Thermally Treated Berberine-Loaded SA/PVA/PEO Electrospun Microfiber Membranes for Antibacterial Wound Dressings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
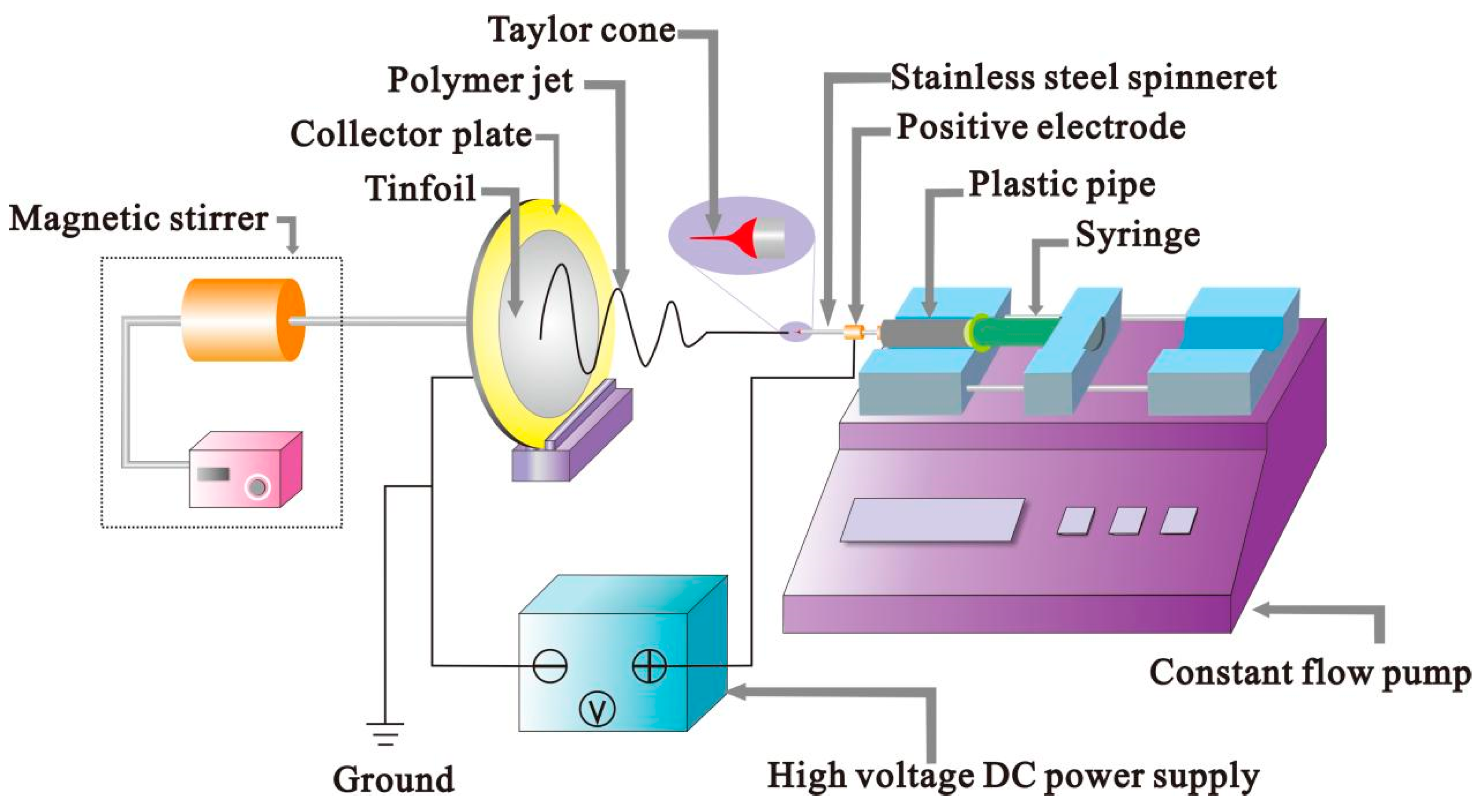
2.2. Equipment
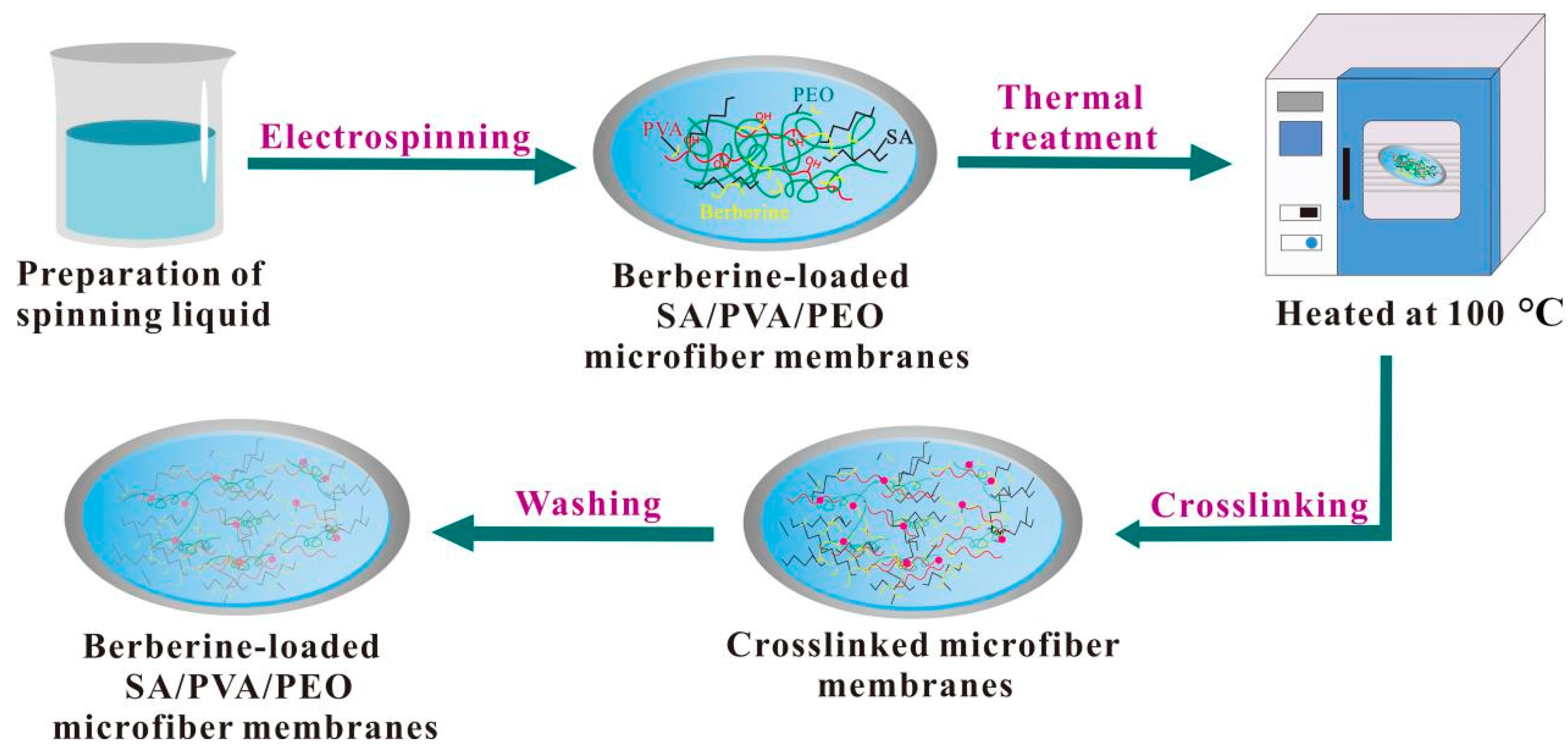
2.3. Preparation of SA/PVA/PEO Microfiber Membranes
2.4. Morphology Observation
2.5. Preparation and Treatment of Berberine-Loaded SA/PVA/PEO Microfiber Membranes
2.6. Antibacterial Activity Test of Berberine-Loaded SA/PVA/PEO Microfiber Membranes
2.7. Water Dissolution Resistance Test of Berberine-Loaded SA/PVA/PEO Microfiber Membranes
2.8. Moisture Content Test of Berberine-Loaded SA/PVA/PEO Microfiber Membranes
2.9. Fracture Strength Test of Berberine-Loaded SA/PVA/PEO Microfiber Membranes
3. Results and Discussion
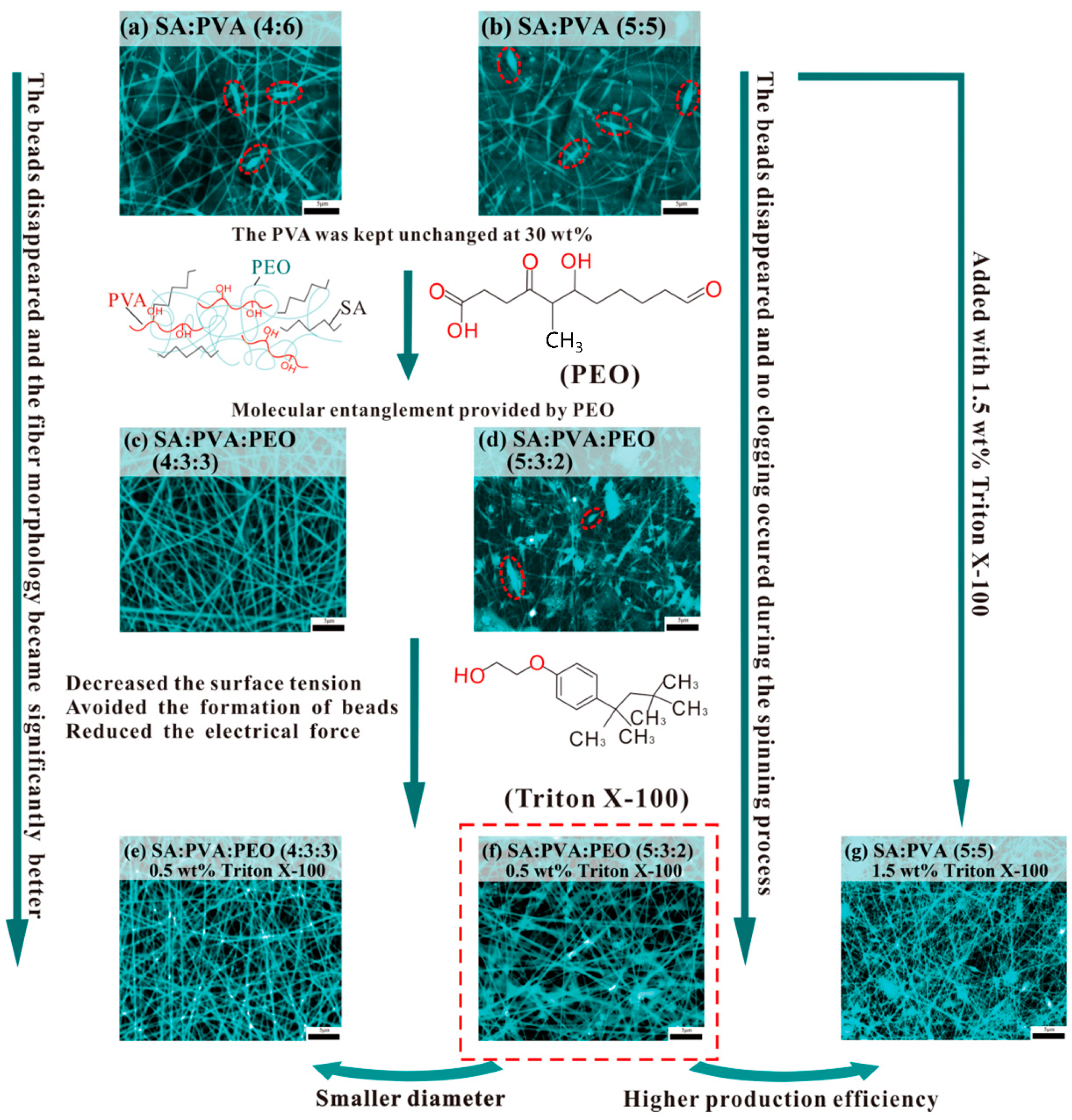
3.1. The Best Ratio of SA/PVA/PEO with a Good Electrostatic Spinning Effect
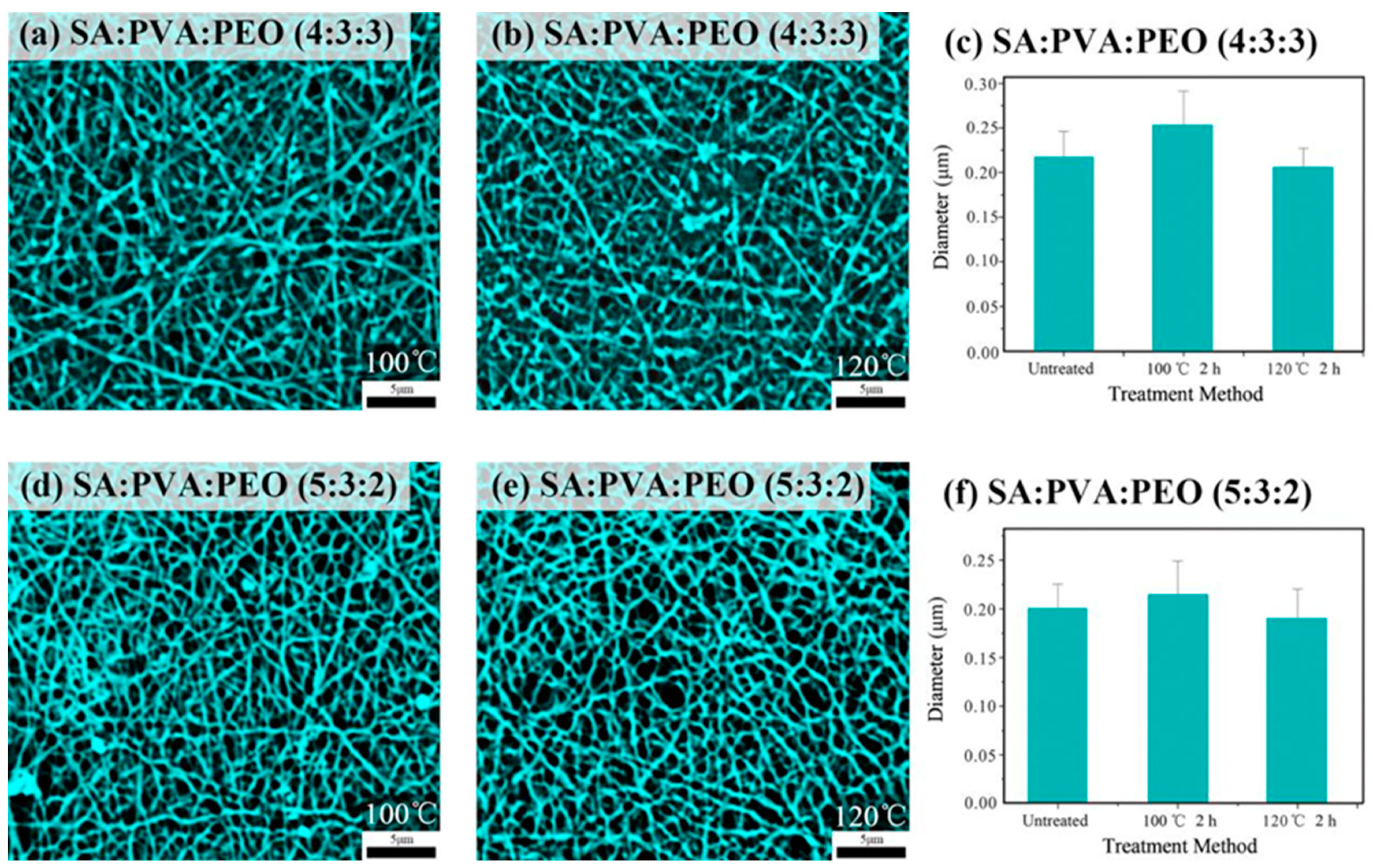
3.2. Thermal Treatment to SA/PVA/PEO Microfiber Membranes
3.3. Antibacterial Efficacy of Berberine-Loaded SA/PVA/PEO Microfiber Membranes
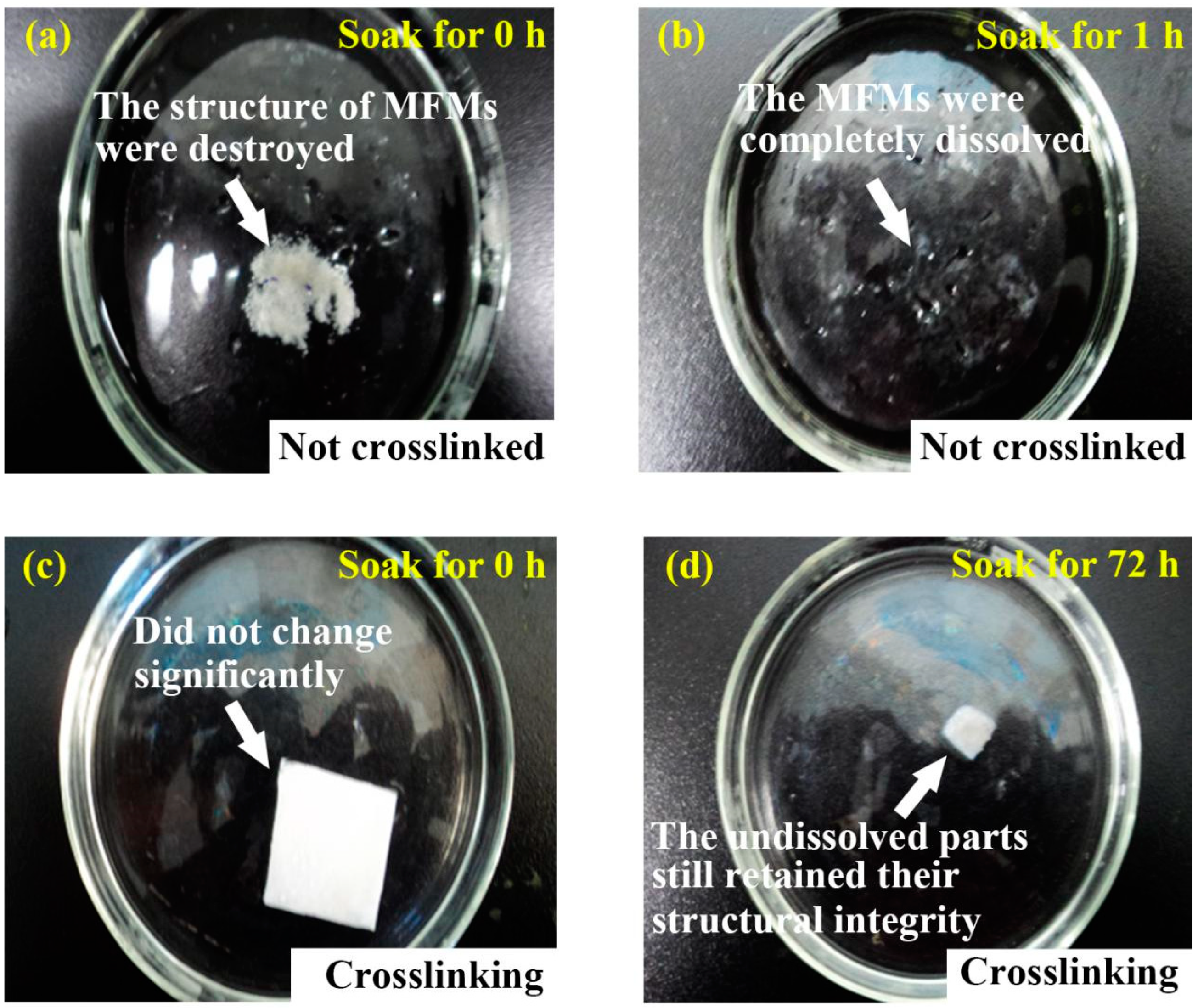
3.4. Water Dissolution Resistance of Berberine-Loaded SA/PVA/PEO Microfiber Membranes
3.5. Moisture Absorption and Fracture Strength of Berberine-Loaded SA/PVA/PEO Microfiber Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, R.; Li, C.; Yu, C.; Xie, H.; Shi, S.; Li, Z.; Wang, Q.; Lu, L. A novel electrospun membrane based on moxifloxacin hydrochloride/poly(vinyl alcohol)/sodium alginate for antibacterial wound dressings in practical application. Drug Deliv. 2016, 23, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.; Henriques, M.; Teixeira, P.; Fernandes, M.M.; Fangueiro, R.; Zille, A. Comfort and Infection Control of Chitosan-impregnated Cotton Gauze as Wound Dressing. Fibers Polym. 2019, 20, 922–932. [Google Scholar] [CrossRef]
- Kim, Y.; Doh, S.J.; Lee, G.D.; Kim, C.; Im, J.N. Composite Nonwovens Based on Carboxymethyl Cellulose for Wound Dressing Materials. Fibers Polym. 2019, 20, 2048–2056. [Google Scholar] [CrossRef]
- Lee, G.D.; Doh, S.J.; Kim, Y.; Im, J.N. Preparation and Characterization of Carboxymethyl Chitosan Nonwovens for Wound Dressing. Fibers Polym. 2020, 21, 1894–1905. [Google Scholar] [CrossRef]
- Gharibi, R.; Yeganeh, H.; Abdali, Z. Preparation of antimicrobial wound dressings via thiolene photopolymerization reaction. J. Mater. Sci. 2018, 53, 1581–1595. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Lee, J. Fabrication of Microfiber Patterns with Ivy Shoot-Like Geometries Using Improved Electrospinning. Materials 2016, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, Z.; Sun, L.; Guo, P.; Zeng, C.; Wang, C.; Zhang, L. Polymerization-induced phase separation fabrication: A versatile microfluidic technique to prepare microfibers with various cross sectional shapes and structures. Chem. Eng. J. 2017, 315, 25–34. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun nanofibers for wound healing. Mat. Sci. Eng. C-Mater. 2017, 76, 1413–1423. [Google Scholar] [CrossRef]
- Mahamuni-Badiger, P.P.; Patil, P.M.; Patel, P.R.; Dhanavade, M.J.; Badiger, M.V.; Marathe, Y.N.; Bohara, R.A. Electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/polyethylene oxide (PEO) microfibers reinforced with ZnO nanocrystals for antibacterial and antibiofilm wound dressing applications. New J. Chem. 2020, 44, 9754–9766. [Google Scholar] [CrossRef]
- Thorvaldsson, A.; Edvinsson, P.; Glantz, A.; Rodriguez, K.; Walkenström, P.; Gatenholm, P. Superhydrophobic behaviour of plasma modified electrospun cellulose nanofiber-coated microfibers. Cellulose 2012, 19, 1743–1748. [Google Scholar] [CrossRef]
- Barhate, R.; Koeppl, S.; Ramakrishna, S. Porous nano- and microfibrous polymeric membrane material for catalytic support. Chem. Eng. Res. Des. 2011, 89, 621–630. [Google Scholar] [CrossRef]
- Lanno, G.-M.; Ramos, C.; Preem, L.; Putrinš, M.; Laidmäe, I.; Tenson, T.; Kogermann, K. Antibacterial Porous Electrospun Fibers as Skin Scaffolds for Wound Healing Applications. ACS Omega 2020, 5, 30011–30022. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-T.; Zhang, J.; Gao, Y.; Liu, X.-F.; Liu, J.-J.; Wang, X.-X.; Xiang, H.-F.; Long, Y.-Z. Self-powered portable melt electrospinning for in situ wound dressing. J. Nanobiotechnol. 2020, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Karayeğen, G.; Koçum, I.C.; Serdaroğlu, D.; Doğan, M. Aligned polyvinylpyrrolidone nanofibers with advanced electrospinning for biomedical applications. Bio-Med. Mater. Eng. 2018, 29, 685–697. [Google Scholar] [CrossRef]
- Chen, K.; Wang, F.; Liu, S.; Wu, X.; Xu, L.; Zhang, D. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int. J. Biol. Macromol. 2020, 148, 501–509. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A promising wound dressing material with excellent cytocompatibility and proangiogenesis action for wound healing: Strontium loaded Silk fibroin/Sodium alginate (SF/SA) blend films. Int. J. Biol. Macromol. 2017, 104, 969–978. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Z.; Zhou, K.; Chen, W.; Tao, J.; Li, C.; Xin, H.; Song, Y.; Ai, F. Preparation and characterization of borosilicate-bioglass-incorporated sodium alginate composite wound dressing for accelerated full-thickness skin wound healing. Biomed. Mater. 2020, 15, 055009. [Google Scholar] [CrossRef]
- Oh, S.-T.; Kim, W.-R.; Kim, S.-H.; Chung, Y.-C.; Park, J.-S. The preparation of polyurethane foam combined with pH-sensitive alginate/bentonite hydrogel for wound dressings. Fibers Polym. 2011, 12, 159–165. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.M.; Jancar, J.; Massoud, D.; Fohlerova, Z.; Elhadidy, H.; Spotz, Z.; Hebeish, A. Novel chitin/chitosan-glucan wound dressing: Isolation, characterization, antibacterial activity and wound healing properties. Int. J. Pharm. 2016, 510, 86–99. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Sun, Y.; Zhao, S.; Feng, M.; Xu, G.; Zhu, H.; Ji, P.; Mao, H.; He, Y.; et al. A double-network polysaccharide-based composite hydrogel for skin wound healing. Carbohydr. Polym. 2021, 261, 117870. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.S.; Ghaffar, A.; Huang, Q. Development and characterization of sodium alginate/poly(sodium 4-styrenesulfonate) composite films for release behavior of ciprofloxacin hydrogen chloride monohydrate. Polym. Polym. Compos. 2021, 29, S143–S153. [Google Scholar] [CrossRef]
- Hu, W.-W.; Yu, H.-N. Coelectrospinning of chitosan/alginate fibers by dual-jet system for modulating material surfaces. Carbohydr. Polym. 2013, 95, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Bonino, C.A.; Krebs, M.D.; Saquing, C.D.; Jeong, S.I.; Shearer, K.L.; Alsberg, E.; Khan, S.A. Electrospinning alginate-based nanofibers: From blends to crosslinked low molecular weight alginate-only systems. Carbohydr. Polym. 2011, 85, 111–119. [Google Scholar] [CrossRef]
- Sharma, A.; Mittal, A.; Puri, V.; Kumar, P.; Singh, I. Curcumin-loaded, alginate–gelatin composite fibers for wound healing applications. 3 Biotech 2020, 10, 464. [Google Scholar] [CrossRef]
- Lu, J.-W.; Zhu, Y.-L.; Guo, Z.-X.; Hu, P.; Yu, J. Electrospinning of sodium alginate with poly(ethylene oxide). Polymer 2006, 47, 8026–8031. [Google Scholar] [CrossRef]
- Vicini, S.; Mauri, M.; Vita, S.; Castellano, M. Alginate and alginate/hyaluronic acid membranes generated by electrospinning in wet conditions: Relationship between solution viscosity and spinnability. J. Appl. Polym. Sci. 2018, 135, 46390. [Google Scholar] [CrossRef]
- Shen, W.; Hsieh, Y.-L. Biocompatible sodium alginate fibers by aqueous processing and physical crosslinking. Carbohydr. Polym. 2014, 102, 893–900. [Google Scholar] [CrossRef]
- Paduraru, A.; Ghitulica, C.; Trusca, R.; Surdu, V.A.; Neacsu, I.A.; Holban, A.M.; Birca, A.C.; Iordache, F.; Vasile, B.S. Antimicrobial Wound Dressings as Potential Materials for Skin Tissue Regeneration. Materials 2019, 12, 1859. [Google Scholar] [CrossRef]
- Saquing, C.D.; Tang, C.; Monian, B.; Bonino, C.A.; Manasco, J.L.; Alsberg, E.; Khan, S.A. Alginate–Polyethylene Oxide Blend Nanofibers and the Role of the Carrier Polymer in Electrospinning. Ind. Eng. Chem. Res. 2013, 52, 8692–8704. [Google Scholar] [CrossRef]
- Surendhiran, D.; Cui, H.; Lin, L. Encapsulation of Phlorotannin in Alginate/PEO blended nanofibers to preserve chicken meat from Salmonella contaminations. Food Packag. Shelf Life 2019, 21, 100346. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.; García-Cela, E.; Magan, N.; Rahatekar, S.S. Electrospinning alginate/polyethylene oxide and curcumin composite nanofibers. Mater. Lett. 2020, 270, 127662. [Google Scholar] [CrossRef]
- Luo, X.; Li, J.; Lin, X. Effect of gelatinization and additives on morphology and thermal behavior of corn starch/PVA blend films. Carbohydr. Polym. 2012, 90, 1595–1600. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Cuadri, A.A.; Franco, J.M. Development and Characterization of Novel Fibers Based on Potato Protein/Polyethylene Oxide Through Electrospinning. Fibers Polym. 2019, 20, 1586–1593. [Google Scholar] [CrossRef]
- Song, Q.; Li, A.; Shi, L.; Qian, C.; Feric, T.G.; Fu, Y.; Zhang, H.; Li, Z.; Wang, P.; Li, Z.; et al. Thermally stable, nano-porous and eco-friendly sodium alginate/attapulgite separator for lithium-ion batteries. Energy Storage Mater. 2019, 22, 48–56. [Google Scholar] [CrossRef]
- Fang, W.; Yang, S.; Yuan, T.-Q.; Charlton, A.; Sun, R.-C. Effects of Various Surfactants on Alkali Lignin Electrospinning Ability and Spun Fibers. Ind. Eng. Chem. Res. 2017, 56, 9551–9559. [Google Scholar] [CrossRef]
- Yao, L.; Haas, T.W.; Guiseppi-Elie, A.; Bowlin, G.L.; Simpson, D.G.; Wnek, G.E. Electrospinning and Stabilization of Fully Hydrolyzed Poly(Vinyl Alcohol) Fibers. Chem. Mater. 2003, 15, 1860–1864. [Google Scholar] [CrossRef]
- Dou, X.; Wang, Q.; Li, Z.; Ju, J.; Wang, S.; Hao, L.; Sui, K.; Xia, Y.; Tan, Y. Seaweed-Derived Electrospun Nanofibrous Membranes for Ultrahigh Protein Adsorption. Adv. Funct. Mater. 2019, 29, 1905610. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Zou, L.; Wang, Y.; Zhou, X.; Yao, L.; Wang, Z.; Li, C.; Qiu, Y. Robust ZIF-8/alginate fibers for the durable and highly effective antibacterial textiles. Colloids Surf. B 2020, 193, 111127. [Google Scholar] [CrossRef]
- Ni, W.-J.; Ding, H.-H.; Tang, L.-Q. Berberine as a promising anti-diabetic nephropathy drug: An analysis of its effects and mechanisms. Eur. J. Pharmacol. 2015, 760, 103–112. [Google Scholar] [CrossRef]
- Jin, J.; Xu, M.; Liu, Y.; Ji, Z.; Dai, K.; Zhang, L.; Wang, L.; Ye, F.; Chen, G.; Lv, Z. Alginate-based composite microspheres coated by berberine simultaneously improve hemostatic and antibacterial efficacy. Colloids Surf. B 2020, 194, 111168. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.Y.; bin Abdullah, A.Y.K.; binti Rozman, N.A.S.; bin Wahid, M.I.A.; Hossain, M.; Ring, L.C.; Lazim, Y.; Tan, W.-N. Antimicrobial wound dressing film utilizing cellulose nanocrystal as drug delivery system for curcumin. Cellulose 2018, 25, 631–638. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural Polymer-Based Antimicrobial Hydrogels without Synthetic Antibiotics as Wound Dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Dinu, M.V. Advances in porous chitosan-based composite hydrogels: Synthesis and applications. React. Funct. Polym. 2020, 146, 104372. [Google Scholar] [CrossRef]
- Subhedar, A.; Bhadauria, S.; Ahankari, S.; Kargarzadeh, H. Nanocellulose in biomedical and biosensing applications: A review. Int. J. Biol. Macromol. 2021, 166, 587–600. [Google Scholar] [CrossRef]
- Pang, Q.; Zheng, X.; Luo, Y.; Ma, L.; Gao, C. A photo-cleavable polyprodrug-loaded wound dressing with UV-responsive antibacterial property. J. Mater. Chem. B 2017, 5, 8975–8982. [Google Scholar] [CrossRef]
- Hedayati, N.; Montazer, M.; Mahmoudirad, M.; Toliyat, T. Ketoconazole and Ketoconazole/β-cyclodextrin performance on cotton wound dressing as fungal skin treatment. Carbohydr. Polym. 2020, 240, 116267. [Google Scholar] [CrossRef]
- Nataraj, D.; Reddy, R.; Reddy, N. Crosslinking electrospun poly (vinyl) alcohol fibers with citric acid to impart aqueous stability for medical applications. Eur. Polym. J. 2020, 124, 109484. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, D.; Zhou, Y.; Ma, G.; Nie, J. Photocrosslinked electrospun chitosan-based biocompatible nanofibers. J. Appl. Polym. Sci. 2008, 109, 3337–3343. [Google Scholar] [CrossRef]
- Xu, X.; Yue, Y.; Cai, D.; Song, J.; Han, C.; Liu, Z.; Wang, D.; Xiao, J.; Wu, H. Aqueous Solution Blow Spinning of Seawater-Stable Polyamidoxime Nanofibers from Water-Soluble Precursor for Uranium Extraction from Seawater. Small Methods 2020, 4, 2000558. [Google Scholar] [CrossRef]
- Islam, S.; Karim, M.R. Fabrication and characterization of poly(vinyl alcohol)/alginate blend nanofibers by electrospinning method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 135–140. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, X.; Zheng, X.; Wang, L. Antimicrobial Properties of Flax Fibers in the Enzyme Retting Process. Fibres Text. East. Eur. 2016, 24, 15–17. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Kahya, N.; Adımcılar, V.; Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Erim, F.B. Antibacterial nano cerium oxide/chitosan/cellulose acetate composite films as potential wound dressing. Eur. Polym. J. 2020, 133, 109777. [Google Scholar] [CrossRef]
- YY/T 0471.1-2004; Test Methods for Primary Wound Dressing—Part 1: Aspects of Absorbency. National Medical Products Administration: Beijing, China, 2004.
- Wu, D.; Wei, W.; Li, H.; Wang, X.; Wang, T.; Tang, S.; Li, Q.; Yao, Y.; Pan, Y.; Wei, J. Blended films containing polybutyrolactam and chitosan for potential wound dressing applications. J. Appl. Polym. Sci. 2018, 135, 46511. [Google Scholar] [CrossRef]
- Pourhojat, F.; Sohrabi, M.; Shariati, S.; Mahdavi, H.; Asadpour, L. Evaluation of poly ε-caprolactone electrospun nanofibers loaded with Hypericum perforatum extract as a wound dressing. Res. Chem. Intermed. 2017, 43, 297–320. [Google Scholar] [CrossRef]
- Safi, S.; Morshed, M.; Hosseini Ravandi, S.A.; Ghiaci, M. Study of electrospinning of sodium alginate, blended solutions of sodium alginate/poly(vinyl alcohol) and sodium alginate/poly(ethylene oxide). J. Appl. Polym. Sci. 2007, 104, 3245–3255. [Google Scholar] [CrossRef]
- Ahire, J.; Robertson, D.; van Reenen, A.; Dicks, L. Surfactin-loaded polyvinyl alcohol (PVA) nanofibers alters adhesion of Listeria monocytogenes to polystyrene. Mater. Sci. Eng. C 2017, 77, 27–33. [Google Scholar] [CrossRef]
- Yu, D.-G.; Chatterton, N.P.; Yang, J.-H.; Wang, X.; Liao, Y.-Z. Coaxial Electrospinning with Triton X-100 Solutions as Sheath Fluids for Preparing PAN Nanofibers. Macromol. Mater. Eng. 2012, 297, 395–401. [Google Scholar] [CrossRef]
- Beigmoradi, R.; Samimi, A.; Mohebbi-Kalhori, D. Fabrication of polymeric nanofibrous mats with controllable structure and enhanced wetting behavior using one-step electrospinning. Polymer 2018, 143, 271–280. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.M.; Jiang, J.; Zhou, D.S. Study on isothermal crystallization kinetics of polyvinyl oxide in restricted state by high-speed scanning calorimetry. Acta Polym. Sin. 2018, 9, 1228–1235. (In Chinese) [Google Scholar] [CrossRef]
- Martín-Alfonso, J.; Cuadri, A.; Greiner, A. The combined effect of formulation and pH on properties of polyethylene oxide composite fiber containing egg albumen protein. Int. J. Biol. Macromol. 2018, 112, 996–1004. [Google Scholar] [CrossRef]
- Medeiros, G.B.; de Souza, P.R.; Retamiro, K.M.; Nakamura, C.V.; Muniz, E.C.; Corradini, E. Experimental design to evaluate properties of electrospun fibers of zein/poly (ethylene oxide) for biomaterial applications. J. Appl. Polym. Sci. 2021, 138, 50898. [Google Scholar] [CrossRef]
- Yang, K.; Chi, Q.; Wang, X.; Jiang, Y.; Li, F.; Xue, B. The role of halloy site on crystallinity, ion conductivity, thermal and mechanical properties of poly(ethylene-oxide)/halloysite nanocomposites. J. Polym. Res. 2019, 26, 138. [Google Scholar] [CrossRef]
- Shahzad, A.; Khan, A.; Afzal, Z.; Umer, M.F.; Khan, J.; Khan, G.M. Formulation development and characterization of cefazolin nanoparticles-loaded cross-linked films of sodium alginate and pectin as wound dressings. Int. J. Biol. Macromol. 2019, 124, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ju, J.; Tan, Y.; Hao, L.; Ma, Y.; Wu, Y.; Zhang, H.; Xia, Y.; Sui, K. Controlled synthesis of sodium alginate electrospun nanofiber membranes for multi-occasion adsorption and separation of methylene blue. Carbohydr. Polym. 2019, 205, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Díez-García, I.; Santamaría-Echart, A.; Eceiza, A.; Tercjak, A. Synthesis and characterization of environmentally-friendly waterborne poly(urethane-urea)s. Eur. Polym. J. 2018, 99, 240–249. [Google Scholar] [CrossRef]
- Akrami-Hasan-Kohal, M.; Tayebi, L.; Ghorbani, M. Curcumin-loaded naturally-based nanofibers as active wound dressing mats: Morphology, drug release, cell proliferation, and cell adhesion studies. New J. Chem. 2020, 44, 10343–10351. [Google Scholar] [CrossRef]
- Samadian, H.; Zamiri, S.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Khastar, H.; Alam, M.; Ai, A.; Derakhshankhah, H.; Allahyari, Z.; et al. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in vivo studies. Sci. Rep. 2020, 10, 8312. [Google Scholar] [CrossRef]



| The Ratio of SA/PVA/PEO | Processing Temperature (°C) | Processing Time (h) |
|---|---|---|
| 4:3:3 | 100 | 2 |
| 4:3:3 | 120 | 2 |
| 5:3:2 | 100 | 2 |
| 5:3:2 | 120 | 2 |
| Serial Number | Chemicals | Weight (g) |
|---|---|---|
| 1 | NaCl | 8.0 |
| 2 | KCl | 0.2 |
| 3 | Na2HPO4·12H2O | 1.96 |
| 4 | KH2PO4 | 0.24 |
| Sample Name | Bacterial Colony Count after Antibacterial (cfu·mL−1) | Bacteriostatic Rate (%) |
|---|---|---|
| Control sample | 394 | - |
| SA MFMs with 0% berberine | 336 | 14.7 |
| SA MFMs with 3% berberine | 28 | 92.9 |
| SA MFMs with 5% berberine | 11 | 97.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, Y.; Wu, H.; Wang, C.; Salleh, K.M.; Li, H.; Zakaria, S. Thermally Treated Berberine-Loaded SA/PVA/PEO Electrospun Microfiber Membranes for Antibacterial Wound Dressings. Polymers 2022, 14, 4473. https://doi.org/10.3390/polym14214473
Zhang J, Li Y, Wu H, Wang C, Salleh KM, Li H, Zakaria S. Thermally Treated Berberine-Loaded SA/PVA/PEO Electrospun Microfiber Membranes for Antibacterial Wound Dressings. Polymers. 2022; 14(21):4473. https://doi.org/10.3390/polym14214473
Chicago/Turabian StyleZhang, Jishu, Yonggang Li, Huawei Wu, Chunhong Wang, Kushairi Mohd Salleh, Hongchang Li, and Sarani Zakaria. 2022. "Thermally Treated Berberine-Loaded SA/PVA/PEO Electrospun Microfiber Membranes for Antibacterial Wound Dressings" Polymers 14, no. 21: 4473. https://doi.org/10.3390/polym14214473
APA StyleZhang, J., Li, Y., Wu, H., Wang, C., Salleh, K. M., Li, H., & Zakaria, S. (2022). Thermally Treated Berberine-Loaded SA/PVA/PEO Electrospun Microfiber Membranes for Antibacterial Wound Dressings. Polymers, 14(21), 4473. https://doi.org/10.3390/polym14214473







