Fabrication of Nylon 6-Montmorillonite Clay Nanocomposites with Enhanced Structural and Mechanical Properties by Solution Compounding
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials Used
2.2. Sample Preparation
2.3. XRD
2.4. Nanoindentation
2.5. Melt Flow Index
2.6. TEM
3. Results and Discussion
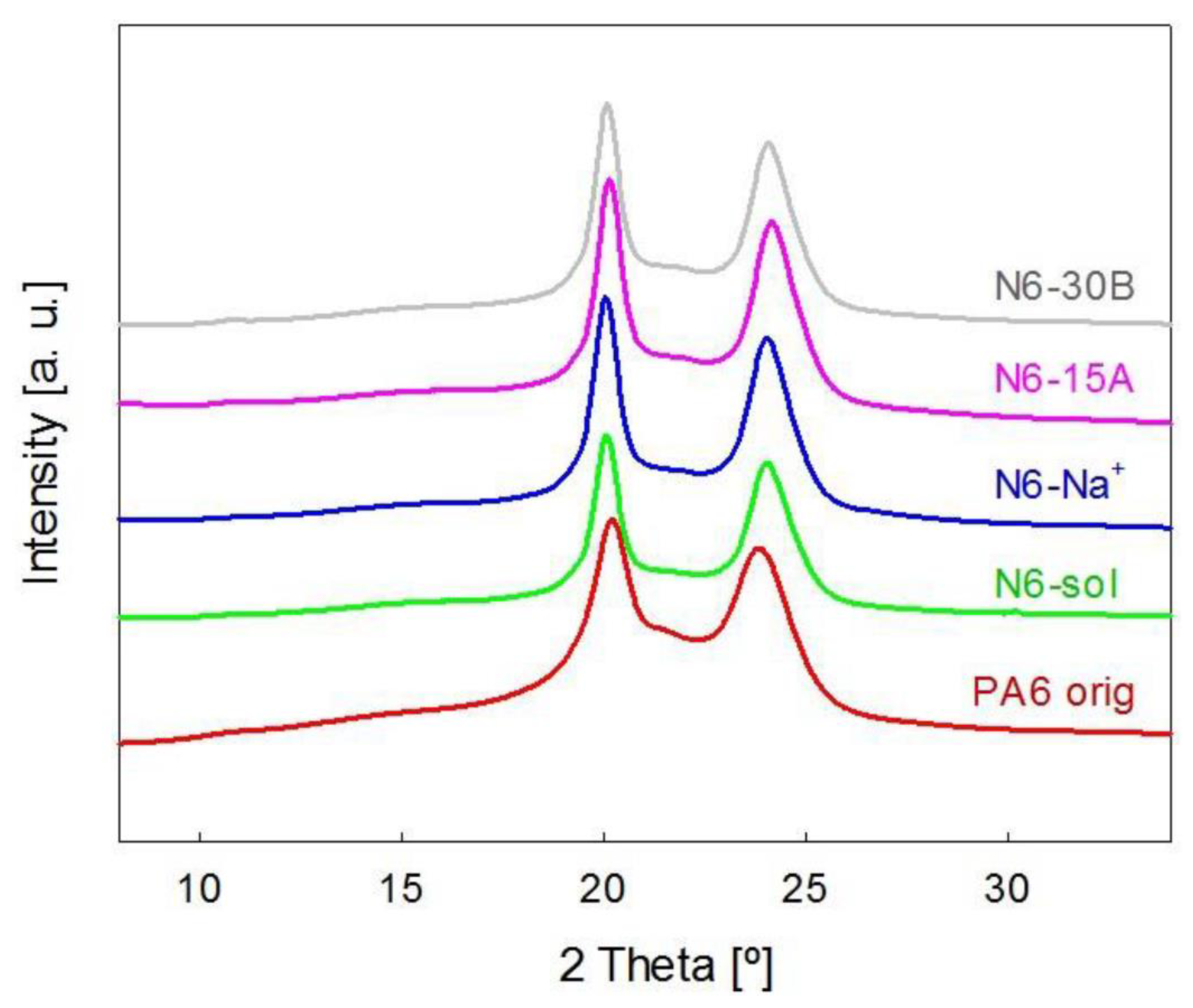
3.1. X-ray Diffraction
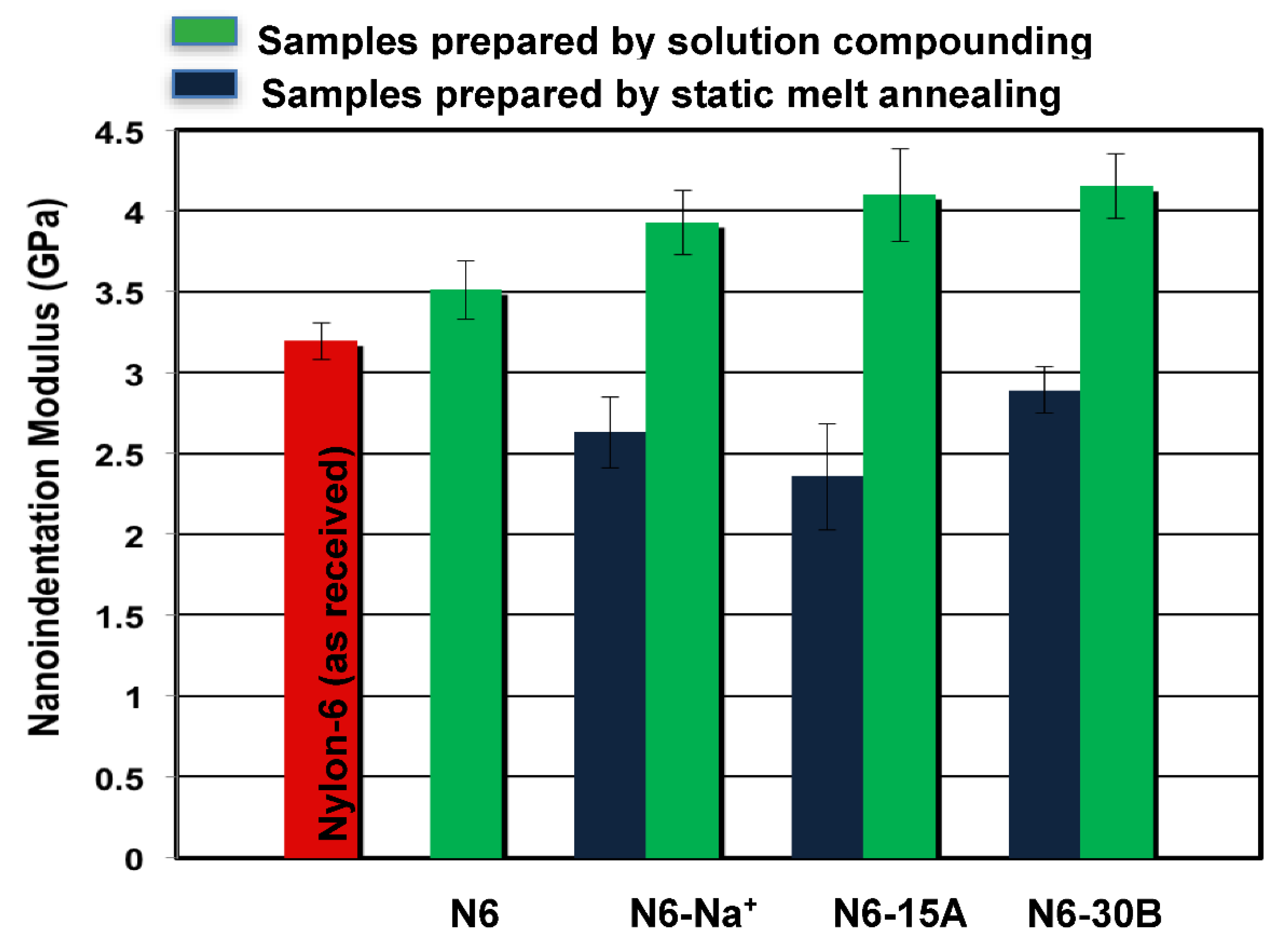
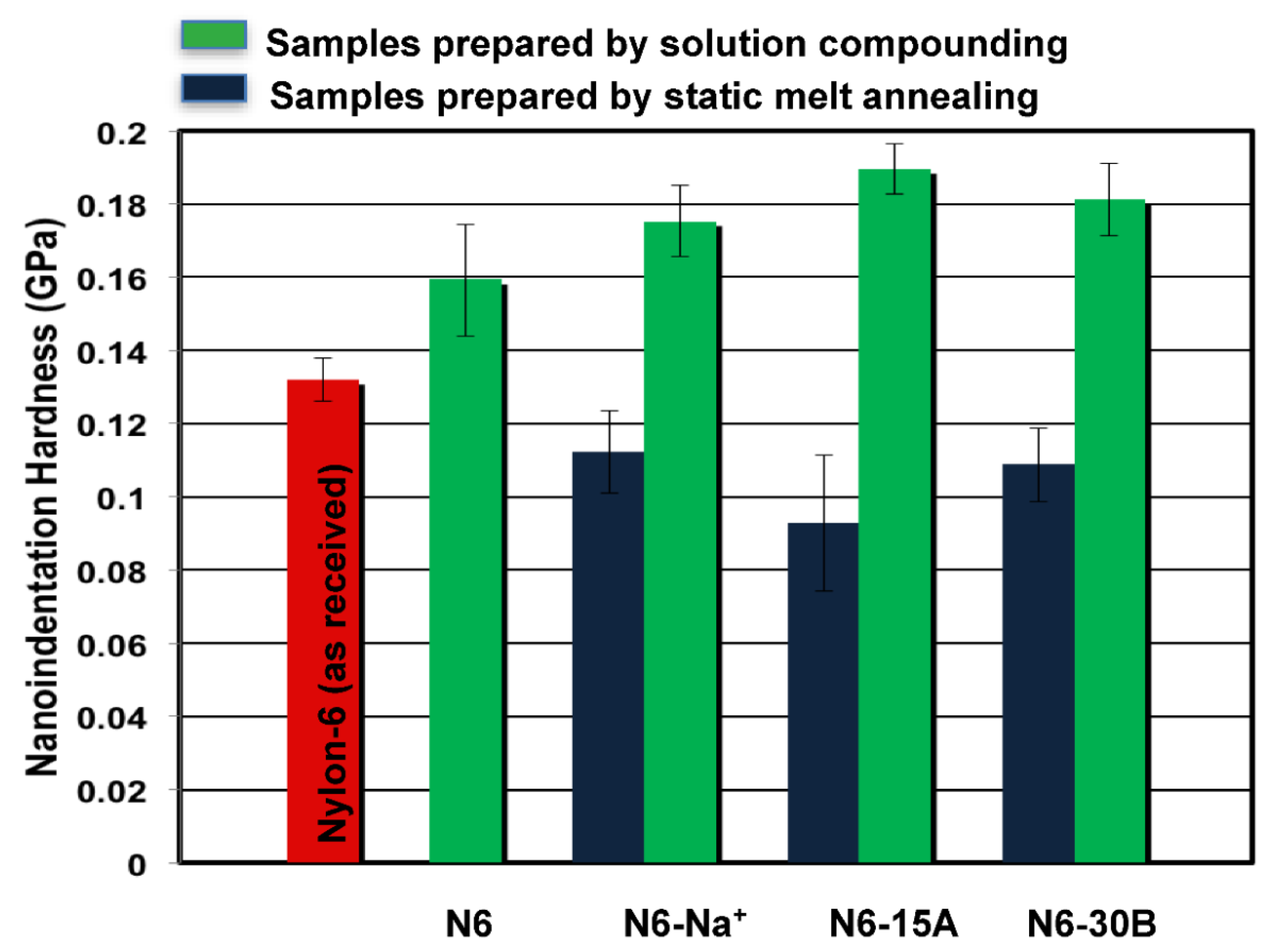
3.2. Nanoindentation
3.3. Melt Flow Index
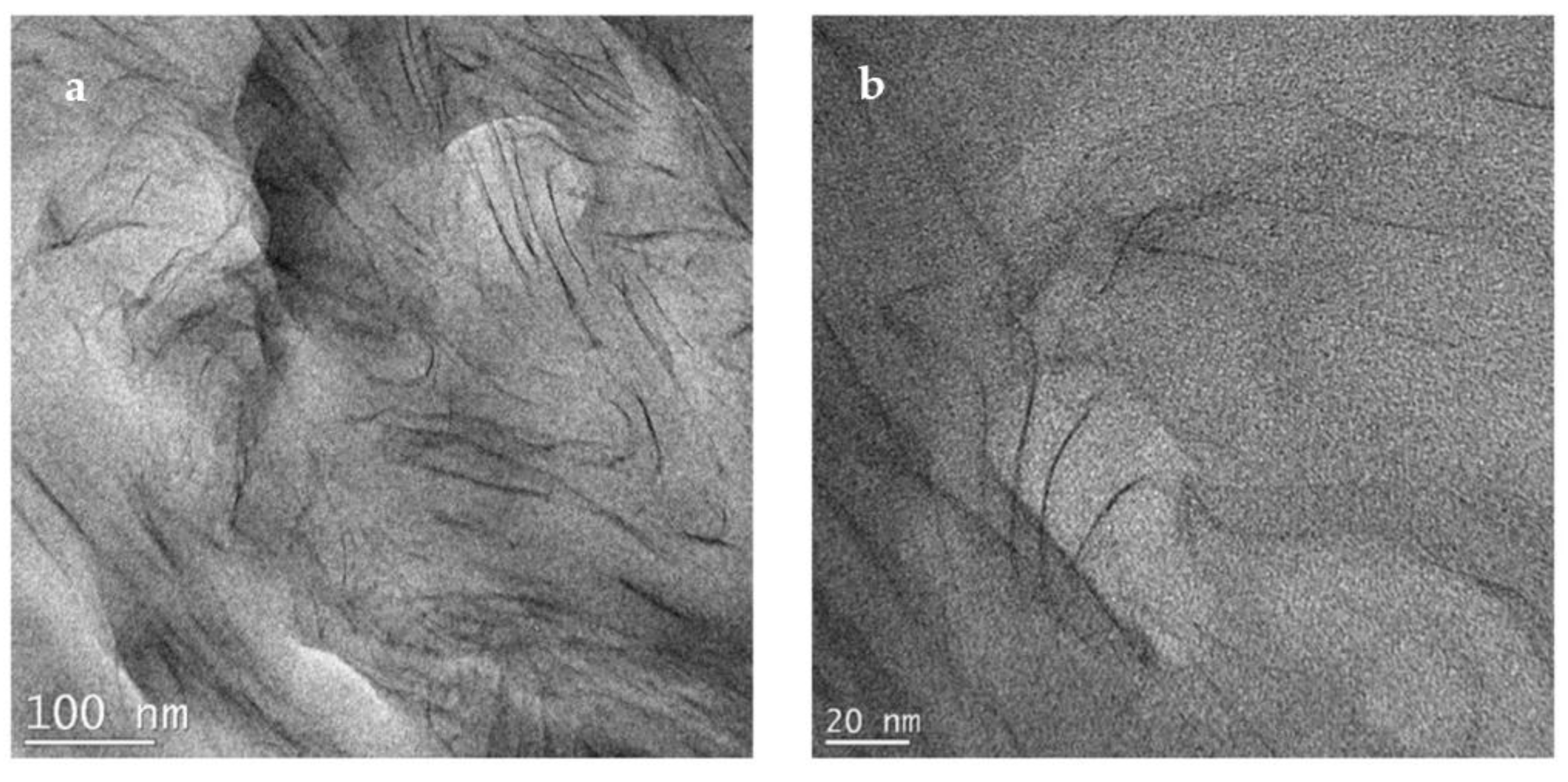
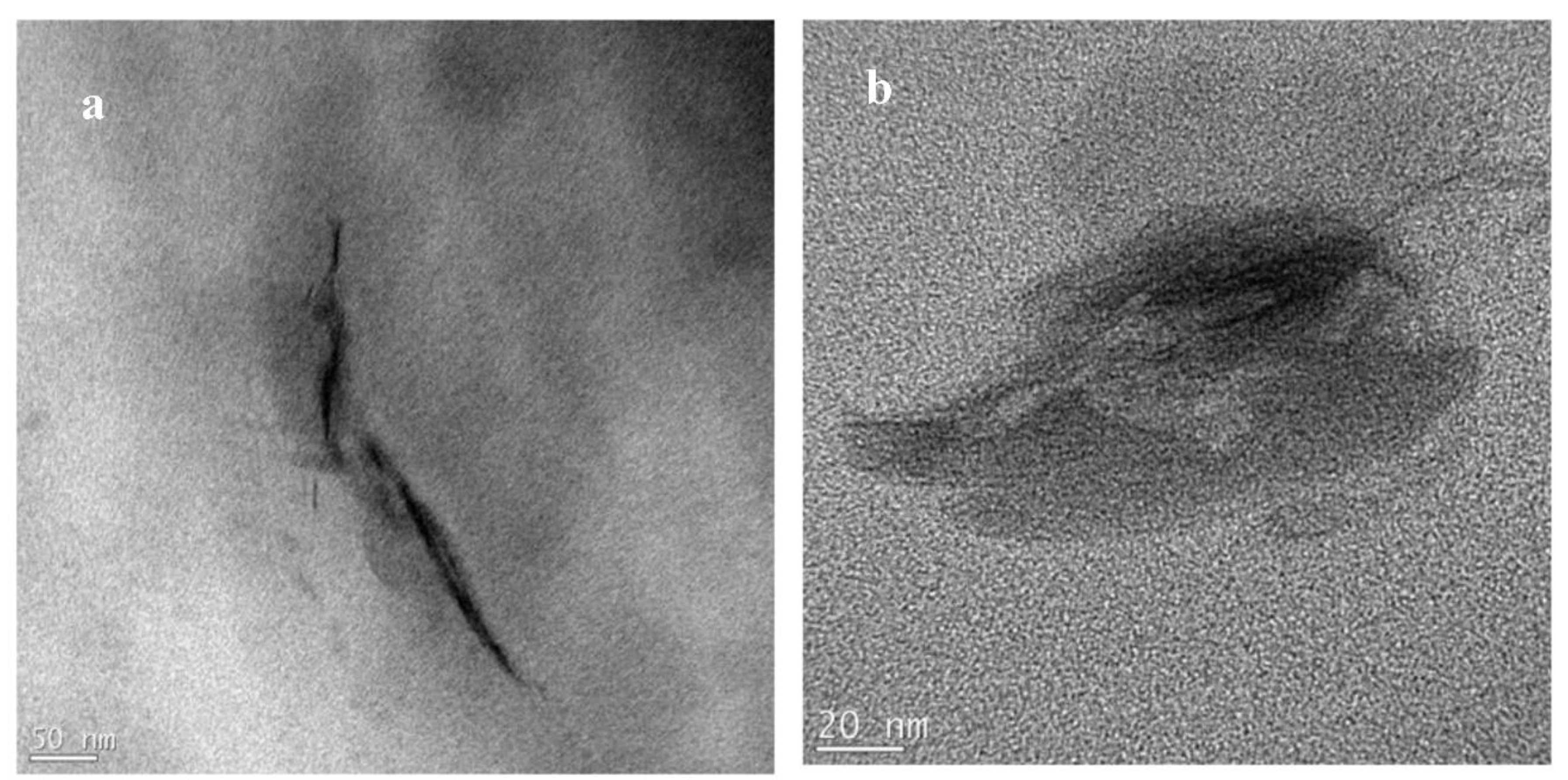
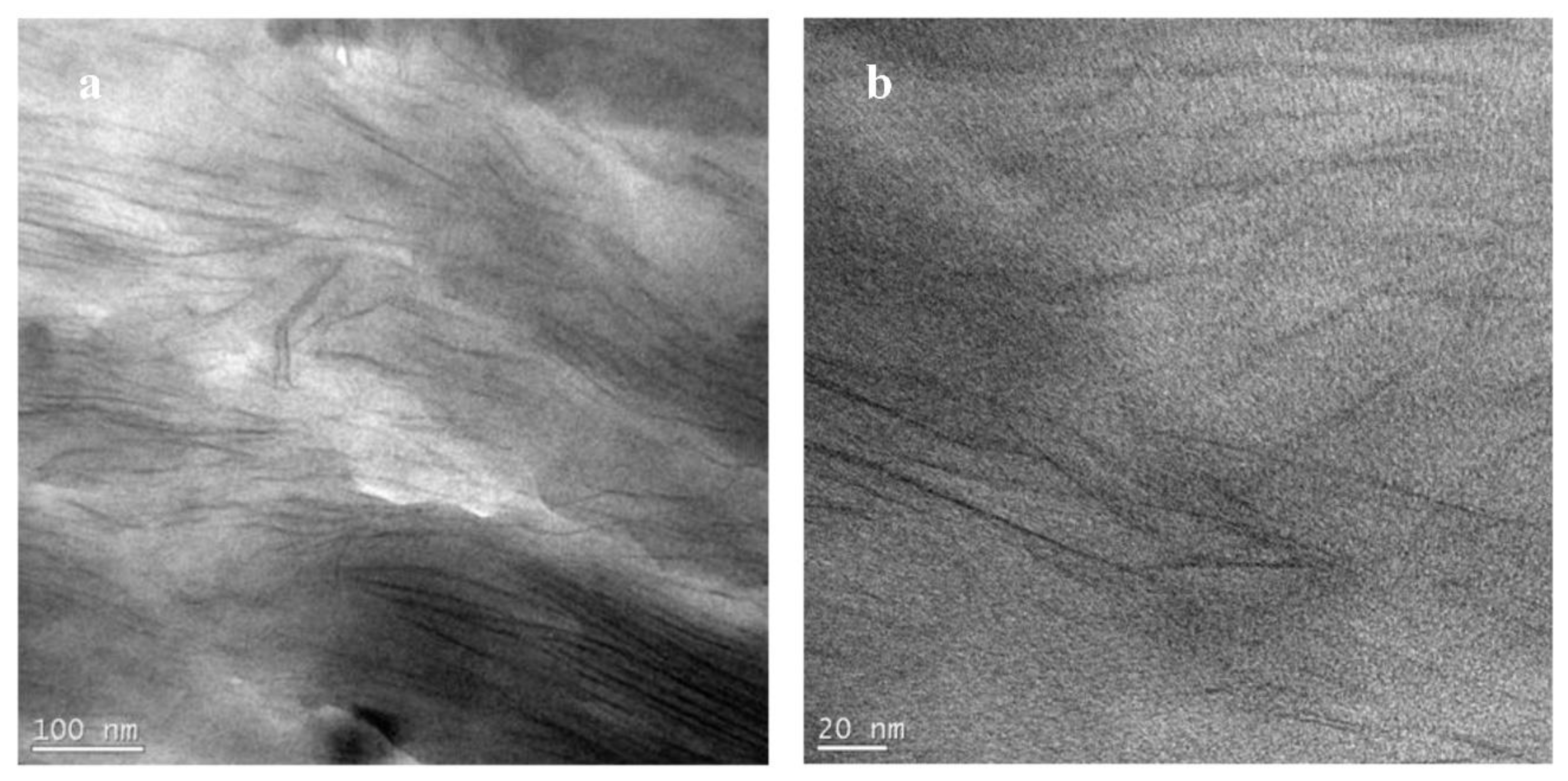
3.4. TEM
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Okada, A.; Kawasumi, M.; Usuki, A.; Kojima, Y.; Kurauchi, T.; Kamigaito, O. Synthesis and properties of nylon-6/clay hybrids. In Polymer Based Molecular Composites, Proceedings of Materials Research Society Symposium, Pittsburgh, PA, USA; Schaefer, D.W., Mark, J.E., Eds.; Cambridge University Press: New York, NY, USA, 1990; Volume 171, p. 45. [Google Scholar]
- Ahmadi, S.; Huang, Y.; Li, W. Synthetic routes, properties and future applications of polymer-layered silicate nanocomposites. J. Mater Sci. 2004, 39, 1919. [Google Scholar] [CrossRef]
- Chen, B. Polymer-clay nanocomposites: An overview with emphasis on interaction mechanisms. Br. Ceram. Trans. 2004, 103, 241. [Google Scholar] [CrossRef]
- Giannelis, E.P. Polymer-layered silicate nanocomposites: Synthesis, properties and applications. Appl. Organomet. Chem. 1998, 12, 675. [Google Scholar] [CrossRef]
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11. [Google Scholar] [CrossRef]
- Abdel Gawad, A.; Esawi, A.; Ramadan, A. Structure and properties of nylon 6–clay nanocomposites: Effect of temperature and reprocessing. J. Mater Sci. 2010, 45, 6677. [Google Scholar] [CrossRef]
- Bhat, A.H.; Rangreez, T.A.; Inamuddin; Chisti, H.-T.-N. Wastewater Treatment and Biomedical Applications of Montmorillonite Based Nanocomposites: A Review. Curr. Anal. Chem. 2022, 18, 269–287. [Google Scholar] [CrossRef]
- Das, P.; Manna, S.; Behera, A.K.; Shee, M.; Basak, P.; Sharma, A.K. Current synthesis and characterization techniques for clay-based polymer nano-composites and its biomedical applications: A review. Environ. Res. 2022, 212, 113534. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhou, C.H.; Kabwe, F.B.; Wu, Q.Q.; Li, C.S.; Zhang, J.R. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66. [Google Scholar] [CrossRef]
- Martinez-Colunga, J.G.; Sanchez-Valdes, S.; Blanco-Cardenas, A.; Ramírez-Vargas, E.; Ramos-De Valle, L.F.; Benavides-Cantu, R.; Espinoza-Martinez, A.B.; Sanchez-Lopez, S.; Lafleur, P.G.; Karami, S.; et al. Dispersion and exfoliation of nanoclays in itaconic acid funcionalized LDPE by ultrasound treatment. J. Appl. Polym. Sci. 2018, 135, 46260. [Google Scholar] [CrossRef]
- Asgari, M.; Abouelmagd, A.; Sundararaj, U. Silane functionalization of sodium montmorillonite nanoclay and its effect on rheological and mechanical properties of HDPE/clay nanocomposites. Appl. Clay Sci. 2017, 146, 439–448. [Google Scholar] [CrossRef]
- Wu, H.; Krifa, M.; Koo, J.H. Flame retardant polyamide 6/nanoclay/intumescent nanocomposite fibers through electrospinning. Text. Res. J. 2014, 84, 1106–1118. [Google Scholar] [CrossRef]
- Fereydoon, M.; Tabatabaei, S.H.; Ajji, A. Rheological, crystal structure, barrier, and mechanical properties of PA6 and MXD6 nanocomposite films. Polym. Eng. Sci. 2014, 54, 2617–2631. [Google Scholar]
- Briesenick, D.; Bremser, W. Synthesis of polyamide-imide-montmorillonite-nanocomposites via new approach of in situ, polymerization and solvent casting. Prog. Org. Coat. 2015, 82, 26–32. [Google Scholar] [CrossRef]
- Osman, A.F.; Kalo, H.; Hassan, M.S.; Hong, T.W.; Azmi, F. Pre–dispersing of montmorillonite nanofiller: Impact on morphology and performance of melt compounded ethyl vinyl acetate nanocomposites. J. Appl. Polym. Sci. 2016, 133, 43204. [Google Scholar] [CrossRef]
- Moreira, J.F.M.; Alves, T.S.; Barbosa, R.; De Carvalho, É.M.; Carvalho, L.H. Effect of cis–13–docosenamide in the properties of compatibilized polypropylene/clay nanocomposites. Macromol. Symp. 2016, 367, 68–75. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Naebe, M. Self-assembly of quaternized chitosan nano-particles within nanoclay layers for enhancement of interfacial properties in toughened polymer nanocomposites. Mater. Des. 2017, 119, 277–289. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Wu, T.; Lin, W.Y.; Tan, Y.B.; Chen, R.Y.; Huang, Z.X.; Yin, X.C.; Qu, J.P. Preparation of polymer/clay nanocomposites via melt intercalation under continuous elongation flow. Compos. Sci. Technol. 2017, 145, 157–164. [Google Scholar] [CrossRef]
- Ammar, A.; Elzatahry, A.; Al-Maadeed, M.; Alenizi, A.M.; Huq, A.F.; Karim, A. Nanoclay compatibilization of phase separated polysulfone/polyimide films for oxygen barrier. Appl. Clay Sci. 2017, 137, 123–134. [Google Scholar] [CrossRef]
- Filippi, S.; Marazzato, C.; Magagnini, P.; Famulari, A.; Arosio, P.; Meille, S.V. Structure and morphology of HDPE-g-MA/organoclay nanocomposites: Effects of the preparation procedures. Eur. Polym. J. 2008, 44, 987–1002. [Google Scholar] [CrossRef]
- Vaia, R.A.; Giannelis, E.P. Lattice model of polymer melt intercalation in organically-modified layered silicates. Macromolecules 1997, 30, 7990. [Google Scholar] [CrossRef]
- Paci, M.; Filippi, S.; Magagnini, P. Nanostructure development in nylon 6-Cloisite® 30B composites. Effects of the preparation conditions. Eur. Polym. J. 2010, 46, 838. [Google Scholar]
- Dennis, H.; Hunter, D.; Chang, D.; Kim, S.; White, J.; Cho, J.; Paul, D. Effect of melt processing conditions on the extent of exfoliation in organoclay-based nanocomposites. Polymer 2001, 42, 9513. [Google Scholar] [CrossRef]
- Shen, Z.; Simon, G.P.; Cheng, Y.-B. Comparison of solution intercalation and melt intercalation of polymer ± clay nanocomposites. Polymer 2002, 43, 4251–4260. [Google Scholar] [CrossRef]
- Strawhecker, K.; Manias, E. Structure and properties of poly (vinyl alcohol)/Na+ montmorillonite nanocomposites. Chem. Mater. 2000, 12, 2943. [Google Scholar] [CrossRef]
- Aranda, P.; Ruiz-Hitzky, E. Poly (ethylene oxide)-silicate intercalation materials. Chem. Mater. 1992, 4, 1395. [Google Scholar] [CrossRef]
- Wu, J.; Lerner, M.M. Structural, thermal, and electrical characterization of layered nanocomposites derived from sodium-montmorillonite and polyethers. Chem. Mater. 1993, 5, 835. [Google Scholar] [CrossRef]
- Ogata, N.; Jimenez, G.; Kawai, H.; Ogihara, T. Structure and thermal/mechanical properties of poly (L-lactide)–clay blend. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 389. [Google Scholar] [CrossRef]
- Yano, K.; Usuki, A.; Okada, A.; Kurauchi, T.; Kamigaito, O. Synthesis and properties of polyimide-clay hybrid. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 2493. [Google Scholar] [CrossRef]
- Magaraphan, R.; Lilayuthalert, W.; Sirivat, A.; Schwank, J.W. Preparation, structure, properties and thermal behavior of rigid-rod polyimide/montmorillonite nanocomposites. Compos. Sci. Technol. 2001, 61, 1253. [Google Scholar] [CrossRef]
- Jeon, H.; Jung, H.T.; Lee, S.; Hudson, S. Morphology of polymer/silicate nanocomposites High density polyethylene and a nitrile copolymer. Polym. Bull. 1998, 41, 107. [Google Scholar] [CrossRef]
- Burnside, S.D.; Giannelis, E.P. Synthesis and properties of new poly (dimethylsiloxane) nanocomposites. Chem. Mater. 1995, 7, 1597. [Google Scholar] [CrossRef]
- Hammersley, A.P.; Svensson, S.O.; Thomson, A. Calibration and correction of spatial distortions in 2D detector systems. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1994, 346, 312–321. [Google Scholar] [CrossRef]
- Samon, J.M.; Schultz, J.M.; Hsiao, B.S. Study of the cold drawing of nylon 6 fiber by in-situ simultaneous small- and wide-angle X-ray scattering techniques. Polymer 2000, 41, 2169–2182. [Google Scholar] [CrossRef]
- Lee, D.; Char, K. Effect of acidity on the deintercalation of organically modified layered silicates. Langmuir 2002, 18, 6445. [Google Scholar] [CrossRef]
- Filippi, S.; Paci, M.; Polacco, G.; Dintcheva, N.T.; Magagnini, P. On the interlayer spacing collapse of Cloisite 30B organoclay. Polym. Degrad. Stab. 2011, 96, 823–832. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef]
- Kojima, Y.; Matsuoka, T.; Takahashi, H.; Kurauchi, T. Crystallization of nylon 6–clay hybrid by annealing under elevated pressure. J. Appl. Polym. Sci. 1994, 51, 683. [Google Scholar] [CrossRef]
- Aharoni, S.M. n-Nylons: Their Synthesis, Structure, and Properties; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Tidick, P.; Fakirov, S.; Avramova, N.; Zachmann, H. Effect of the melt annealing time on the crystallization of nylon-6 with various molecular weights. Colloid Polym. Sci. 1984, 262, 445. [Google Scholar] [CrossRef]
- Ramadan, A.R.; Esawi, A.M.K.; Gawad, A.A. Effect of ball milling on the structure of Na+-montmorillonite and organo-montmorillonite (Cloisite 30B). Appl. Clay Sci. 2010, 47, 196. [Google Scholar] [CrossRef]
- Mani, G. Size reduction of clay particles in nanometer dimensions. Mater. Res. Soc. Symp. Proc. 2003, 740, I3.23. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Shen, L.; Tjiu, W.C.; Liu, T. Nanoindentation and morphological studies on injection-molded nylon-6 nanocomposites. Polymer 2005, 46, 11969–11977. [Google Scholar] [CrossRef]
- Cho, J.W.; Paul, D.R. Nylon 6 nanocomposites by melt compounding. Polymer 2001, 42, 1083–1094. [Google Scholar] [CrossRef]
- Ranade, A.; D′Souza, N.A.; Gnade, B.; Dharia, A. Nylon-6 and Montmorillonite-Layered Silicate (MLS) Nanocomposites. J. Plast. Film Sheeting 2003, 19, 271–286. [Google Scholar] [CrossRef]
- Fornes, T.D.; Yoon, P.J.; Keskkula, H.; Paul, D.R. Nylon 6 nanocomposites: The effect of matrix molecular weight. Polymer 2001, 42, 9929–9940. [Google Scholar] [CrossRef]
- Gonzalez, T.V.; Salazar, C.G.; De la Rosa, J.R.; Gonzalez, V.G. Nylon 6/organoclay nanocomposites by extrusion. J. Appl. Polym. Sci. 2008, 108, 2923–2933. [Google Scholar] [CrossRef]
- Tsai, J.-L.; Huang, J.-C. Strain Rate Effect on Mechanical Behaviors of Nylon 6–Clay Nanocomposites. J. Compos. Mater. 2006, 40, 925–938. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, S.; Zhao, B.; Qin, H.; Wang, F.; Yang, M. Tensile fracture morphologies of nylon-6/montmorillonite nanocomposites. Polym. Int. 2005, 54, 1673–1680. [Google Scholar] [CrossRef]
- Okada, A.; Usuki, A. Polymer-layered silicate nanocomposites: An overview. Macromol. Mater. Eng. 2006, 291, 1449–1476. [Google Scholar] [CrossRef]
- Yan, T.; Chen, D.; Zhao, B.; Jiang, X.; Wang, L.; Li, Y. Percolation Network Formation in Nylon 6/Montmorillonite Nanocomposites: A Critical Structural Insight and the Impact on Solidification Process and Mechanical Behavior. Polymers 2022, 14, 3672. [Google Scholar] [CrossRef]
- Bazmara, M.; Silani, M.; Dayyani, I. Effect of functionally-graded interphase on the elasto-plastic behavior of nylon-6/clay nanocomposites; a numerical study. Def. Technol. 2021, 17, 177–184. [Google Scholar] [CrossRef]
- Yao, W.H. The preparation of modified polyamide clay nanocomposite/recycled maleic anhydride polyamide 6 and blending with low density polyethylene for film blowing application. Polym. Polym. Compos. 2021, 29 (Suppl. 9), S631–S643. [Google Scholar] [CrossRef]
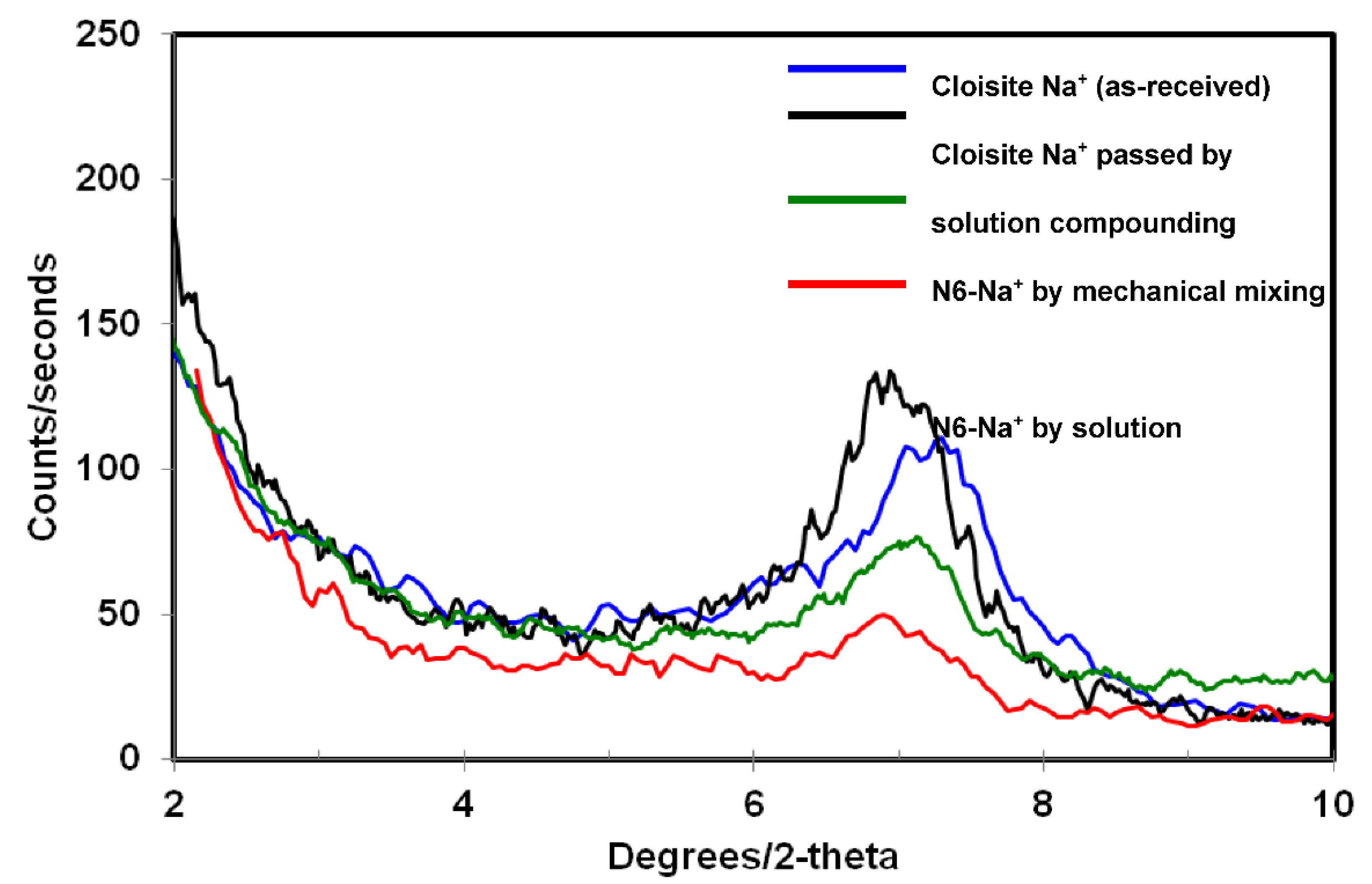

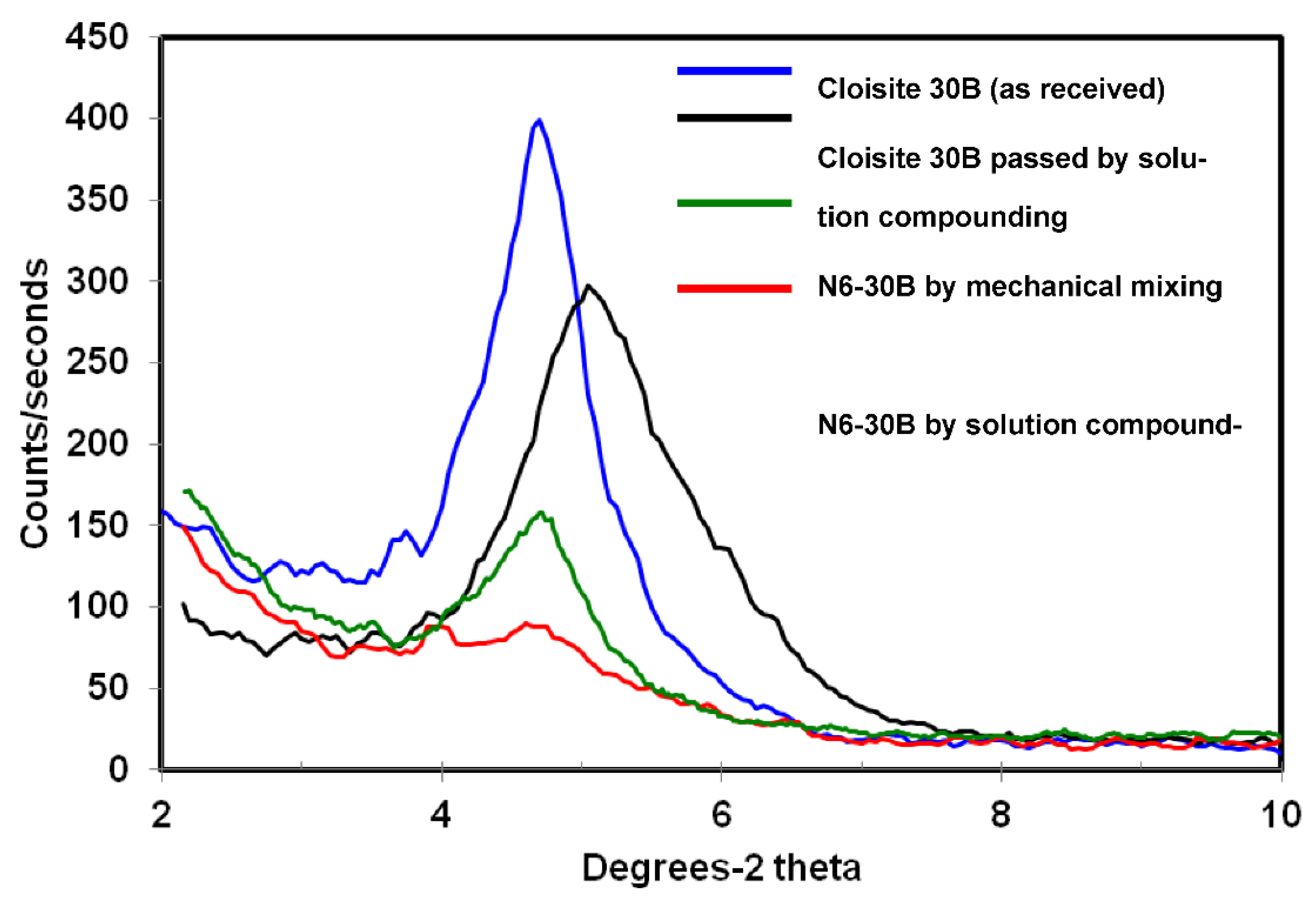




| Clay | Compatibilizer | Gallery d-Spacing d001 (Å) | Organic Content (% Mass) |
|---|---|---|---|
| Cloisite Na+ | - | 11.7 | - |
| Cloisite 15A | 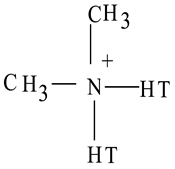 T = (~65% C18; ~30% C16; ~5% C14) | 31.5 | 43% |
| Cloisite 30B | 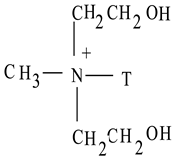 T = (~65% C18; ~30% C16; ~5% C14) | 18.5 | 28% |
| Sample | Degree of Crystallinity by WAXS, Xc |
|---|---|
| PA6 orig | 0.36 |
| N6-sol | 0.58 |
| N6-Na+ | 0.57 |
| N6-15A | 0.58 |
| N6-30B | 0.58 |
| Modulus Enhancement | Hardness Enhancement | |||
|---|---|---|---|---|
| I ◊ | II * | I ◊ | II * | |
| N6-Na+ | 12% | 11% | 10% | 8% |
| N6-15A | 17% | 15% | 19% | 15% |
| N6-30B | 18% | 16% | 14% | 11% |
| Clay Type | Epolymer (GPa) | Ecomposite (GPa) | Preparation Method | Modulus Evaluation Technique | Reference |
|---|---|---|---|---|---|
| Cloisite 30 B | 3.5 | 4.2 | Solution compounding | nanoindentation | Current study |
| Cloisite 15A | 3.5 | 4.15 | Solution compounding | nanoindentation | Current study |
| Cloisite Na+ | 3.5 | 3.9 | Solution compounding | nanoindentation | Current study |
| Cloisite 30B | 3 | 3.7 (+23%) | Melt compounding | nanoindentation | [7] |
| Cloisite 15A | 3 | 3.25 (+8.3%) | Melt compounding | nanoindentation | [7] |
| Cloisite Na+ | 3 | 3.2 (+6.7%) | Melt compounding | nanoindentation | [7] |
| organically modified clay (Nanomerw I.30TC) | 1.06 | 2 (+88.7%) | Melt compounding | nanoindentation | [44] |
| Cloisite Na+ | 2.66 | 3.01 (+13.2%) | Melt compounding | Tensile testing | [45] |
| Organoclay SCPX 2004 | 2.66 | 3.66 (+37.6%) | Melt compounding | Tensile testing | [45] |
| Cloisite 30B | 1.2 | 1.3 (8.3%) | Melt compounding | Tensile testing | [46] |
| MMT | 1.11 | 1.93 (+73.9%) | (in situ polymerization) | [47] | |
| MMT | 2.82 | 4.2–4.8 (+59.6%) | Melt intercalation | [47] | |
| Orgomodified MMT | 1.99 | 3.12 (+57%) | Melt compounding | Tensile testing | [48] |
| Natural MMT | 1.99 | 2.04 (+2.5%) | Melt compounding | Tensile testing | [48] |
| Organoclay | 0.73–1.2 | 1.05–1.6 (+43%) | Melt compounding | Compression testing | [49] |
| OrganoMMT | 2.9 | 4.1 (+41.4%) | Melt compounding | Tensile testing | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Gawad, A.M.; Ramadan, A.R.; Flores, A.; Esawi, A.M.K. Fabrication of Nylon 6-Montmorillonite Clay Nanocomposites with Enhanced Structural and Mechanical Properties by Solution Compounding. Polymers 2022, 14, 4471. https://doi.org/10.3390/polym14214471
Abdel-Gawad AM, Ramadan AR, Flores A, Esawi AMK. Fabrication of Nylon 6-Montmorillonite Clay Nanocomposites with Enhanced Structural and Mechanical Properties by Solution Compounding. Polymers. 2022; 14(21):4471. https://doi.org/10.3390/polym14214471
Chicago/Turabian StyleAbdel-Gawad, Ahmed M., Adham R. Ramadan, Araceli Flores, and Amal M. K. Esawi. 2022. "Fabrication of Nylon 6-Montmorillonite Clay Nanocomposites with Enhanced Structural and Mechanical Properties by Solution Compounding" Polymers 14, no. 21: 4471. https://doi.org/10.3390/polym14214471
APA StyleAbdel-Gawad, A. M., Ramadan, A. R., Flores, A., & Esawi, A. M. K. (2022). Fabrication of Nylon 6-Montmorillonite Clay Nanocomposites with Enhanced Structural and Mechanical Properties by Solution Compounding. Polymers, 14(21), 4471. https://doi.org/10.3390/polym14214471




