Electrical Properties of Polyetherimide-Based Nanocomposites Filled with Reduced Graphene Oxide and Graphene Oxide-Barium Titanate-Based Hybrid Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
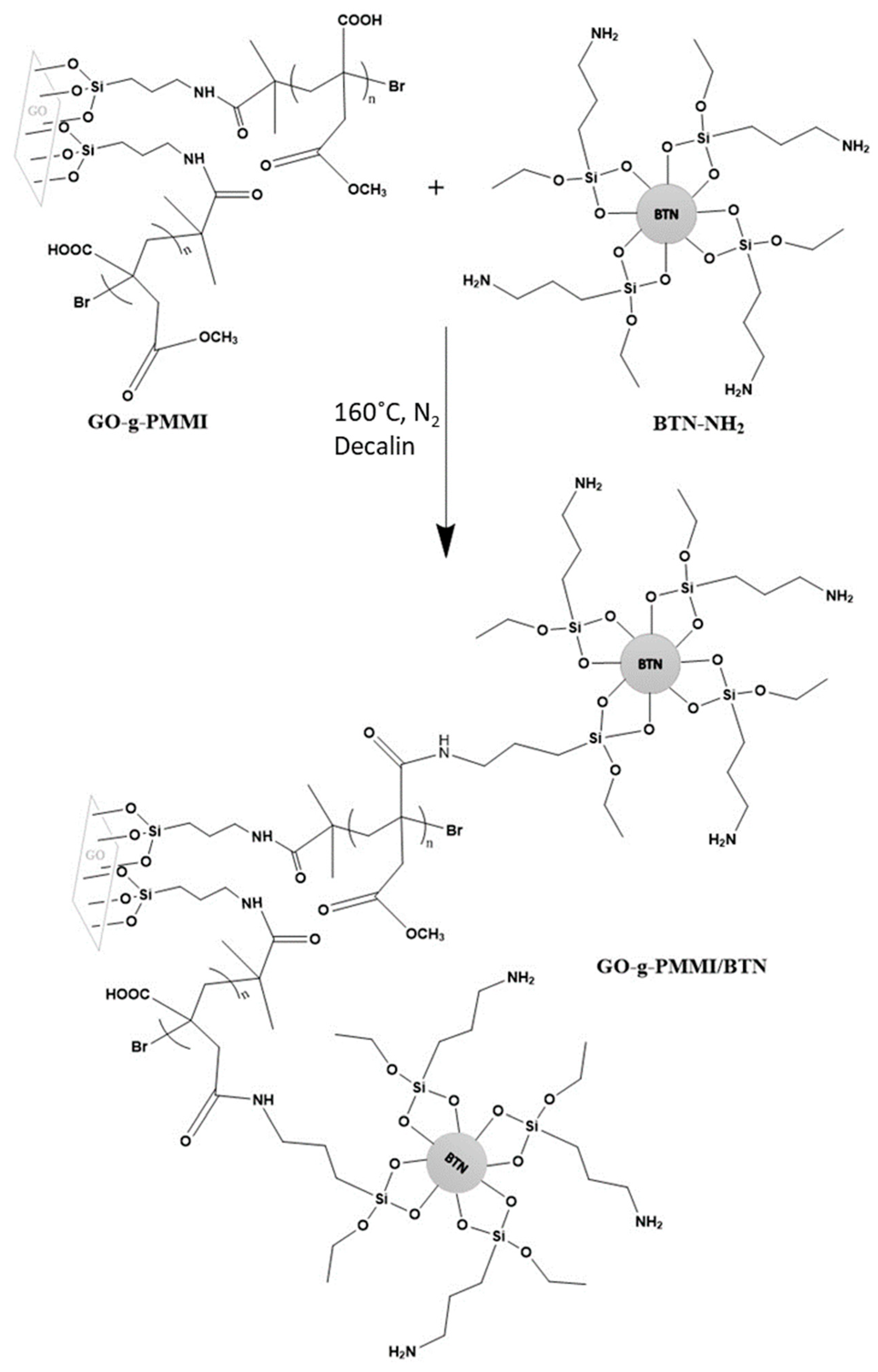
2.2. Synthesis of Hybrid Nanomaterial Based on Graphene Oxide Grafted with PMMI and Barium Titanate Nanoparticles (GO-g-PMMI/BTN)
2.2.1. Hydroxylation of Barium Titanate Nanoparticles
2.2.2. Silanization of Barium Titanate Nanoparticles
2.2.3. Synthesis of the GO-g-PMMI/BTN
2.3. Preparation of Nanocomposites
2.4. Characterization
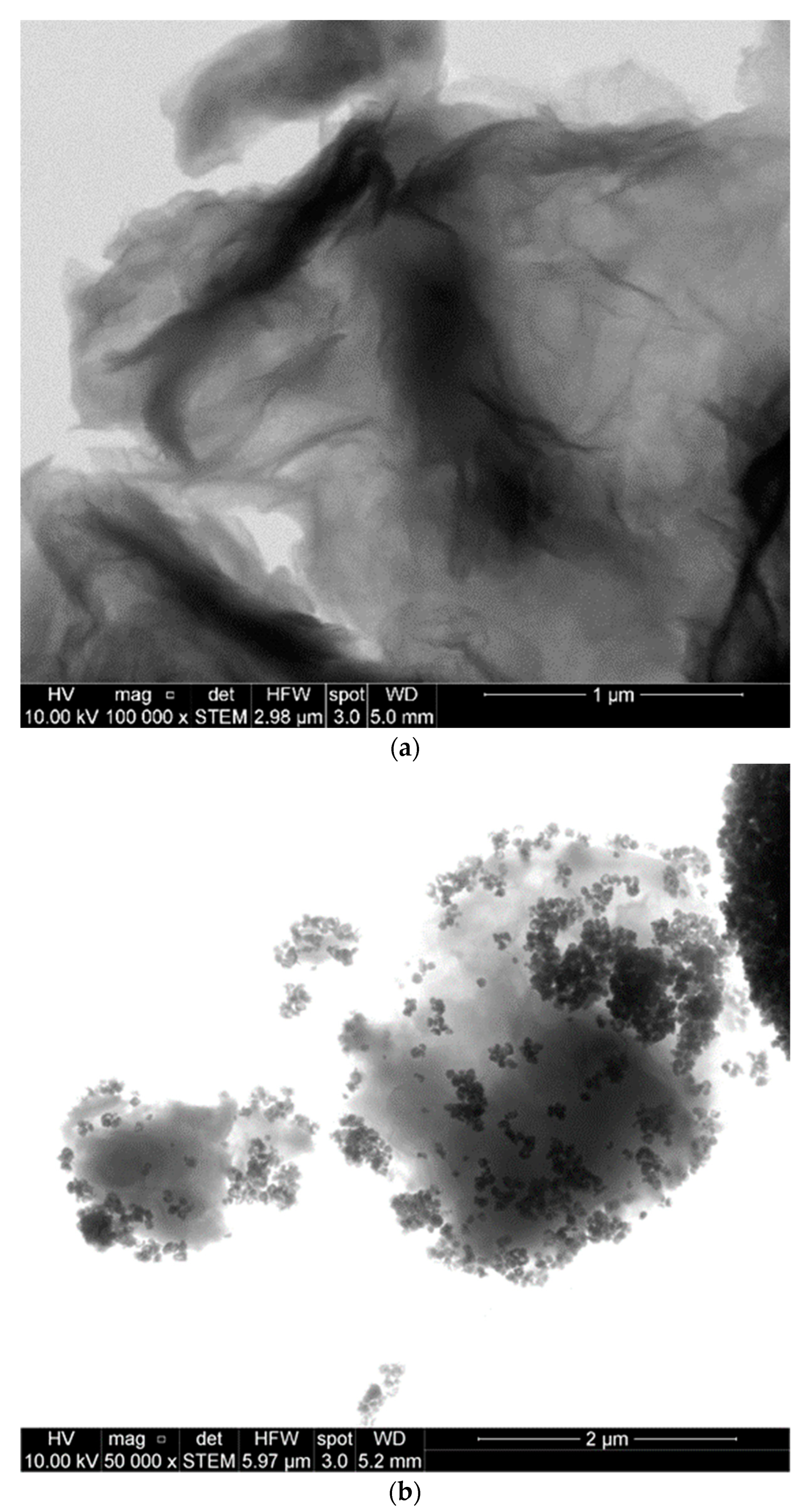
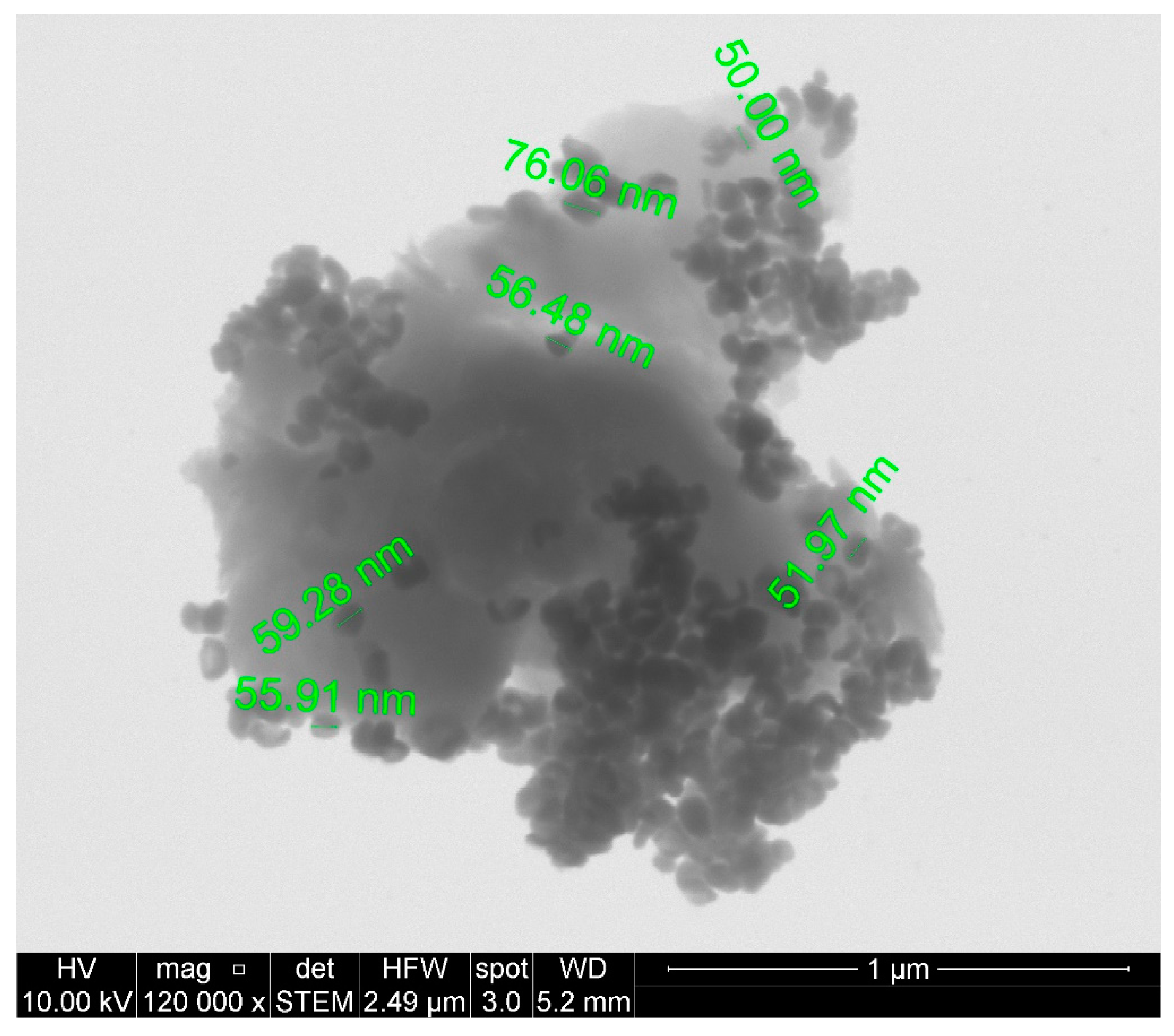
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguilar-Bolados, H.; Vargas-Astudillo, D.; Yazdani-Pedram, M.; Acosta-Villavicencio, G.; Fuentealba, P.; Contreras-Cid, A.; Verdejo, R.; López-Manchado, M.A. Facile and Scalable One-Step Method for Amination of Graphene Using Leuckart Reaction. Chem. Mater. 2017, 29, 6698–6705. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Contreras-Cid, A.; Yazdani-Pedram, M.; Acosta-Villavicencio, G.; Flores, M.; Fuentealba, P.; Neira-Carrillo, A.; Verdejo, R.; López-Manchado, M.A. Synthesis of fluorinated graphene oxide by using an easy one-pot deoxyfluorination reaction. J. Colloid Interface Sci. 2018, 524, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Bolados, H.; Yazdani-Pedram, M.; Verdejo, R. Thermal, electrical, and sensing properties of rubber nanocomposites. In High-Performance Elastomeric Materials Reinforced by Nano-Carbons; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–175. [Google Scholar] [CrossRef]
- Yang, T.; Lin, H.; Loh, K.P.; Jia, B. Fundamental Transport Mechanisms and Advancements of Graphene Oxide Membranes for Molecular Separation. Chem. Mater. 2019, 31, 1829–1846. [Google Scholar] [CrossRef]
- Faysal Hossain, M.D.; Akther, N.; Zhou, Y. Recent advancements in graphene adsorbents for wastewater treatment: Current status and challenges. Chinese Chem. Lett. 2020, 31, 2525–2538. [Google Scholar] [CrossRef]
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Y.; He, C.; Gao, Y.; Jia, N.; Chen, Z.; Song, W. The role of sp2/sp3 hybrid carbon regulation in the nonlinear optical properties of graphene oxide materials. RSC Adv. 2017, 7, 53643–53652. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approach. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Rubio, N.; Au, H.; Leese, H.S.; Hu, S.; Clancy, A.J.; Shaffer, M.S.P. Grafting from versus Grafting to Approaches for the Functionalization of Graphene Nanoplatelets with Poly(methyl methacrylate). Macromolecules 2017, 50, 7070–7079. [Google Scholar] [CrossRef]
- Eskandari, P.; Abousalman-Rezvani, Z.; Roghani-Mamaqani, H.; Salami-Kalajahi, M.; Mardani, H. Polymer grafting on graphene layers by controlled radical polymerization. Adv. Colloid Interface Sci. 2019, 273, 102021. [Google Scholar] [CrossRef]
- Zhao, M.; Lu, X.; Zong, H.; Li, J.; Zhuge, B. Itaconic acid production in microorganisms. Biotechnol. Lett. 2018, 40, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Itapu, B.M.; Jayatissa, A.H. A Review in Graphene/Polymer Composites. Chem. Sci. Int. J. 2018, 23, 1–16. [Google Scholar] [CrossRef]
- Tewatia, K.; Sharma, A.; Sharma, M.; Kumar, A. Factors affecting morphological and electrical properties of Barium Titanate: A brief review. Mater. Today Proc. 2020, 44, 4548–4556. [Google Scholar] [CrossRef]
- Romasanta, L.J.; Lopez-Manchado, M.A.; Verdejo, R. Increasing the performance of dielectric elastomer actuators: A review from the materials perspective. Prog. Polym. Sci. 2015, 51, 188–211. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Yazdani-Pedram, M.; Quinteros-Jara, E.; Cuenca-Bracamonte, Q.; Quijada, R.; Carretero-González, J.; Avilés, F.; Lopez-Manchado, M.A.; Verdejo, R. Synthesis of sustainable, lightweight and electrically conductive polymer brushes grafted multi-layer graphene oxide. Polym. Test. 2021, 93, 106986. [Google Scholar] [CrossRef]
- Zheng, M.S.; Zheng, Y.T.; Zha, J.W.; Yang, Y.; Han, P.; Wen, Y.Q.; Dang, Z.M. Improved dielectric, tensile and energy storage properties of surface rubberized BaTiO3/polypropylene nanocomposites. Nano Energy 2018, 48, 144–151. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Zhang, L.; Zong, C.; Xu, A.; Zhang, Y.; Geng, B.; Zhang, S. Enhanced breakdown strength and suppressed dielectric loss of polymer nanocomposites with BaTiO3 fillers modified by fluoropolymer. RSC Adv. 2020, 10, 7065–7072. [Google Scholar] [CrossRef]
- Yaqoob, U.; Iftekhar Uddin, A.S.M.; Chung, G.S. The effect of reduced graphene oxide on the dielectric and ferroelectric properties of PVDF–BaTiO 3 nanocomposites. RSC Adv. 2016, 6, 30747–30754. [Google Scholar] [CrossRef]
- Wang, S.; Chi, H.; Chen, L.; Li, W.; Li, Y.; Li, G.; Ge, X. Surface Functionalization of Graphene Oxide with Polymer Brushes for Improving Thermal Properties of the Polymer Matrix. Adv. Polym. Technol. 2021, 2021, 5591420. [Google Scholar] [CrossRef]
- Molecular, L.D.F. Properties of polyelectrolytes: Poly(mono-methyl itaconate). conformational and viscometric behaviour in dilute soluton. Eur. Polym. J. 1989, 25, 1059–1063. [Google Scholar] [CrossRef]
- Al-Mufti, S.M.S.; Almontasser, A.; Rizvi, S.J.A. Influence of temperature variations on the dielectric parameters of thermally reduced graphene oxide. Mater. Today Proc. 2022, 57, 1713–1718. [Google Scholar] [CrossRef]
- Johra, F.T.; Jung, W.G. Hydrothermally reduced graphene oxide as a supercapacitor. Appl. Surf. Sci. 2015, 357, 1911–1914. [Google Scholar] [CrossRef]
- Bychko, I.; Abakumov, A.; Didenko, O.; Chen, M.; Tang, J.; Strizhak, P. Differences in the structure and functionalities of graphene oxide and reduced graphene oxide obtained from graphite with various degrees of graphitization. J. Phys. Chem. Solids 2022, 164, 110614. [Google Scholar] [CrossRef]
- Chang, S.J.; Liao, W.S.; Ciou, C.J.; Lee, J.T.; Li, C.C. An efficient approach to derive hydroxyl groups on the surface of barium titanate nanoparticles to improve its chemical modification ability. J. Colloid Interface Sci. 2009, 329, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Guo, M.; Zhong, L.; Fu, H. In situ growth of BaTiO3 nanotube on the surface of reduced graphene oxide: A lightweight electromagnetic absorber. J. Alloys Compd. 2019, 773, 423–431. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Zou, Y.; Qian, Q.; Jin, Y.; Li, G.; Jiang, K.; Fan, S. The dependence of graphene Raman D-band on carrier density. Nano Lett. 2013, 13, 6170–6175. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, J.; Kang, P.; Wu, P.; Zhou, Z.; Chen, G.X.; Li, Q. Improve the dielectric property and breakdown strength of composites by cladding a polymer/BaTiO3 composite layer around carbon nanotubes. Polymer 2020, 188, 122157. [Google Scholar] [CrossRef]
- Li, L.; Zheng, S. Enhancement of dielectric constants of epoxy thermosets via a fine dispersion of barium titanate nanoparticles. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Woudenberg, F.C.M.; Sager, W.F.C.; Ten Elshof, J.E.; Verweij, H. Nanostructured barium titanate thin films from nanoparticles obtained by an emulsion precipitation method. Thin Solid Films 2005, 471, 134–139. [Google Scholar] [CrossRef]
- Chen, B.K.; Su, C.T.; Tseng, M.C.; Tsay, S.Y. Preparation of polyetherimide nanocomposites with improved thermal, mechanical and dielectric properties. Polym. Bull. 2006, 57, 671–681. [Google Scholar] [CrossRef]
- Oliveira, R.; Georgieva, P.; Feyo de Azevedo, S. Plant and Equipment | Instrumentation and Process Control: Instrumentation. In Encyclopedy of Diary Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 234–241. ISBN 978-0-12-374407-4. [Google Scholar]
- Cuenca-bracamonte, Q.; Yazdani-pedram, M.; Hernandez Santana, M.; Aguilar-Bolados, H. Electrical Properties of Poly(Monomethyl Itaconate)/Few-Layer Functionalized Graphene Oxide/Lithium Ion Nanocomposites. Polymers 2020, 12, 2673. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E. Negative Electrical Conductivity Metamaterials. Preprints 2022, 2022030357. [Google Scholar] [CrossRef]
- Schön, J.H. Chapter 8—Electrical Properties. In Physical Properties of Rocks; Schön, J.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 65, pp. 301–367. ISBN 0376-7361. [Google Scholar]
- Wang, D.; Zhang, X.; Zha, J.W.; Zhao, J.; Dang, Z.M.; Hu, G.H. Dielectric properties of reduced graphene oxide/polypropylene composites with ultralow percolation threshold. Polymer 2013, 54, 1916–1922. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, C.; Wang, K.; Deng, L.; Ma, M.; Xu, G. Negative dielectric constant manifested by static electricity. Appl. Phys. Lett. 2013, 102, 62904. [Google Scholar] [CrossRef]



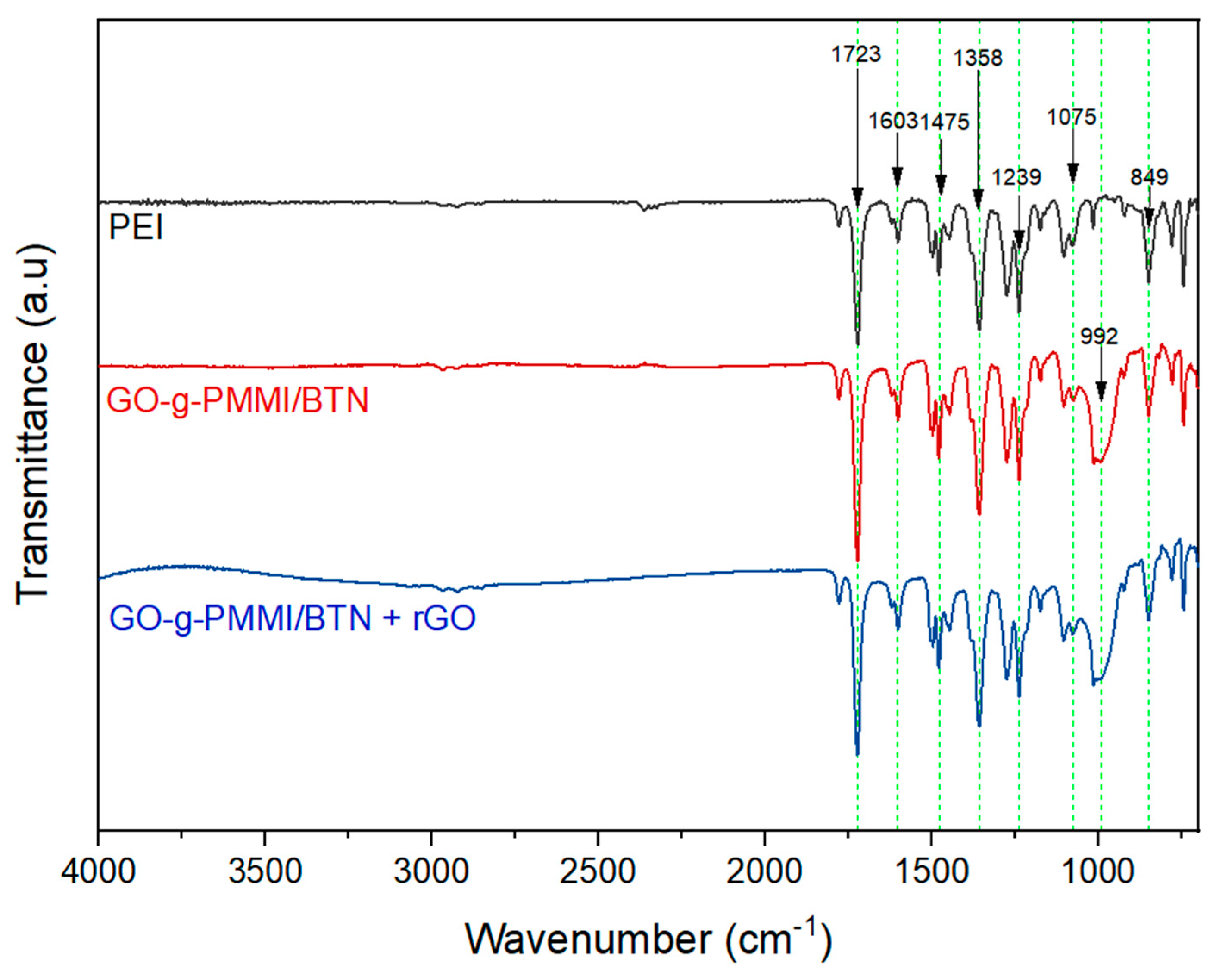
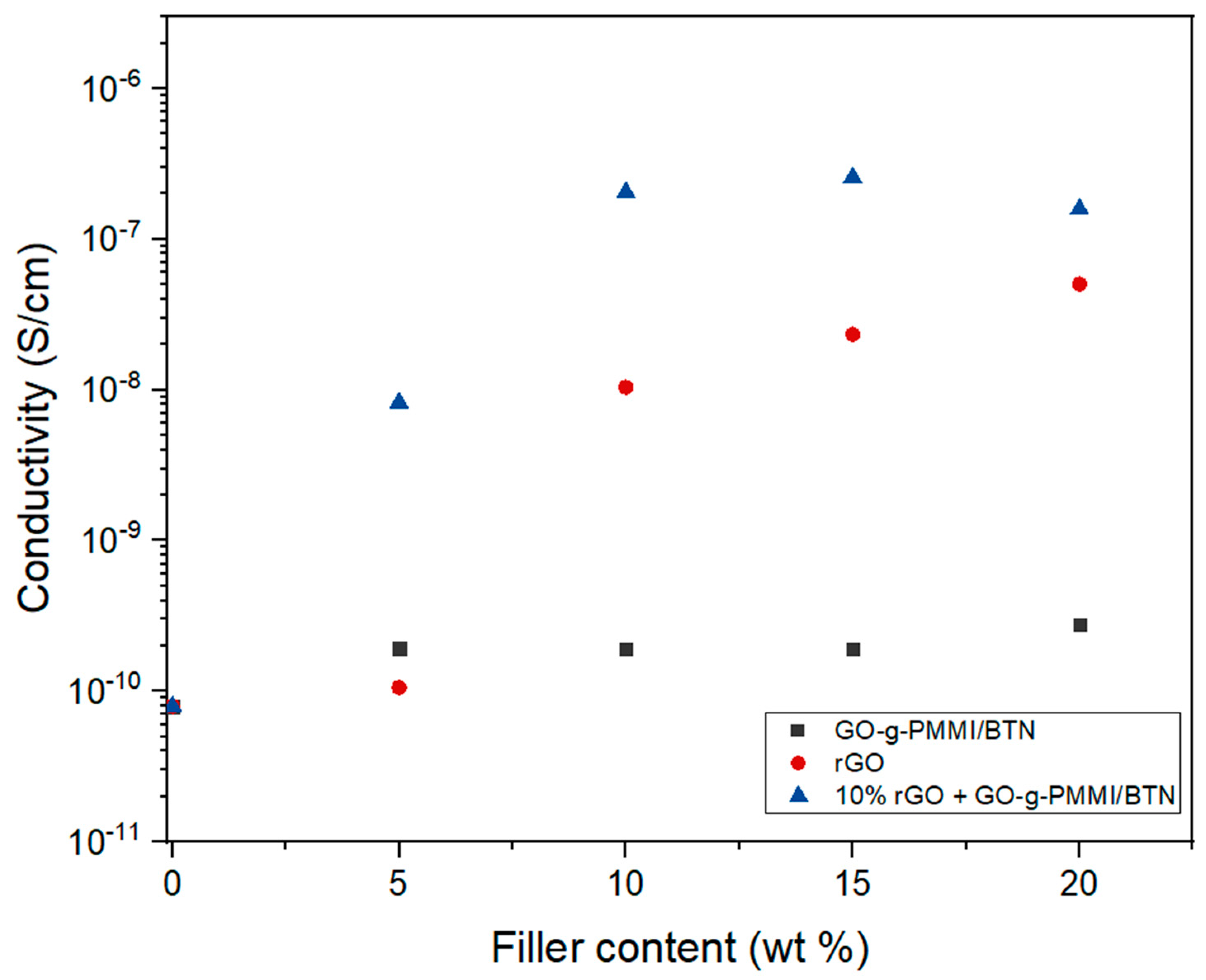


| Compound | PEI (g) | GO-g-PMMI/BTN (g) | rGO (g) |
|---|---|---|---|
| PEI | 0.9000 | 0 | 0 |
| GO-g-PMMI/BTN | 0.9200 | 0.0937 | 0 |
| rGO | 0.9300 | 0 | 0.0936 |
| GO-g-PMMI/BTN + rGO | 0.9300 | 0.0946 | 0.0937 |
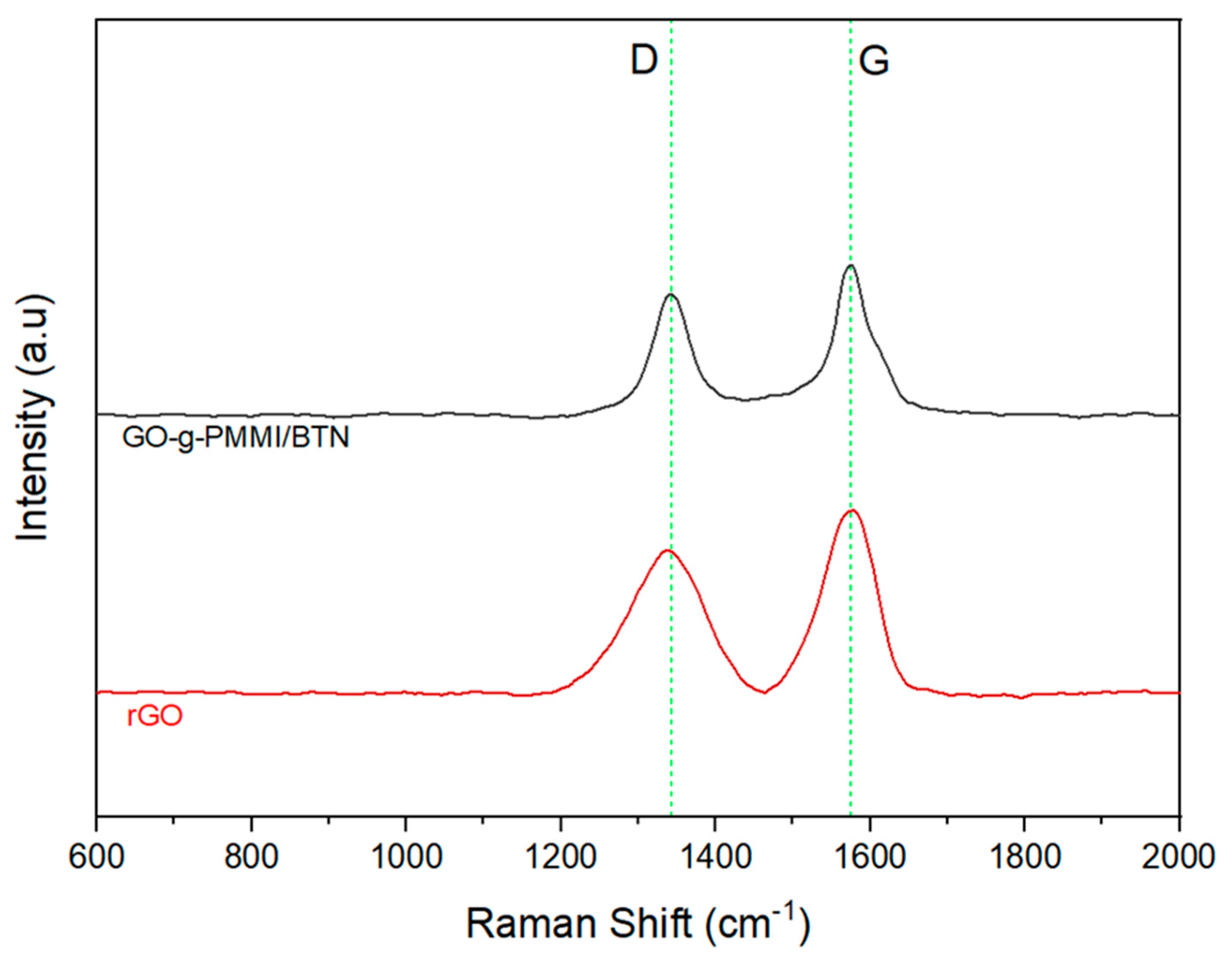
| Sample | Raman Shift (cm−1) | FWHM | ID/IG | ||
|---|---|---|---|---|---|
| D | G | D | G | ||
| rGO | 1335 | 1576 | 58.898 | 83.643 | 0.837 |
| GO-g-PMMI/BTN | 1343 | 1574 | 51.320 | 54.610 | 0.962 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuenca-Bracamonte, Q.; Yazdani-Pedram, M.; Aguilar-Bolados, H. Electrical Properties of Polyetherimide-Based Nanocomposites Filled with Reduced Graphene Oxide and Graphene Oxide-Barium Titanate-Based Hybrid Nanoparticles. Polymers 2022, 14, 4266. https://doi.org/10.3390/polym14204266
Cuenca-Bracamonte Q, Yazdani-Pedram M, Aguilar-Bolados H. Electrical Properties of Polyetherimide-Based Nanocomposites Filled with Reduced Graphene Oxide and Graphene Oxide-Barium Titanate-Based Hybrid Nanoparticles. Polymers. 2022; 14(20):4266. https://doi.org/10.3390/polym14204266
Chicago/Turabian StyleCuenca-Bracamonte, Quimberly, Mehrdad Yazdani-Pedram, and Héctor Aguilar-Bolados. 2022. "Electrical Properties of Polyetherimide-Based Nanocomposites Filled with Reduced Graphene Oxide and Graphene Oxide-Barium Titanate-Based Hybrid Nanoparticles" Polymers 14, no. 20: 4266. https://doi.org/10.3390/polym14204266
APA StyleCuenca-Bracamonte, Q., Yazdani-Pedram, M., & Aguilar-Bolados, H. (2022). Electrical Properties of Polyetherimide-Based Nanocomposites Filled with Reduced Graphene Oxide and Graphene Oxide-Barium Titanate-Based Hybrid Nanoparticles. Polymers, 14(20), 4266. https://doi.org/10.3390/polym14204266






