface Sci. 2005, 288, 444–448. [Google Scholar] [CrossRef]
- Xia, X.; Yang, M.; Wang, Y.; Zheng, Y.; Li, Q.; Chen, J.; Xia, Y. Quantifying the coverage density of poly(ethylene glycol) chains on the surface of gold nanostructures. ACS Nano 2012, 6, 512–522. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Zhou, W.; Sun, L.; Samanta, D.; Mirkin, C. Corner-, edge-, and facet-controlled growth of nanocrystals. Sci. Adv. 2021, 7, eabf1410. [Google Scholar] [CrossRef]
- Papastefanaki, F.; Jakovcevski, I.; Poulia, N.; Djogo, N.; Schulz, F.; Martinovic, T.; Ciric, D.; Loers, G.; Vossmeyer, T.; Weller, H.; et al. Intraspinal delivery of polyethylene glycol-coated gold nanoparticles promotes functional recovery after spinal cord injury. Mol. Ther. 2015, 23, 993–1002. [Google Scholar] [CrossRef]
- Li, X.; Lenhart, J.J.; Walker, H.W. Aggregation kinetics and dissolution of coated silver nanoparticles. Langmuir 2012, 28, 1095–1104. [Google Scholar] [CrossRef]
- Li, X.A.; Lenhart, J.J.; Walker, H.W. Dissolution-accompanied aggregation kinetics of silver nanoparticles. Langmuir 2010, 26, 16690–16698. [Google Scholar] [CrossRef]
- Zhang, X.; Servos, M.R.; Liu, J. Ultrahigh nanoparticle stability against salt, pH, and solvent with retained surface accessibility via depletion stabilization. J. Am. Chem. Soc. 2012, 134, 9910–9913. [Google Scholar] [CrossRef] [PubMed]
- Radziuk, D.; Skirtach, A.; Sukhorukov, G.; Shchukin, D.; Möhwald, H. Stabilization of silver nanoparticles by polyelectrolytes and poly(ethylene glycol). Macromol. Rapid Commun. 2007, 28, 848–855. [Google Scholar] [CrossRef]
- Kvítek, L.; Prucek, R.; Panáček, A.; Novotny, R.; Hrbáč, J.; Zbořil, R. The influence of complexing agent concentration on particle size in the process of SERS active silver colloid synthesis. J. Mater. Chem. 2005, 15, 1099–1105. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Guragain, S.; Yusa, S.; Nakashima, K. Novel synthesis route for Ag@SiO2 core-shell nanoparticles via micelle template of double hydrophilic block copolymer. Rsc Adv. 2012, 2, 5938–5940. [Google Scholar] [CrossRef]
- Tezuka, Y.; Oike, H. Topological polymer chemistry. Prog. Polym. Sci. 2002, 27, 1069–1122. [Google Scholar] [CrossRef]
- Zhang, L.H.; Elupula, R.; Grayson, S.M.; Torkelson, J.M. Major impact of cyclic chain topology on the T-g-confinement effect of supported thin films of polystyrene. Macromolecules 2016, 49, 257–268. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. Cyclic Polymers: Synthetic strategies and physical properties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 251–284. [Google Scholar] [CrossRef]
- Zhang, S.S.; Cheng, X.X.; Wang, J.Z.; Zhang, Z.B.; Zhang, W.; Zhu, X.L. Synthesis of a cyclic-brush polymer with a high grafting density using activated ester chemistry via the “grafting onto” approach. Polym. Chem. 2018, 9, 5155–5163. [Google Scholar] [CrossRef]
- Jeong, Y.; Jin, Y.; Chang, T.; Uhlik, F.; Roovers, J. Intrinsic viscosity of cyclic polystyrene. Macromolecules 2017, 50, 7770–7776. [Google Scholar] [CrossRef]
- Honda, S.; Yamamoto, T.; Tezuka, Y. Topology-directed control on thermal stability: Micelles formed from linear and cyclized amphiphilic block copolymers. J. Am. Chem. Soc. 2010, 132, 10251–10253. [Google Scholar] [CrossRef]
- Honda, S.; Yamamoto, T.; Tezuka, Y. Tuneable enhancement of the salt and thermal stability of polymeric micelles by cyclized amphiphiles. Nat. Commun. 2013, 4, 1574. [Google Scholar] [CrossRef]
- Watanabe, T.; Chimura, S.; Wang, Y.B.; Ono, T.; Isono, T.; Tajima, K.; Satoh, T.; Sato, S.; Ida, D.; Yamamoto, T. Cyclization of PEG and pluronic surfactants and the effects of the topology on their interfacial activity. Langmuir 2021, 37, 6974–6984. [Google Scholar] [CrossRef]
- Wang, Y.; Quinsaat, J.E.Q.; Ono, T.; Maeki, M.; Tokeshi, M.; Isono, T.; Tajima, K.; Satoh, T.; Sato, S.; Miura, Y.; et al. Enhanced dispersion stability of gold nanoparticles by the physisorption of cyclic poly(ethylene glycol). Nat. Commun. 2020, 11, 6089. [Google Scholar] [CrossRef]
- Oziri, O.J.; Wang, Y.B.; Watanabe, T.; Uno, S.; Maeki, M.; Tokeshi, M.; Isono, T.; Tajima, K.; Satoh, T.; Sato, S.I.; et al. PEGylation of silver nanoparticles by physisorption of cyclic poly(ethylene glycol) for enhanced dispersion stability, antimicrobial activity, and cytotoxicity. Nanoscale Adv. 2022, 4, 532–545. [Google Scholar] [CrossRef]
- Cooke, J.; Viras, K.; Yu, G.-E.; Sun, T.; Yonemitsu, T.; Ryan, A.J.; Price, C.; Booth, C. Large cyclic poly(oxyethylene)s: Chain folding in the crystalline state studied by Raman spectroscopy, X-ray scattering, and differential scanning calorimetry. Macromolecules 1998, 31, 3030–3039. [Google Scholar] [CrossRef]
- Panacek, A.; Prucek, R.; Hrbac, J.; Nevecna, T.; Steffkova, J.; Zboril, R.; Kvitek, L. Polyacrylate-assisted size control of silver nanoparticles and their catalytic activity. Chem. Mater. 2014, 26, 1332–1339. [Google Scholar] [CrossRef]
- Sun, T.; Yu, G.E.; Price, C.; Booth, C.; Cooke, J.; Ryan, A.J. Cyclic polyethers. J. Polymer 1995, 36, 3775–3778. [Google Scholar] [CrossRef]
- Hirose, Y.; Taira, T.; Sakai, K.; Sakai, H.; Endo, A.; Imura, T. Structures and surface properties of "Cyclic" polyoxyethylene alkyl ethers: Unusual behavior of cyclic surfactants in water. Langmuir 2016, 32, 8374–8382. [Google Scholar] [CrossRef]
- Lonsdale, D.E.; Bell, C.A.; Monteiro, M.J. Strategy for rapid and high-purity monocyclic polymers by CuAAC “Click” reactions. Macromolecules 2010, 43, 3331–3339. [Google Scholar] [CrossRef]
- Pressly, E.D.; Amir, R.J.; Hawker, C.J. Rapid synthesis of block and cyclic copolymers via click chemistry in the presence of copper nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 814–819. [Google Scholar] [CrossRef]
- Nam, S.; Parikh, D.V.; Condon, B.D.; Zhao, Q.; Yoshioka-Tarver, M. Importance of poly(ethylene glycol) conformation for the synthesis of silver nanoparticles in aqueous solution. J. Nanopart. Res. 2011, 13, 3755–3764. [Google Scholar] [CrossRef]
- Shin, H.S.; Yang, H.J.; Kim, S.B.; Lee, M.S. Mechanism of growth of colloidal silver nanoparticles stabilized by polyvinyl pyrrolidone in γ-irradiated silver nitrate solution. J. Colloid Interface Sci. 2004, 274, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.; Kim, Y.Y.; Kitazawa, Y.; Kim, M.; Jin, K.S.; Yamamoto, T.; Ree, M. Structural characteristics of amphiphilic cyclic and linear block copolymer micelles in aqueous solutions. ACS Macro Lett. 2014, 3, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Ree, B.J.; Satoh, T.; Yamamoto, T. Micelle Structure Details and Stabilities of Cyclic Block Copolymer Amphiphile and Its Linear Analogues. Polymers 2019, 11, 163. [Google Scholar] [CrossRef]
- Quinsaat, J.E.Q.; Testino, A.; Pin, S.; Huthwelke, T.; Nuesch, F.A.; Bowen, P.; Hofmann, H.; Ludwig, C.; Opris, D.M. Continuous production of tailored silver nanoparticles by polyol synthesis and reaction yield measured by X-ray absorption spectroscopy: Toward a growth mechanism. J. Phys. Chem. C 2014, 118, 11093–11103. [Google Scholar] [CrossRef]
- Tadano, T.; Zhu, R.; Muroga, Y.; Hoshi, T.; Sasaki, D.; Yano, S.; Sawaguchi, T. A new mechanism for the silica nanoparticle dispersion-agglomeration transition in a poly(methyl methacrylate)/silica hybrid suspension. Polym. J. 2014, 46, 342–348. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Z.; Zhang, H.; Zhang, Q. Control of heat integrated pressure-swing-distillation process for separating azeotropic mixture of tetrahydrofuran and methanol. Ind. Eng. Chem. Res. 2015, 54, 1646–1655. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).