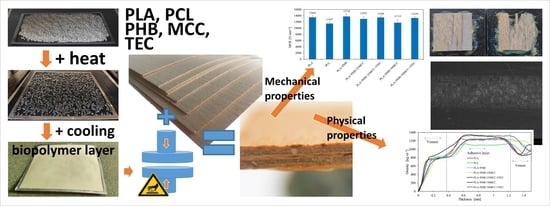
Selected Properties of Bio-Based Layered Hybrid Composites with Biopolymer Blends for Structural Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biopolymer Blend Elaboration
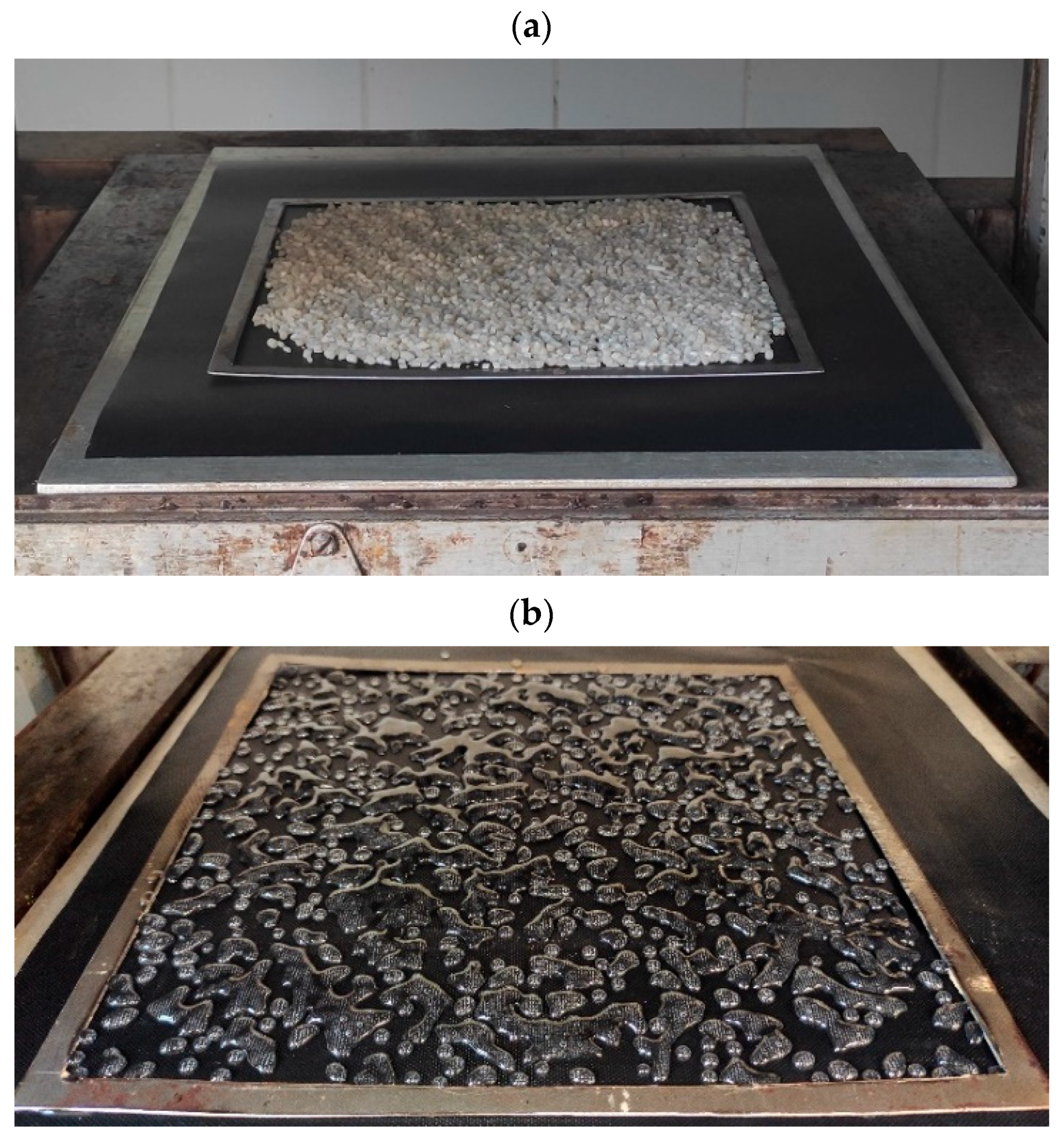
2.3. Manufacturing of Biopolymer Adhesive Layers
2.4. Layered Composite Manufacturing
2.5. Mechanical and Physical Properties
2.6. Statistical Analysis
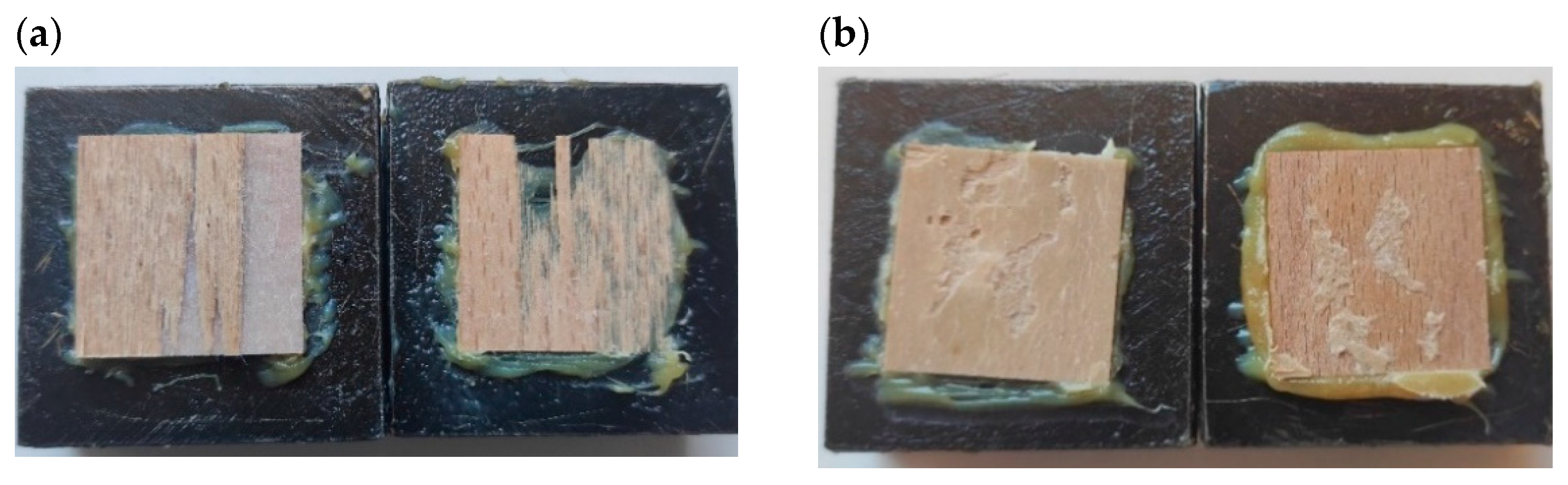
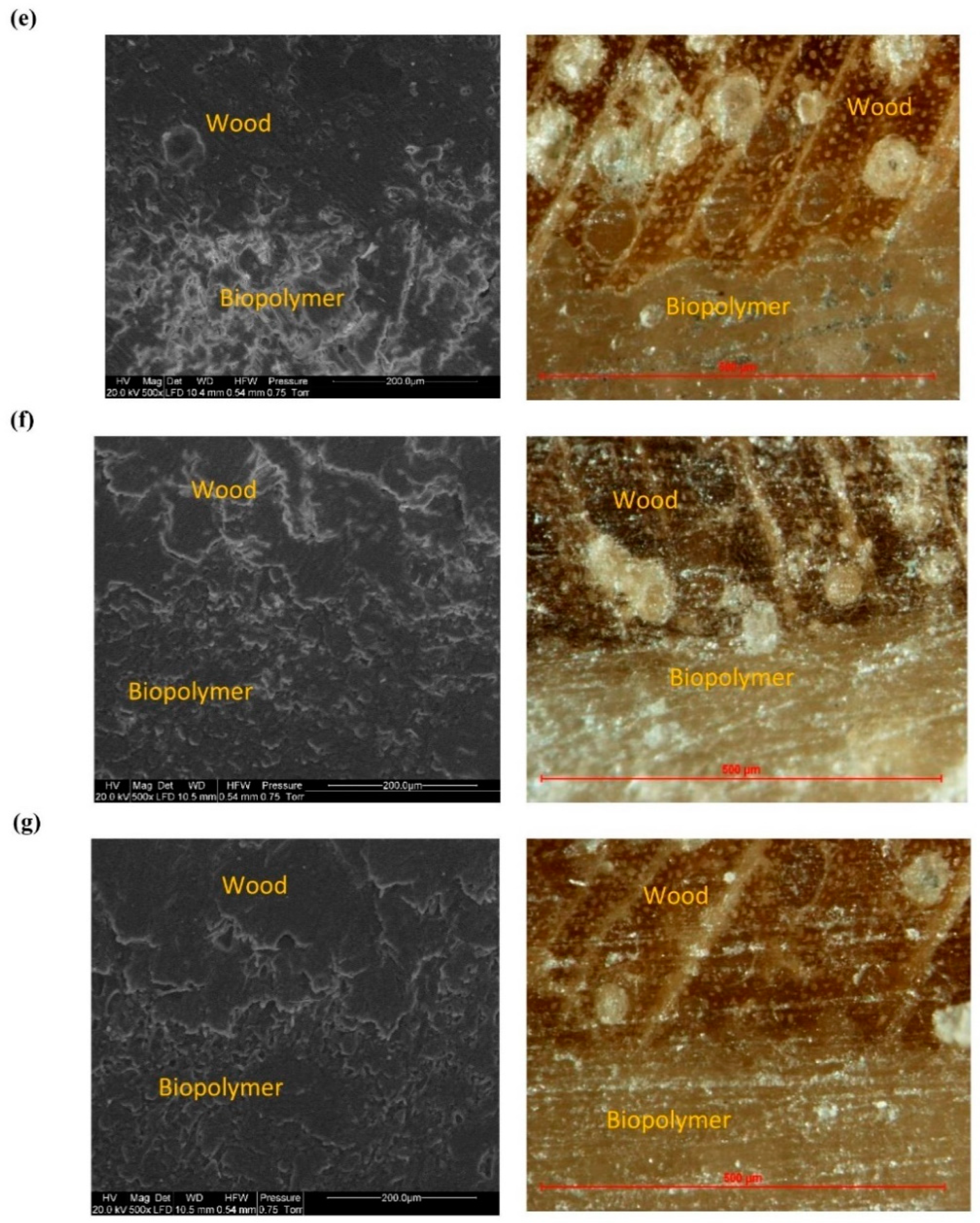
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brito, F.M.S.; Bortoletto Júnior, G.; Paes, J.B.; Belini, U.L.; Tomazello-Filho, M. Technological characterization of particleboards made with sugarcane bagasse and bamboo culm particles. Constr. Build. Mater. 2020, 262, 120501. [Google Scholar] [CrossRef]
- Aydin, I.; Demirkir, C.; Colak, S.; Colakoglu, G. Utilization of bark flours as additive in plywood manufacturing. Eur. J. Wood Wood Prod. 2017, 75, 63–69. [Google Scholar] [CrossRef]
- Rajeshkumar, G.; Seshadri, S.A.; Devnani, G.L.; Sanjay, M.R.; Siengchin, S.; Maran, J.P.; Al-Dhabi, N.A.; Karuppiah, P.; Mariadhas, V.A.; Sivarajasekar, N.; et al. Environment friendly, renewable and sustainable poly lactic acid (PLA) based natural fiber reinforced composites—A comprehensive review. J. Clean. Prod. 2021, 310, 127483. [Google Scholar] [CrossRef]
- Pędzik, M.; Janiszewska, D.; Rogoziński, T. Alternative lignocellulosic raw materials in particleboard production: A review. Ind. Crops Prod. 2021, 174, 114162. [Google Scholar] [CrossRef]
- Lee, S.H.; Lum, W.C.; Boon, J.G.; Kristak, L.; Antov, P.; Pędzik, M.; Rogoziński, T.; Taghiyari, H.R.; Lubis, M.A.R.; Fatriasari, W.; et al. Particleboard from agricultural biomass and recycled wood waste: A review. J. Mater. Res. Technol. 2022, 20, 4630–4658. [Google Scholar] [CrossRef]
- França, W.T.; Barros, M.V.; Salvador, R.; de Francisco, A.C.; Moreira, M.T.; Piekarski, C.M. Integrating life cycle assessment and life cycle cost: A review of environmental-economic studies. Int. J. Life Cycle Assess. 2021, 26, 244–274. [Google Scholar] [CrossRef]
- Hammiche, D.; Boukerrou, A.; Azzeddine, B.; Guermazi, N.; Budtova, T. Characterization of polylactic acid green composites and its biodegradation in a bacterial environment. Int. J. Polym. Anal. Charact. 2019, 24, 236–244. [Google Scholar] [CrossRef]
- Couret, L.; Irle, M.; Belloncle, C.; Cathala, B. Extraction and characterization of cellulose nanocrystals from post-consumer wood fiberboard waste. Cellulose 2017, 24, 2125–2137. [Google Scholar] [CrossRef]
- Haag, A.P.; Maier, R.M.; Combie, J.; Geesey, G.G. Bacterially derived biopolymers as wood adhesives. Int. J. Adhes. Adhes. 2004, 24, 495–502. [Google Scholar] [CrossRef]
- Soubam, T.; Gupta, A.; Sharma, S.; Shima Jamari, S. Mechanical property study of plywood bonded with dimethylol dihydroxy ethylene urea crosslinked rice starch-natural rubber latex-based adhesive. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Kristak, L.; Antov, P.; Bekhta, P.; Lubis, M.A.R.; Iswanto, A.H.; Reh, R.; Sedliacik, J.; Savov, V.; Taghiyari, H.R.; Papadopoulos, A.N.; et al. Recent progress in ultra-low formaldehyde emitting adhesive systems and formaldehyde scavengers in wood-based panels: A review. Wood Mater. Sci. Eng. 2022, 1–20. [Google Scholar] [CrossRef]
- Yu, C.W.F.; Crump, D.R. Testing for formaldehyde emission from wood-based products—A review. Indoor Built Environ. 1999, 8, 280–286. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Neykov, N. Sustainable bio-based adhesives for eco-friendly wood composites a review. Wood Res. 2020, 65, 51–62. [Google Scholar] [CrossRef]
- Islam, M.N.; Rahman, F.; Das, A.K.; Hiziroglu, S. An overview of different types and potential of bio-based adhesives used for wood products. Int. J. Adhes. Adhes. 2022, 112, 102992. [Google Scholar] [CrossRef]
- Halász, K.; Hosakun, Y.; Csóka, L. Reducing Water Vapor Permeability of Poly(lactic acid) Film and Bottle through Layer-by-Layer Deposition of Green-Processed Cellulose Nanocrystals and Chitosan. Int. J. Polym. Sci. 2015, 2015, 954290. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA-PHB-Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Shi, G.; Cai, Q.; Wang, C.; Lu, N.; Wang, S.; Bei, J. Fabrication and Biocompatibility of Cell Scaffolds of Poly(L-lactic acid) and Poly(L-lactic-co-glycolic acid). Polym. Adv. Technol. 2002, 13, 227–232. [Google Scholar] [CrossRef]
- Robles, E.; Urruzola, I.; Labidi, J.; Serrano, L. Surface-modified nano-cellulose as reinforcement in poly(lactic acid) to conform new composites. Ind. Crops Prod. 2015, 71, 44–53. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Auras, R.A.; Harte, B.; Selke, S.; Hernandez, R. Mechanical, Physical, and Barrier Properties of Poly(Lactide) Films. J. Plast. Film Sheeting 2003, 19, 123–135. [Google Scholar] [CrossRef]
- Kose, R.; Kondo, T. Size effects of cellulose nanofibers for enhancing the crystallization of poly(lactic acid). J. Appl. Polym. Sci. 2013, 128, 1200–1205. [Google Scholar] [CrossRef]
- Martino, V.P.; Jiménez, A.; Ruseckaite, R.A.; Avérous, L. Structure and properties of clay nano-biocomposites based on poly(lactic acid) plasticized with polyadipates. Polym. Adv. Technol. 2011, 22, 2206–2213. [Google Scholar] [CrossRef]
- Fortunati, F.; Armentano, I.; Iannoni, A.; Barbale, M.; Zaccheo, S.; Scavone, M.; Visai, L.; Kenny, J.M. New Multifunctional Poly(lactide acid) Composites:Mechanical, Antibacterial, and Degradation Properties. J. Appl. Polym. Sci. 2012, 124, 87–98. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.N.; Ford, C.V.; He, Z. Evaluation of polyblends of cottonseed protein and polycaprolactone plasticized by cottonseed oil. Int. J. Polym. Anal. Charact. 2019, 24, 389–398. [Google Scholar] [CrossRef]
- Mina Hernandez, J.H. Effect of the incorporation of polycaprolactone (Pcl) on the retrogradation of binary blends with cassava thermoplastic starch (tps). Polymers 2021, 13, 38. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Zuhri, M.Y.M.; Norrrahim, M.N.F.; Misenan, M.S.M.; Jenol, M.A.; Samsudin, S.A.; Nurazzi, N.M.; Asyraf, M.R.M.; Supian, A.B.M.; Bangar, S.P.; et al. Natural Fiber-Reinforced Polycaprolactone Green and Hybrid Biocomposites for Various Advanced Applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Chee, J.-Y.; Yoga, S.-S.; Lau, N.; Ling, S.; Abed, R.M.M.; Sudesh, K. Bacterially Produced Polyhydroxyalkanoate (PHA): Converting Renewable Resources into Bioplastics. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 1395–1404. [Google Scholar]
- Calvão, P.S.; Chenal, J.-M.; Gauthier, C.; Demarquette, N.R.; Bogner, A.; Cavaille, J.Y. Understanding the mechanical and biodegradation behaviour of poly(hydroxybutyrate)/rubber blends in relation to their morphology. Polym. Int. 2012, 61, 434–441. [Google Scholar] [CrossRef]
- Malinová, L.; Brožek, J. Mixtures poly((R)-3-hydroxybutyrate) and poly(l-lactic acid) subjected to DSC. J. Therm. Anal. Calorim. 2011, 103, 653–660. [Google Scholar] [CrossRef]
- Mendizábal, E.; Candia, J.M.; Jasso, C.F.; Cruz, L. Comparison of the time-temperature curing dependence of industrial and dry blend modified plastisols. J. Vinyl Addit. Technol. 1992, 14, 202–206. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Blending polylactic acid with polyhydroxybutyrate: The effect on thermal, mechanical, and biodegradation properties. Adv. Polym. Technol. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Tsuyoshi, F.; Harumi, S.; Rumi, M.; Jianming, Z.; Yong-Xin, D.; Isao, N.; Shukichi, O.; Yukihiro, O. Structure, Dispersibility, and Crystallinity of Poly(hydroxybutyrate)/Poly(l-lactic acid) Blends Studied by FT-IR Microspectroscopy and Differential Scanning Calorimetry. Macromolecules 2005, 38, 6445–6454. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Castro-López, M.D.M.; Rayón, E.; Barral-Losada, L.F.; López-Vilariño, J.M.; López, J.; González-Rodríguez, M.V. Plasticized Poly(lactic acid)–Poly(hydroxybutyrate) (PLA–PHB) Blends Incorporated with Catechin Intended for Active Food-Packaging Applications. J. Agric. Food Chem. 2014, 62, 10170–10180. [Google Scholar] [CrossRef]
- Vogel, C.; Siesler, H.W. Thermal Degradation of Poly(ɛ-caprolactone), Poly(L-lactic acid) and their Blends with Poly(3-hydroxy-butyrate) Studied by TGA/FT-IR Spectroscopy. Macromol. Symp. 2008, 265, 183–194. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA-PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; López, J.; Jiménez, A. Combined Effect of Poly(hydroxybutyrate) and Plasticizers on Polylactic acid Properties for Film Intended for Food Packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- EN 310 Wood-Based Panels; Determination of Modulus of Elasticity in Bending and of Bending Strength. European Committee for Standardization: Brussels, Belgium, 1993.
- EN 319 Particleboards and Fibreboards; Determination of Tensile Strength Perpendicular to the Plane of the Board. European Committee for Standardization: Brussels, Belgium, 1993.
- Gumowska, A.; Robles, E.; Kowaluk, G. Evaluation of functional features of lignocellulosic particle composites containing biopolymer binders. Materials 2021, 14, 7718. [Google Scholar] [CrossRef]
- Reis, K.C.; Pereira, L.; Melo, I.C.N.A.; Marconcini, J.M.; Trugilho, P.F.; Tonoli, G.H.D. Particles of coffee wastes as reinforcement in polyhydroxybutyrate (PHB) based composites. Mater. Res. 2015, 18, 546–552. [Google Scholar] [CrossRef]
- Dasan, Y.K.K.; Bhat, A.H.H.; Faiz, A.; Ahmad, F.; Faiz, A. Polymer blend of PLA/PHBV based bionanocomposites reinforced with nanocrystalline cellulose for potential application as packaging material. Carbohydr. Polym. 2017, 157, 1323–1332. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Nicolae, C.A.; Frone, A.N.; Chiulan, I.; Stanescu, P.O.; Draghici, C.; Iorga, M.; Mihailescu, M. Plasticized poly(3-hydroxybutyrate) with improved melt processing and balanced properties. J. Appl. Polym. Sci. 2017, 134, 44810. [Google Scholar] [CrossRef]
- Auriga, R.; Gumowska, A.; Szymanowski, K.; Wronka, A.; Robles, E.; Ocipka, P.; Kowaluk, G. Performance properties of plywood composites reinforced with carbon fibers. Compos. Struct. 2020, 248, 112533. [Google Scholar] [CrossRef]
- Jorda, J.; Cesprini, E.; Barbu, M.-C.; Tondi, G.; Zanetti, M.; Král, P. Quebracho Tannin Bio-Based Adhesives for Plywood. Polymers 2022, 14, 2257. [Google Scholar] [CrossRef]
- Martin, O.; Avérous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Soto-Valdez, H.; Auras, R.; Peralta, E. Fabrication of Poly(lactic acid) Films with Resveratroland the Diffusion of Resveratrol into Ethanol. J. Appl. Polym. Sci. 2011, 121, 970–978. [Google Scholar] [CrossRef]
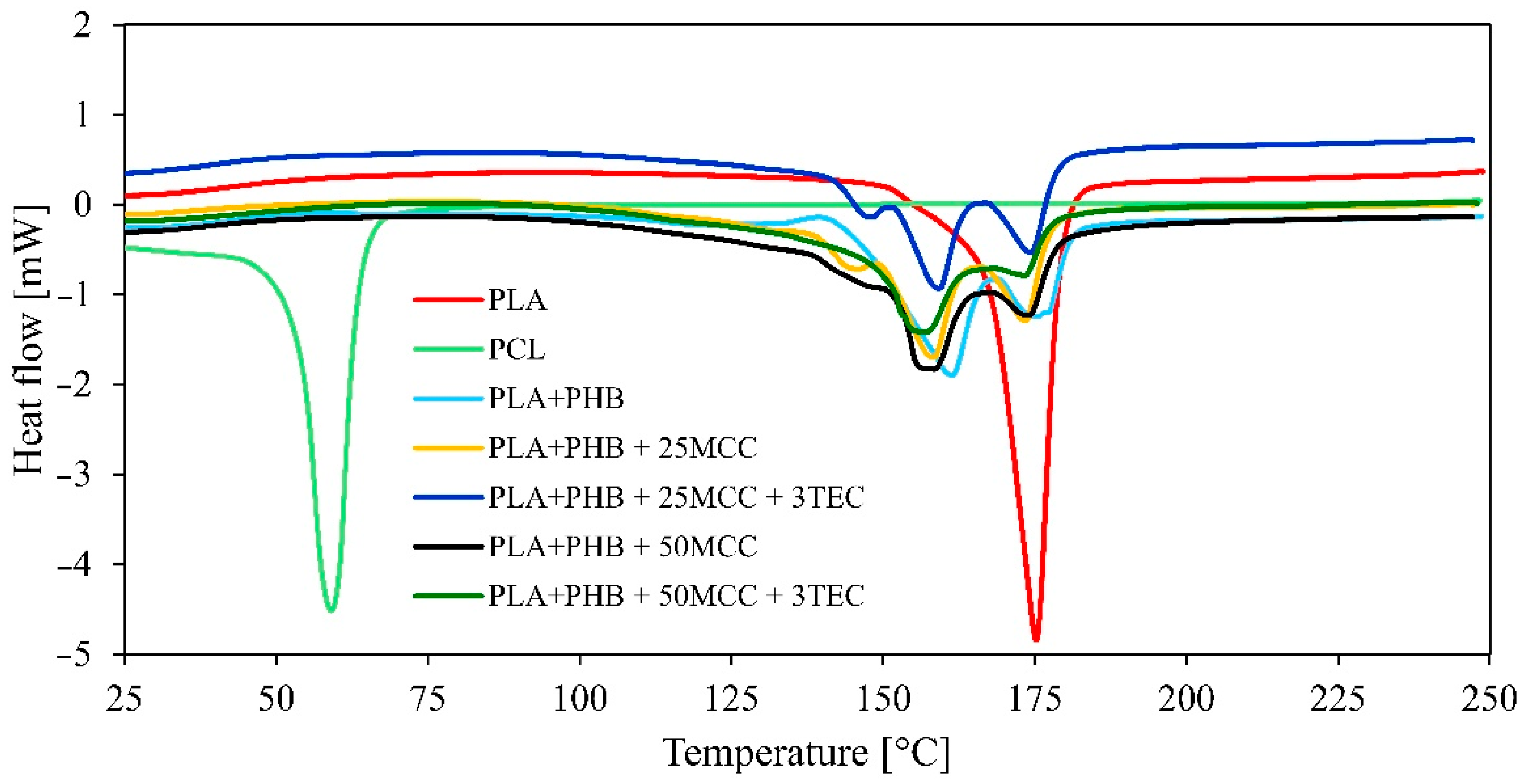
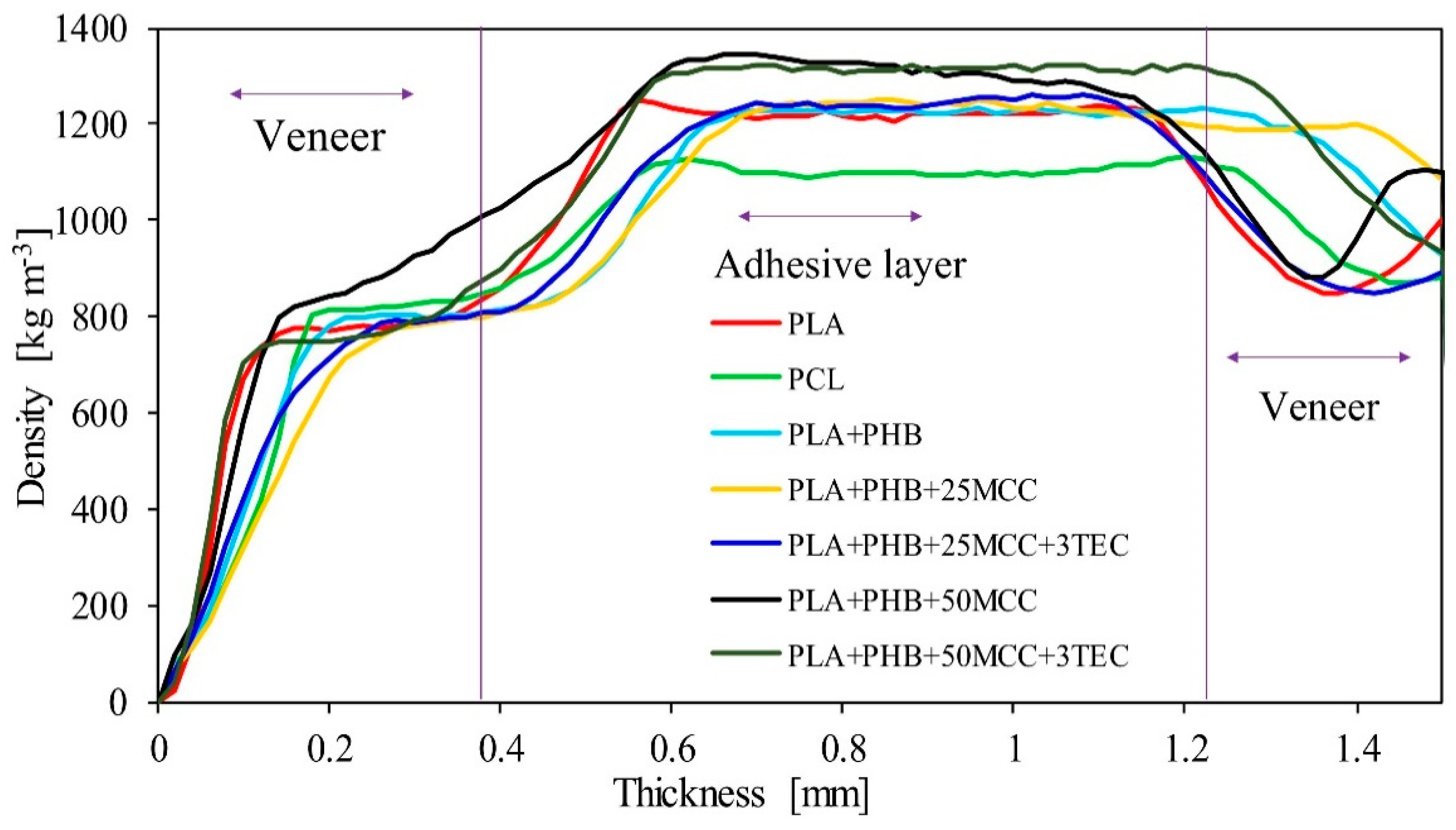
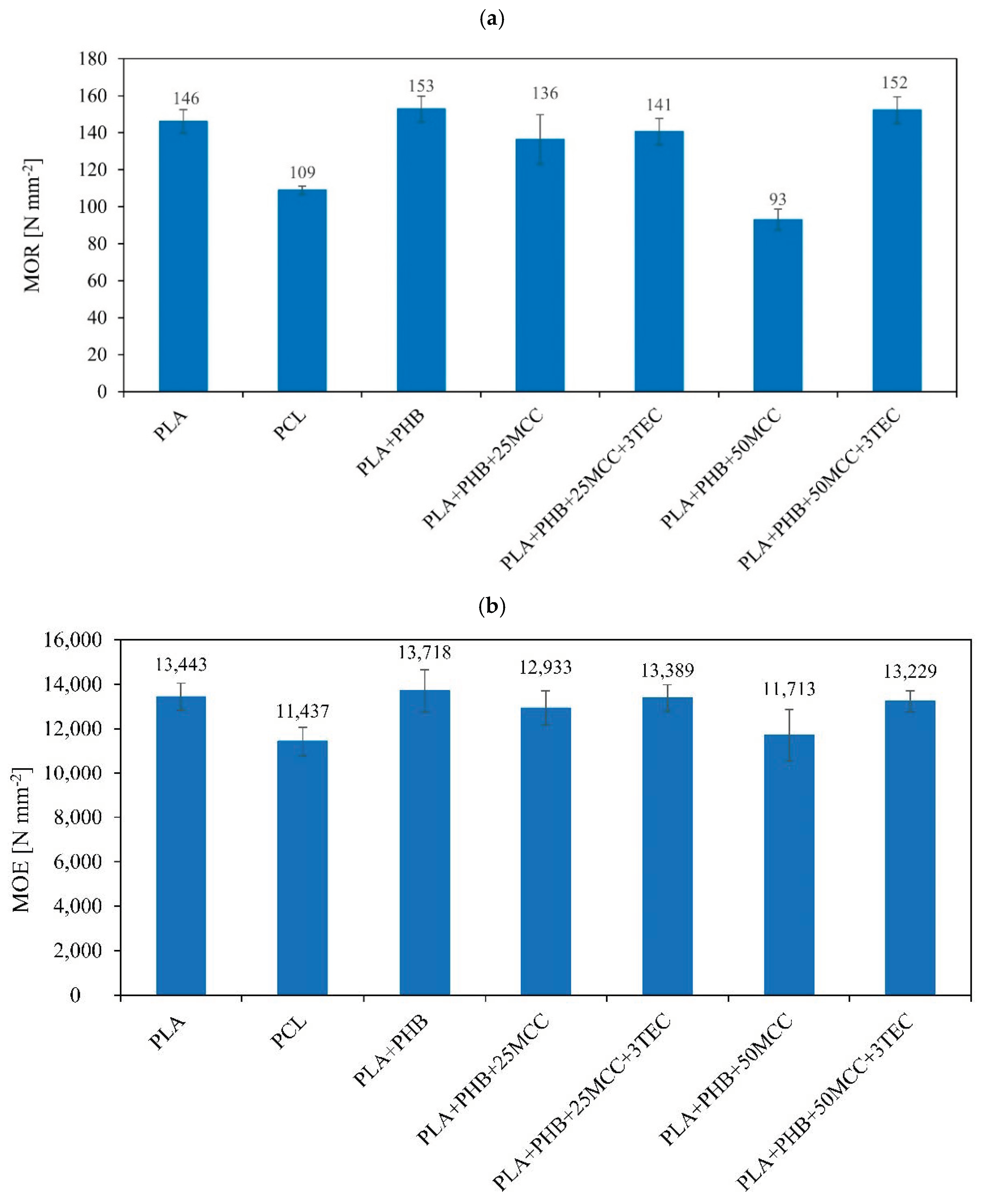
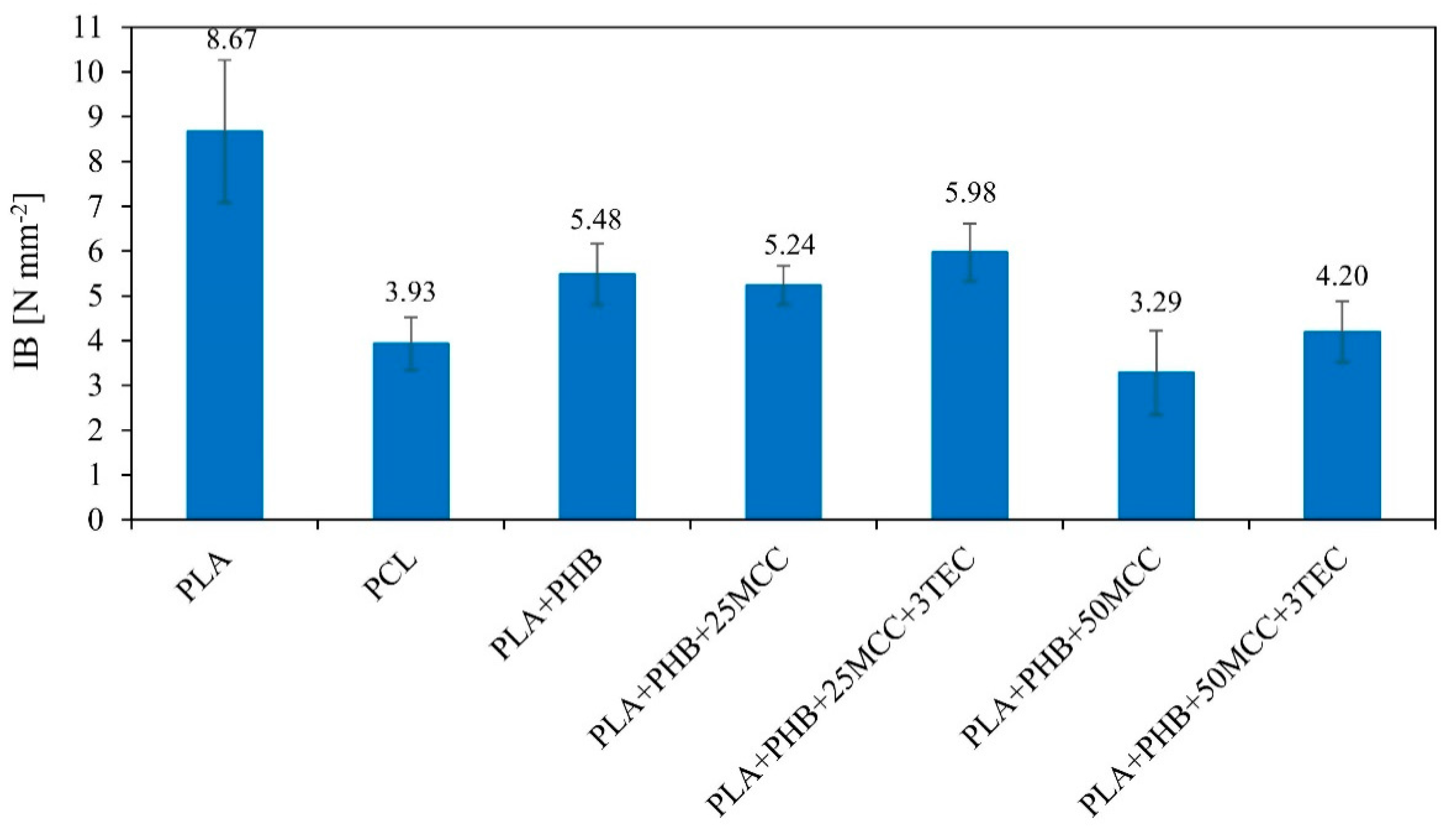

| Matrix | PLA [%] | PHB [%] | MCC [%] | TEC [%] |
|---|---|---|---|---|
| PLA + PHB (75:25) | 75.0000 | 25.0000 | 0.0000 | 0.0000 |
| PLA + PHB (75:25) | 54.5625 | 18.1875 | 24.2500 | 0.0000 |
| PLA + PHB (75:25) | 55.5000 | 18.5000 | 25.0000 | 3.0000 |
| PLA + PHB (75:25) | 37.5000 | 12.5000 | 50.0000 | 0.0000 |
| PLA + PHB (75:25) | 36.3750 | 12.1250 | 48.5000 | 3.0000 |
| Biopolymer Layers | Sample |
|---|---|
| Polylactide | PLA |
| Polycaprolactone | PCL |
| PLA + PHB | PLA + PHB |
| PLA + PHB + 25% microcrystalline cellulose (MCC) | PLA + PHB + 25MCC |
| PLA + PHB + 25% MCC + 3% triethyl citrate (TEC) | PLA + PHB + 25MCC + 3TEC |
| PLA + PHB + 50% MCC | PLA + PHB + 50MCC |
| PLA + PHB + 50% MCC + 3% TEC | PLA + PHB + 50MCC + 3TEC |
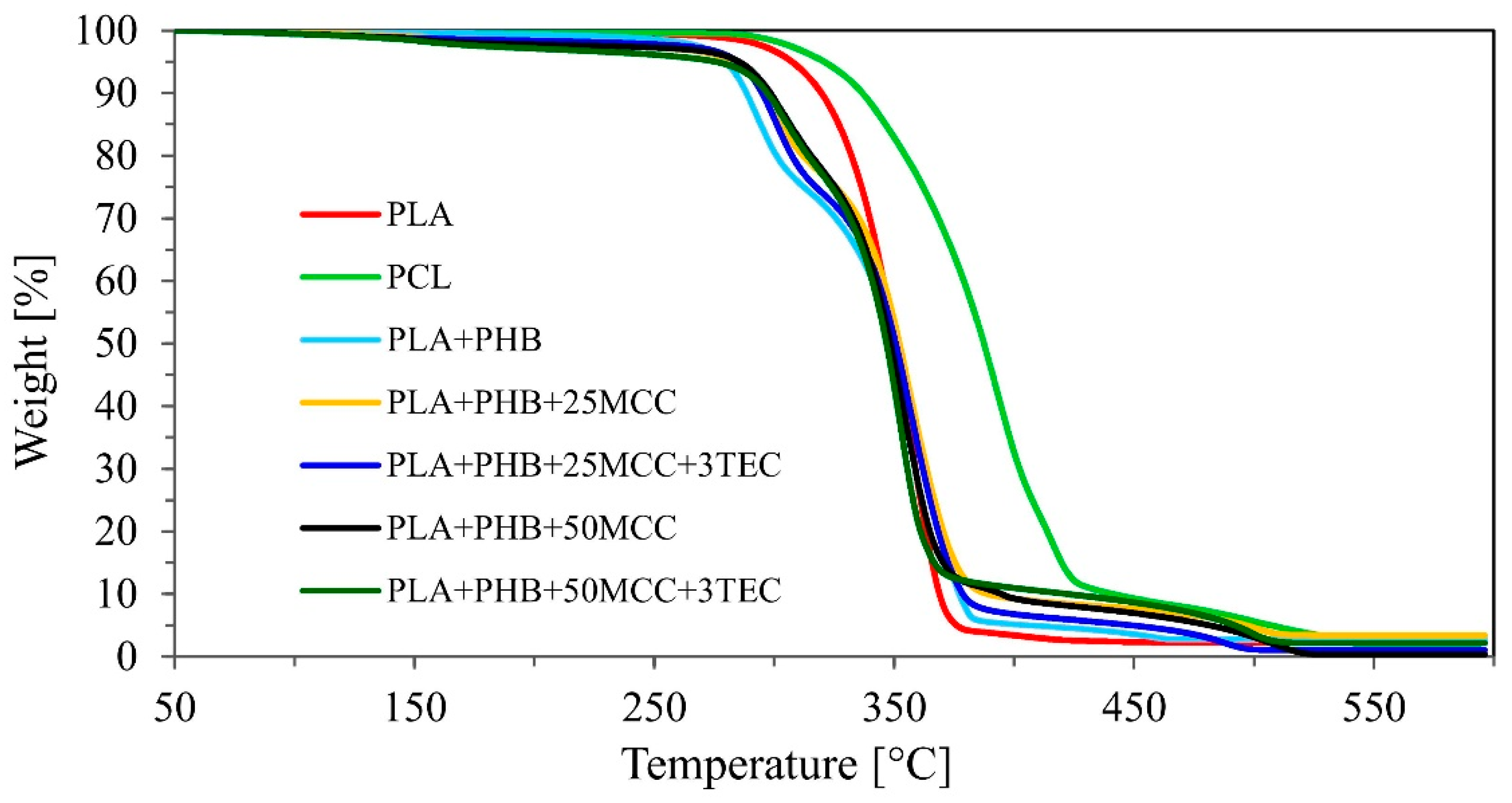
| Tested Materials | Mass Loss | |
|---|---|---|
| 50% | 80% | |
| °C | ||
| PLA | 349.3 | 363.3 |
| PCL | 387.4 | 413.8 |
| MB | 350.3 | 369.7 |
| MB + C1 | 352.2 | 370.5 |
| MB + C2 | 350.6 | 368.3 |
| MB + C3 | 348.5 | 364.9 |
| MB + C4 | 346.8 | 361.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumowska, A.; Robles, E.; Bikoro, A.; Wronka, A.; Kowaluk, G. Selected Properties of Bio-Based Layered Hybrid Composites with Biopolymer Blends for Structural Applications. Polymers 2022, 14, 4393. https://doi.org/10.3390/polym14204393
Gumowska A, Robles E, Bikoro A, Wronka A, Kowaluk G. Selected Properties of Bio-Based Layered Hybrid Composites with Biopolymer Blends for Structural Applications. Polymers. 2022; 14(20):4393. https://doi.org/10.3390/polym14204393
Chicago/Turabian StyleGumowska, Aneta, Eduardo Robles, Arsene Bikoro, Anita Wronka, and Grzegorz Kowaluk. 2022. "Selected Properties of Bio-Based Layered Hybrid Composites with Biopolymer Blends for Structural Applications" Polymers 14, no. 20: 4393. https://doi.org/10.3390/polym14204393
APA StyleGumowska, A., Robles, E., Bikoro, A., Wronka, A., & Kowaluk, G. (2022). Selected Properties of Bio-Based Layered Hybrid Composites with Biopolymer Blends for Structural Applications. Polymers, 14(20), 4393. https://doi.org/10.3390/polym14204393