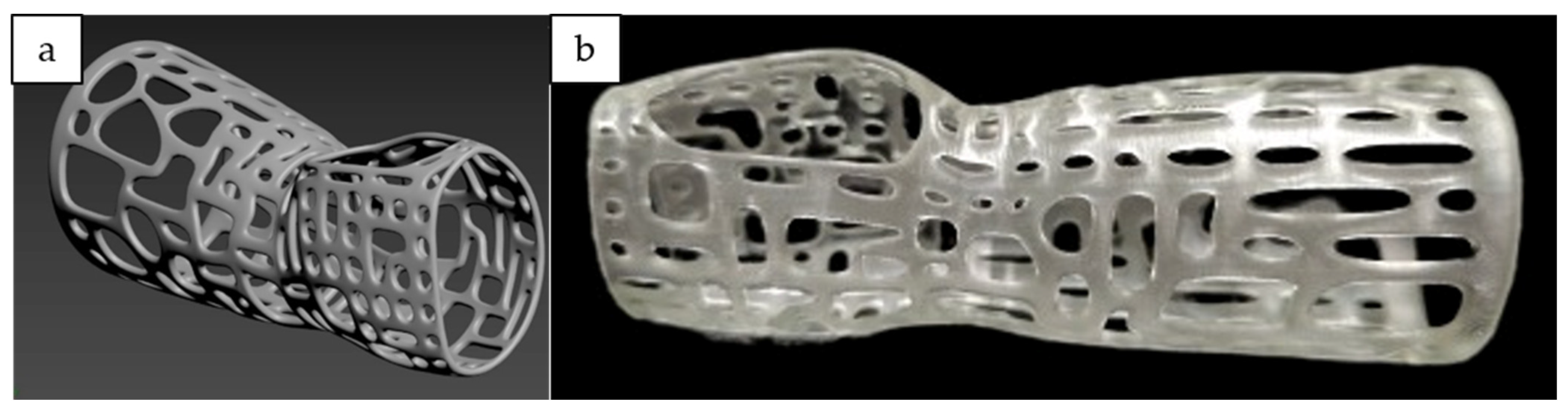
1. Introduction
In recent decades, a wide range of biopolymers has stimulated researchers’ interest to explore and utilize their benefits to create a sustainable material for the sake of environmental issues. Polyhydroxyalkanoates (PHA) are those among biopolymers that can act as an alternative to the synthetic polymers derived from petrochemicals [
1]. PHA are biopolymers that are naturally derived and accumulated by different families of microorganisms such as Azotobacter, Bacillus and Pseudomonas [
2,
3,
4]. PHA are classified according to their basic structural chain length: short chain length (consists of 3–5 carbons), medium chain length (6–14 carbons) and lastly long chain length (consists of more than 15 carbons) [
5]. Polyhydroxybutyrate (PHB) falls under short chain length in the PHA family and is also the most common form of PHA [
6].
Polyhydroxybutyrate (PHB) is a microbial aliphatic polyester created through the deprivation of nitrogen, phosphorus, or oxygen in the availability of excess carbon sources [
7]. Highly crystallized PHB due to its stereo-chemical regularity of the structure contributed towards its excellent mechanical properties; high elasticity of modulus ranging between 3 to 3.5 GPa and tensile strength of 20–40 MPa [
8]. In addition, its water-insoluble property differentiates it from most other currently available biodegradable plastics that have undergone hydrolytic degradation [
9]. Indeed, the mechanical properties of this polymer are comparable to those of petroleum-based polypropylene [
10]. Nevertheless, mass production of PHB products has been limited to myriad challenges due to its narrow thermal processing window [
11]. Thermal degradation could occur when PHB is exposed to a higher temperatures during the manufacturing process [
12]. The utilization of this polymer has become potential in biomedical applications due to its biocompatibility and biodegradability [
13]. The manufacturing of such biomedical applications can be done via additive manufacturing (AM) technology. At the moment, AM technology (better known as 3D printing) has been applied in functional biomaterials for tissue engineering, fabrication of anatomical and pharmacological models, and production of medical instruments [
14,
15,
16].
AM technology is a process of depositing materials by layering to construct a three-dimensional (3D) object by using a computer-aided design (CAD) file where novel customization can be utilized [
17]. It has become one of the key components that have been highlighted in the Industrial Revolution 4.0 (IR4.0), as it is considered the future of the manufacturing sector [
18]. The process of additive manufacturing has been categorized into six types: vat-photopolymerization, material extrusion, powder bed fusion, material jetting, directed energy deposition, and sheet lamination [
19,
20,
21]. Each of these categories has its own type of materials, advantages and drawbacks that should be given greater attention so they can be fully utilized according to the proposed applications. The commonly used biomaterials for AM are polymers and composites due to their diversity and high compatibility with different types of AM processes. The polymer materials are usually used in the form of solutions for vat-photopolymerization, thermoplastic filaments for material extrusion and powder beads for powder-bed fusion. Most leading polymers used for 3D printable biomaterials are polylactic acid (PLA), acrylonitrile butadiene styrene (ABS), polyurethane (PU) and polycaprolactone (PCL) [
22,
23,
24]. However, the choices for biocompatible materials in AM technology are still limited. Thus, studies towards broadening those choices of materials could be a great advancement in the medical sector by integrating AM technology. Hence, the selection of AM technique and its materials are crucial to ensure the products complement the specifications needed for the applications.
Currently stereolithography (SLA), which falls under the vat-photopolymerization technique in AM processes, can produce parts with high dimensional accuracy with very intricate details [
25]. Thus, it is the most favorable method used in the medical field, such as in surgical tools, temporary replacement medical devices, and fracture-bone casts [
26]. It uses a single laser to cure light-sensitive polymer (photopolymer) directed at a particular point, building up layer upon layer contained in a vat/tank. SLA comprises three main components: the printer, material, and CAD file [
27]. Any adjustment of these components will give a different outcome to the mechanical properties of 3D structures, especially on the materials side, and particularly the photopolymer resin.
The SLA process is a 3D printing technique based on the principle of resin photo-polymerization. Photopolymer resin comprises monomers, oligomers, and photo-initiators. During the photo-polymerization process, the laser will activate the photo-initiators to release free radicals, which will then induce cross-linking reactions between the functionalized monomers and oligomers to create a solidified structure [
28]. Several commonly used monomers are bisphenol A-glycidyl dimethacrylate (Bis-GMA), bisphenol A ethoxylated dimethacrylate (Bis-EMA), urethane dimethacrylate (UDMA) and triethylene glycol dimethacrylate (TEGDMA) [
29]. Since the after-products of degradation of Bis-GMA and Bis-EMA generate bisphenol A (BPA), which has been proven to have an estrogenic effect on human health, UDMA has been developed with BPA-free formulation [
30]. The polymerization rate in UDMA resin was the highest, even though TEGDMA obtained the highest degree of double bond conversion (DC). The resulting more-robust polymer network exhibited the highest flexural strength compared to TEGDMA. The better mechanical properties presented by UDMA were probably attributed to the cross-linking of stronger hydrogen bonding and less cyclization [
31].
UDMA resin could be utilized for medical application purposes by integrating with SLA. SLA demonstrates greater versatility and has the highest fabrication resolution, which is crucial in medical applications [
32]. In addition, its customizability to create a custom-fitted design to maintain the fracture bone alignment according to the patients has captivated many researchers to explore the potential of this technology to be embedded in the medical applications [
33]. Studies have proven that 3D printed casts could minimize interference, reduce the risk of pressure-related complications and improve ventilation [
34]. However, the idea of this research only focused on the material itself.
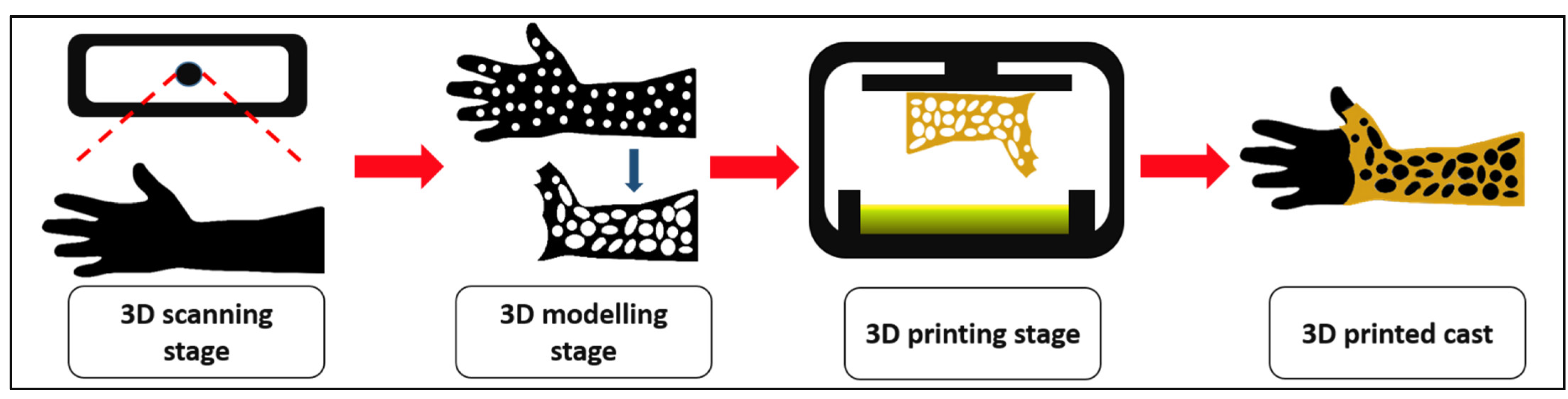
In treating fractured-bone patients, it is crucial to encase partially, or in a surrounding rigid form called a cast. The immobilized limbs are usually encased by a rigid structure for long periods, often for as long as six weeks or more. However, due to prolonged pressure and poor ventilation of conventional casts, patients have a high tendency toward irritation and muscle fatigue [
35]. Moreover, by employing 3D printing technology, the idea to create customized casts for patients that are properly fitted and have a vented structure could be an effective alternative to tackle these problems. To our concern, the integration of biopolymer PHB in the UDMA resin by utilizing 3D printing techniques for casting has not been explored yet. This research aims to study the 3D printability and mechanical properties of PHB/UDMA resin blends and their potential in medical applications as casting for fractured-bone patients.
2. Materials and Methods
2.1. Materials
Polyhyroxybutyrate (PHB) powder was obtained from Biomer Incorporation (Krailing, Germany), referenced P309, and used as received. Urethane dimethacrylate (UDMA) based resin was purchased from Formlabs Incorporation (Formlabs, Somerville, MA, USA). Isopropanol (IPA) used for cleaning the residual after printing the sample was obtained from Sigma Aldrich (Burlington, MA, USA).
2.2. Preparation of PHB/UDMA Resin Blends
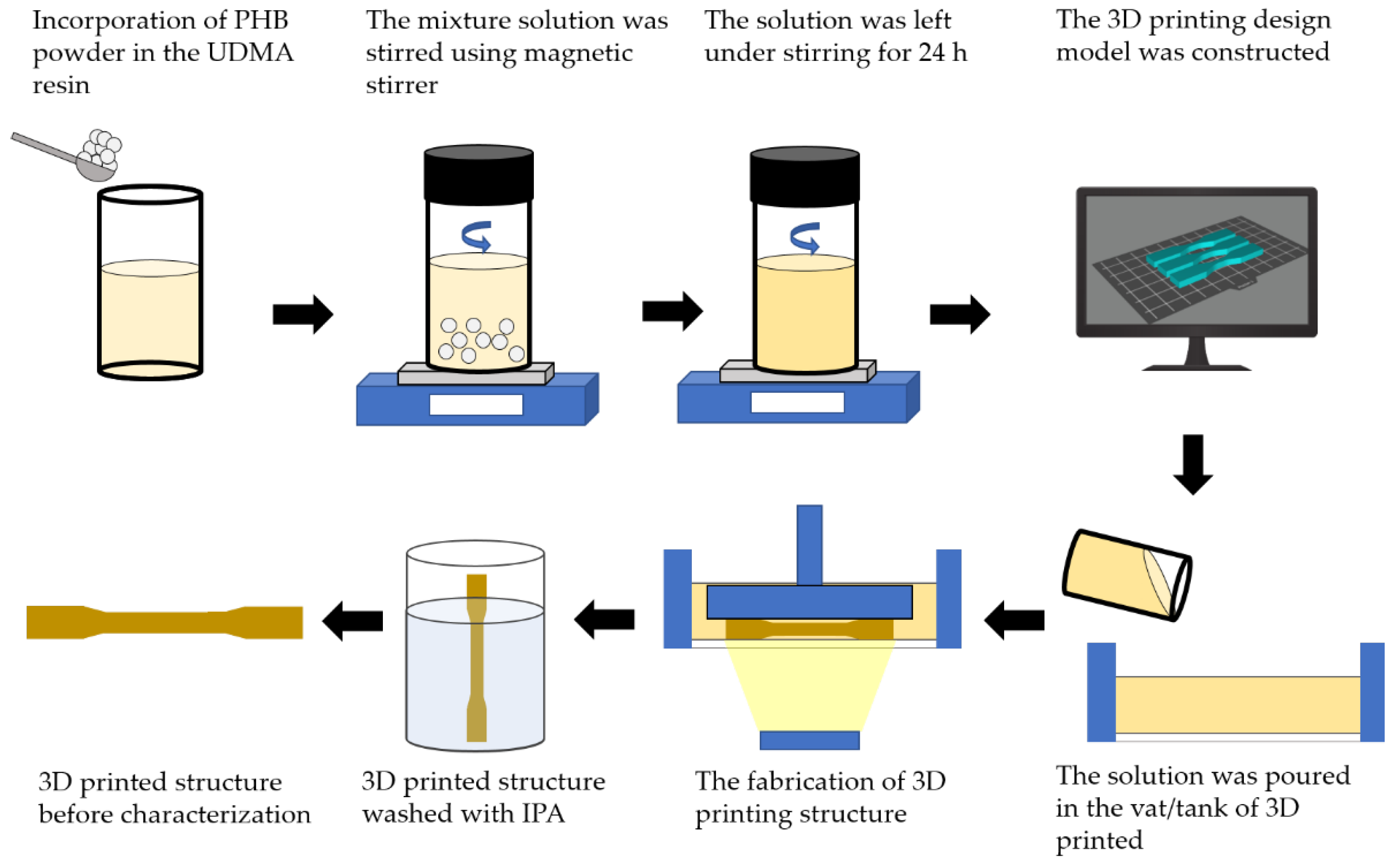
Four compositions of weight ratio of polyhydroxybutyrate (PHB): 0, 3, 7, 11 wt.% were incorporated within the urethane dimethacrylate (UDMA) based resin as shown in
Table 1. The PHB powder was put inside the vacuum oven for 24 h to remove any moisture. Then, PHB powder and UDMA resin were weighed by using analytical balance according to the weight percentage of compositions that had been decided. They were stirred and stored in the amber veils to prevent any light exposure. The mixture solution was stirred using a magnetic stirrer (WiseStir MSH-20D, Witeg, Germany) at a lower rotation per minute (rpm) at 300 rpm to discard any possibilities for bubble formation. The solutions were left for 24 h under stirring to achieve a homogenous mixture of all components. The resulting mixture was then cooled to room temperature for about ten minutes prior printing.
2.3. 3D Printing Design
The design of the 3D printing model was constructed in CAD software (Blender, Amsterdam, The Netherlands). A dog-bone shape (63.5 mm × 9.53 mm × 3.2 mm) was used to evaluate the tensile properties according to ASTM D638 (Type V), whilst a rectangular shape with V-notched (63.5 mm × 12.7 mm × 3.2 mm) was used for impact properties according to ASTM D256. Then, a circle shape (diameter = 10 mm; thickness = 1.5 mm) was used for Fourier transform infrared (FTIR) (Thermo Fisher Scientific, Waltham, MA, USA), field emission scanning electron microscopy (FESEM) (Jeol JSM-IT800 Schottky IT800, Tokyo, Japan) and thermogravimetric analysis (TGA) (TGA/DSC 3+ Mettler Toledo, Columbus, OH, USA); meanwhile, a rectangular shape (19.5 mm × 19.5 mm × 0.5 mm) was used in X-ray diffraction (XRD) analysis (Rigaku Miniflex 600, Tokyo, Japan). The completed design was then rendered and exported into a standard triangulated language (STL) file. Photon S Slicer was used to convert the STL file into a readable file for the SLA 3D printer (Anycubic Photon S, Shenzhen, China) prior to printing.
2.4. 3D Printing of PHB/UDMA Resin Blends
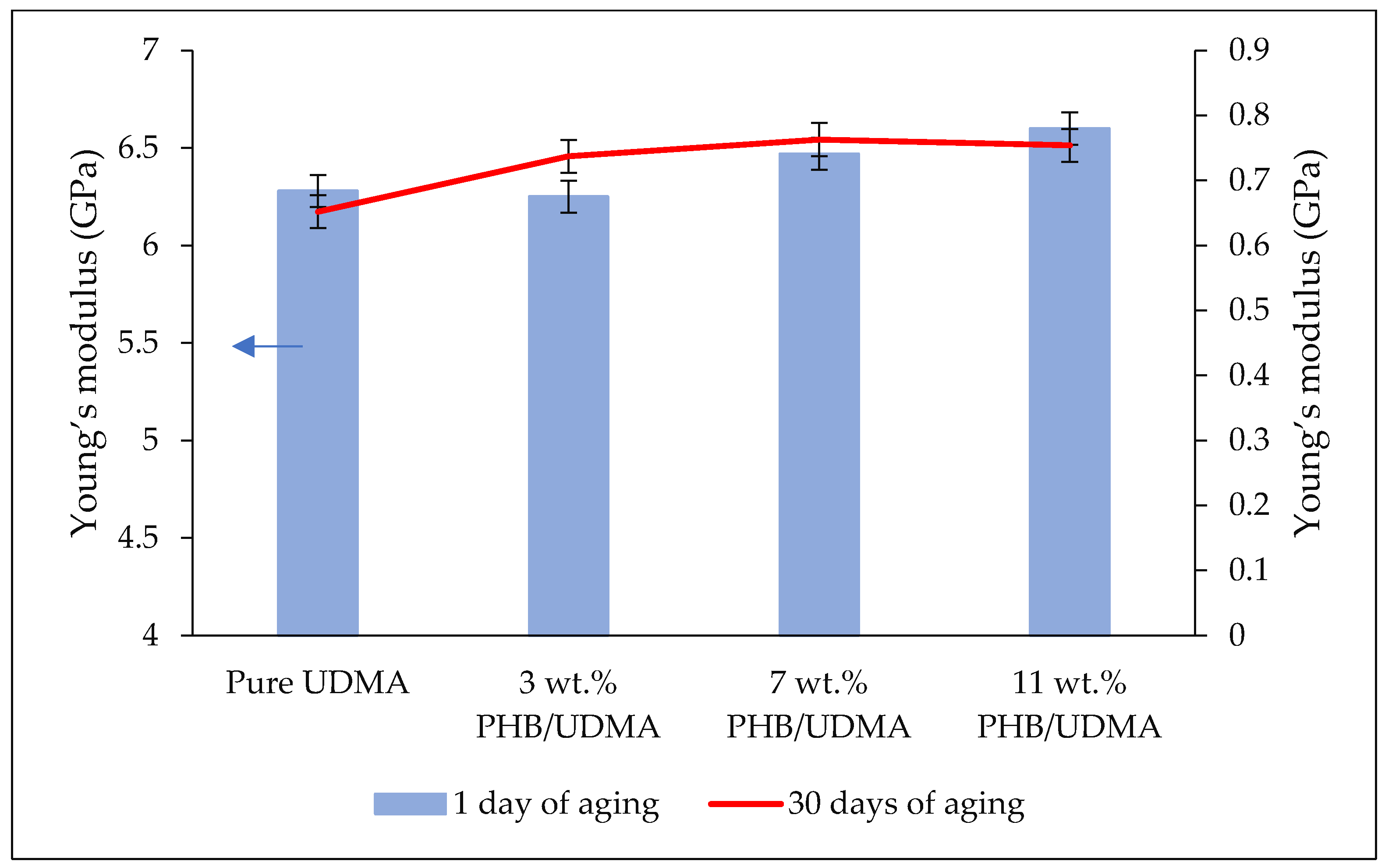
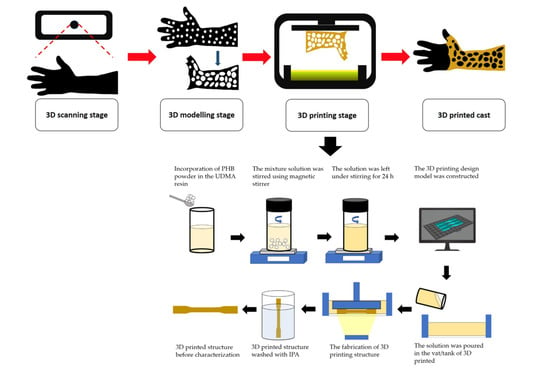
Triplicate samples were printed for each composition by one-time printing using an SLA 3D Printer with an exposure time of 60 s. The samples were cleaned with isopropanol (IPA) to remove any residual resin that had not fully cured on the surface of the samples. The samples were then cured for 60 m at 60 °C in an ultraviolet (UV) cure machine (Form Cure, Formlabs, Somerville, MA, USA). A total of 24 samples for each tensile and impact test were printed. The samples were then aged in a desiccator for a day (12 samples) and 30 days (12 samples) to evaluate the mechanical properties of 3D printed PHB/UDMA. All other analyses (FTIR, FESEM, XRD, TGA) were performed after a day of aging in desiccator. The overview process flow is shown in
Figure 1.
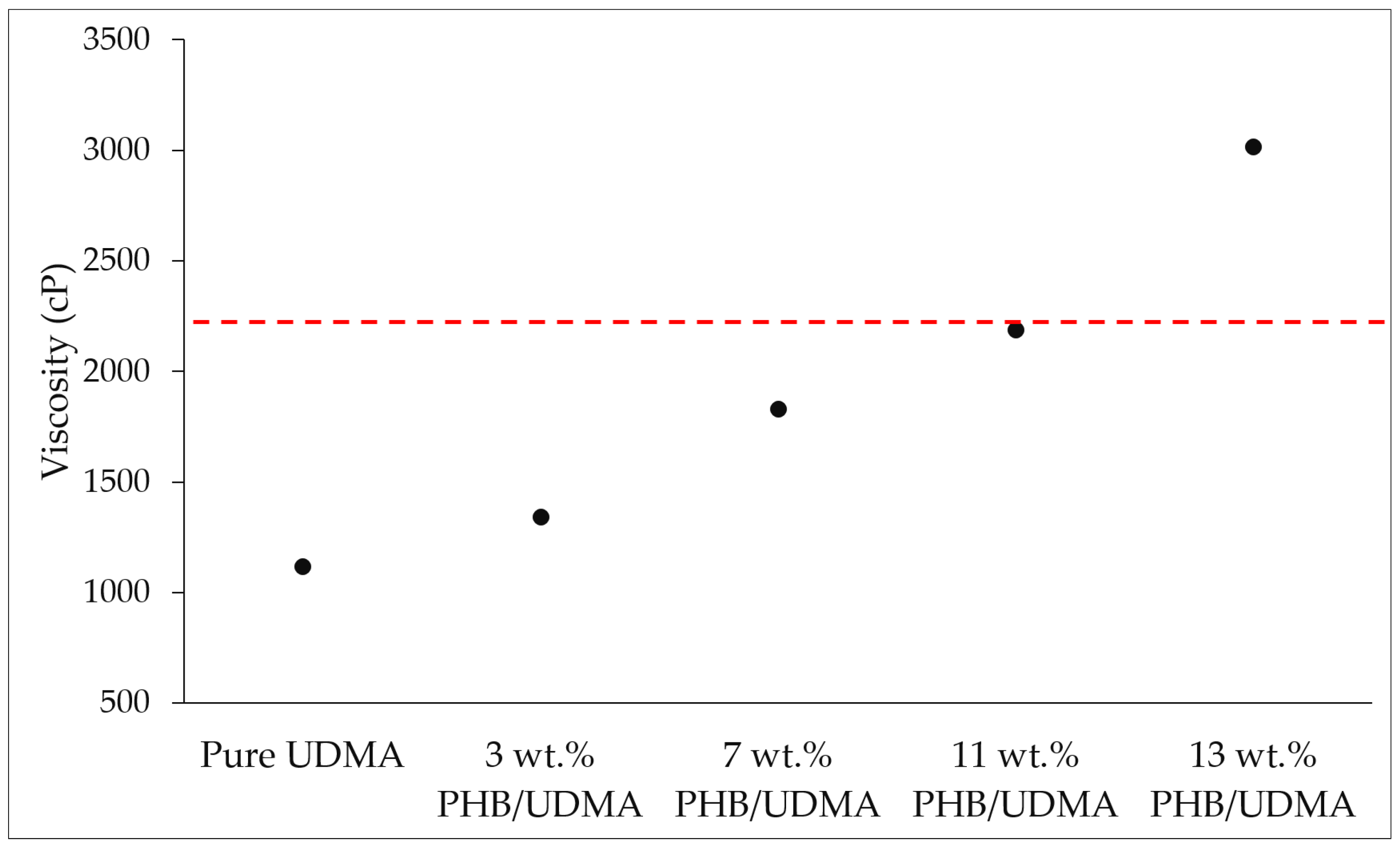
2.5. Viscosity Measurement for Pure UDMA and PHB/UDMA Resin Blends
The viscosity of the pure UDMA resin and PHB/UDMA resin blends were measured by using a rheometer (DVNext Rheometer, AMETEK Brookfield, Middleborough, MA, USA). The spindle used was RV-04 set at 50 rotations per minute (rpm). All the measurements were taken at room temperature.
2.6. Tensile Test
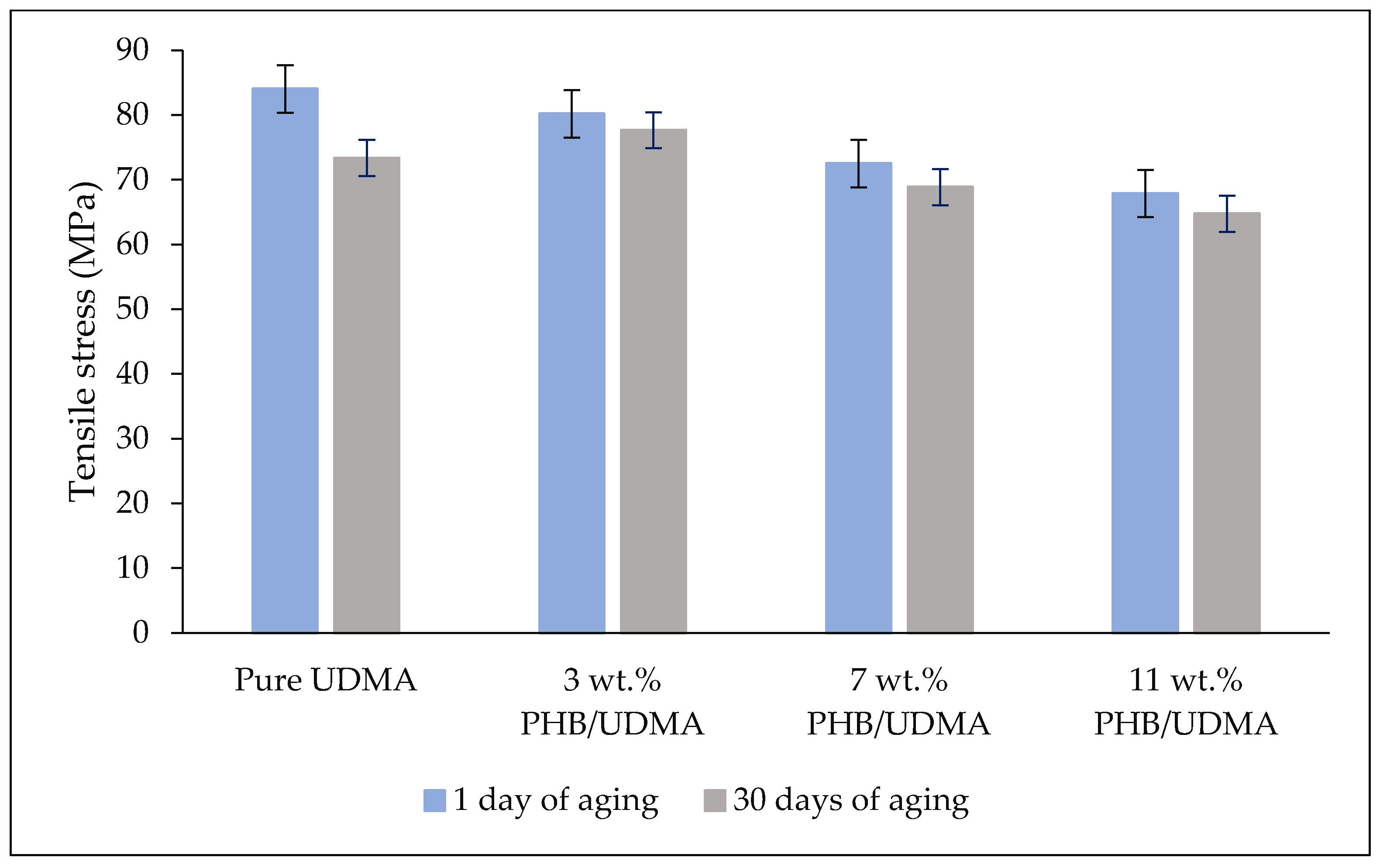
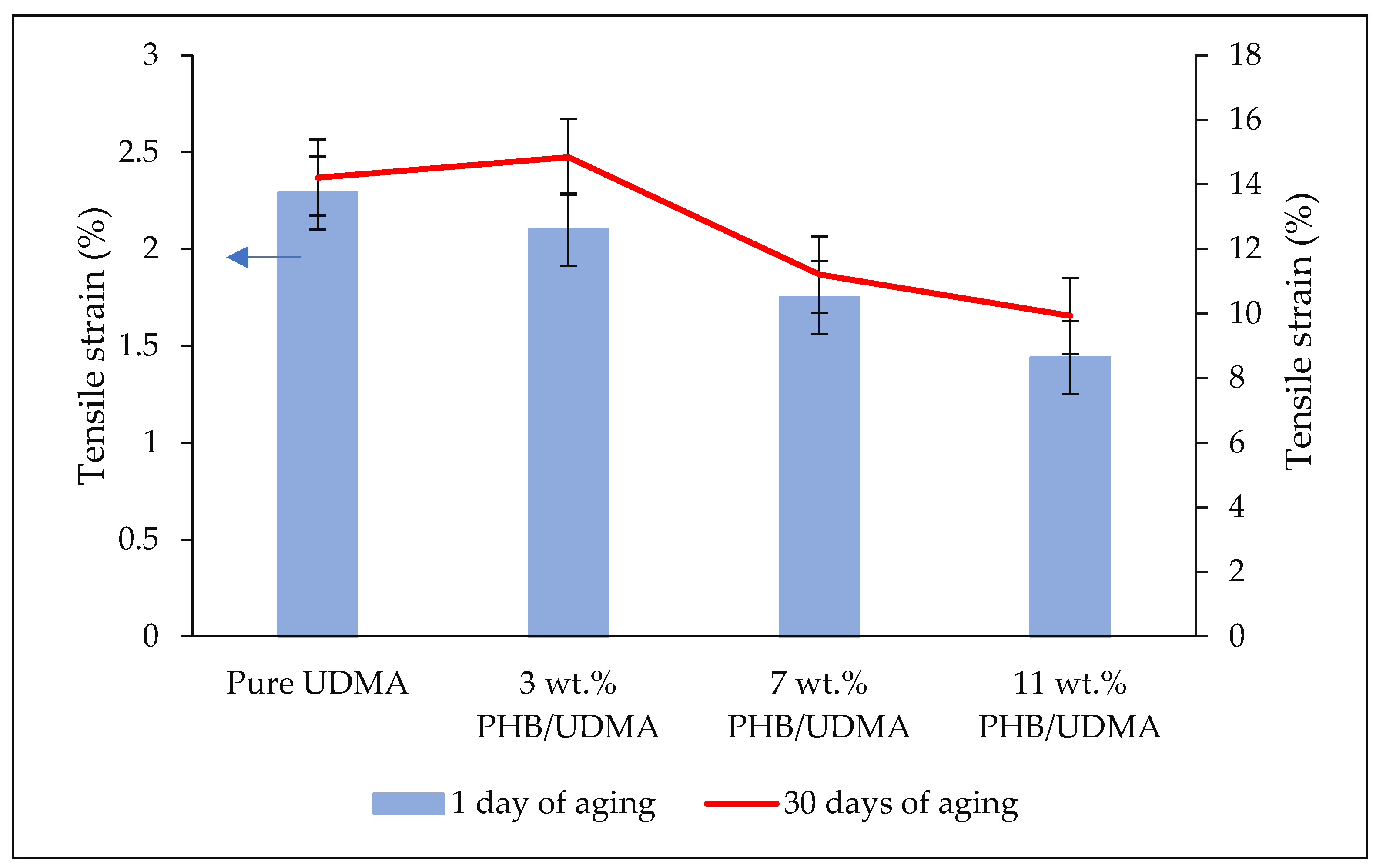
Tensile tests were performed using the dog-bone samples based on Type V of ASTM D638 (63.5 mm × 9.53 mm × 3.2 mm) using a universal testing machine (Instron 5566, Instron Corporation, Norwood, MA, USA) with a crosshead speed of 5 mm/min and equipped with 10 kN load cell. The tests were conducted at ambient temperature and 50% of relative humidity until the failure of the samples and the stress-strain curve was obtained.
2.7. Impact Test
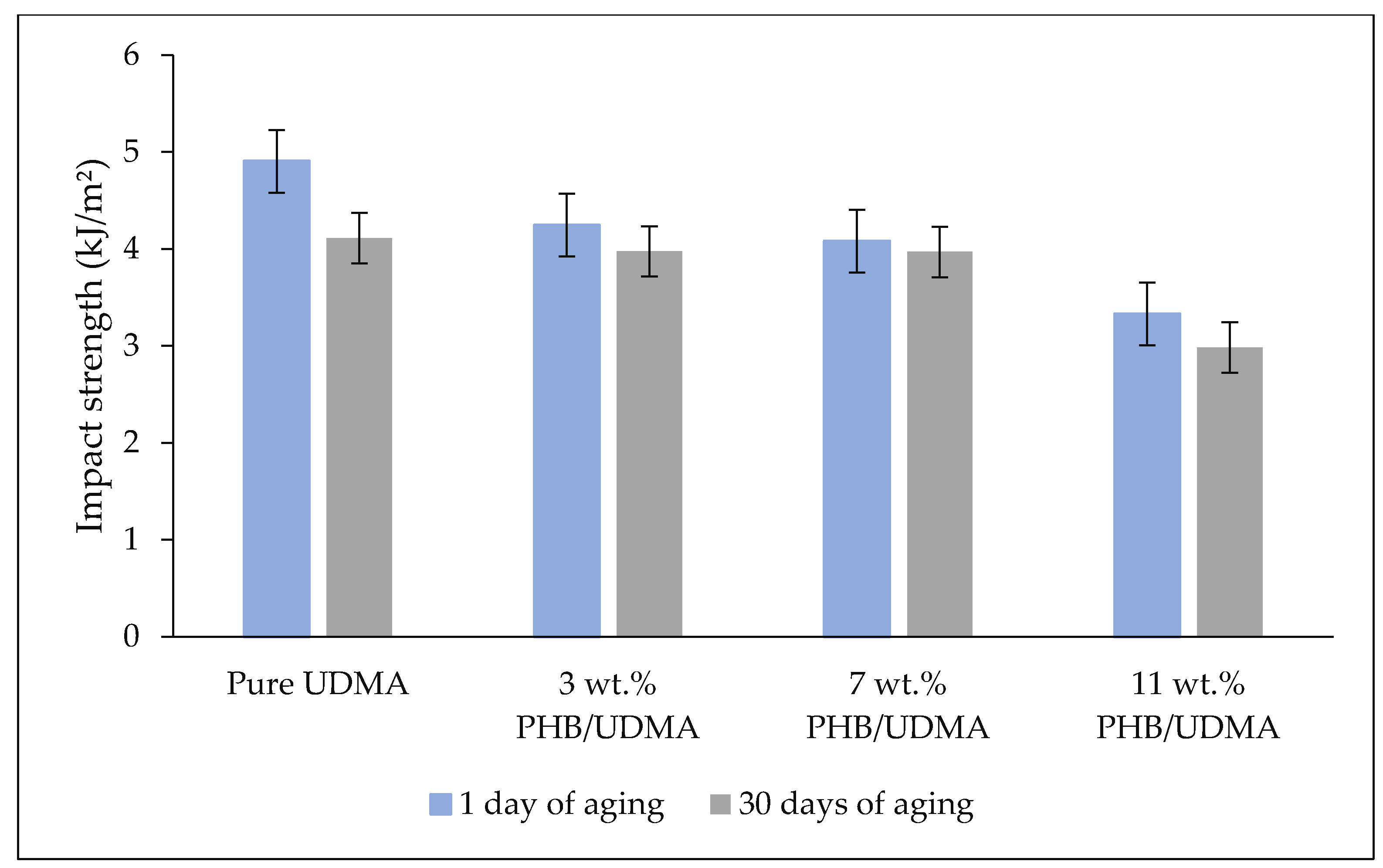
Impact tests were measured using a rectangular shape with a V-notch according to ASTM D256 (63.5 mm × 12.7 mm × 3.2 mm) by using an Impact Test with Notcher (Instron Ceast 9050, Instron Corporation, Norwood, MA, USA) with nominal impact energy at 11 J and impact velocities of 3.5 m/s.
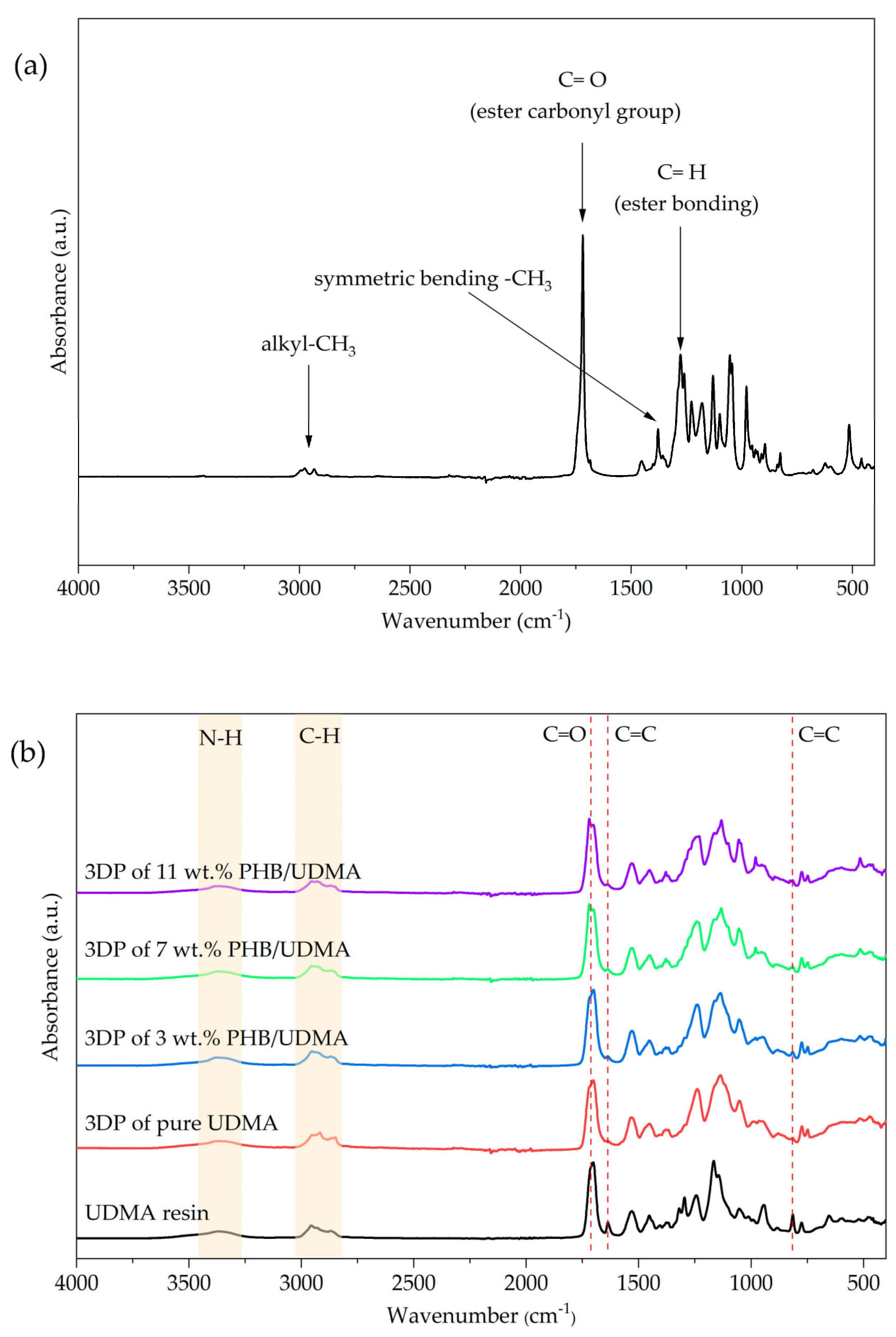
2.8. Fourier Transform Infrared (FTIR)
Fourier Transform Infrared (FTIR) spectrum test was performed to obtain specific information about chemical bonds and molecular structure of PHB powder, UDMA resin, 3D printed UDMA, and 3D printed PHB/UDMA blends composites. An FTIR spectrometer (Nicolet iS50, Thermo Fisher Scientific, Waltham, MA, USA) was used to analyze the changes in spectra within a range of 400 cm
−1–4000 cm
−1. The degree of double bond conversion (DC %) was obtained for each compositions by using Equation (1) [
36]:
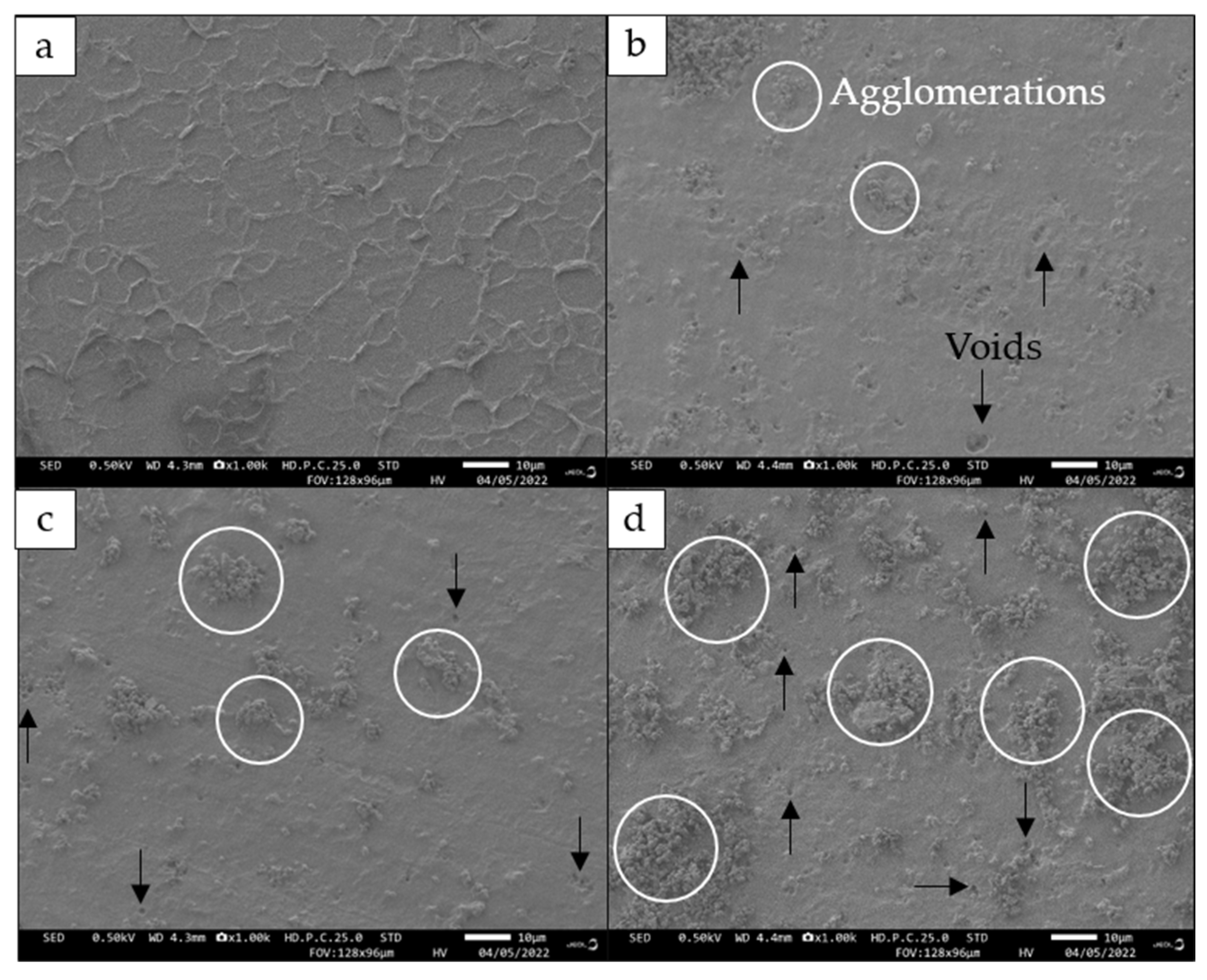
2.9. Field-Emission Scanning Electron Microscopy (FESEM)
The morphology of 3D printed UDMA and PHB/UDMA blends surface were studied using Field Emission Scanning Electron Microscopy (Jeol JSM-IT800 Schottky IT800, Tokyo, Japan). The observation and microphotographs for all compositions of 3D printed samples were taken at 1K resolution.
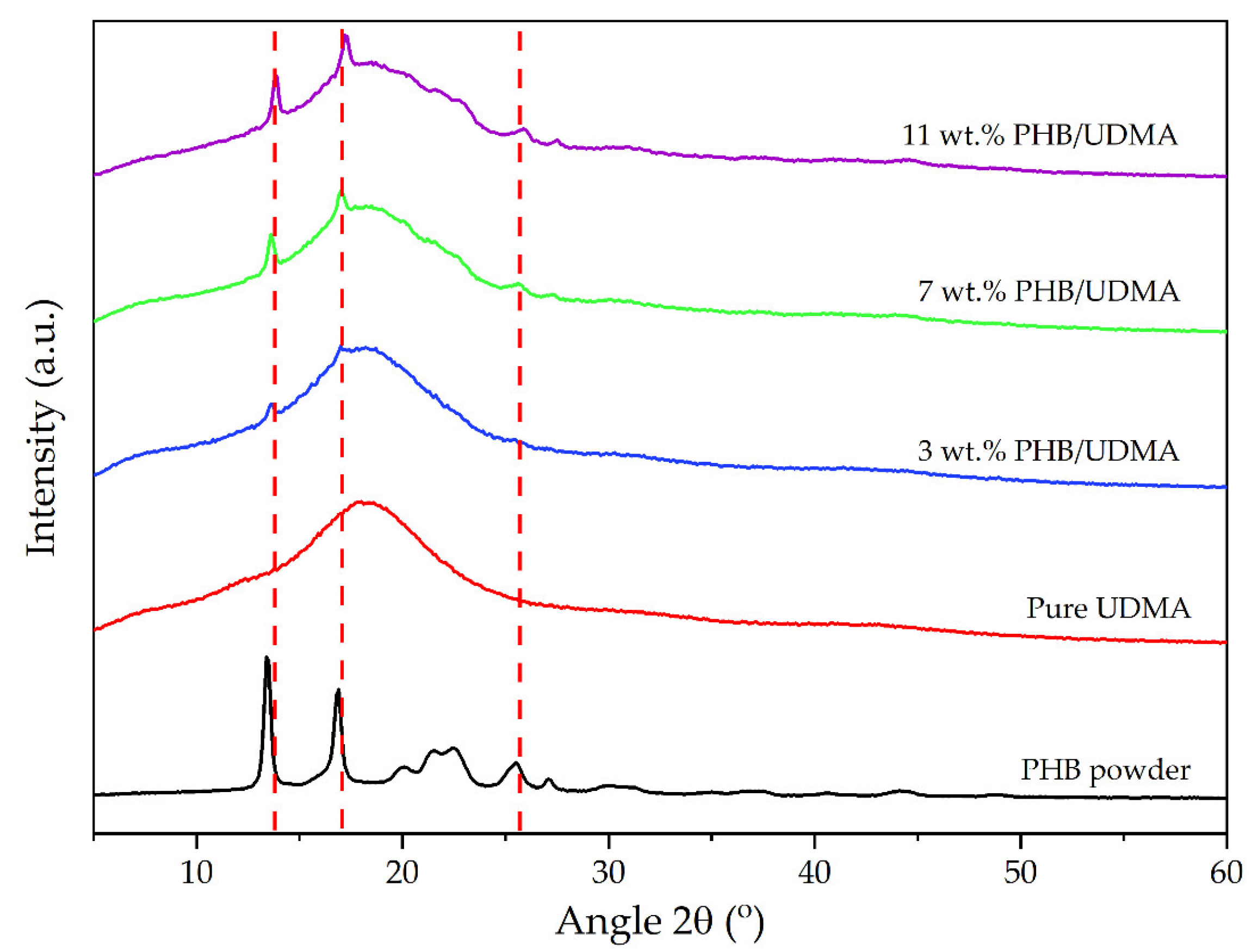
2.10. X-ray Diffraction (XRD)
The structural characterization of the PHB powder, 3D printed UDMA and PHB/UDMA were performed by an X-ray diffraction instrument (Rigaku Miniflex 600, Tokyo, Japan). The X-Ray diffractometer was regulated at 40 kV and 15 mA, with Cu
Kα radiation (λ = 0.154 nm). The scattering angle was set from 2.5° to 30° whilst the step duration was set at 10° s
−1. All the peaks were observed and identified in concordance with the International Center for Diffraction Data (ICDD). The crystallinity index for each composition of 3D printed samples was obtained using OriginPro (OriginLab Corporation, Northampton, MA, USA) according to the Equation (2):
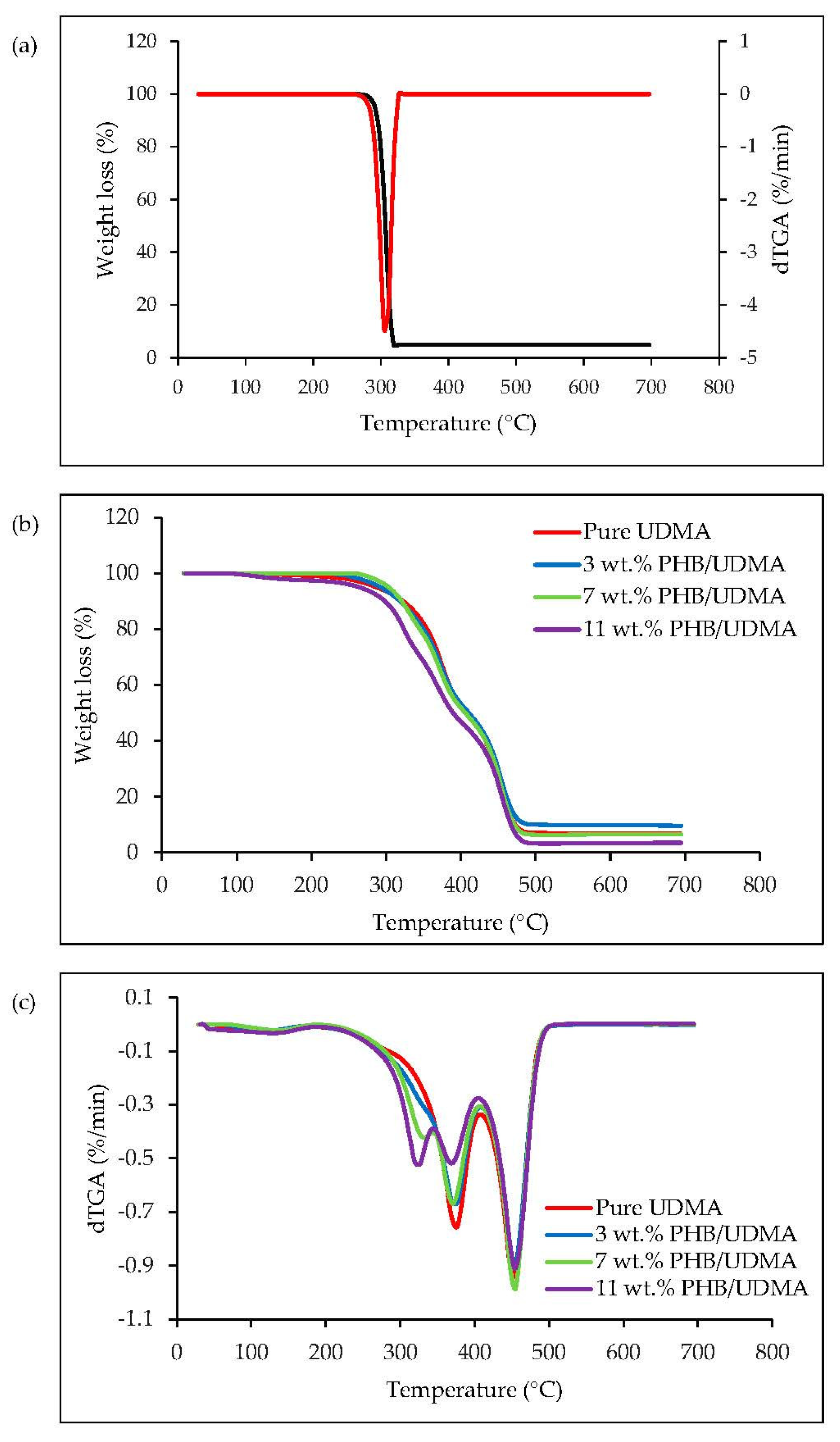
2.11. Thermogravimetric Analysis (TGA)
Thermal stability of PHB powder, 3D printed UDMA and 3D printed PHB/UDMA were studied using a thermogravimetric analyzer by observing the weight loss of the samples upon heating (TGA/DSC 3+ Mettler Toledo, Columbus, OH, USA). The measurements were conducted in a nitrogen, N2 atmosphere at a constant flow rate of 20 mL/min, the heating scan rate of 20 °C/min, and the temperature was elevated from 25 °C until 700 °C.
2.12. Statistical Analysis
Statistical analysis of tensile properties (Young’s modulus, tensile stress and tensile strain) and impact strength values were performed through the Statistical Package for the Social Sciences (SPSS v.21) (IBM Corporation, Armonk, NY, USA). As the data were found to be consistent with a normal distribution (p > 0.05), the data were then analyzed using two-way analysis of variance (ANOVA) to study the interaction between the varied composition of PHB (wt.%) incorporation within UDMA resin and the aging duration of 3D printed PHB/UDMA towards mechanical properties.